Abstract
Essentials.
Venous thromboembolism (VTE) is a known complication in chronic pediatric heart disease (CPHD).
The effect of certain VTE risk factors on VTE and its health care burden in CPHD is unknown.
VTE in CPHD is associated with significantly increased health care resource utilization.
Recent cardiac or noncardiac surgery is a risk factor that infers the highest VTE risk in CPHD.
Abstract
Background
Venous thromboembolism (VTE) is a complication in children with chronic pediatric heart disease (CPHD). The influence of acute VTE risk factors and the health care burden associated with VTE in CPHD is unknown.
Methods
Children <18 years of age with a CPHD diagnostic code were identified from the 2003‐2013 MarketScan Commercial Databases. VTE diagnoses were identified either concomitantly with initial CPHD diagnoses or during a 6‐month follow‐up. The associations between demographic and clinical characteristics and VTE among children with CPHD, stratified by recent cardiac surgery, were assessed by multivariable logistic regression models. Estimates of health care utilization were compared using Wilcoxon rank‐sum tests.
Results
VTE events occurred in 957 of 120 884 children with CPHD (0.8%). In‐hospital mortality was significantly higher in children with VTE. Single‐ventricle physiology had the highest VTE rate (2.3%). All comorbid conditions were significantly associated with VTE, but the prevalence was highest in children with recent cardiac (11.1%) or noncardiac surgery (7.8%). The magnitude of association between noncardiac comorbidities and acquired acute cardiovascular conditions and VTE were larger for children without a recent cardiac surgery. Children with VTE had significantly higher health care utilization.
Conclusions
VTE in CPHD is associated with significantly increased health care resource utilization and in‐hospital mortality. All of the comorbid conditions examined were significantly associated with VTE, but a recent surgical procedure, especially cardiac surgery, conferred the highest VTE risk. Although confounding inherently limits observational studies, these findings provide practical information about the health care costs among patients with CPHD and VTE.
Keywords: cardiac surgery, congenital, health care costs, heart defects, venous thromboembolism
1. INTRODUCTION
The population scale incidence of pediatric venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism, has been estimated to be 0.07 to 0.95 per 10 000 children.1, 2, 3 The incidence of VTE is increasing across all pediatric populations; however, pediatric VTE is most common among infants and adolescents.4 Most pediatric VTEs occur among children with at least 1 complex chronic medical condition who are admitted to a tertiary care pediatric hospital.1, 2, 4, 5 The estimated incidence of VTE among hospitalized children is 40 to 53 per 10 000.4, 5
Chronic pediatric heart diseases (CPHD), including congenital heart disease, cardiomyopathy (acquired or congenital), conduction disorders (acquired or congenital), and acquired chronic cardiac conditions, are associated with the highest rate of VTE development in children.4, 5 The reported incidence of extracardiac thrombosis in patients aged 0 to 17 years with cardiomyopathy or congenital heart disease is approximately 1%, with a higher frequency in patients suffering from cardiomyopathy (3.4%‐23.1%).6, 7 Although the relationship of cardiomyopathy and congenital heart disease to VTE development in pediatric patients has been described, the contribution of other CPHD to VTE development has not been well defined.
It is estimated that between 14% and 29% of all inpatient childhood VTEs occur in patients with CPHD.1, 2, 4, 5 CPHD has been associated with the second‐highest rate of hospital‐acquired VTE among all complex chronic medical conditions in children.8 Pediatric cardiac surgery has been associated with an increased risk of VTE, with a reported incidence of up to 11.4%.9, 10 Children with single‐ventricle physiology cardiac lesions seem to be at even higher risk for VTE development following cardiac surgery.11 Age, heart transplantation, unscheduled intensive care admissions, extracorporeal membrane oxygenation (ECMO), and cardiopulmonary bypass are all factors associated with increased VTE risk in relation to pediatric cardiac surgery. VTE associated with pediatric cardiac surgery has been shown to lead to longer intensive care and hospital stays, increased risk of cardiac arrest, earlier reoperation or percutaneous cardiac catheter reintervention, and increased in‐hospital mortality rates.9, 11, 12, 13
A more thorough understanding of the epidemiology and risk factors associated with VTE development in CPHD is an essential next step toward prevention and management of this life‐threatening complication. Although information exists on VTE risk for children with CPHD, many of the published studies are small, retrospective, and inadequately powered to assess the potential impact of noncardiac acute and chronic comorbidities on VTE risk. The impact of noncardiac complex chronic conditions (ncCCCs), recent cardiac or noncardiac surgery, acute trauma, or the presence of acute acquired cardiac conditions on the likelihood of VTE in CPHD may lead to the identification of high‐risk groups of children who are most likely to benefit from thromboprophylactic anticoagulation.
In this study, we used an administrative data set to examine VTE prevalence, estimate the health care burden, and delineate risk factors associated with VTE in a large cohort of children with CPHD. We hypothesized that acute and chronic comorbidities would increase the likelihood of VTE and that children with VTE would have higher health care utilization and expenditures than children without VTE.
2. MATERIALS AND METHODS
2.1. Data source
Data from the 2003‐2013 Truven Health MarketScan Commercial Databases were used for this study.14 This database was created for billing purposes and includes all health insurance claims for employees and their dependents covered under both regional health plans and large self‐insured employers. Claims for prescription drugs, outpatient services, and inpatient admissions are included, and patient‐level information can be linked longitudinally unless enrollees change employers. Payments on all billed services, including inpatient and outpatient claims, as well as those for prescription drugs, were captured along with diagnostic codes, which allows for a true cost analysis as opposed to provider charge estimates.15, 16 In 2013, the database included information on approximately 43 million enrollees and their dependents from over 150 contributing employers and 25 contributing health plans.
