Abstract
Pulmonary embolism (PE) is a major cause of morbidity and mortality in the United States. Although new therapeutic tools and strategies have recently been developed for the diagnosis and treatment of patients with PE, the outcomes for patients who present with massive or high‐risk PE remain dismal. To address this crisis, pulmonary embolism response teams (PERTs) are being created around the world in an effort to immediately and simultaneously engage multiple specialists to determine the best course of action and coordinate the clinical care for patients with acute PE. The scope of this review is to describe the PERT model and purpose, present the structure and organization, examine the available evidence for efficacy and usefulness, and propose future directions for research that is needed to demonstrate the value of PERT and determine if this multidisciplinary approach represents a new standard of care.
Keywords: advanced therapies, catheter‐directed thrombolysis, follow‐up care, multidisciplinary, pulmonary embolism, pulmonary embolism response team
Essentials.
Pulmonary embolism (PE) is a major cause of morbidity and mortality in the United States, and the management of acute PE remains poorly standardized.
PE response teams (PERTs) are being established around the world to provide rapid, individualized, and expert‐based care for patients with acute PE.
Preliminary results from early adopters suggest that PERTs feasible and facilitates access to advanced therapies.
Research is needed to determine if the PERT approach improves survival, reduces long‐term complications, and is cost‐effective.
1. INTRODUCTION
Pulmonary embolism (PE) is a major cause of morbidity and mortality.1, 2, 3 In the United States, there are approximately 900 000 cases of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and PE, every year. Of those, 150 000 to 250 000 are PE‐related hospitalizations and 60 000 to 100 000 deaths, making it the third most common cause of cardiovascular death.4, 5 The diagnosis and treatment of acute PE can be challenging, as it has a heterogenous range of presentations, from an asymptomatic incidentally identified PE to one that causes hemodynamic instability or even sudden death. Over the past decade, there has been a surge of new therapeutic tools and strategies designed to treat PE patients. Despite these developments, the mortality rate for patients who present with massive or high‐risk PE remains high.6, 7 Additionally, there is a lack of robust evidence investigating or comparing each strategy as well as current standardized guidelines in the treatment of acute PE. Consequently, treatment decisions are often based on expert opinion, which can be inconsistent and variable. To address this need, multidisciplinary rapid response teams focused on PE have developed across the world. These pulmonary embolism response teams (PERTs) aim to expeditiously engage multiple experts simultaneously to generate a thoughtful, coordinated, and comprehensive treatment plan for each PE patient. Recently, these teams formed the PERT Consortium, an international nonprofit organization, whose goal is to guide and influence PE care. The purpose of this article is to define the PERT concept and purpose, describe the structure and operation, review the evidence for efficacy, and explore future directions for research that is needed to solidify the idea.
2. CASE PRESENTATION
A 64‐year‐old woman presented to the emergency department complaining of 1 week of cough and increasing dyspnea on exertion. Her symptoms had progressively worsened in the 24 hours before presentation, with associated scant hemoptysis and dull substernal chest pressure. She had a past medical history of hypertension, hypothyroidism, DVT, and PE 8 years prior but had been off anticoagulation for the past 6 months. On presentation, her vital signs were: temperature 37°C, blood pressure (BP) 109/56 mm Hg, heart rate 108 bpm, respiratory rate 22, and oxygen saturation of 92% on room air. Electrocardiogram showed sinus tachycardia. Labs were remarkable for a troponin of 0.46 ng/mL (normal <0.1 ng/mL). A contrast‐enhanced chest computed tomography angiogram was ordered (Figure 1) which showed a saddle PE with extensive extension into bilateral pulmonary arterial branches and segments, and an enlarged right ventricle. She was immediately started on unfractionated heparin infusion (UFH) and transferred to the intensive care unit (ICU) for closer observation. Although low‐molecular‐weight heparin with its rapid and reliable bioavailability and excellent safety profile was considered, her initial providers preferred UFH, as they believed it allowed for optimal management flexibility, especially since advanced therapies may be considered. On arrival at the ICU, the patient's vital signs were: temperature 36.1°C, BP 136/76 mm Hg, heart rate 140 bpm, respiratory rate 34, and oxygen saturation of 96% on 4‐L nasal cannula. On examination, she appeared to be in mild distress, tachypneic, tachycardic, with clear lungs sounds, and without lower extremity edema. A transthoracic echocardiogram was done promptly on arrival at the ICU (Figure 2). It showed severe right ventricular (RV) enlargement with hypokinesis, interventricular septal flattening, and McConnell's sign (RV free wall dysfunction with sparing of apex).
Figure 1.
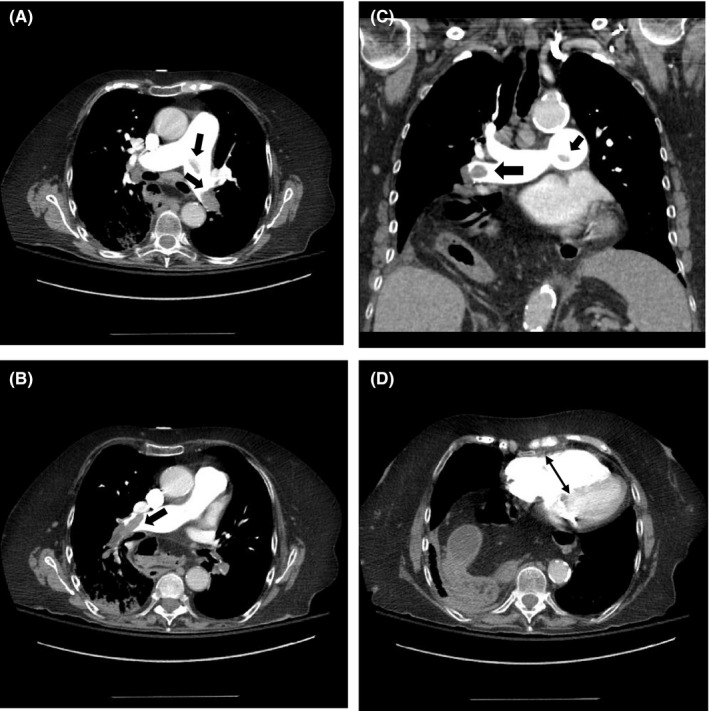
Contrast‐enhanced chest CTA showing saddle pulmonary embolism (thick arrows) (A–C), and enlarged right ventricle (thin arrow) (D). CTA, computed tomography angiography
Figure 2.
