Abstract
Introduction
VTE‐BLEED is a validated score for identification of patients at increased risk of major bleeding during extended anticoagulation for venous thromboembolism (VTE). It is unknown whether VTE‐BLEED high‐risk patients also have an increased risk for recurrent VTE, which would limit the potential usefulness of the score.
Methods
This was a post hoc analysis of the randomized, double‐blind, placebo‐controlled PADIS‐PE trial that randomized patients with a first unprovoked pulmonary embolism (PE) initially treated during 6 months to receive an additional 18‐month of warfarin vs. placebo. The primary outcome of this analysis was recurrent VTE during 2‐year follow‐up after anticoagulant discontinuation, that is, after the initial 6‐month treatment in the placebo arm and after 24 months of anticoagulation in the active treatment arm. This rate, adjusted for study treatment allocation, was compared between patients in the high‐ vs. low‐risk VTE‐BLEED group.
Results
In complete case analysis (n = 308; 82.4% of total population), 89 (28.9%) patients were classified as high risk; 44 VTE events occurred after anticoagulant discontinuation during 668 patient‐years. The cumulative incidence of recurrent VTE was 16.4% (95% confidence interval [CI], 10.0%‐26.1%; 14 events) and 14.6% (95% CI, 10.4%‐20.3%; 30 events) in the high‐risk and low‐risk VTE‐BLEED groups, respectively, for an adjusted hazard ratio of 1.16 (95% CI, 0.62‐2.19).
Conclusion
In this study, patients with unprovoked PE classified at high risk of major bleeding by VTE‐BLEED did not have a higher incidence of recurrent VTE after cessation of anticoagulant therapy, supporting the potential yield of the score for making management decisions on the optimal duration of anticoagulant therapy.
Keywords: anticoagulation therapy, bleeding, prediction score, recurrence, venous thromboembolism
Essentials.
VTE‐BLEED is a validated score for identification of patients at higher risk of major bleeding.
This was a post hoc analysis of the PADIS‐PE trial to assess the association of VTE‐BLEED and recurrent venous thromboembolism (VTE).
Patients classified at high risk by VTE‐BLEED did not have a higher incidence of recurrent VTE.
VTE‐BLEED may be useful for determination of the optimal duration of anticoagulant therapy.
1. INTRODUCTION
One of the most debated challenges of management of patients with venous thromboembolism (VTE) is the determination of the optimal duration of anticoagulation. As the risk of recurrent VTE is low after VTE associated with a major transient risk factor (eg, major surgery), current international guidelines recommend cessation of anticoagulant treatment after a minimum of 3 months.1, 2, 3, 4, 5 On the other hand, patients with active cancer‐associated VTE are candidates for extended anticoagulation in light of the substantial risk for recurrence. However, the benefit of long‐term anticoagulation is not fully established because of their concomitant high risk of bleeding.1, 2, 6, 7, 8
The risk of recurrent VTE after anticoagulant discontinuation in patients with unprovoked VTE is reported to be high as well, with a 20‐year rate of 30% to 50%.3, 9, 10 Therefore, indefinite anticoagulant therapy after unprovoked VTE events is usually recommended, with the important exception of patients at high risk of bleeding.1, 2 Although several risk prediction scores have been developed over the years for the identification of VTE patients at a high risk of bleeding, these tools present major limitations for use in routine practice, including the fact that they were derived from observational data of moderate quality, lack proper validation in adequately powered studies with adjudication of the events, and mostly focus on the acute phase of treatment with vitamin K antagonist (VKA) anticoagulation, rather than long‐term treatment with DOACs. Furthermore, many of these scores were originally derived using data of patients outside of VTE populations (eg, atrial fibrillation, cardiac surgery) and may thereby not be completely relevant in the context of the VTE patients.11 Most importantly, these score variables largely overlap with predictors of VTE recurrence demonstrating that these scores are not useful for decision making regarding optimal duration of anticoagulation in unprovoked VTE patients.