2.2. Study population
Children aged 0 to 17 years of age at the time of their initial CPHD diagnostic code were identified for inclusion if they also had obtainable information on health plan enrollment and outpatient pharmaceutical claims. CPHD was defined using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes for cardiomyopathies (acquired or congenital), single‐ventricle physiology congenital heart disease, 2‐ventricle physiology congenital heart disease, conduction disorders and dysrhythmias (acquired or congenital), and acquired chronic cardiac conditions (Table 1). These groupings were not mutually exclusive, and overlap may exist. The ICD‐9‐CM classifies congenital heart disease by anatomic diagnosis and not by physiologic palliation. Many anatomic diagnoses can be classified as either single‐ventricle or 2‐ventricle physiology following palliation. However, several complex congenital heart disease anatomic ICD‐9‐CM diagnoses may be described with codes that can be associated with either single‐ or 2‐ventricle physiology following palliation (ie, 745.11, Double‐outlet right ventricle). For the purposes of this study, patients with ICD‐9‐CM codes for which palliative options were unclear were classified as single‐ventricle physiology if certain Current Procedural Terminology (CPT) codes (33468, 33615‐33617, 33619, 33766‐33768) were reported for a patient with ICD‐9‐CM codes for single‐ or 2‐ventricle physiology only.
Table 1.
Diagnostic classifications and specific ICD‐9‐CM codes
| Diagnostic classifications | Subclassifications | ICD‐9‐CM codes | CPT codes |
|---|---|---|---|
| Chronic cardiac diseases | Cardiomyopathies | 425.0‐425.4, 429.1 | |
| Single‐ventricle physiology | 397.0, 745.3, 745.7, 746.1, 746.7, 746.89 | ||
| Single‐ or double‐ventricle physiology | 745.11, 745.60, 746.01, 746.2, 746.84, 747.22 | 33468, 33615‐33617, 33619, 33766‐33768a | |
| Double‐ventricle physiology | 745.0, 745.1, 745.2, 745.4, 745.5, 745.61‐745.69, 745.9, 746.02, 746.09, 746.3‐746.6, 747.1‐747.20, 747.29‐747.49 | ||
| Conduction disorders/dysrhythmias | 426.0, 426.13, 426.7, 426.8, 427.0‐427.4, 427.6‐427.9 | ||
| Acquired chronic cardiac conditions | 393, 394.0‐394.4, 395.0‐395.9, 396.0‐396.9, 397.0‐397.9, 398.0, 398.90‐398.91 | ||
| Venous thromboembolism | Cerebral sinus thrombosis | 325 | 33910, 33915, 33916, 34401, 34421, 34451, 34471, 34490, 37187, 37188 |
| Pulmonary embolism | 415.1, 415.11, 415.12, 415.19 | ||
| Lower‐extremity DVT | 451.1x, 451.2, 451.81, 453.4x | ||
| Upper‐extremity DVT | 451.83, 451.84, | ||
| Renal vein thrombosis | 453.3 | ||
| Other DVT | 451.89, 451.9, 452, 453, 453.2, 453.8, 453.9, 572.1 | ||
| Thrombectomy | 38.07, 38.09 | ||
| Noncardiac CCCa | Cardiac codes omitted | ||
| Trauma | 800‐904.9, 925‐929.9, 940.0‐959.9 | ||
| Cardiac surgery | 35.00‐35.99, 36.0‐36.99, 37.0‐37.49, 37.52‐37.99, 38.00‐38.99, 39.0‐39.99 | Table S1 | |
| Heart transplantation | 37.51 | ||
| ECMO/CPB | 39.61, 39.65 | ||
| Acquired cardiac conditions | 391.0‐391.9, 392.0, 410.0‐410.9, 411.0‐411.89, 413.0‐413.9, 415.0, 420.0‐420.99, 421.0‐421.9, 422.0‐422.99, 425.9, 428.1, 428.21, 428.23, 428.31, 428.33, 428.41, 428.43, 441.0‐441.03, 441.1, 441.3, 441.5, 441.6, 446.1 | ||
| Infections | Septicemia | MEPS Category 2, 38.12, 771.81, 995.1, 995.92 | |
| Pneumonia | MEPS Category 122, 487.0, 482.42 | ||
| Urinary tract infections | MEPS Category 159 | ||
| Cellulitis | MEPS Category 197, 528.3 | ||
| Abdominal infections | MEPS Category 142 and 148, 567.31 | ||
| Skeletal infections | MEPS Category 201 |
CCC, complex chronic conditions; CPB, cardiopulmonary bypass; CPT, Current Procedural Terminology; DVT, deep vein thrombosis; ECMO, extracorporeal membranous oxygenation; ICD‐9‐CM, International Classification of Diseases, 9th Revision, Clinical Modification; MEPS, Medical Expenditure Panel Survey.
CPT codes were used to classify patients with single‐ or double‐ventricle physiology ICD‐9 codes. If CPT code was also present, patients were classified as single‐ventricle. If not present, patients were classified as double‐ventricle.
Inpatient ICD‐9‐CM codes for CPHD were considered valid, as previously described.15 Outpatient codes without a matching inpatient diagnosis were considered only if 2 or more codes were recorded at least 30 days apart. Children with an initial CPHD diagnosis reported after June 30, 2013, were excluded to enable a 6‐month accrual window for the event data described below. To reduce the likelihood of capturing preexisting cardiac diagnoses, children needed to be continuously enrolled for at least 6 months prior to the initial claim with a CPHD diagnosis (except for infants) and at least 6 months after the claim with a CPHD diagnosis (unless an in‐hospital death was reported); otherwise, they were excluded.