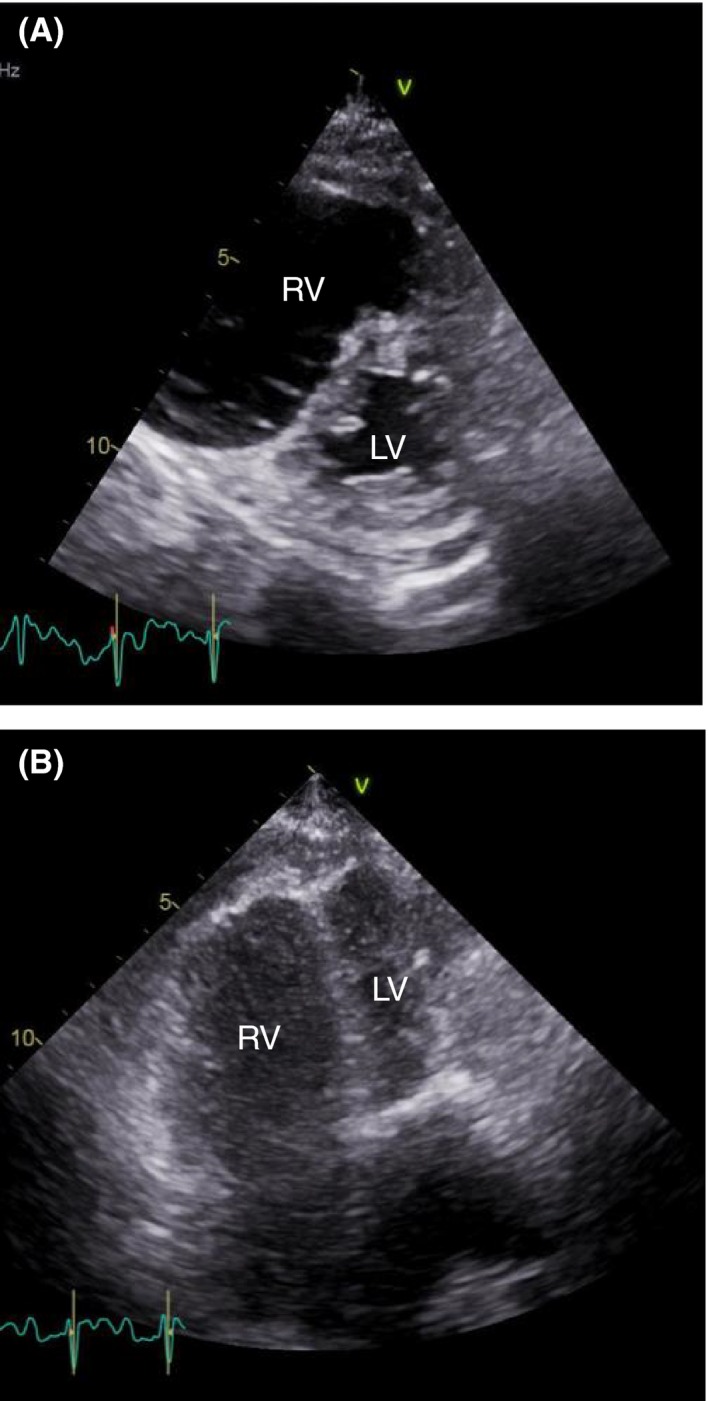
Transthoracic echocardiogram showing severe right ventricular enlargement and interventricular septal compression. LV, left ventricle; RV, right ventricle
The pulmonary and critical care fellow and attending physician evaluated the patient at the bedside and activated their PERT. After careful review of the patient's presenting history, comorbidities, bleeding risks, imaging, and labs, a multidisciplinary PERT meeting took place via telephone call, via a secure hospital call center. The meeting included members from PERT (fellow and attending), pulmonary and critical care team (fellow and attending), and interventional cardiology (attending) and had a duration of approximately 10 minutes. The patient was risk stratified as intermediate–high‐risk (submassive) PE, as she had signs of RV dilation on imaging and positive cardiac biomarkers. A detailed discussion ensued where each member of the team opined on various treatment options, reviewing the risks and benefits for this patient, and proposed a recommendation. Ultimately, a consensus decision was made to perform catheter‐directed thrombolysis (CDT). This plan was decided on and urgently executed given that the patient had an intermediate–high‐risk PE and signs of impending hemodynamic decompensation, based on clinical appearance, increasing heart rate (HR, 108‐140 bpm) and escalating supplemental oxygen requirements (O2 increased from room air to 4‐L nasal cannula), despite a few hours on therapeutic anticoagulation, adequate fluid resuscitation, and no contraindications to thrombolysis. An initial bolus of 1 mg of tissue plasminogen activator was given, followed by an infusion at 1 mg/h for 12 hours via bilateral catheters. UFH infusion was also continued with a low‐intensity protocol targeting an anti‐Xa range of 0.2 to 0.5 units/mL. The next morning, her vitals were: temperature 36.5°C, BP 142/82 mm Hg, heart rate 98 bpm, respiratory rate 18, oxygen saturation of 95% on 1‐L nasal canula. She appeared more comfortable and reported feeling better. The catheters were removed, hemoglobin remained stable, and she was transitioned to oral anticoagulation and discharged home on day 4.
