Abstract
Background
Low‐molecular‐weight heparin has been the preferred treatment of cancer‐associated thrombosis (CAT); however, emerging data support the use of direct oral anticoagulants (DOACs).
Objectives
The Memorial Sloan Kettering Cancer Center Clinical Pathway has served as the institutional guideline for the use of rivaroxaban to treat CAT since 2014. Key elements are to recommend against use of a DOAC in patients with active gastrointestinal (GI) or genitourinary tract lesions, and a prespecified dose reduction in the elderly (75+ years old). We present our institutional experience for treatment of CAT.
Methods
From January 2014 through September 2016, 1072 patients began rivaroxaban treatment for CAT; 91.9% had a solid tumor, 8.1% had hematologic malignancies, and 75% of patients with solid tumors had metastatic disease. All patients with CAT treated with rivaroxaban were included in this analysis, regardless of adherence to the Clinical Pathway.
Results
The 6‐month cumulative incidence of recurrent venous thromboembolism and major bleeding were 4.2% (95% confidence interval [CI], 2.7%‐5.7%) and 2.2% (95% CI, 1.1%‐3.2%), respectively. The incidence of clinically relevant non–major bleeding leading to discontinuation of rivaroxaban for at least 7 days was 5.5% (95% CI, 3.7%‐7.1%), and 73.3% of major bleeds occurred in the GI tract. The 6‐month cumulative mortality rate was 22.2% (95% CI, 19.4%‐24.9%). The elderly had similar rates of recurrent thrombosis and bleeding as those aged under 75 years.
Conclusion
Our institutional experience suggests that in appropriately selected patients, rivaroxaban may be used for treatment of CAT with promising safety and efficacy.
Keywords: aged, hemorrhage, neoplasms, rivaroxaban, venous thromboembolism
Essentials.
Rivaroxaban is effective treatment of cancer‐associated thrombosis (CAT) but with increased bleeding.
We describe results of an institutional protocol for CAT treatment with rivaroxaban.
We recommended avoiding rivaroxaban in patients with gastrointestinal or genitourinary tract lesions, and dose reduction for age ≥ 75.
Results showed acceptable efficacy and safety.
1. INTRODUCTION
Venous thromboembolism (VTE) is major source of morbidity and mortality in cancer patients.1, 2 Incidence rates of cancer‐associated thrombosis (CAT) vary with cancer type, stage, treatment, and comorbidities, but it is estimated that approximately 15% to 20% of cancer patients will develop a venous thromboembolic episode at some point during the course of their illness.3, 4
Treatment of CAT is particularly challenging, with higher rates of recurrence and major bleeding than for non–cancer patients with VTE.5 Low‐molecular‐weight heparins (LMWHs) have been shown to be superior to vitamin K antagonists such as warfarin,6 although LMWHs are expensive and the injections are burdensome to patients, leading to poor compliance.7 Across several studies of an LMWH to treat CAT, the rates of VTE recurrence and major bleeding with LWMH are approximately 7% to 8% and 4% to 5%, respectively.6, 7, 8
There is a growing body of data supporting the effective use of direct oral anticoagulants (DOACs) for treatment of CAT. Rivaroxaban was the first DOAC approved by the US Food and Drug Administration (FDA) for treatment of VTE, in 2012. The approval did not address the specific niche of cancer, either supporting use or cautioning against use, as the 2 pivotal phase III trials leading to approval included approximately 5.6% of cancer patients in the rivaroxaban‐treated arms.9, 10 A subsequent subgroup analysis of the EINSTEIN trials of cancer patients did not indicate any signal of particular risk in the cancer patients.11
In 2013, we designed a Clinical Pathway to guide use of rivaroxaban in cancer patients within Memorial Sloan Kettering Cancer Center. The key criteria were to recommend against use of rivaroxaban in patients with active luminal gastrointestinal (GI) tract or genitourinary (GU) tract lesions. In addition, we employed a modest dose reduction in the elderly. In 2017, we published outcomes of our first 200‐patient cohort of patients with CAT treated with rivaroxaban, following our Clinical Pathway, and demonstrated both low rates of recurrent VTE (4.4%; 95% confidence interval [CI], 1.4%‐7.4%) and major bleeding (2.2%; 95% CI, 0%‐4.2%) at 6 months. 12
Since our first report of our single institutional experience, 2 randomized clinical trials (RCTs) comparing a DOAC with an LMWH have been published, the HOKUSAI VTE Cancer trial of edoxaban13 and the SELECT‐D trial of rivaroxaban.14 Both studies demonstrated a trend toward lower rates of recurrent VTE with the DOAC but with higher rates of bleeding, particularly in the GI and GU tracts.13, 14
We now report on efficacy and safety outcomes in an expanded cohort of 1072 patients with CAT, who received rivaroxaban for treatment. To our knowledge, this is the largest reported population of cancer patients treated with a DOAC.