2.3. Venous thromboembolism
Children included in the study were categorized into the VTE group if an ICD‐9‐CM diagnosis code for VTE or CPT code for a VTE‐associated procedure was found concomitantly with their first claim with a CPHD diagnosis or during the following 6‐month follow‐up period.4, 5, 15 Consistent with previous studies using administrative data, all inpatient VTE codes were considered valid; however, outpatient codes without a matching inpatient diagnostic code were included only if there was evidence of a filled anticoagulant prescription within 90 days following the VTE diagnosis.15, 16
2.4. Comorbid conditions
All ncCCC diagnoses were ascertained using ICD‐9‐CM codes and were included if they occurred between 3 months prior to the first claim with a CPHD diagnosis and 6 months after that claim (irrespective of VTE diagnosis in that time frame), as previously described.15, 17 The ncCCCs included neuromuscular, renal, respiratory, gastrointestinal, hematology and immunodeficiency, metabolic, genetic, and malignant chronic conditions. Again, all inpatient codes were considered valid, whereas outpatient codes without a corresponding inpatient diagnosis were considered valid only if 2 or more codes were recorded at least 30 days apart within the 9‐month reporting range. Subject accrual began April 1, 2003, to account for the prediagnosis 3‐month comorbidity time frame.
Acute‐onset comorbidities were ascertained during the 3 months prior to VTE diagnosis or 3 months prior and 6 months after CPHD diagnosis in those lacking a VTE diagnosis.5 They were organized into trauma, noncardiac surgery, cardiac procedure/surgery, heart transplantation, postoperative ECMO/cardiopulmonary bypass, infections, and acquired acute cardiovascular conditions. These are major risk factors known to be associated with pediatric VTE in general as well as VTE in CPHD patients.5, 9 ICD‐9‐CM codes indicative of trauma were used, as previously published (Table 1).5, 15, 18 Noncardiac surgery was identified using diagnosis‐related group codes, as previously described.15, 16 Heart transplantation, ECMO, and acquired acute cardiovascular conditions were identified using ICD‐9‐CM diagnosis codes (see Table 1). Cardiac procedures/surgery were identified using ICD‐9‐CM and CPT codes (Table S1). The Medical Expenditure Panel Survey, HC‐120: Appendix 3, Clinical Classification Code to ICD‐9‐CM Code Crosswalk from the US Department of Health and Human Services Agency for Healthcare Research and Quality19 was used to identify infections, with minor modifications as described in Table 1 and as reported previously.15 For the purposes of this study, all ICD‐9‐CM “V” codes were ignored due to their known lack of specificity.15
2.5. Statistics
The distributions of demographic and clinical characteristics of children with CPHD with and without a VTE diagnosis were compared using chi‐square and 2‐tailed Student's t‐tests. The prevalence of VTE for each CPHD category and for children with heart transplant and ECMO was also calculated. Mean and median values for health care utilization and expenditures were calculated for inpatient admissions, outpatient visits, and pharmaceutical claims for children with and without VTE; the Wilcoxon rank‐sum test was used to compare distributions. Outpatient visit claims were aggregated by date of service. Generalized linear models with a log‐link function and gamma distribution were used to estimate adjusted total mean and median expenditures, after controlling for age at first CPHD claim, ncCCC, cardiac surgery, noncardiac surgery, trauma, infection, and acquired acute cardiovascular conditions.
Unadjusted and adjusted odds ratios and 95% confidence intervals (CIs) were used to assess the association between demographic and clinical characteristics with VTE among children with CPHD. The variables included in the initial multivariable models were age at first CPHD claim, ncCCC, cardiac surgery, noncardiac surgery, trauma, infection, and acquired acute cardiovascular conditions. Statistically significant interactions were noted for cardiac surgery and ncCCCs, trauma, infection, and other noncardiac surgery. Due to this finding, final models were stratified by cardiac surgery. SAS Version 9.4 (Cary, NC) and Stata 14 (College Station, TX) were used for analyses. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographics
Between April 1, 2003, and June 30, 2013, there were 120 884 children 0 to 17 years of age with a CPHD diagnosis (Table 2). VTE occurred in 957 (0.8%) of these children, 802 (83.8%) of which were isolated DVT and 155 (16.2%) were pulmonary embolisms with or without concomitant DVT. Although the age distribution differed for children with and without VTE (P < 0.0001), the mean age of the groups was not significantly different. The proportion of males was higher in children with VTE compared to those without (60.8% vs. 52.5%; P < 0.0001). In‐hospital mortality was considerably higher in children with VTE vs. those without (10.8% vs. 1.3%; P < 0.0001). The prevalence of VTE increased from 0.19% (2003) to 1.63% (2013), which is consistent with previously reported overall pediatric VTE prevalence data.4
Table 2.