In a previous study, we derived VTE‐BLEED, a simple 6‐variable risk score designed to predict major bleeding in patients with VTE on stable, long‐term anticoagulation (Table 1).12 This score was found to identify VTE patients at a 3‐ to 5‐fold increased risk of bleeding during therapy with either VKA, direct thrombin inhibitors, or direct factor Xa inhibitors, both in a clinical trial setting and in a large practice‐based cohort.12, 13, 14, 15 The score demonstrated good predictive ability, with the C‐statistic reaching 0.78. That being said, it is currently unknown whether implementation of the VTE‐BLEED into clinical practice will present physicians with a confounding effect between risk of bleeding and risk of recurrence similar to that described above. In light of this question, we performed a post hoc analysis of the PADIS‐PE trial16 to assess and compare the incidence of recurrent VTE after discontinuation of anticoagulant treatment in patients in the high‐ vs. low‐risk VTE‐BLEED category in order to determine whether there was an association between risk classification according to the VTE‐BLEED and VTE recurrence in these patients.
Table 1.
The VTE‐BLEED score with original definition of variables12
| Factor | Score |
|---|---|
| Active cancera | 2 |
| Male with uncontrolled arterial hypertensionb | 1 |
| Anemiac | 1.5 |
| History of bleedingd | 1.5 |
| Age ≥60 y old | 1.5 |
| Renal dysfunctione | 1.5 |
| Classification of patients with the VTE‐BLEED score | |
| Low bleeding risk | Total score <2 |
| High bleeding risk | Total score ≥2 |
Cancer diagnosed within 6 mo before diagnosis of VTE (excluding basal‐cell or squamous‐cell carcinoma of the skin), recently recurrent or progressive cancer, or any cancer that required anticancer treatment within 6 mo before the VTE was diagnosed.
Males with uncontrolled arterial hypertension were defined by values of systolic blood pressure ≥140 mm Hg at baseline.
Hemoglobin <13 g/dL in men or <12 g/dL in women.
Including prior major or nonmajor clinically relevant bleeding event, rectal bleeding, frequent nose bleeding, or hematuria.
The estimated glomerular filtration rate (eGRF) <60 mL/min defined the presence of renal dysfunction: eGRF was calculated at baseline with the Cockcroft‐Gault formula, which includes serum creatinine, age, and body weight.
2. METHODS
2.1. Study setting and patients
The design of the PADIS‐PE study and patients’ selection criteria are detailed in the original publication.16 In short, PADIS‐PE was a randomized, double‐blind, placebo‐controlled trial in patients with a first unprovoked pulmonary embolism (PE). The trial aimed at determining the benefits and harms of 6 vs. 24 months of anticoagulation with VKA. Patients were randomized after an initial 6‐month course of anticoagulants to either continuation of anticoagulant treatment or placebo. The primary outcome was the composite of recurrent VTE or major bleeding at 18 months after randomization. One of the secondary outcomes was the incidence of the primary outcome after a follow‐up period of 42 months. Symptomatic recurrent VTE was diagnosed upon clinical suspicion and objective confirmation by ultrasonography, ventilation‐perfusion lung scanning, spiral computed tomographic angiography, pulmonary angiography, or autopsy, and in the event of a sudden death for which no other cause could be identified.17 Definition of major bleeding was based on the ISTH recommendations.18 All outcomes were adjudicated by an independent central adjudication committee.16
For the current analysis, we considered all patients with complete baseline data (complete case analysis) in whom VTE‐BLEED could be calculated.
2.2. VTE‐BLEED
The 6‐variable VTE‐BLEED score (Table 1) was calculated from the baseline variables. Renal insufficiency was defined as an estimated creatinine clearance (Cockcroft‐Gault) <60 mL/min, and uncontrolled arterial hypertension as a systolic blood pressure ≥140 mm Hg. A score of ≥2 points served to identify those patients at a predicted high risk of bleeding.12
2.3. Study aim
The aim of the current analysis was to assess and compare the incidence of recurrent VTE after discontinuation of anticoagulant treatment in patients stratified according to the VTE‐BLEED score (high risk [≥2 points] vs. low risk [<2 points]). For each included patient, incidence of recurrence was measured during the 24 months following the individual's personal date of treatment discontinuation. For the patients in the placebo group of the PADIS‐PE study, all events occurred during the 24 months following randomization (ie, the initial 18‐month study period plus the 6‐month follow‐up period after discontinuation of the study drug) were considered. For the patients randomized to active treatment, only the events that occurred during the 24 months after study treatment discontinuation were included. Therefore, in patients having discontinued active treatment prematurely for any reason (eg, patient's decision, bleeding), the 24‐month period was counted starting from the date of premature treatment discontinuation.