3. DEFINITION AND PURPOSE OF PERT
This case illustrates the complexity with which PE patients can present and the urgency with which treatment decisions must be made. Recently, many promising and novel strategies have emerged to treat patients with acute PE. These treatments are wide ranging and include anticoagulation, systemic thrombolysis, CDT, catheter embolectomy, surgical embolectomy, and/or mechanical circulatory support devices. Deciding which therapy is most appropriate for each patient can be challenging. As demonstrated in this case, PERTs can bring together a multidisciplinary group of clinicians with expertise in the diagnosis and management of acute PE who collaborate to improve patient care. PERTs are often called to treat patients who present with submassive or massive PE; however, PERTs may also be helpful in determining the best course of treatment in low‐risk PE patients who have significant comorbidities such as intracranial hemorrhage where anticoagulation may be contraindicated. In the first 30 months at Massachusetts General Hospital (MGH), for example, the number of PERT activations for low‐risk PE patients increased over time, which may reflect the value of a multidisciplinary team approach in low‐risk but ill PE patients for whom management decisions are complex.8 It is important to emphasize that the initiation of anticoagulation is paramount in the treatment of PE and must not be delayed unless contraindicated. With the emergence of this new approach to treating PE, concurrent management recommendations need to be implemented simultaneously with PERT activations and discussions.
The optimal structure of a PERT remains unknown, and its members vary by institution.9, 10 It may involve critical care, pulmonary medicine, vascular medicine, emergency medicine, interventional and noninterventional cardiology, interventional radiology, vascular surgery, cardiac surgery, hematology, and pharmacy (Figure 3). Each member has a crucial role in PERT. The initial steps in acute PE care, such as diagnosis and risk stratification, are often managed by emergency medicine specialists, although acute PE may also be diagnosed after a patient has been admitted. Then, either critical care, pulmonary medicine, vascular medicine, or cardiology provide an initial PERT consultation and gather relevant patient information. Endovascular procedures, such as CDT or catheter embolectomy, if warranted, are usually provided by interventional cardiology or radiology or vascular surgery. If a surgical thrombectomy is indicated, this is done by cardiac surgery. The hematologist on the team can provide expertise in anticoagulation selection and, if appropriate and feasible, duration as well as thrombophilia evaluation, if indicated. Finally, a clinical pharmacist is usually overseeing to ensure that dosing of different drugs, such as thrombolytics and anticoagulants, are appropriate and adequate. Importantly, these roles may vary by sites and country.
Figure 3.
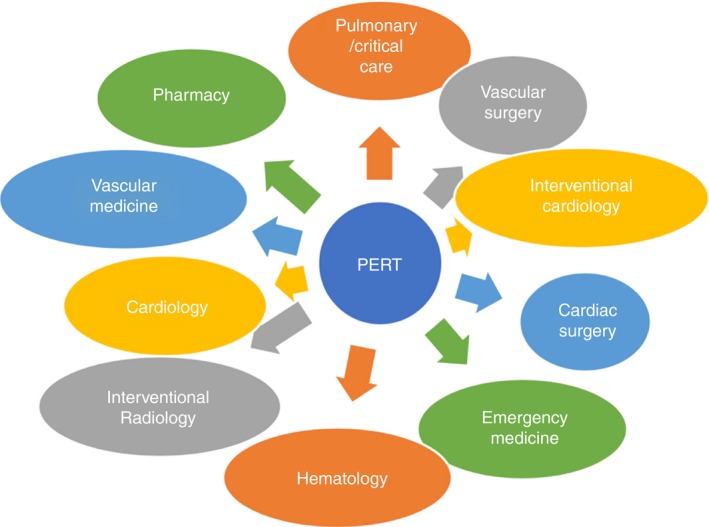
Example of key PERT participants. PERT, pulmonary embolism response team
The first PERT was created in 2012 at MGH.11 Thereafter, other hospitals formed their own PERTs. Given this rising interest, an inaugural meeting of over 30 institutions, convened on May 21, 2015, in Boston, Massachusetts, and the National PERT Consortium was established; the definition of a PERT was crafted and adopted (Table 1).12 Since this time, the Consortium has become international, with members from Europe, Asia, South America, and Australia. The vision of the PERT Consortium is to guide and influence PE care worldwide. With the goal of multicenter collaboration in education, research, and clinical guidelines, PERT created a framework of committees: Governance, Research, Education, Clinical Practice and Protocols, Development, and Communication. Each committee collaborates to create the infrastructure for advancement of PE care.
Table 1.
Definition of PERT according PERT Consortium Guidelines
PERT is an institutionally based multidisciplinary team that must meet the following criteria:
|
PERT, Pulmonary embolism response team.
4. STRUCTURE AND OPERATION OF PERT
There are many ways to create a PERT and the organization and structure of each one largely depends on the resources of the institution, the interests of its members, and the clinical demands of the community. Because the definition of PERT includes the ability to exercise a full range of medical, surgical, and endovascular therapies, several specialists are often involved in the system. A recent survey of 31 PERT programs found that pulmonary/critical care, interventional cardiology, and emergency medicine are the most commonly involved specialists, followed by cardiac surgery, interventional radiology, noninterventional cardiology, hematology, and vascular medicine.9, 10 Most PERTs have 3 to 5 specialists involved in their program, with 1 having 10 specialists. This survey also demonstrated that the institutional setting and organizational structure of each PERT varies. Although the majority of PERTs surveyed are in academic hospitals (71%), one quarter of the programs are in community hospitals. The size and management of teams also differ and range from a small intimate group to a large inclusive group and from 1 physician in charge of running the team to a steering committee overseeing the entire enterprise, respectively.