Characteristics of children with CPHD (N = 120 884) with and without VTE in the MarketScan Commercial Databases for 2003‐2013
| VTE (n = 957) n (%) | No VTE (n = 119 927) n (%) | P value | |
|---|---|---|---|
| Age (y) | |||
| <1 | 501 (52.4) | 52 969 (44.2) | <0.0001 |
| 1‐4 | 58 (6.1) | 12 134 (10.1) | |
| 5‐9 | 43 (4.5) | 15 364 (12.8) | |
| 10‐14 | 123 (12.9) | 21 380 (17.8) | |
| 15‐17 | 232 (24.2) | 18 080 (15.1) | |
| Mean age (SD) | 6.0 (7.1) | 5.7 (6.3) | 0.27 |
| Male gender | 582 (60.8) | 62 918 (52.5) | <0.0001 |
| Type of CPHDa | |||
| Single‐ventricle physiology | 262 (27.4) | 11 186 (9.3) | <0.0001 |
| Two‐ventricle physiology | 364 (38.0) | 71 836 (59.9) | <0.0001 |
| Cardiomyopathies | 37 (3.9) | 2255 (1.9) | <0.0001 |
| Conduction disorders and dysrhythmias | 291 (30.4) | 34 090 (28.4) | 0.18 |
| Acquired chronic cardiovascular conditions | 101 (10.6) | 5411 (4.5) | <0.0001 |
| In‐hospital mortality | 103 (10.8) | 1582 (1.3) | <0.0001 |
| Co‐occurring conditions and procedures | |||
| Noncardiac complex chronic conditions | 618 (64.6) | 25 815 (21.5) | <0.0001 |
| Neuromuscular | 242 (25.3) | 7093 (5.9) | <0.0001 |
| Respiratory | 165 (17.2) | 5398 (4.5) | <0.0001 |
| Renal | 84 (8.8) | 2207 (1.8) | <0.0001 |
| Gastrointestinal | 79 (8.3) | 2032 (1.7) | <0.0001 |
| Hematology and immunodeficiency | 77 (8.1) | 1699 (1.4) | <0.0001 |
| Metabolic | 93 (9.7) | 2297 (1.9) | <0.0001 |
| Other congenital or genetic defect | 220 (23.0) | 10 183 (8.5) | <0.0001 |
| Malignancy | 153 (16.0) | 3621 (3.0) | <0.0001 |
| Recent trauma | 182 (19.0) | 6502 (5.4) | <0.0001 |
| Recent infection | 370 (38.7) | 9725 (8.1) | <0.0001 |
| Recent noncardiac surgery | 478 (50.0) | 5663 (4.7) | <0.0001 |
| Recent cardiac surgery | 511 (53.4) | 4106 (3.4) | <0.0001 |
| Acquired acute cardiovascular conditions | 123 (12.9) | 1724 (1.4) | <0.0001 |
| Evidence of end‐stage cardiac disease | |||
| Heart transplantation | 4 (0.4) | 1 (0.0) | <0.0001 |
| Extracorporeal membranous oxygenation | 22 (2.3) | 83 (0.07) | <0.0001 |
CPHD, chronic pediatric heart disease; SD, standard deviation; VTE, venous thromboembolism.
Categories not mutually exclusive.
With the exception of the Conduction Disorders and Dysrhythmias category, the distribution of each CPHD type was significantly different for children with and without VTE (Table 2). The prevalence of VTE in children with single‐ventricle physiology was 2.3%, whereas the prevalence of VTE in other categories ranged from 0.5% to 1.9% (Figure 1). Children who experienced VTE were significantly more likely to have an ncCCC (64.6% vs. 21.5%; P < 0.0001), recent trauma (19.0% vs. 5.4%; P < 0.0001), recent noncardiac surgery (50.0% vs. 4.7%; P < 0.0001), recent cardiac surgery (53.4% vs. 3.4%; P < 0.0001), or an acute acquired cardiovascular condition (12.9% vs. 1.4%; P < 0.0001) than children without VTE. VTEs were also more frequent in patients who underwent heart transplant (80%; 4 of 5), recognizing the small sample size, or recent ECMO (21%; 22 of 105) (P < 0.0001).
Figure 1.
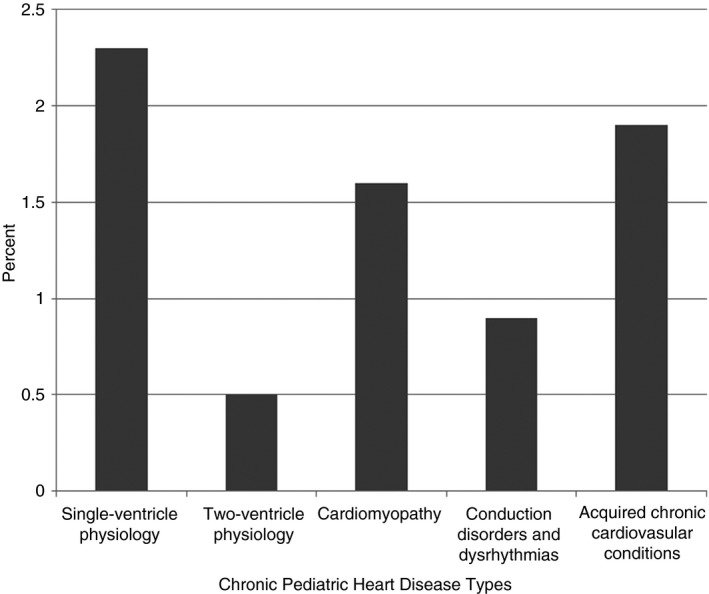
Rate of VTE in children with chronic pediatric heart disease by type
3.2. Multivariable analysis
Age, ncCCC, recent trauma, recent infection, recent noncardiac surgery, and acquired acute cardiovascular conditions were significantly associated with VTE after stratifying for the presence or absence of recent cardiac surgery (Table 3). The magnitude of the association between VTE and presence of an ncCCC was considerably larger for children without recent cardiac surgery vs. those with (adjusted OR [aOR], 4.33 [95% CI, 3.54‐5.30] vs. 1.34 [95% CI, 1.09‐1.65]). Similar patterns were observed for the association between VTE and recent trauma, recent infection, and acquired cardiovascular conditions. The presence of a recent noncardiac surgery was associated with a higher VTE risk for children, regardless of whether they had undergone recent cardiac surgery (aOR, 4.18 [95% CI, 3.36‐5.19] and 3.11 [95% CI, 2.45‐3.95]), respectively.
Table 3.