2. MATERIALS AND METHODS
2.1. Clinical pathway
The Clinical Pathway was designed to help guide clinician use of rivaroxaban for CAT within our institution (Appendix S1). Key points of the Clinical Pathway include patient selection. Active luminal lesions of the GI or GU tract were considered contraindications. Bioavailability of rivaroxaban is approximately > 80%,15 but active drug remains in the upper GI lumen. Similarly, rivaroxaban and other DOACs are excreted in the urine at biologically active concentrations.16 In the presence of known luminal lesions of the GI or GU tract, an LMWH was recommended instead. Untreated central nervous system neoplasms were also a considered a contraindication to the use of rivaroxaban, as at the time there were no published data on safety in that setting.
In general, we followed the FDA‐approved dosing guidelines, including contraindication in the presence of renal insufficiency (with creatinine clearance values < 30 mL/min) and possible drug interactions involving the inhibitors or inducers of cytochrome P450 3A4 and P‐glycoprotein efflux pump. However, for patients aged 75 years or older, we recommended a reduced dose of 10 mg orally twice a day for 3 weeks, followed by 15 mg daily. This was based on the known 4‐fold increased risk of life‐threatening and fatal hemorrhagic complications in patients aged 80 years or older, with the superimposed increased risk of life‐threatening hemorrhage in cancer patients.5, 17, 18
2.2. Patients and outcomes
All patients at Memorial Sloan Kettering Cancer Center treated with rivaroxaban are monitored in an ongoing institutional quality assessment (QA) project. From January 2014 through September 2016, 2013 patients received rivaroxaban for all indications. From that cohort, we identified 1072 individuals with active cancer who received rivaroxaban for at least part of their course of treatment for CAT (Figure 1). For CAT, we include any proximal or symptomatic distal lower extremity deep vein thrombosis (DVT) and/or incidental or symptomatic pulmonary embolism. Symptomatic distal DVTs were included, as these are routinely treated in cancer patients, and a recent study has confirmed that in cancer patients, distal DVTs have a comparable risk of VTE recurrence as proximal DVTs.19
Figure 1.
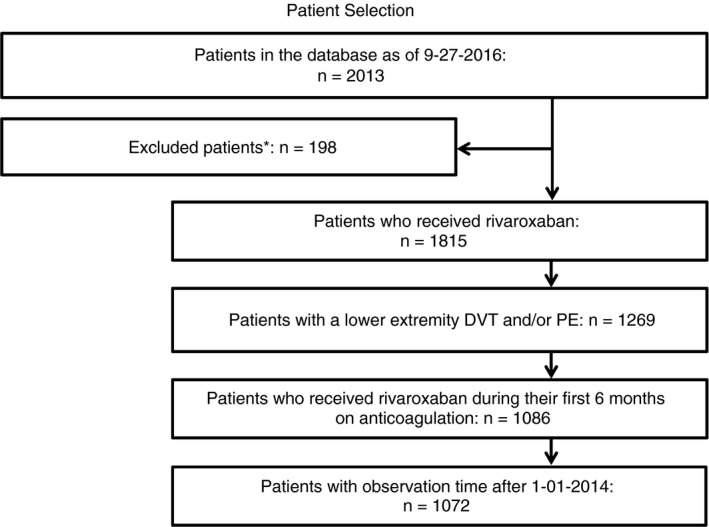
Patient selection. Our target was to identify and characterize all cases of CAT treated with rivaroxaban within the first 2000 patients at our institution who were prescribed rivaroxaban for any indication. From January 1, 2014, through September 2016, 2013, patients had rivaroxaban ordered. Of these, 198 were excluded, as they were prescribed rivaroxaban but never received a dose. We derived a cohort of 1072 patients with CAT who received rivaroxaban for at least part of their anticoagulation course, starting after January 1, 2014. DVT, deep vein thrombosis; PE, pulmonary embolism
Other patients who received rivaroxaban for cardiac indications, received post–orthopedic surgery prophylaxis, had an upper extremity thrombosis, or did not have cancer were not included in this analysis. Outcomes of interest were captured up to 6 months from initiation of anticoagulation and while the patient was on rivaroxaban. Outcomes were defined a priori, consisting of recurrent VTE (using the definition from the CLOT study6), major bleeding (ISTH definition20), and clinically relevant nonmajor bleeding (CRNMB, ISTH definition21) and all‐cause mortality. Due to conditions underlying data capture, we were not confident that we could capture all CRNMB events, so we chose to focus only on CRNMB episodes leading to discontinuation of rivaroxaban for at least 7 days. All‐cause mortality while on rivaroxaban was also an end point. Many patients, upon transfer to hospice care, discontinue anticoagulation with rivaroxaban or other anticoagulation. Therefore, the 6‐month death rate was captured for all patients within the cohort, including those who discontinued rivaroxaban for any reason. Baseline characteristics of patients were retrieved from the electronic medical record (EMR), including internal and external records.