2.4. Statistical analysis
For the presentation of the baseline characteristics, continuous variables are described with means and standard deviation, and categorical variables are presented as proportions (n/N) and percentage (%). The absolute number and cumulative incidence of the primary and secondary outcomes are presented with corresponding 95% confidence intervals (CIs). A Cox regression model using VTE‐BLEED and treatment group as covariates was applied to compare the incidence of recurrent VTE in the 2 VTE‐BLEED risk categories, adjusted for study treatment allocation. Statistical analyses were performed using SAS V9.4 software (SAS Institute, Cary, NC).
3. RESULTS
3.1. Patients
From all 374 patients included in the original study, 3 withdrew informed consent, and data for calculation of the VTE‐BLEED score were missing in 63 (16%; see characteristics in Table S1): data on the presence of anemia in 44 patients, on hypertension in 8 patients, on renal dysfunction in 2, and on anemia plus renal dysfunction in 9 patients. Those patients were excluded. The mean age of the 308 (82.4%) patients left for analysis was 58.7 years (±18.0), 164 (53.2%) patients were women, 21 (6.8%) patients had an estimated creatinine clearance (Cockcroft) <50 mL/min and 13 (4.2%) patients had a history of cancer (Table 2).
Table 2.
Characteristics of analyzed patients
| All (n = 308) | |
|---|---|
| Age, mean (SD), y | 58.7 (18.0) |
| Women, n (%) | 164 (53.2) |
| Body mass index, mean (SD) | 27.3 (5.6) |
| ≥30, n (%) | 71 (23.1) |
| Estimated creatinine clearance (Cockroft‐Gault) category, n (%) | |
| <30 mL/min | 0 |
| ≥30‐<50 mL/min | 21 (6.8) |
| ≥50 mL/min | 287 (93.2) |
| Comorbidities, n (%) | |
| Previous cancer | 13 (4.2) |
| Previous distal deep vein thrombosis or superficial vein thrombosis | 25 (8.1) |
| Chronic heart failure | 11 (3.6) |
| Chronic respiratory failure | 70 (22.7) |
| Thrombophilia, n (%) | |
| Minor | 44 (14.7) |
| Major | 62 (20.7) |
| Treatment of pulmonary embolism prior to randomization | |
| Warfarin, n (%) | 220 (71.4) |
| Fluindione, n (%) | 90 (29.2) |
| Acenocoumarol, n (%) | 5 (1.6) |
| Duration of initial anticoagulation, mean (SD), mo | 6.3 (0.5) |
| Percentage of time in therapeutic INR range, mean (SD) | 68.0 (23.0) |
| Use of compression stockings, n (%) | 189 (61.4) |
| Main concomitant treatments, n (%) | |
| Antiplatelet agent | 24 (7.8) |
| Statins | 56 (18.2) |
Previous cancer was defined as cancer resolved more than 2 y before patient inclusion; thrombophilia was defined as minor if patients had heterozygous factor V Leiden or heterozygous G20210A prothrombin gene variant or elevated factor VIII (90th percentile); thrombophilia was defined as major if patients had antithrombin or protein C or protein S deficiency or anticardiolipin antibodies (99th percentile) or lupus anticoagulant or homozygous factor V Leiden or combined thrombophilia.
INR, international normalized ratio; SD, standard deviation.
3.2. VTE‐BLEED
Of the 308 patients included in the current analysis, 89 (28.9%) were classified as VTE‐BLEED high risk and 219 (71.1%) as VTE‐BLEED low risk. Most prevalent VTE‐BLEED variable was age ≥60 years (163, 52.9%); 59 patients had uncontrolled hypertension at randomization (19.2%), 44 anemia (14.3%), 38 renal dysfunction (12.3%) and 14 history of bleeding (4.5%; Table 3). Being an exclusion criterion of the original PADIS‐PE trial, none of the patients had active cancer.
Table 3.