Distribution of VTE risk factors stratified for recent cardiac surgery
| Crude OR | 95% CI | P value | Adjusteda OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Recent cardiac surgery = yes (n = 4617) | ||||||
| Age (y) | ||||||
| <1 | 1.91 | 1.53‐2.39 | <0.0001 | 3.07 | 2.40‐3.91 | <0.0001 |
| 1‐14 | 1.00 | 1.00 | ||||
| 15‐17 | 1.33 | 0.99‐1.77 | 0.06 | 1.40 | 1.03‐1.89 | 0.03 |
| Noncardiac complex chronic conditions | 1.68 | 1.38‐2.05 | <0.0001 | 1.34 | 1.09‐1.65 | 0.006 |
| Recent trauma | 1.61 | 1.28‐2.03 | <0.0001 | 1.79 | 1.37‐2.34 | <0.0001 |
| Recent infection | 2.05 | 1.70‐2.47 | <0.0001 | 1.90 | 1.56‐2.32 | <0.0001 |
| Recent noncardiac surgery | 3.91 | 3.18‐4.80 | <0.0001 | 4.18 | 3.36‐5.19 | <0.0001 |
| Acquired acute cardiovascular conditions | 2.76 | 2.11‐3.60 | <0.0001 | 2.60 | 1.95‐3.48 | <0.0001 |
| Recent cardiac surgery = no (n = 116 267) | ||||||
| Age | ||||||
| <1 | 1.74 | 1.37‐2.20 | <0.0001 | 1.54 | 1.21‐1.96 | 0.0005 |
| 1‐14 | 1.00 | 1.00 | ||||
| 15‐17 | 3.68 | 2.86‐4.74 | <0.0001 | 3.73 | 2.88‐4.82 | <0.0001 |
| Noncardiac complex chronic conditions | 5.97 | 4.93‐7.22 | <0.0001 | 4.33 | 3.54‐5.30 | <0.0001 |
| Recent trauma | 3.92 | 3.07‐5.01 | <0.0001 | 2.88 | 2.21‐3.77 | <0.0001 |
| Recent infection | 5.79 | 4.74‐7.08 | <0.0001 | 3.59 | 2.91‐4.44 | <0.0001 |
| Recent noncardiac surgery | 8.47 | 6.78‐10.59 | <0.0001 | 3.11 | 2.45‐3.95 | <0.0001 |
| Acquired acute cardiovascular conditions | 7.94 | 5.74‐10.99 | <0.0001 | 3.65 | 2.60‐5.13 | <0.0001 |
CI, confidence interval; OR, odds ratio; VTE, venous thromboembolism.
Adjusted for all covariates in this table.
We further examined differences in the type of chronic conditions among patients with and without VTE, stratified by recent cardiac surgery (Table 4). All of the ncCCCs assessed in this analysis were significantly associated with VTE in the cohort of patients who did not have recent cardiac surgery. For patients with ncCCC and recent cardiac surgery, significant VTE association was seen with all ncCCCs except gastrointestinal disease (P = 0.09) and malignancy (P = 0.22) (Table 4).
Table 4.
Distribution of noncardiac CCC types stratified for recent cardiac surgery
| Recent cardiac surgery = yes | Recent cardiac surgery = no | |||||
|---|---|---|---|---|---|---|
| VTE (n = 511) n (%) | No VTE (n = 4106) n (%) | P value | VTE (n = 446) n (%) | No VTE (n = 115 821) n (%) | P value | |
| Noncardiac complex chronic conditions | 349 (68.3) | 2306 (56.2) | <0.0001 | 269 (60.3) | 23 509 (20.3) | <0.0001 |
| Neuromuscular | 129 (25.2) | 759 (18.5) | 0.0003 | 113 (25.3) | 6334 (5.5) | <0.0001 |
| Respiratory | 92 (18.0) | 581 (14.2) | 0.02 | 73 (16.4) | 4817 (4.2) | <0.0001 |
| Renal | 51 (10.0) | 234 (5.7) | 0.0001 | 33 (7.4) | 1973 (1.7) | <0.0001 |
| Gastrointestinal | 50 (9.8) | 314 (7.6) | 0.09 | 29 (6.5) | 1718 (1.5) | <0.0001 |
| Hematology and immunodeficiency | 44 (8.6) | 197 (4.8) | 0.0003 | 33 (7.4) | 1502 (1.3) | <0.0001 |
| Metabolic | 51 (10.0) | 258 (6.3) | 0.002 | 42 (9.4) | 2039 (1.8) | <0.0001 |
| Other congenital or genetic defect | 138 (27.0) | 627 (15.3) | <0.0001 | 82 (18.4) | 9556 (8.2) | <0.0001 |
| Malignancy | 81 (15.8) | 741 (18.1) | 0.22 | 72 (16.1) | 2880 (2.5) | <0.0001 |
CCC, complex chronic condition; VTE, venous thromboembolism.
3.3. Health care utilization and expenditures
All measures of health care utilization were higher for patients with VTE vs. those without VTE (Table 5). For example, children with VTE had over 3.5‐times more admissions (2.2 vs. 0.6; P < 0.0001) and they stayed in the hospital more than 6‐times longer (55.1 vs. 8.7 days; P < 0.0001). Likewise, children with VTE had 2.5 times the number of outpatient visits than those without VTE (28.1 vs. 11.2; P < 0.0001) and filled almost 3 times more prescriptions (13.3 vs. 4.7; P < 0.0001). After adjustment, VTE diagnosis was associated with more than 2‐fold higher total mean expenditures compared to the non‐VTE group ($93 780 vs. $42 725; P < 0.0001).
Table 5.
Health care utilization and expenditures for CPHD with and without VTE (6 mo following first CPHD diagnosis)
| VTE | No VTE | P value | |||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| Utilization | |||||
| Inpatient admissions (n) | 2.2 (1.7) | 2 | 0.6 (1.0) | 0 | <0.0001a |
| Length of stay (d) | 55.1 (62.7) | 31 | 8.7 (24.7) | 0 | <0.0001a |
| Outpatient visits (n) | 28.1 (23.4) | 23 | 11.2 (12.9) | 7 | <0.0001a |
| Pharmaceutical claims (n) | 13.3 (14.0) | 9 | 4.7 (7.3) | 2 | <0.0001a |
| Expendituresb | |||||
| Inpatient admissions | $316 379 (465 967) | $113 386 | $29 678 (118 303) | $0 | <0.0001a |
| Outpatient visits | $25 866 (43 160) | $13 570 | $6 838 (18 085) | $2556 | <0.0001a |
| Pharmaceutical claims | $2561 (6835) | $486 | $585 (3322) | $36 | <0.0001a |
| Total expenditures | $344 807 (471 323) | $152 339 | $37 102 (123 180) | $4517 | <0.0001 |
| Total expenditures (adjusted)b | $93 780 (235 613) | $51 543 | $42 725 (107 343) | $23 483 | <0.0001 |
CPHD, chronic pediatric heart disease; SD, standard deviation; VTE, venous thromboembolism.