Compliance with the Clinical Pathway was not required or considered for inclusion in the final cohort. Patients were observed from the time they started rivaroxaban up to 6 months (180 days) after the start of anticoagulation. No assessment was made of potential events occurring on an alternate anticoagulant prior to initiation of rivaroxaban. Clinical notes were downloaded from the EMR for the periods of interest and scanned for keywords indicative of VTE or bleeding (Appendix S2). All charts that were flagged with at least 1 keyword were reviewed by a study hematologist and events recorded as indicated. Dates of death were obtained from the EMR.
2.3. Statistical analysis
The 6‐month incidence of a recurrent VTE, major bleed, and CRNMB were separately estimated using cumulative incidence functions. Each of the 3 event types was considered a competing risk for the remaining event types; in addition, death in the absence of each event was considered a competing risk. In the primary analysis, patients entered the risk set upon starting rivaroxaban following the index VTE. A sensitivity analysis was conducted among patients who started rivaroxaban immediately following the VTE. Patients without an event were censored upon discontinuation of rivaroxaban or upon completion of the 6‐month analysis window, whichever came first. The 6‐month cumulative incidence of mortality was similarly estimated; however, for this end point, there were no competing events, and patients were not censored upon discontinuation of rivaroxaban if occurring before the 6‐month time point.
These end points were also estimated among patients above and below age 75. All analyses were done in the R v3.3.3. (R Foundation for Statistical Computing, Vienna, Austria.)
3. RESULTS
A total of 1072 individuals were included in the final cohort, representing rivaroxaban start dates from August 8, 2013, to September 2, 2016. Characteristics of patients are shown in Table 1. The mean age was 63 years old (range, 18‐92), and 17% were aged ≥75 years at the time of the index VTE episode. The most common cancers were lung, gynecologic, pancreas, colorectal, breast, and hematologic. Of the patients for whom cancer stage was available (excluding hematologic and brain), 86% had locally advanced or metastatic disease. Although our Clinical Pathway recommended against use of rivaroxaban in the presence of untreated primary or metastatic brain cancer, 17 patients with brain tumors were treated, and their outcome is included in our analysis.
Table 1.
Baseline patient characteristics
| Characteristic (n = 1072) | N | % |
|---|---|---|
| Male | 468 | 43.7 |
| Female | 604 | 56.3 |
| Age at date of VTE (y) | ||
| Mean, (range, SD) | 63 (18‐92, 12.7) | |
| 75+ | 182 | 17.0 |
| Cancer types | ||
| Heme (lymphoma/myeloma, leukemia, myeloproliferative neoplasm) | 87 | 8.1 |
| Solid tumor | 985 | 91.9 |
| Lung | 173 | 16.1 |
| Gynecologic | 143 | 13.3 |
| Pancreas | 134 | 12.5 |
| Colorectal | 100 | 9.3 |
| Breast | 91 | 8.5 |
| Genitourinary | 62 | 5.8 |
| Sarcoma | 50 | 4.7 |
| Hepatobiliary | 43 | 4.0 |
| Prostate | 41 | 3.8 |
| Gastric/Esophagus | 40 | 3.7 |
| Head and neck | 24 | 2.2 |
| Brain | 17 | 1.6 |
| Cancer stage (not including hematologic or brain,a N = 968) | ||
| 1 | 12 | 1.2 |
| 2 | 33 | 3.4 |
| 3 | 94 | 9.7 |
| 4 | 722 | 74.6 |
| Not available | 21 | 2.2 |
| No evidence of disease (after cancer surgery or while receiving adjuvant chemotherapy) | 86 | 8.9 |
| Index VTE event | ||
| Lower extremity DVT—calf | 156 | 14.5 |
| Lower extremity DVT—at or above popliteal fossa | 326 | 30.4 |
| PE with or without DVT | 590 | 55.0 |
| Index VTE event—symptomatic vs. incidental | ||
| Lower extremity DVT | ||
| Symptomatic | 395 | 36.8 |
| Incidental | 85 | 7.9 |
| Unclear | 2 | 0.2 |
| PE (with or without DVT) | ||
| Symptomatic | 325 | 30.3 |
| Incidental | 263 | 24.5 |
| Unclear | 2 | 0.2 |
Brain tumors and most hematologic malignancies are not routinely staged.