Similar to the diverse composition and organization of PERTs, the operation of each PERT differs across programs. Each PERT has an infrastructure that can rapidly identify appropriate patients, activate the team, and assemble necessary resources if advanced interventions are warranted. Often, teams will also arrange for comprehensive follow‐up care. Most programs have the entire multidisciplinary team respond to the initial PERT activation, and a tiered approach whereby a single physician consults on the initial response followed by a multidisciplinary team discussion for complex cases is less often practiced. The way teams communicate also vary. PERT‐specific or individual clinicians’ pagers and telephone alerts are used to notify the point person on the team of a potential patient. Teams then use phone calls, conference calls, or virtual meetings to discuss the case. Occasionally, and if feasible, the patient or a family member is included in those conversations. To date, there are 32 published descriptions or reviews of how various PERTs establish, organize, operate, and/or manage their programs (Table 2).9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 To identify published studies on PERT, we conducted a systematic search of the literature for publications in MEDLINE and EMBASE from inception to November 2018 using the following search strategy: (pulmonary) and (embolism) and (response) and (team or teams) or (PERT) or (PERTs). Articles were eligible for inclusion if the primary focus of the report was a description or review of PERTs. In total, we reviewed 68 published papers, and 32 were selected for inclusion.
Table 2.
Surveys and descriptive reviews of PERT programs
| Author | Year | Type of Article | Number of participants | Main findings |
|---|---|---|---|---|
| Surveys | ||||
| Todoran | 2018 | Survey: In‐person administered during the second annual meeting of PERT Consortium | 100 | Presentation of clinical practice questions and clinical vignettes. There was overall agreement with regard to criteria used for risk stratification of PE patients, but there was substantial variation in treatment strategies, the latter highlighting the needs for more clinical trial data. |
| Barnes | 2017 | Survey: Online to PERT Consortium members | 31 PERT institutions | Questions centered on core components of functioning PERTs (ie, type of institution, number of specialists on team, setup of activations). While all programs incorporate team‐based multidisciplinary care into their core structure, several different models exist with varying personnel and resource utilization. Understanding how different PERT programs impact clinical care remains to be investigated. |
| Barnes | 2016 | Survey: Online to PERT Consortium members | 31 PERT institutions | Questions pertaining to the organizational structure of PERT. Responses demonstrate the diversity of PERT programs, structure, and characteristics. |
| Descriptive reviews | ||||
| Porres‐Aguilar | 2018 | Review | N/A | Discussion of the current role and strategies on how to leverage the strength of PERTs and their possible adoption worldwide. |
| Rosovsky | 2018 | Review | N/A | Description of how to organize and structure a PERT, review of importance and reasons for creating a follow‐up clinic for PE patients after discharge, and exploration of how PERT programs are changing the landscape of PE treatment and may represent a new standard of care. |
| Friedman | 2018 | Review | N/A | Narrative of how PERT can be timely, unify recommendations, and optimize care for PE patients. |
| Rodríguez Chiaradía | 2018 | Review | N/A | Portrayal of role of pulmonologist in PERT. |
| Rali | 2018 | Review | N/A | Definition, risk stratification, management approach, and outcomes of submassive PE and the role of PERT in the management of these patients. |
| Root | 2018 | Review | N/A | Presentations of several cases to describe variations in PERTs currently in operation at different institutions as well as potential difficulties in forming a PERT. |
| Giri | 2018 | Review | N/A | Critical appraisal of current literature on PERT and a call for clinical outcome‐driven trials to justify implementation of the PERT model. |
| Nosher | 2017 | Review | N/A | Description of tools available for endovascular therapy of PE, with review of literature available to date on these methods and description of function of PERT. |
| Galmer | 2017 | Review | N/A | Report on how PERT programs are being creatively customized in terms of their methods of operation, team structures, and practice patterns to meet needs of individual institutions based on available resources, skills, personnel, and institutional goals. |
| Merli | 2017 | Review | N/A | Review of major trials using peripheral thrombolysis and insight into need for a team approach to pulmonary care (PERT), standardization of pulmonary classification, and need for trials designed for both short‐ and long‐term outcomes using thrombolysis for select PE populations. |
| Ozcinar | 2017 | Letter | N/A | Letter querying whether surgical pulmonary embolectomy can be performed with acceptable outcomes without a PERT. |
| Huisman | 2017 | Editorial | N/A | Discussion of potential role of PERTs in Dutch hospitals. Hypothesizing that main advantage of PERT could be uniform management strategy that is supported by a multidisciplinary team including all key specialists in treatment of severe PE. |
| Witkin | 2017 | Review | N/A | Description of rationale for and structure of PERTS, with focus on recognition and treatment of patients with persistent morbidity following PE, particularly those who may have symptomatic chronic pulmonary embolism or chronic thromboembolic pulmonary hypertension. |
| Zern | 2017 | Review | N/A | Review of initial experiences of MGH PERT, creation of the PERT Consortium and discussion of future directions. |
| Fasanya | 2017 | Review | N/A | Overview of venous thromboembolism and PERTs. |