Wilcoxon rank‐sum test.
Estimate adjusted for age, noncardiac complex chronic conditions, recent trauma, recent infection, recent cardiac surgery, recent noncardiac surgery, and acquired acute cardiovascular conditions.
To determine if these utilization patterns were driven by the higher acuity of single‐ventricle physiology rather than VTE, we confirmed these observations in the single‐ventricle subcohort (Table 6). Similar to the overall CPHD group, patients with single‐ventricle physiologywith VTE had 2.5 times more inpatient admissions (2.4 vs. 0.95; P < 0.0001), 5.5 times longer hospital stays (80.8 vs. 14.6; P < 0.0001), 1.9 times more outpatient visits (26.6 vs. 14.3; P < 0.0001), and 1.6 times more prescription claims (10.3 vs. 6.3; P < 0.0001). After adjustment, total mean expenditures for children with VTE were 1.5 times those of children without VTE ($195 577 vs. $126 878; P = 0.003).
Table 6.
Single‐ventricle CPHD patient health care utilization and expenditures with and without VTE (6 mo following first CPHD diagnosis)
| VTE (n = 262) | No VTE (n = 11 186) | P value | |||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| Utilization | |||||
| Inpatient admissions (n) | 2.4 (1.7) | 2 | 0.95 (1.3) | 1 | <0.0001a |
| Length of stay (d) | 80.8 (73.3) | 60 | 14.6 (33.9) | 1 | <0.0001a |
| Outpatient visits (n) | 26.6 (22.5) | 23 | 14.3 (15.5) | 9 | <0.0001a |
| Pharmaceutical claims (n) | 10.3 (11.0) | 7 | 6.3 (8.6) | 3 | <0.0001a |
| Expendituresb | |||||
| Inpatient admissions | $589 200 (594 474) | $411 401 | $83.998 (219 937) | $363 | <0.0001a |
| Outpatient visits | $25 089 (42 229) | $15 136 | $10 862 (125 113) | $4151 | <0.0001a |
| Pharmaceutical claims | $1494 (3098) | $259 | $725 (3100) | $56 | <0.0001a |
| Total expenditures | $615 783 (596 234) | $436 395 | $95 586 (226 742) | $11 137 | <0.0001 |
| Total expenditures (adjusted)b | $195 577 (444 167) | $91 881 | $126 878 (288 147) | $59 607 | 0.003 |
CPHD, chronic pediatric heart disease; SD, standard deviation; VTE, venous thromboembolism.
Wilcoxon rank‐sum test.
Estimate adjusted for age, noncardiac complex chronic conditions, recent trauma, recent infection, recent cardiac surgery, recent noncardiac surgery, and acquired acute cardiovascular conditions.
4. DISCUSSION
In this large cohort of children with CPHD, approximately 0.8% were affected by VTE. This is lower than the previously reported VTE prevalence in pediatric heart disease (1.3%‐2.4%), but this is likely reflective of the inclusion of lower‐risk CPHD conditions such as conduction disorders.2, 4, 6, 7 The observed prevalence was similar to previous reports for specific CPHD conditions (ie, single‐ventricle and cardiomyopathy).6, 7 We found that conditions reflecting single‐ventricle physiology had the highest prevalence of CPHD‐associated VTE (2.3%), whereas previous reports suggest that children with cardiomyopathy are most likely to develop VTE.6, 7
Approximately 65% of children experiencing a VTE also had an ncCCC, which is similar to previous reports on VTE in children with chronic renal disease.16 All of the co‐occurring conditions, including acute conditions and procedures, were significantly associated with VTE, and VTE occurred most frequently in children with an ncCCC and recent surgery (cardiac and noncardiac). The prevalence of VTE was much higher in children with recent cardiac and noncardiac surgery (11.1% and 7.8%, respectively), suggesting that recent surgery poses substantial VTE risk. VTE was also associated with significantly increased in‐hospital mortality and higher health care resource utilization and costs, although other unmeasured comorbidities among children with VTE may also influence these parameters.
Over 53% of patients with VTE had a recent cardiac surgery, and 50% had a recent noncardiac surgery in this study. VTE as a complication following surgery has been understood for many years, yet very few data exist on the incidence, risk factors, and outcomes specifically regarding recent cardiac and noncardiac surgery in children with CPHD.5, 20 There is recent evidence showing that the rate of recognized VTE in congenital heart disease following cardiac surgery is increasing and that it is associated with younger age (<28 days).21 In our study, recent cardiac surgery seemed to interact with many of the other risk factors, suggesting that it may have accounted for a large proportion of increased VTE risk in patients with multiple risk factors. When stratifying for recent cardiac surgery, the adjusted effect estimates for associations between VTE and age, ncCCC, acute trauma, acute infection, and acquired acute cardiovascular conditions were smaller in magnitude compared with the estimates among children without recent cardiac surgery. Likewise, when the distribution of ncCCCs were examined among children with and without VTE diagnoses and stratified by recent cardiac surgery, all ncCCCs were significantly associated with VTE if no recent cardiac surgery had been performed, but with recent cardiac surgery some of the ncCCCs were no longer significantly associated with VTE. Interestingly, in the adjusted models, the risk for VTE development in children with both a recent cardiac and noncardiac surgery was higher than those with only a recent noncardiac surgery, suggesting a synergistic effect in children that require both cardiac and noncardiac surgeries.