DVT, deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation; VTE, venous thrombolism.
3.1. Clinical end points
Frequencies of clinical end points occurring while the patients were on rivaroxaban are presented in Table 2. No patients were lost to follow‐up. The cumulative incidence analysis is presented in Table 3 and plots for competing end points are shown in Figure 1. In the unadjusted analysis (Table 2), 8.1% of patients experienced 1 of the prespecified end points of recurrent VTE, major bleeding, CRNMB, or death while receiving rivaroxaban. The small number of deaths (N = 6) in patients receiving rivaroxaban reflects, at least in part, the common practice for anticoagulation to be discontinued when a cancer patient's care is transitioned to hospice/comfort measures.
Table 2.
Frequencies of end points while on rivaroxaban
| End point | N | % |
|---|---|---|
| Censored without an event at 6 ma | 530 | 49.4 |
| Censored before 6 mo for other reasons | 287 | 26.8 |
| Recurrent VTE | 29 | 2.7 |
| PE | 13 | 1.2 |
| Proximal DVT | 14 | 1.3 |
| Distal DVT | 2 | 0.2 |
| Bleeding end point | 52 | 4.9 |
| Major bleed | 15 | 1.4 |
| CRNMBb | 37 | 3.5 |
| Death, while on rivaroxabanc | 6 | 0.6 |
| Death/Hospice, within 6 mo from index VTE (includes events after discontinuation of rivaroxaban)c | 174 | 16.2 |
Censoring before 6 mo without an end point occurred when a patient discontinued rivaroxaban for medical reasons, such as development of contraindications (GI/GU process), completed planned course of anticoagulation, or transferred to an outside institution.
Clinically relevant nonmajor bleeding. Only CRNMB events leading to discontinuation of rivaroxaban for ≥7 days are included.
The routine practice at Memorial Sloan Kettering Cancer Center is to discontinue anticoagulation when patients are transferred to hospice, or otherwise cease cancer‐directed therapy. See Figure 1 for overall mortality rate of the cohort.
CRNMB, clinically relevant nonmajor bleeding; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thrombolism.
Table 3.
Day 180 analysis adjusting for competing risks
|
Overall (N = 1072) mean (95% CI) |
<75 yo (N = 890) mean (95% CI) |
≥75 yo (N = 182) mean (95% CI) |
|
|---|---|---|---|
| Recurrent VTE | 4.2% (2.7‐5.7) | 4.1% (2.5‐5.8) | 4.5% (0.6‐8.3) |
| Major bleed | 2.2% (1.1‐3.2) | 2.2% (1.0‐3.4) | 1.8% (<1‐4.2) |
| CRNMB | 5.5% (3.7‐7.1) | 5.5% (3.6‐7.3) | 5.5% (1.1‐9.7) |
| Death/Hospice | 22.2% (19.4‐24.9) | 21.3% (18.3‐24.2) | 26.7% (19.2‐33.6) |
See Statistical Analysis3.2 in Methods for full description. Recurrent VTE, major bleed, and CRNMB leading to discontinuation of rivaroxaban for ≥7 days were separately estimated using cumulative incidence functions. For the 6‐mo cumulative incidence of mortality end point, there were no competing events, and patients were not censored upon discontinuation of rivaroxaban if occurring before the 6‐mo time point.
CI, confidence interval; CRNMB, clinically relevant nonmajor bleeding; VTE, venous thrombolism.
In a cumulative incidence analysis (Table 3 and Figure 2), the 6‐month incidence of a recurrent VTE was 4.2% (95% CI, 2.7‐5.7), major bleeding was 2.2% (95% CI, 1.1‐3.2), and CRNMB was 5.5% (95% CI, 3.7‐7.1). The remaining patients were censored at 6 months or censored due to discontinuation of rivaroxaban for a reason other than reaching 1 of the prespecified clinical end points.