| Serhal | 2017 | Review | N/A | Overview of treatment guidelines for PE and of results from recent clinical trials involving patients with submassive PE as well as an outline of Cleveland Clinic approach and use of PERT. |
| Kabrhel | 2017 | Review | N/A | Discussion of ways to integrate multiple specialists, with diverse perspectives and skills, into a cohesive PERT. Detailed description of purpose of forming a PERT, strengths of different PERT specialties, strategies to leverage these strengths to optimize participation and cooperation across team members, as well as unresolved challenges. |
| Dudzinski | 2017 | Review | N/A | Review of various modalities available to treat the many phenotypes of PE and how PERTs can combine expertise from many specialties to generate consensus for treatment plans. |
| Rodriguez‐Lopez | 2017 | Review | N/A | Description of PERTs. |
| Dudzinski | 2017 | Review | N/A | Description of start‐up, organization, and performance of PERTs for diagnosis and treatment of acute PE. |
| Huber | 2017 | Review | N/A | Description and discussion of the potential impact of a multidisciplinary treatment algorithm. |
| Monteleone | 2016 | Review | N/A | Case‐based approach to demonstrate how PERT concept and system generates a multidisciplinary treatment plan that encompasses goals and concerns of all clinicians involved and provides a forum for a coherent strategy to be vetted and carried out. |
| Witkin | 2016 | Review | N/A | Description and rationale for creation and implementation of PERTs. |
| Corrigan | 2016 | Review | N/A | Discussion of clinical challenges of PE diagnosis, risk stratification, and treatment that emergency physicians face every day and introduction of role of PERTs. |
| McDaniel | 2016 | Review | N/A | Description of PERTs. |
| Jaber | 2016 | Review | N/A | Discussion of the formation of PERTs and description of available treatment options beyond anticoagulation, with a focus on the interventional approach. |
| Dudzinski | 2016 | Review | N/A | Description of PERTs. |
| Reza | 2015 | Review | N/A | Description of PERTs, novel approach to PE care modeled after existing rapid response and collaborative teams. |
| Provias | 2014 | Review | N/A | One of the first descriptions of a PERT at MGH, detailing the structure and function, importance of research and educational activities, and the creation of the PERT Consortium. |
MGH, Massachusetts General Hospital; N/A, not applicable; PE, pulmonary embolism; PERT, pulmonary embolism response team.
As an example, to activate the PERT at MGH, any referring medical provider from either inside the hospital or an outside institution can call a 24‐hour/7‐days‐a‐week telephone number with the last 4 digits being PERT (7378). This call is then sent to the PERT fellow, who gathers pertinent information about the patient to help determine the severity of the PE (Figure 4). The fellow and attending physician of record will decide if the case warrants activation of the entire multidisciplinary team. If the whole team is needed, a page and email are sent to each specialist with a link to a web‐based virtual electronic meeting. During that meeting, the fellow outlines the patient's clinical course, and the team discusses various available diagnostic and treatment options. During that discourse, the team generates recommendations and then assembles the appropriate resources to carry out those recommendations, especially if advanced therapies are felt to be warranted. On discharge, patients are scheduled in a multidisciplinary follow‐up clinic to help bridge the gap between the patient's in‐hospital stay and their outpatient follow‐up care. During the follow‐up clinic visit, many issues can be addressed, including further inquiry into the cause of the PE, thrombophilia testing if appropriate, age‐appropriate cancer screening, anticoagulation management, scheduling removal of an inferior vena cava (IVC) filter if placed, and screening for long‐term PE complications such as pulmonary hypertension, post‐PE syndrome, and chronic thromboembolic pulmonary hypertension. An essential feature of each PERT is the ability to include nontraditional PERT members when necessary, such as an obstetrician for pregnant patients or a neurosurgeon for patients with intracranial hemorrhage. Creating an infrastructure that can accommodate all the various aspects of each PERT is critical for its success.
Figure 4.
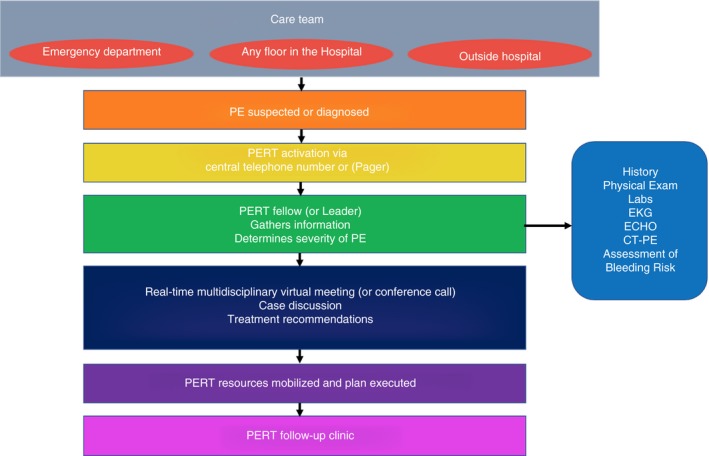
Activation of PERT at Massachusetts General Hospital. Parentheses indicate other ways to engage in a PERT activation besides what is done at Massachusetts General Hospital. PERT, pulmonary embolism response team
5. EVIDENCE FOR EFFICACY OF PERT
Most patients with PE are successfully treated with anticoagulation alone, and many will have no lasting consequences.41 However, there is a subset of patients at increased risk of rapid hemodynamic decompensation. Guidelines have been developed to help identify patients at risk for short‐term mortality and clinical deterioration. The American College of Chest Physicians,42 American Heart Association (AHA),43 and the European Society of Cardiology (ESC)44 recommend advanced therapy (other than anticoagulation alone) for patients with acute PE and sustained hypotension, classifying them as having massive PE (AHA) or at high risk (ESC); massive PE patients represent 5% of the PE population and have a 58% mortality risk.45 However, the appropriate management is less clear for normotensive patients with evidence of RV dysfunction and/or elevated cardiac biomarkers. These patients are classified as having submassive PE (AHA) or at intermediate risk (ESC); submassive PE patients represent 25% to 40% of the PE population with a mortality risk ranging from 2% to 3% to 21% at 3 months.45, 46 Both the AHA and ESC defer management to clinical judgment in patients with a submassive/intermediate‐risk PE, a decision that is not straightforward given patient complexity and potential risks of advanced therapy. To this end, PERTs may provide the necessary clinical expertise.