Recent infections are a known VTE risk factor and our findings confirmed this association. A distinct bimodal age distribution was noted, with the <1‐year‐old age group having the highest prevalence, consistent with previous reports.1, 2 Significant coagulopathy has been described in CPHD, especially children with congenital heart disease, who have been shown to have significantly lower natural anticoagulant levels compared to age matched controls, both at baseline and following surgical repair.22, 23, 24, 25, 26 Although many VTE risk factors have been identified in children with CPHD, our data suggests that recent surgery, especially recent cardiac surgery, may play the most formidable role in VTE development. Thus, optimizing care for all other acute risk factors before proceeding with any surgical procedure may help reduce the risk of VTE development in this already high‐risk population.
All health care utilization measures were higher for patients with CPHD and VTE vs. those without VTE, which is similar to previous findings in children undergoing surgery for congenital heart disease and those with chronic kidney disease.15, 21 Although children with single‐ventricle physiology likely require the highest acuity of care among children with heart disease, VTE was still associated with substantially higher resource utilization. Longer cumulative central venous catheter (CVC) use, baseline oxygen saturations <85%, and history of previous thrombus are all significant VTE risk factors seen in association with cardiac surgery.9 Postoperative ECMO and heart transplantation have been shown to correlate with the likelihood of VTE, which was also demonstrated in this study.9 Prolonged hospital stays have been associated with increased risk of VTE development, and VTE has been associated with significantly longer intensive care unit and overall hospital stays, as well as a higher likelihood of developing cardiac arrest, requiring cardiac catheter reintervention, and/or reoperation.9 These clinical findings may, in part, explain the much higher health care costs and utilization among CPHD patients with VTE, but it remains unclear whether the higher expenditures are due to the VTE or the fact that the patients may have been generally more ill. Nonetheless, optimizing care to reduce and manage VTE risk factors for children with CPHD, especially single‐ventricle congenital heart disease, is important to prevent morbidity and mortality and potentially reduce health care utilization and expenditures.
Although in‐hospital mortality was significantly higher in patients with CPHD and VTE, it is not possible to determine mortality causes with secondary analyses of claims data. Thus, these observations may reflect that VTEs are more likely among the most critically ill children. However, up to 21% of deaths in CPHD patients undergoing cardiac surgery have been directly attributed to VTE development and higher mortality rates (12.3% vs. 0.8%; P < 0.001) have been described previously in patients with congenital heart disease who develop VTE compared to those that do not.20, 21
This study was designed to detect VTEs that were reported with a concurrent CPHD diagnosis or in the 6 months following the CPHD diagnosis; however, because hemostatic abnormalities may persist, especially in congenital heart disease, late and/or recurrent VTEs may not have been captured in these analyses.25 The study strategy also excluded the ability to capture recurrent or subsequent VTEs, so the influence of this complication on the utilization data could not be ascertained. Also, the CPHD categories were not mutually exclusive, suggesting that overlap between the groups may exist.
Observational studies are subject to selection bias. Misclassification in regards to CPHD and/or VTE diagnosis due to insufficient sensitivity and specificity with ICD‐9‐CM coding systems could result in information bias.27, 28 To limit this bias, multiple codes, including CPT codes, were used to help discern appropriate CPHD diagnoses and eliminate overlap in the various groups, and multiple diagnostic identifiers were used to aid in identifying VTE, as previously described.15 It is important to note that this data set is representative of commercially insured individuals and does not include children enrolled in Medicaid. Thus, our findings may be generalizable only to privately insured children. Also, certain known pediatric VTE risk factors, including thrombophilia and CVC placement, cannot be accurately discerned from claims data. CVCs are often used to care for children with various forms of CPHD and, thus, may play an important role in CPHD‐related VTE. As such, residual confounding due to unmeasured confounders may have influenced our results. Future epidemiologic research of CPHD‐associated VTE should focus on defining the clinical attributes (ie, CVC type and anatomic location, infections, age, etc.) within the high‐risk groups defined here to determine which CPHD patients are most likely to benefit from thromboprophylactic interventions.
In summary, this study demonstrates that VTE in CPHD patients is a life‐threatening complication of CPHD that also significantly impacts health care resource utilization and cost. Despite the limitation that confounding for illness severity cannot be completely controlled for in a claims data study, these data reveal important information regarding VTE risk stratification in children with CPHD. In particular, those undergoing surgical procedures, especially cardiac surgery, are at greatest risk for VTE development, which may inform future VTE prevention approaches. These data indicate that children with CPHD who require a surgical procedure, especially with the presence of other comorbid illnesses including ncCCC, may benefit from randomized trials of thromboprophylactic interventions.
RELATIONSHIP DISCLOSURE
BAK received research funding from CSL Behring Foundation and Novo Nordisk A/S unrelated to this study. The other authors state that they have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
All of the authors have seen and approved the final version of the manuscript and are willing to accept responsibility for this work. The first author, Dr. Woods, worked with Drs. Kerlin and Boulet to conceive the study, participated in data analysis, and functioned as the main author of the manuscript. Dr. Boulet helped conceive the study, collected the data, performed the statistical analyses, and participated in drafting and revising the manuscript. Drs. Texter and Yates participated in drafting and revising the manuscript. Dr. Kerlin conceived the study and both supervised and participated in data collection and analysis, and participated in drafting and revising the manuscript.
Supporting information
ACKNOWLEDGMENTS
BAK is supported by funding from the National Institutes of Health (K08DK103982 and L40DK103299).
Woods GM, Boulet SL, Texter K, Yates AR, Kerlin BA. Venous thromboembolism in chronic pediatric heart disease is associated with substantial health care burden and expenditures. Res Pract Thromb Haemost. 2019;3:372–382. 10.1002/rth2.12205
Contributor Information
Gary M. Woods, Email: gary.woods@choa.org.