Prevalence of the VTE‐BLEED score items among the study population
|
VTE‐BLEED Low risk (n = 219) |
VTE‐BLEED High risk (n = 89) |
All (n = 308) |
|
|---|---|---|---|
| Male with uncontrolled arterial hypertension, n (%) | 21 (9.6) | 38 (42.7) | 59 (19.2) |
| Anemia, n (%) | 12 (5.5) | 32 (36.0) | 44 (14.3) |
| History of bleeding, n (%) | 5 (2.3) | 9 (10.1) | 14 (4.5) |
| Age ≥60 y, n (%) | 75 (34.2) | 88 (98.9) | 163 (52.9) |
| Renal dysfunction (Cockcroft), n (%) | 0 | 38 (42.7) | 38 (12.3) |
| Active cancer | 0 | 0 | 0 |
3.3. Recurrent VTE
A total of 44 adjudicated VTE events occurred after anticoagulant discontinuation during 668 patient‐years, that is, from the date of randomization in the placebo group and from the date of study treatment discontinuation in the active treatment group. Median individual follow‐up time was 753 days. Of 44 patients with recurrent VTE events, 7 had symptomatic DVT, and 37 had symptomatic PE (28 without associated DVT and 9 in association with DVT). Recurrent PE was fatal in 4 (1%; 95% CI, 0.52‐3.7) patients: 1 fatal PE event occurred in the VTE‐BLEED low‐risk group and 3 in the VTE‐BLEED high‐risk group. The cumulative incidence of recurrent VTE was 16% (95% CI, 10.0%‐26.1%; 14 events) in the high‐risk VTE‐BLEED group and 15% (95% CI, 10.4%‐20.3%; 30 events) in the low‐risk VTE‐BLEED group (adjusted hazard ratio [HR], 1.16; 95% CI, 0.62‐2.19; Figure 1). All episodes were unexplained sudden deaths considered to be fatal PE by the adjudication committee.
Figure 1.
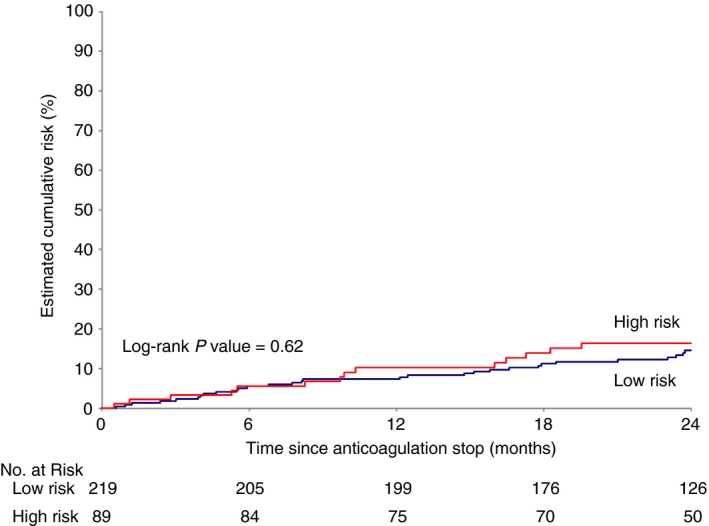
Kaplan Meier curve showing estimated cumulative incidence of recurrent deep vein thrombosis and/or pulmonary embolism in the first 2 years after treatment cessation according to the VTE‐BLEED score (high: red curve; low: blue curve). Number of patients left in analysis are indicated in the table
4. DISCUSSION
Our main finding is that, after anticoagulant discontinuation, patients with unprovoked VTE in the VTE‐BLEED high‐risk category (29% of all patients) did not have a higher risk of developing recurrent VTE than patients in the low‐risk VTE‐BLEED category (adjusted HR, 1.16; 95% CI, 0.62‐2.19). This observation supports the use of the VTE‐BLEED score in future management studies of patients with unprovoked VTE for whom the duration of anticoagulant treatment is driven by risk stratification according to the individual bleeding risk.