Survivors of acute PE are also at risk for lasting adverse effects.47, 48 In a recent prospective cohort study, almost half of PE patients have exercise limitation at 1 year, defined by percent‐predicted VO2max <80% on cardiopulmonary exercise testing, which influences their quality of life and degree of dyspnea.49, 50 Other cohort studies evaluating the degree of functional impairment following acute PE found that 45% to 52% of surviving patients exhibit a New York Heart Association heart failure score of ≥2 at 6 months to 3 years of follow‐up.51, 52, 53 It is believed that the elevated RV and pulmonary arterial pressures caused by occlusive thrombus cause cardiovascular damage and subsequent maladaptive remodeling, decreasing the ability to adapt to exercise. It has been postulated that the rapid unloading of the right ventricle and increased thrombus clearance through advanced therapy may help prevent such remodeling. The limited evidence has so far been inconsistent. Data from the MOPETT (Moderate Pulmonary Embolism Treated With Thrombolysis) trial showed significant immediate reduction in pulmonary arterial pressure that was maintained at 28 months’ follow‐up in PE patients receiving thrombolytic therapy vs. anticoagulation only.54 However, data from the PEITHO (Pulmonary Embolism International Thrombolysis Study) trial showed that thrombolytic treatment did not affect long‐term mortality rates, residual dyspnea, or RV dysfunction vs. anticoagulation alone.41
Data from a recent multicenter registry indicate that management of acute PE remains poorly standardized, and advanced therapy (other than anticoagulation alone) is underutilized. Only 2% of all patients with PE and 9% of patients with massive PE are treated with thrombolysis.3 The PERT model aims to streamline the care of patients with PE and, in particular, expeditiously identify patients with massive (high‐risk) or submassive (intermediate‐risk) PE who may benefit from advanced therapies and initiate and coordinate appropriate treatment.
It remains to be determined whether the PERT approach leads to improved survival, reduces long‐term complications, or is cost‐effective. PERT is still a novel concept. As such, there are no randomized controlled trial data or robust clinical evidence evaluating the PERT approach, though efforts are under way to bridge this gap.55 Current available data from 12 prospective and retrospective studies are summarized in Table 3.8, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 To identify these published studies on PERT, we conducted a similar systematic search of the literature as we did for Table 2. Articles were eligible for inclusion if the primary focus of the report was a prospective or retrospective study involving PERTs. In total, we reviewed 68 published papers, and 12 were selected for inclusion.
Table 3.
Retrospective and prospective studies of PERT programs
| Author | Year | Type of article | Study population | Time span | Treatments administered | Main findings |
|---|---|---|---|---|---|---|
| Rosovsky | 2018 | Interrupted time series analysis | 440 patients; 212 pre‐PERT and 228 post‐PERT | 10 y 2006‐2012 pre‐PERT and 2012‐2016 post‐PERT |
Pre‐PERT: Systemic intravenous thrombolysis (5%), CDT (1%) surgical thrombectomy (4%) Post‐PERT: Systemic intravenous thrombolysis (5%), CDT (14%), surgical thrombectomy (4%) |
More patients underwent catheter directed therapy (1% vs. 14%, P < 0.0001) or any advanced therapy (19 [9%] vs. 44 [19%], P = 0.002) post‐ PERT. Interrupted time series analysis demonstrated that this increase was sudden and coincident with implementation of PERT, and most noticeable among patients with submassive PE. There were no differences in major bleeding or mortality pre‐and post‐PERT implementation. |
| Al‐Bawardy | 2018 | Prospective cohort series | 13 patients with PERT activation who required ECMO within 3 d | Since initiation of PERT in 2012 | 8 patients received systemic thrombolysis, 3 received CDT, and 4 received surgical embolectomy (2/4 also had systemic thrombolysis) | Patients with massive PE who suffer cardiac arrest may undergo ECMO in conjunction with systemic thrombolysis or CDT, or as a bridge to surgical embolectomy. |
| Mahar | 2018 | Retrospective chart review | 134 patients | 1 y, 11 mo October 2014‐September 2016 | 65 (55%) patients received anticoagulation only, 14 (12%) CDT, 16 (13%) systemic half‐dose rtPA, 6 (5%) systemic full‐dose rtPA, 6 (5%) surgical embolectomy, and 4 (3%) mechanical thrombectomy. | The majority of PERT activations that took place were for intermediate‐risk PE (68%). There were no bleeding events among patients who received systemic half‐dose or full‐dose rtPA; however, 3 of the 14 patients receiving CDT experienced bleeding events. Overall, 8.3% of patients receiving thrombolytic therapy had bleeding events. |
| Elbadawi | 2018 | Questionnaire | Survey of 73 trainee physicians at large academic institution | Administered at end of academic year after PERT had been functioning for 1 y | Trainee physicians at a large academic institution perceived an enhanced educational experience while managing PE following PERT implementation. Comparing before and after PERT implementation, residents and fellows perceived enhanced confidence in identifying (P < 0.001), and managing (P = 0.003) submassive/massive PE, and increased knowledge of indications for systemic thrombolysis and surgical embolectomy (P = 0.04 and P < 0.001, respectively). Respondents self‐reported an increased fund of knowledge of high‐risk PE pathophysiology (77%), and 71% favored broad implementation of a PERT similar to an acute myocardial infarction team. | |