Figure 2.
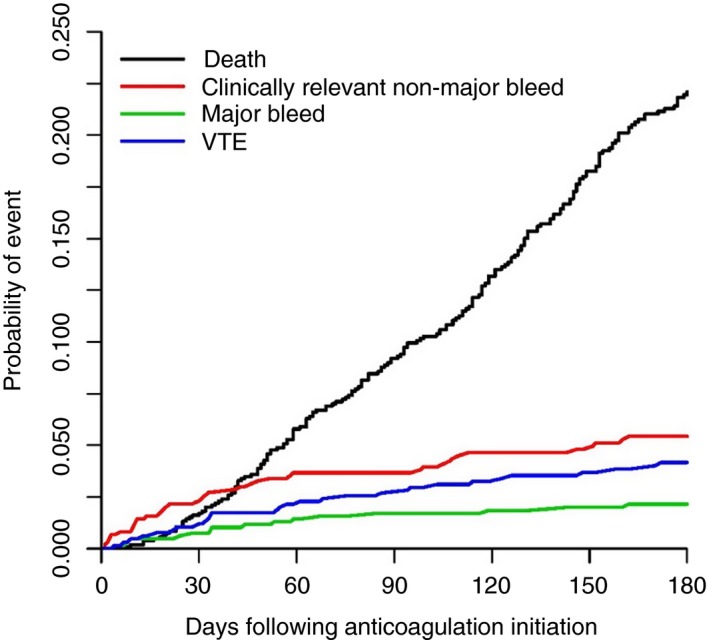
Cumulative incidence of primary end points. Competing risk analysis while patients were on rivaroxaban. End points were recurrent thrombosis, major bleeding, and CRNMB leading to discontinuation of rivaroxaban for at least 7 days. Death was also a competing end point. However, most deaths occurred when patients were transferred to hospice or only receiving supportive care, and rivaroxaban was discontinued. To more meaningfully represent the overall mortality rate of the cohort, all deaths are included, including after rivaroxaban was discontinued. VTE, venous thromboembolism
To determine the mortality cumulative incidence, we needed to take into account that a large number of patients would be censored shortly before reaching this end point, as anticoagulation is typically discontinued upon admission to a hospice program. Therefore, we determined the incidence of death at any time during the 6‐month period after the index VTE event, whether the patient was on rivaroxaban or another anticoagulant or had already reached an end point of recurrent thrombosis or bleeding. The 6‐month incidence of death in the whole cohort was 22.2% (95% CI, 19.3%‐24.8%).
All bleeding episodes were reviewed (Table 4). GI bleeding accounted for 32% of CRNMB and 73% of major bleeds. Cancers of the GI lumen did not appear to be overrepresented for major bleeding in general, nor the GI tract specifically (Table 5). Only 1 of the 100 patients with colorectal cancer and 1 of the 40 with gastric/esophageal cancer experienced a major bleed. There were no fatal hemorrhages and no intracranial hemorrhages. And although not primary end points, there were no recognized episodes of known myocardial infarction or ischemic stroke.
Table 4.
Characterization of all bleeding episodes
| All bleeding (N = 52) | CRNMB (N = 37) | Major bleeds (N = 15) | |
|---|---|---|---|
| Upper GIB | 10 | 4 | 6 |
| Lower GIB | 13 | 8 | 5 |
| Urinary tract | 9 | 9 | 0 |
| Epistaxis | 4 | 4 | 0 |
| Hemoptysis/Lung | 7 | 6 | 1 |
| Gynecologic | 5 | 4 | 1 |
| Multisource/Unclear Source | 1 | 0 | 1 |
| Gingival bleed | 1 | 1 | 0 |
| Other | 2 | 1 | 1 |
CRNMB, clinically relevant nonmajor bleeding; GIB, gastrointestinal bleed.
Table 5.
Cancer types associated with bleeding
| All bleeding (N = 52) | CRNMB (N = 37) | Major bleeds (N = 15) | |
|---|---|---|---|
| Breast | 4 | 2 | 2 |
| Colorectal | 3 | 2 | 1 |
| Gastric/Esophagus | 2 | 1 | 1 |
| Genitourinary | 2 | 2 | 0 |
| Gynecologic | 16 | 12 | 4 |
| Lung | 12 | 9 | 3 |
| Lymphoma/Myeloma | 1 | 0 | 1 |
| Other | 3 | 3 | 0 |
| Pancreas | 8 | 5 | 3 |
| Sarcoma | 0 | 0 | 0 |
| Skin | 1 | 1 | 0 |
CRNMB, clinically relevant nonmajor bleeding.