To ensure usefulness in clinical practice, prediction scores aiming at determining the optimal duration of anticoagulant therapy for VTE should demonstrate consistency and straightforwardness in their application. In the case of the present clinical question, this may be complicated by the time‐varying nature of numerous bleeding risk predictors included in scores such as the HAS‐BLED and the American College of Chest Physicians score.2, 19, 20, 21, 22, 23 In contrast, the VTE‐BLEED includes only 1 potentially inconstant variable, namely, “male with uncontrolled arterial hypertension,” while all other variables are clearly defined and largely objective and tend to be fairly constant over time in most patients. This suggests more consistent and thereby likely reliable estimates of bleeding risks and broader functionality of the VTE‐BLEED than other available scores. Furthermore, in contrast with other currently available bleeding risk scores, the VTE‐BLEED was evaluated and validated both in nonselected VTE patients and in patients with unprovoked VTE, as well as for all currently available classes of oral anticoagulants.12, 13, 14, 19, 20, 21, 22, 23, 24, 25, 26, 27 Additionally, the binary categorization used in the VTE‐BLEED limits the commonly encountered ambiguity surrounding clinical management of patients classified at “intermediate risk” according to other bleeding prediction scores. Results of the present analysis strengthen the currently available arguments in favor of the use of the VTE‐BLEED in clinical practice by demonstrating the absence of the previously discussed common and problematic confounding effect of predictions of anticoagulant‐related bleeding and VTE recurrence.
It remains challenging for clinicians to determine whether the risk of bleeding in patients on long‐term anticoagulant therapy exceeds risk of recurrence after prompt discontinuation of treatment. One meta‐analysis of recent studies (>4500 patients with unprovoked VTE who discontinued treatment) reported a pooled rate of fatal recurrent VTE at 0.17 (95% CI, 0.047‐0.33) per 100 patient‐years with an associated case fatality rate of 2.6% (95% CI, 0.86‐5.0), while another found a pooled rate of fatal VKA‐associated bleeding at 1.31 (95% CI, 1.30‐1.32) per 100 patient‐years with a case fatality of 13.4% (95% CI, 9.4‐17.4).10, 28 While observation of a higher case‐fatality rate of major bleeding than that of recurrent VTE has been proven for VKA treatment, reliable practice‐based numbers are unavailable for long‐term direct oral anticoagulant (DOAC) treatment, although a lower rate may be anticipated than for VKA.29 Moreover, because recurrent VTE occurs mostly in the first years after anticoagulant cessation and diminishes over time, while in contrast the risk of bleeding during stable anticoagulation increases with advancing age, only an adequately powered randomized controlled trial with long‐term follow‐up can answer the question of optimal duration of anticoagulation for unprovoked VTE.
Strengths of this analysis include the randomized design of the study as well as the blinded adjudication of primary and secondary end points. The main limitation of our study rests in its post hoc nature, resulting in a relatively high proportion of patients with missing data for the calculation of the VTE‐BLEED score, potentially introducing selection bias and resulting in a relatively small sample size that did not allow us to evaluate bleeding events and/or a net clinical benefit outcome. However, the VTE‐BLEED has previously been validated in 3 independent high‐quality cohorts totaling over 17 000 patients12, 13, 15 in which results were deemed to be sufficiently conclusive and validation of the score was therefore not part of the aims of the present analysis. Further, our results cannot be generalized to patients with provoked PE or those with DVT or treated with DOACs, although we do not anticipate relevant differences in the 2 latter patient categories.
In conclusion, the current analysis shows that, after stopping anticoagulant therapy, patients with a first unprovoked PE and a high risk of major bleeding by VTE‐BLEED did not have a higher incidence of recurrent VTE than did patients in the VTE‐BLEED low‐risk category. These results support the appropriateness of the score for making management decisions on the optimal duration of anticoagulant therapy. Whereas long‐term anticoagulant treatment has been suggested to be safe and appropriate in patients with unprovoked VTE in the VTE‐BLEED low‐risk category, further outcome studies should explore the optimal duration of anticoagulant therapy of VTE‐BLEED high‐risk patients.