| Kolkailah | 2018 | Retrospective chart review | 133 patients with submassive PE | 14 y, 7 mo October 1999‐May 2015 | 62 (47%) patients received CDT, and 71 (53%) pulmonary embolectomy. | PERT helped determine the most appropriate treatment. Follow‐up echocardiography was performed in 61% of the cohort, 76.5% of which demonstrated resolution of RV dysfunction. |
| Sista | 2018 | Retrospective chart review | 124 patients | 1 y, 8 mo January 2013‐August 2014 | CDT was administered to 25 (20%) patients, systemic thrombolysis to 6 (5%), and anticoagulation alone to 54 (44%). | PERT activations increased after the first 10 mo, and the majority of activations were for patients with submassive PE (90.8%). Rates of bleeding and mortality did not correlate with treatment. Major bleeding occurred in 2 of 31 (6.4%) patients receiving thrombolytic therapy. |
| Carroll | 2017 | Retrospective registry review | 72 patients | 13 mo August 2015‐September 2016 | Patients were managed with anticoagulation alone in 65%, systemic thrombolysis in 11%, CDT in 18%, and ECMO in 3%. An IVC filter was placed in 15%. | The majority of PERT activations were for submassive PE (83%); 13% experienced a major bleed with no intracranial hemorrhage. Major bleeding occurred in 6% of patients receiving thrombolytic therapy. Survival to discharge was 89%. |
| Deadmon | 2017 | Prospective cohort series | 561 patients enrolled of which 446 had confirmed PE and location: 283 from ED, 100 from floors, 63 from ICUs | All PERT patients with telephone request for activation in longitudinal registry | Across all locations, 276 (66%) patients received anticoagulation alone, 48 (11.5%) CDT, and 20 (4.7%) systemic thrombolysis. ICU patients were most likely to be treated with thrombectomy or thrombolysis and had highest rates of IVC filter placement (34%). | PERT activations from different clinical locations (ED, floor, ICU) differ in terms of patient presentation, PE confirmation rates, treatments, and outcomes. Activations from the ED or floor were more likely to be for confirmed PE than from the ICU. Among confirmed PE, ICU patients had more severe PE with greater hemodynamic instability. PERTs should be customized to support the different needs of each clinical area. |
| McNeil | 2017 | Letter | 457 PERT activations; 317 during day and 140 at night | Not specified | CDT accounted for 81% of interventions in the night group but only 55% of the day group. Systemic thrombolysis and surgical embolectomy were more common in the day group. | No statistically significant difference in the median time to intervention, the rate of interventions within 24 h of activation, or 30‐d mortality between the day and night groups. |
| Kabrhel | 2016 | Retrospective cohort series | 394 patients | 2 y, 6 mo since initiation of PERT in 2012 | The majority (69%) were treated with anticoagulation alone. CDT was performed in 28 (9%) patients, systemic thrombolysis in 14 (5%), surgical thrombectomy in 8 (3%) patients, and suction thrombectomy in 1 (0.3%) patient. IVC filters were placed in 47 (15%) patients, and 8 (2%) patients were placed on ECMO. | The PERT paradigm was rapidly adopted with activations increasing 16% every 6 mo after implementation. Bleeding complications were similar among patients treated with CDT and anticoagulation alone, both 4%. |
| Bloomer | 2015 | Retrospective chart review | 31 patients treated with CDT | 2 y, 5 mo January 2012‐May 2014 | All 31 patients were treated with CDT. | Report of an innovative treatment approach to 31 patients with acute PE that incorporated a PERT and implemented a regional referral system to facilitate patient transport and reduce time to intervention. |
| Kabrhel | 2013 | Retrospective review | 30 patients | 12 wk since initiation of PERT in 2012 | 2 (8%) received CDT, 5 (20%) had an IVC filter placed. | The first published description of the novel PERT at MGH. The initial experience suggests that an innovative, multidisciplinary PERT can streamline the care of patients with severe PE and that there is high demand for this approach. |
CDT, catheter‐directed thrombolysis; ECMO, extracorporeal membrane oxygenation; ED, emergency department; ICU, intensive care unit; IVC, inferior vena cava; MGH, Massachusetts General Hospital; PE, pulmonary embolism; PERT, pulmonary embolism response team; rTPA, recombinant tissue plasminogen activator.
Preliminary results from early adopters suggest that PERT facilitates access to advanced therapies. An interrupted time series analysis demonstrates an increase in the proportion of PE patients undergoing any advanced therapy, from 9% to 19%, after the introduction of PERT.65 This increase is largely attributed to greater utilization of CDT, which grew from 1% to 14%, and reports from other PERT institutions show similar use of CDT.8, 58, 63, 66 Possible reasons for the increase in CDT cases include (1) greater awareness and recognition of severe PE, (2) inclusion of interventional specialties in the management of PE, (3) evidence from the PEITHO trial that systemic thrombolysis prevents hemodynamic decompensation in intermediate‐risk PE,46 and (4) the perception that CDT causes less bleeding than systemic thrombolysis.67 The increased use of advanced therapies did not seem to result in increased major bleeding or mortality in these series.65, 66 This may be due to continued improvements in catheter technology and technique, more stringent monitoring of patients receiving thrombolytic therapy, or improved protocols. However, the sample sizes in these studies may not have been large enough to accurately describe CDT's bleeding risk.