As this was a QA initiative, we reviewed all cases of major bleeding (Table 6). An anatomic lesion was identified, either prior to the hemorrhage or in the course of evaluation in 6 of the 11 total major GI bleeding episodes overall, and in 2 of the 4 other major bleeding events. Thus, in the entire cohort, there were only 7 major bleeds that occurred in the absence of an identified anatomic lesion.
Table 6.
Anatomic contribution to major bleeding
| Major bleeds (N = 15) | Anatomic lesion identified | Lesion identified prior to initiation of rivaroxaban | |
|---|---|---|---|
| Upper GIB | 7 | 4 | 3 |
| Lower GIB | 4 | 2 | 2 |
| Urinary tract | 0 | NA | NA |
| Other | 4 | 2 | 2 |
| Total | 15 | 8 | 7 |
Upper GIB: tumor invading the small bowel (N = 1); metastatic breast cancer to stomach (N = 1); gastric ulcer (N = 1); malignant esophageal stricture with stent (N = 1). Lower GIB: malignant rectovaginal fistula, with rectal stent (N = 1); large rectosigmoid primary tumor, still present (N = 1). Other: vaginal bleeding: extensive involvement from cervical cancer (N = 1); hemoptysis; extensive metastatic lung cancer (N = 1).
GIB, gastrointestinal bleed.
3.2. Analysis of elderly
One hundred eighty‐two patients (17%) of the cohort were aged ≥75 years at the time of the index VTE episode. The cumulative incidences of recurrent VTE, major bleeding, CRNMB, and all‐cause 6‐month mortality did not differ significantly for patients under 75 years of age vs. older individuals (Table 3).
4. DISCUSSION
To our knowledge, this is the largest prospective cohort study assessing the use of rivaroxaban for CAT. Our goal was to assess and validate our Clinical Pathway for the use of rivaroxaban in treatment of CAT. The estimated 6‐month risks of recurrent VTE and major bleeding are similar to the results of our earlier, smaller series.12 We maintained low rates of both recurrent VTE and major bleeding.
One essential aspect of our Clinical Pathway was to guide the appropriate choice of anticoagulant for CAT, by anticipating situations where rivaroxaban would be expected to be associated with a higher risk of bleeding than an LMWH. We recommended avoidance of rivaroxaban use in patients with active GI or GU tract lesions. Arguably, any anticoagulant would increase the risk of GI bleeding in the setting of a lesion of the GI lumen. However, a DOAC would pose a particularly high risk of GI bleeding if there were also an anatomic lesion, as the drug is present at biologically active concentrations within the upper GI tract. LMWHs, being parenteral, would not have that particular risk and are recommended as a substitute to rivaroxaban in our Clinical Pathway for patients with active GI lesions. Our Clinical Pathway also recommended LMWH over rivaroxaban in patients with urinary tract lesions. Rivaroxaban and other DOACs are cleared in part and are active in the urine.16 LMWHs are also cleared in the urine but are inactive without the protein antithrombin, which is not normally present in the urine. Therefore, we recommend an LMWH as the preferable anticoagulant in the setting of GI or GU lesions.
Our Clinical Pathway, developed and applied beginning in 2014, recommended against use of rivaroxaban in patients with untreated primary or metastatic cancer of the brain. At the time, there were no data addressing the relative safety of rivaroxaban vs. LMWH in those patients. However, more recent reports support the use of a DOAC in patients with primary or metastatic cancer of the brain.22
Our initial experience with our Clinical Pathway was reported in early 2017.12 Since then, 2 prospective RCTs of a DOAC vs. LWMH for CAT have been reported. Any comparison of outcomes from our single‐arm QA study with results from randomized, prospective clinical trials is limited, as the analysis we conducted was on‐treatment for most end points, in contrast to intention to treat for prospective trials. With that limitation acknowledged, the 6‐month rate of recurrent VTE observed in our cohort was similar to results reported in the recent clinical trials of rivaroxaban and edoxaban.13, 14
A major difference in the results of the RCTs and our single‐arm cohort results is in the bleeding outcomes. The HOKUSAI VTE Cancer trial reported a significantly higher rate of major bleeding with edoxaban, compared with LMWH, particularly in patients with GI or GU cancer.13 The authors did not specify if the primary GI or GU lesions were still present at the time of anticoagulation start. During an interim analysis in the SELECT‐D trial, “The DSMC (Data and Safety Monitoring Committee) also noted a nonsignificant difference in major bleeding between arms in the 19 patients with cancer of the esophagus or gastroesophageal junction. These cancers were subsequently excluded from enrollment as a precautionary measure.”14 The higher rates of GI or GU bleeding in the 2 recent RCTs, which had not excluded patients based on GI or GU tract pathology, supports our Clinical Pathway recommendations in this area.