RELATIONSHIP DISCLOSURE
FK reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD, Actelion, the Dutch Heart Foundation, and the Dutch Thrombosis Association. SB has received congress and travel payments from Daiichi‐Sankyo and financial support for the printing costs of his PhD thesis from Pfizer BV, CSL Behring bv, Sanquin Plasma Products, Boehringer Ingelheim bv, Aspen Netherlands, and Bayer bv. SK reports having received consultancy and lecture honoraria from Bayer HealthCare, Boehringer Ingelheim, Daiichi‐Sankyo, and Pfizer–Bristol‐Myers Squibb; payment for travel accommodation/ meeting expenses from Bayer HealthCare; and institutional grants from Boehringer Ingelheim, Bayer HealthCare, and Daiichi Sankyo. OS reports having received research grant support from Bayer, Daiichi Sankyo, and Portola Pharmaceuticals, and fees or nonfinancial support for consultancy activities from Actelion, GlaxoSmithKline, Boehringer Ingelheim, and Chiesi. PG reports having received personal fees and nonfinancial support from Bayer and Leo Pharma. PM reports having received research grants from Bayer; fees for board memberships from Bayer, Bristol‐Myers Squibb/Pfizer, and Daiichi Sankyo; for lectures from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, Daiichi Sankyo, and Sanofi; and for development of educational presentations from Bayer and Bristol‐Myers Squibb/Pfizer. SL reports having received research grant support from Bayer and Sanofi, and fees for board memberships or consultancy from Bayer, Boehringer Ingelheim, Leo Pharma, and Sanofi. GM reports having received research grant support from Bayer, Boehringer Ingelheim, Leo Pharma, and Sanofi; having been an uncompensated board member and a consultant for Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Leo Pharma, and Pfizer; and having received travel support from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Leo Pharma, and Sanofi. CL reports having received research grant support from Pfizer; fees for board memberships or symposia from Bayer and Astra Zeneca; and travel support from Bayer, Daiichi Sankyo, Leo Pharma, Intermune, and Actelion. FC reports having received research grant support from Pfizer; fees for board memberships or symposia from Bayer, Bristol‐Myers Squibb/Pfizer, and Astra Zeneca; and travel support from Bayer, Bristol‐Myers Squibb/Pfizer, Daiichi Sankyo, Boehringer Ingelheim, Leo Pharma, Intermune, and Actelion. EP, CT, GP, LR, PR, PYL, CH, SM, and LB report nothing to disclose.
AUTHOR CONTRIBUTIONS
All authors have contributed significantly to this manuscript and take responsibility for the analyses.
Supporting information
ACKNOWLEDGMENTS
The work of Frederikus Klok, Stefano Barco, and Stavros Konstantinides was supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503). All authors are responsible for the contents of this publication. The study was supported by grants from the “Programme Hospitalier de Recherche Clinique” (French Department of Health), and the sponsor was the University Hospital of Brest. The funding source was not involved in designing or conducting the study; collecting, managing, analyzing, or interpreting the data; preparing, reviewing or approving the manuscript; or deciding to submit the manuscript for publication.
APPENDIX 1.
PADIS‐PE Study Group
Members of the PADIS‐PE Study Group (all in France) were as follows: Steering Committee – F. Couturaud (Chair), P. Mismetti, C. Leroyer, G. Meyer, O. Sanchez, P. Jego, G. Pernod, E. Duhamel, K. Provost, F. Parent, L. Bertoletti, C. Tromeur, D. Mottier; Coordinating Committee – F. Couturaud (Chair), M. Guégan, S. Mélac, A. Le Hir; Independent Central Adjudication Committee (Critical Events) – P. Girard (Chair), S. Lenoir, C. Lamer; Data Safety Monitoring Board – J.F. Bergmann (Chair), D. Wahl, L. Drouet; Statistical Analysis: E. Presles, S. Laporte; Data Management (ClinInfo, Lyon) – P. Chevarier, N. Monte; Operation team (Brest University Hospital) – F. Morvan, V. Kouassi, N. Ibrir, G. El Asri; Lung Scintigraphy Panel – P.Y. Salaun, P. Robin, P.Y. Le Roux; Ultrasound Panel – L. Bressollette, P. Quéhé, S. Gestin; Computerised Tomography Scan Panel – M. Nonent, J. Bahuon, L. Deloire, C. Tromeur, B. Planquette; Echocardiography Panel – Y. Jobic, Y. Etienne, R. Didier, F. Leven; Central Laboratory – L. Leroux, H. Galinat, C. Le Maréchal, L. Gourhant, F. Mingant; Investigators (by city and in order of the number of patients enrolled) – Brest (198 patients): F. Couturaud, C. Leroyer, C. Tromeur, F. Leven, K. Lacut, E. Lemoigne, L. De Saint Martin, A. Delluc, G. Le Gal, N. Paleiron, R. Le Mao, D. Mottier; Paris (53 patients): O. Sanchez, G. Meyer, B. Planquette; Grenoble (33 patients) G. Pernod, C. Pison; Rennes (33 patients): P. Jego, P. Guéret; Saint‐Etienne (21 patients): P. Mismetti, H. Décousus, C. Lassagne, L. Bertoletti; Saint‐Brieux (9 patients): E. Duhamel; Lannion (8 patients): K. Provost; Le Kremlin‐Bicêtre (5 patients): F. Parent; Quimper (3 patients): B. Pan‐Petesh; Toulouse (2 patients): A. Bura‐Riviere; Tours (2 patients): B. Delahousse, Y. Gruel; Paris (2 patients): C. Lorut; Clermont‐Ferrand (1 patient): J. Schmidt; Nantes (1 patient): J. Connault.