Bryce A. Kerlin, @scouter_trp843.
REFERENCES
- 1. Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7. [PubMed] [Google Scholar]
- 2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two‐year registry in The Netherlands. J Pediatr. 2001;139:676–81. [DOI] [PubMed] [Google Scholar]
- 3. Boulet SL, Grosse SD, Thornburg CD, Yusuf H, Tsai J, Hooper WC. Trends in venous thromboembolism‐related hospitalizations, 1994‐2009. Pediatrics. 2012;130:e812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. [DOI] [PubMed] [Google Scholar]
- 5. Setty BA, O'Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer. 2012;59:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gurgey A, Ozyurek E, Gumruk F, Celiker A, Ozkutlu S, Ozer S, et al. Thrombosis in children with cardiac pathology: frequency of factor V Leiden and prothrombin G20210A mutations. Pediatr Cardiol. 2003;24:244–8. [DOI] [PubMed] [Google Scholar]
- 7. Alioglu B, Avci Z, Tokel K, Atac FB, Ozbek N. Thrombosis in children with cardiac pathology: analysis of acquired and inherited risk factors. Blood Coagul Fibrinolysis. 2008;19:294–304. [DOI] [PubMed] [Google Scholar]
- 8. Takemoto CM, Sohi S, Desai K, Bharaj R, Khanna A, McFarland S, et al. Hospital‐associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164:332–8. [DOI] [PubMed] [Google Scholar]
- 9. Manlhiot C, Menjak IB, Brandao LR, Gruenwald CE, Schwartz SM, Sivarajan VB, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124:1511–9. [DOI] [PubMed] [Google Scholar]
- 10. Tran M, Shein SL, Ji X, Ahuja SP. Identification of a “VTE‐rich” population in pediatrics: critically ill children with central venous catheters. Thromb Res. 2018;161:73–7. [DOI] [PubMed] [Google Scholar]
- 11. Hanson SJ, Punzalan RC, Christensen MA, Ghanayem NS, Kuhn EM, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children with cardiac disease. Pediatr Cardiol. 2012;33:103–8. [DOI] [PubMed] [Google Scholar]
- 12. Petaja J, Peltola K. Venous thrombosis in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1997;11:889–94. [DOI] [PubMed] [Google Scholar]
- 13. Petaja J, Lundstrom U, Sairanen H, Marttinen E, Griffin JH. Central venous thrombosis after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;112:883–9. [DOI] [PubMed] [Google Scholar]
- 14. Hansen LG, Chang S. Health research data for the real world: the Thomson Reuters MarketScan® databases. Thomson Reuters; 2011. [Google Scholar]
- 15. Kerlin BA, Smoyer WE, Tsai J, Boulet SL. Healthcare burden of venous thromboembolism in childhood chronic renal diseases. Pediatr Nephrol. 2015;30:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boulet SL, Amendah D, Grosse SD, Hooper WC. Health care expenditures associated with venous thromboembolism among children. Thromb Res. 2012;129:583–7. [DOI] [PubMed] [Google Scholar]
- 17. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–7. [DOI] [PubMed] [Google Scholar]
- 18. American College of Surgeons . National Trauma Data Bank (NTDB). [Accessed 2018 September 01] Available from https://www.facs.org/quality-programs/trauma/tqp/centerprograms/ntdb
- 19. Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey, HC‐120: Appendix 3, Clinical Classification Code to ICD‐9‐CM Code Crosswalk. [Accessed 2018 September 01] Available from https://meps.ahrq.gov/data_stats/download_data/pufs/h104/h104doc.pdf#search=infectious%20diseaseURLPERC;
- 20. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;128:2622–703. [DOI] [PubMed] [Google Scholar]
- 21. Silvey M, Hall M, Bilynsky E, Carpenter SL. Increasing rates of thrombosis in children with congenital heart disease undergoing cardiac surgery. Thromb Res. 2018;162:15–21. [DOI] [PubMed] [Google Scholar]
- 22. Odegard KC, McGowan FX Jr, DiNardo JA, Castro RA, Zurakowski D, Connor CM, et al. Coagulation abnormalities in patients with single‐ventricle physiology precede the Fontan procedure. J Thorac Cardiovasc Surg. 2002;123:459–65. [DOI] [PubMed] [Google Scholar]
- 23. Odegard KC, McGowan FX Jr, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Coagulation factor abnormalities in patients with single‐ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. 2002;73:1770–7. [DOI] [PubMed] [Google Scholar]
- 24. Odegard KC, Zurakowski D, DiNardo JA, Castro RA, McGowan FX Jr, Neufeld EJ, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through Fontan completion. J Thorac Cardiovasc Surg. 2009;137:934–41. [DOI] [PubMed] [Google Scholar]
- 25. Odegard KC, Zurakowski D, Hornykewycz S, DiNardo JA, Castro RA, Neufeld EJ, et al. Evaluation of the coagulation system in children with two‐ventricle congenital heart disease. Ann Thorac Surg. 2007;83:1797–803. [DOI] [PubMed] [Google Scholar]
- 26. Jahangiri M, Kreutzer J, Zurakowski D, Bacha E, Jonas RA. Evaluation of hemostatic and coagulation factor abnormalities in patients undergoing the Fontan operation. J Thorac Cardiovasc Surg. 2000;120:778–82. [DOI] [PubMed] [Google Scholar]
- 27. White RH, Garcia M, Sadeghi B, Tancredi DJ, Zrelak P, Cuny J, et al. Evaluation of the predictive value of ICD‐9‐CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–7. [DOI] [PubMed] [Google Scholar]
- 28. Branchford BR, Gibson E, Manco‐Johnson MJ, Goldenberg NA. Sensitivity of discharge diagnosis ICD‐9 codes for pediatric venous thromboembolism is greater than specificity, but still suboptimal for surveillance and clinical research. Thromb Res. 2012;129:662–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