Our detailed analysis of all major bleeding episodes was notable in how few truly “spontaneous” major hemorrhages there were. Of the 15 major bleeding episodes, 8 had an identified anatomic lesion that made the patient particularly at risk for bleeding, such as cancer directly involving the gastrointestinal lumen. Only 7 patients experienced a major bleed without an identified anatomic lesion contributing. The fact that most of the anatomic risks were identified and known before the hemorrhage also suggests we can further optimize safety.
For our analysis, we prespecified CRNMB leading to discontinuation of rivaroxaban for at least 7 days, a different definition from the 2 recent RCTs.13, 14 Therefore, no comparison of rates of CRNMB can be made between our results and the SELECT‐D and HOKUSAI VTE Cancer trial.
The 1 key area where our Clinical Pathway diverges from the FDA‐approved package insert/dosing guidelines is our approach to dose reduction in the elderly. We felt this was appropriate due to the known 4‐fold increased risk of fatal or life‐threatening hemorrhage in the elderly with the additional risk of bleeding associated with cancer.5, 17, 18 The elderly had similar rates of recurrent thrombosis, major bleeding, and CRNMB as the patients under 75 years old, supporting our practice of dose modification in the elderly.
Based on our Clinical Pathway and our institutional experience, for cancer patients without GI or GU lesions and contraindications, rivaroxaban may be an effective and safe choice. Plus, rivaroxaban spares the cost and burden to the patient of LMWH injections. However, for patients with GI or GU lesions, an LMWH may be the most appropriate choice. Drug interactions with rivaroxaban may also be a consideration in some patients.
There are additional questions to address. We do not have validation of a rivaroxaban dose modification strategy in the setting of thrombocytopenia, as has been provided for enoxaparin.12 Other questions include what proportion of patients with CAT meet the eligibility criteria set forth in our Clinical Pathway. This is a subject of our ongoing work. But we estimate the majority of episodes of CAT may be treated with rivaroxaban. Further, we do not yet have data on the clinical outcomes for patients with CAT and a contraindication to rivaroxaban, who are treated with an LMWH. Patients with a relative contraindication to rivaroxaban due to anatomic risks for bleeding would likely have a higher risk of bleeding on any anticoagulant. Our goal is to develop a strategy of patient selection and anticoagulant choice, in order to ultimately offer each individual with CAT the drug that will maximize safety and efficacy.
5. LIMITATIONS
There are several limitations to our cohort study. Selection bias could come from potential risk factors for bleeding not listed in the Clinical Pathway which, in the mind of the treating physician, would prompt them to use LMWH instead. A perceived (real or imagined) higher risk of recurrent VTE could also tilt the balance in favor of starting the time‐proven LMWH standard in some individuals. However, the choice of initial anticoagulant was usually made by an emergency department physician or a treating oncologist. They were educated about the Clinical Pathway, but ultimately it was their choice of anticoagulant.
Detection bias could have occurred due to software or observer error when assessing clinical notes for end points. However, the keyword list used for the initial computer‐driven review of the EMR is extensive. Further, after electronic capture, all relevant notes were then reviewed by at least 2 members of the investigator team. In addition, all radiographic or other imaging studies were reviewed for thrombosis, and the pharmacy records were reviewed for anticoagulant orders.
Finally, as this was an institutional cohort study, adjudication was performed by the treating physicians, not an independent team.
6. CONCLUSIONS
Our new QA initiative report has further validated the effective and safe use of rivaroxaban for treatment of cancer‐associated thrombosis. The risk of recurrent thrombosis is as low or lower than historical controls with LMWH. Based on the pharmacokinetics of rivaroxaban, we have developed and validated a Clinical Pathway to identify when rivaroxaban should or should not be used.