Klok FA, Presles E, Tromeur C, et al.; for the PADIS‐PE Investigators . Evaluation of the predictive value of the bleeding prediction score VTE‐BLEED for recurrent venous thromboembolism. Res Pract Thromb Haemost. 2019;3:364–371. 10.1002/rth2.12214
Contributor Information
Frederikus A. Klok, Email: f.a.klok@LUMC.nl, @__Klok.
for the PADIS‐PE Investigators:
P. Jego, E. Duhamel, K. Provost, F. Parent, D. Mottier, M. Guégan, A. Le Hir, S. Lenoir, C. Lamer, J.F. Bergmann, D. Wahl, L. Drouet, P. Chevarier, N. Monte, F. Morvan, V. Kouassi, N. Ibrir, G. El Asri, P.Y. Salaun, L. Bressollette, P. Quéhé, S. Gestin, M. Nonent, J. Bahuon, L. Deloire, B. Planquette, Y. Jobic, Y. Etienne, R. Didier, F. Leven, L. Leroux, H. Galinat, C. Le Maréchal, L. Gourhant, F. Mingant, K. Lacut, E. Lemoigne, L. De Saint Martin, A. Delluc, G. Le Gal, N. Paleiron, R. Le Mao, C. Pison, P. Guéret, H. Décousus, C. Lassagne, B. Pan‐Petesh, A. Bura‐Riviere, B. Delahousse, Y. Gruel, C. Lorut, J. Schmidt, and J. Connault
REFERENCES
- 1. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al.; The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) . 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 3. Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. [DOI] [PubMed] [Google Scholar]
- 4. White RH, Murin S, Wun T, Danielsen B. Recurrent venous thromboembolism after surgery‐provoked versus unprovoked thromboembolism. J Thromb Haemost. 2010;8:987–97. [DOI] [PubMed] [Google Scholar]
- 5. Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- 6. van der Hulle T, den Exter PL, van den Hoven P, van der Hoeven JJ, van der Meer FJ, Eikenboom J, et al. Cohort study on the management of cancer‐associated venous thromboembolism aimed at the safety of stopping anticoagulant therapy in patients cured of cancer. Chest. 2016;149:1245–51. [DOI] [PubMed] [Google Scholar]
- 7. Jara‐Palomares L, Solier‐Lopez A, Elias‐Hernandez T, Asensio‐Cruz MI, Blasco‐Esquivias I, Sanchez‐Lopez V, et al. D‐dimer and high‐sensitivity C‐reactive protein levels to predict venous thromboembolism recurrence after discontinuation of anticoagulation for cancer‐associated thrombosis. Br J Cancer. 2018;119:915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee AYY. When can we stop anticoagulation in patients with cancer‐associated thrombosis? Hematology Am Soc Hematol Educ Program. 2017;2017(1):128-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:364–7. [DOI] [PubMed] [Google Scholar]
- 10. van der Wall SJ, van der Pol LM, Ende‐Verhaar YM, Cannegieter SC, Schulman S, Prandoni P, et al. Fatal recurrent VTE after anticoagulant treatment for unprovoked VTE: a systematic review. Eur Respir Rev. 2018;27(150) pii:180094 10.1183/16000617.0094-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klok FA, Kooiman J, Huisman MV, Konstantinides S, Lankeit M. Predicting anticoagulant‐related bleeding in patients with venous thromboembolism: a clinically oriented review. Eur Respir J. 2015;45:201–10. [DOI] [PubMed] [Google Scholar]
- 12. Klok FA, Hosel V, Clemens A, Yollo WD, Tilke C, Schulman S, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48:1369–76. [DOI] [PubMed] [Google Scholar]
- 13. Klok FA, Barco S, Konstantinides SV. External validation of the VTE‐BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb Haemost. 2017;117:1164–70. [DOI] [PubMed] [Google Scholar]