7. RELATIONSHIP DISCLOSURE
This research was funded in part from Janssen Scientific Affairs through an Investigator Initiated Study grant to Gerald A Soff MD. Additional funding was from the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
8. AUTHOR CONTRIBUTION
GAS oversaw the project, wrote the Clinical Pathway with SM, and wrote the first draft of the manuscript. JM contributed to review of medical records to extract end point data and contributed to writing and editing the manuscript. CW contributed to review of medical records to extract end point data and contributed to writing and editing the manuscript. SD was primarily responsible for the statistical analysis and contributed to writing and editing the manuscript. EHL contributed to review of medical records to extract end point data and contributed to writing and editing the manuscript. JW was responsible for extraction of the pertinent notes from the electronic medical record and contributed to writing and editing the manuscript. DMS reviewed and adjudicated the radiologic reports and contributed to writing and editing the manuscript. KJ was primarily responsible for extraction of text from the radiological reports and contributed to writing and editing the manuscript. MS was responsible for extraction of the pertinent notes from the electronic medical record and contributed to writing and editing the manuscript. YM contributed to review of medical records to extract end point data and contributed to writing and editing the manuscript. JB contributed to review of medical records to extract end point data and contributed to writing and editing the manuscript. SM co‐wrote the Clinical Pathway, contributed to the overall study design and oversight, and contributed to writing and editing the manuscript.
Supporting information
Soff GA, Mones J, Wilkins C, et al. Rivaroxaban treatment of cancer‐associated venous thromboembolism: Memorial Sloan Kettering Cancer Center institutional experience. Res Pract Thromb Haemost. 2019;3:349–356. 10.1002/rth2.12215
Funding information
This research was funded, in part, through support from Janssen Scientific Affairs, LLC. This research was also funded, in part, through the NIH/NCI Cancer Center Support Grant P30 CA008748.
REFERENCES
- 1. Ay C, Pabinger I, Cohen AT. Cancer‐associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219–30. [DOI] [PubMed] [Google Scholar]
- 2. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 3. Deitcher SR. Cancer‐related deep venous thrombosis: clinical importance, treatment challenges, and management strategies. Semin Thromb Hemost. 2003;29:247–58. [DOI] [PubMed] [Google Scholar]
- 4. Prandoni P. How I treat venous thromboembolism in patients with cancer. Blood. 2005;106:4027–33. [DOI] [PubMed] [Google Scholar]
- 5. Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–363. [DOI] [PubMed] [Google Scholar]
- 6. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- 7. Khorana AA, McCrae KR, Milentijevic D, Fortier J, Nelson WW, Laliberte F, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: a Randomized Clinical Trial. JAMA. 2015;314:677–86. [DOI] [PubMed] [Google Scholar]
- 9. Investigators E, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 10. Investigators E‐P, Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 11. Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN‐DVT and EINSTEIN‐PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1:e37–46. [DOI] [PubMed] [Google Scholar]
- 12. Mantha S, Laube E, Miao Y, Sarasohn DM, Parameswaran R, Stefanik S, et al. Safe and effective use of rivaroxaban for treatment of cancer‐associated venous thromboembolic disease: a prospective cohort study. J Thromb Thrombolysis. 2017;43:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raskob GE, Van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378:615–24. [DOI] [PubMed] [Google Scholar]
- 14. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36:2017–23. [DOI] [PubMed] [Google Scholar]
- 15. Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol. 2012;26:27–32. [DOI] [PubMed] [Google Scholar]
- 16. Harenberg J, Du S, Wehling M, Zolfaghari S, Weiss C, Kramer R, et al. Measurement of dabigatran, rivaroxaban and apixaban in samples of plasma, serum and urine, under real life conditions: an international study. Clin Chem Lab Med. 2016;54:275–83. [DOI] [PubMed] [Google Scholar]
- 17. Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–52. [DOI] [PubMed] [Google Scholar]
- 18. Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–9. [DOI] [PubMed] [Google Scholar]
- 19. Galanaud J‐P, Sevestre M‐A, Pernod G, Genty C, Richelet S, Kahn SR, et al. Long‐term outcomes of cancer‐related isolated distal deep vein thrombosis: the OPTIMEV study. J Thromb Haemos. 2017;15:907–16. [DOI] [PubMed] [Google Scholar]
- 20. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 21. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26. [DOI] [PubMed] [Google Scholar]
- 22. Carney BJ, Uhlmann EJ, Puligandla M, Mantia C, Weber GM, Neuberg DS, et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019;17:72–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