- 14. Klok FA, Barco S, Konstantinides SV. Evaluation of VTE‐BLEED for predicting intracranial or fatal bleeding in stable anticoagulated patients with venous thromboembolism. Eur Respir J. 2018;51 pii:1800077 10.1183/13993003.00077-2018. [DOI] [PubMed] [Google Scholar]
- 15. Klok FA, Barco S, Turpie AGG, Haas S, Kreutz R, Mantovani LG, et al. Predictive value of venous thromboembolism (VTE)‐BLEED to predict major bleeding and other adverse events in a practice‐based cohort of patients with VTE: results of the XALIA study. Br J Haematol. 2018;183:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Couturaud F, Sanchez O, Pernod G, Mismetti P, Jego P, Duhamel E, et al.; PADIS‐PE Investigators . Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: the PADIS‐PE randomized clinical trial. JAMA. 2015;314:31–40. [DOI] [PubMed] [Google Scholar]
- 17. Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013;11:412–22. [DOI] [PubMed] [Google Scholar]
- 18. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 19. Kooiman J, van Hagen N, Iglesias Del Sol A, Planken EV, Lip GY, van der Meer FJ, et al. The HAS‐BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PLoS One. 2015;10:e0122520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klok FA, Niemann C, Dellas C, Hasenfuss G, Konstantinides S, Lankeit M. Performance of five different bleeding‐prediction scores in patients with acute pulmonary embolism. J Thromb Thrombolysis. 2016;41:312–20. [DOI] [PubMed] [Google Scholar]
- 21. Riva N, Bellesini M, Di Minno MN, Mumoli N, Pomero F, Franchini M, et al. Poor predictive value of contemporary bleeding risk scores during long‐term treatment of venous thromboembolism. A multicentre retrospective cohort study. Thromb Haemost. 2014;112:511–21. [DOI] [PubMed] [Google Scholar]
- 22. van Es N, Wells PS, Carrier M. Bleeding risk in patients with unprovoked venous thromboembolism: a critical appraisal of clinical prediction scores. Thromb Res. 2017;152:52–60. [DOI] [PubMed] [Google Scholar]
- 23. Brown JD, Goodin AJ, Lip GYH, Adams VR. Risk stratification for bleeding complications in patients with venous thromboembolism: application of the HAS‐BLED bleeding score during the first 6 months of anticoagulant treatment. J Am Heart Assoc. 2018;7 pii:e007901 10.1161/jaha.117.007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Nisio M, Ageno W, Rutjes AW, Pap AF, Buller HR. Risk of major bleeding in patients with venous thromboembolism treated with rivaroxaban or with heparin and vitamin K antagonists. Thromb Haemost. 2016;115:424–32. [DOI] [PubMed] [Google Scholar]
- 25. Di Nisio M, Raskob G, Buller HR, Grosso MA, Zhang G, Winters SM, et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost. 2017;117:784–93. [DOI] [PubMed] [Google Scholar]
- 26. Palareti G, Antonucci E, Ageno W, Mastroiacovo D, Poli D, Tosetto A. The American College of Chest Physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism: reply. J Thromb Haemost. 2018;16:2539–40. [DOI] [PubMed] [Google Scholar]
- 27. Palareti G, Antonucci E, Mastroiacovo D, Ageno W, Pengo V, Poli D, et al. The American College of Chest Physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism. J Thromb Haemost. 2018;16:1994–2002. [DOI] [PubMed] [Google Scholar]
- 28. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta‐analysis. Ann Intern Med. 2003;139:893–900. [DOI] [PubMed] [Google Scholar]
- 29. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:320–371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