As a consult service, PERT has been well received by adopting institutions. Several institutions report increasing numbers of PERT activations over time after implementation,8, 58, 65, 66 which may be an indirect measure of success. PERTs are readily activated and respond rapidly irrespective of the time of day; an analysis of 457 PERT activations comparing daytime vs. nighttime activations showed no significant difference in the median time to intervention, rate of intervention within 24 hours after activation, or 30‐day mortality.64 Providers value the expedited and individually tailored treatment plans formed by the consensus of expert opinion. The majority (89%) of surveyed trainee physicians at a large PERT institution believe that a multidisciplinary team improves the care of patients with high‐risk PE, and 71% favor broad implementation of PERT.60
6. FUTURE DIRECTIONS FOR RESEARCH
While the growth of the PERT model has been exponential, and numerous manuscripts have been published describing the development of similar teams (Tables 2 and 3), objective evidence confirming the effectiveness of the approach is still limited. Most of the research studies published to date are descriptive in nature.8, 63, 66 While these studies have been helpful in describing the PERT model and expected effect of implementation, several key questions remain to be answered. The most important of these are (1) do advanced therapies provided by PERTs improve clinical outcomes? and (2) does the multidisciplinary decision‐making process integral to the PERT approach change clinical care in a positive way? Future research should be directed toward answering these questions. In addition, work is required to show that the PERT approach is cost‐effective and consistent across centers.
6.1. Does PERT improve clinical outcomes?
6.1.1. Interventional therapies for PE
Early therapeutic anticoagulation in acute PE is crucial, and even when additional therapeutic modalities are considered, anticoagulation should not be delayed unless contraindicated. Several studies provide evidence that PERTs frequently employ interventional approaches in the treatment of PE. For example, reports from 3 PERT centers (MGH, Cleveland Clinic, and New York University) indicate that CDT is used in 5% to 32% of high‐risk PE, and 9% to 27% of intermediate‐risk PE.8, 63, 66 A single‐center report from MGH also found a significant increase in the use of advanced therapy and CDT after the implementation of the PERT program.65
However, there are no data to date correlating the increased use of these therapies with improved clinical outcomes. This is partly because there are no large‐scale clinical trials demonstrating a clinical benefit of CDT for PE. Data in support of CDT are thus far limited to 1 placebo‐controlled clinical trial (ULTIMA [Ultrasound Accelerated Thrombolysis of Pulmonary Embolism]) of 59 patients,68 a clinical trial (OPTALYSE PE [Optimum Dose and Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate‐Risk Pulmonary Embolism]), comparing different doses of thrombolytic without a nonthrombolytic control group,69 and several case series.70, 71 In both clinical trials, the primary end point was change in right ventricular to left ventricular (RV:LV) ratio. The clinical importance of this outcome to patients is unclear. Moreover, in ULTIMA, while there was improvement in RV:LV ratio 24 hours after CDT, there was no difference at 90 days. Similarly, the OPTALYSE PE trial showed improvement in RV:LV ratio and thrombus burden within 48 hours of treatment. However, there was no difference in this outcome with varying doses of thrombolytics. The lack of an observed dose‐effect could be interpreted as evidence that lower doses of CDT are as effective as higher doses. However, dose‐response is one of the Bradford‐Hill criteria for causation,72 so lack of such an effect could also be interpreted as evidence that CDT is not the basis for improvement in RV:LV ratio. Of course, therapies provided by PERTs are not limited to CDT. Multidisciplinary teams also facilitate access to surgical thromboembolectomy, percutaneous mechanical thrombolectomy, and extracorporeal membrane oxygenation (ECMO).56, 73, 74 Data supporting these therapies are even more limited than data supporting CDT, and reports are entirely limited to case series. Thus, the first step in demonstrating the effectiveness of the PERT approach may be demonstrating the effectiveness of therapies provided by PERTs. Otherwise, PERTs may be viewed as merely causing overtreatment with potential harmful and expensive interventions that are not proven beneficial. Determining whether specific therapies improve outcomes is a prerequisite for determining whether PERTs improve the appropriate selection of therapies. Establishing the appropriate timing of therapeutic intervention will also be important, as studies have yet to show that early intervention is associated with improved outcomes.64 It will be challenging but critically important that studies are powered to assess patient‐centered clinically important outcomes. Fortunately, outcomes like inpatient and 30‐day mortality, recurrent VTE, and bleeding are rare. Long‐term morbidity is also difficult to assess, and no gold‐standard criterion exists for its measurement. To address these challenges, novel methods and large, Consortium‐based studies will be necessary to demonstrate benefit of advanced therapies and the PERT approach.
6.2. Does the PERT decision‐making process improve PE care?
6.2.1. The multidisciplinary rapid‐response team
More than simply facilitating access to advanced therapies, the main innovation of the PERT approach is the provision of real‐time, rapid, multidisciplinary consultation and discussion. The concept of rapid response teams is not new, and similar teams have been applied to the treatment of stroke, ST‐elevation myocardial infarction (STEMI), trauma, and shock.75, 76, 77, 78, 79, 80 For patients with impending shock, cardiovascular collapse, or airway or respiratory compromise, rapid response teams have been shown to reduce mortality in both adults and children.64, 81, 82 It is therefore logical to extrapolate this evidence to the PERT.
In contrast to most PERTs, stroke and STEMI teams are typically composed of specialists from a single specialty (eg, neurology or cardiology). Thus, these teams are less notable for their multidisciplinary nature than their ability to expedite access to a single treatment (eg, thrombolysis or percutaneous angioplasty). In contrast, PERTs bring together a multidisciplinary team of medical and surgical specialists, each with their own expertise and perspective. PERTs rely on their ability to achieve consensus or at least an agreed‐upon plan of care. Whether the multidisciplinary exchange of ideas improves the care of patients with PE is not yet known. This is especially true since, unlike some other rapid response teams, it is less clear that interventional therapy for PE is beneficial or time dependent.
While the benefit of bringing together multiple perspectives seems intuitive, doing so is resource intensive. The activation of a PERT and multidisciplinary discussion requires infrastructure, albeit minimal, and specialists must be available and willing to contribute their time. Research is required to show that this real‐time multidisciplinary decision making of PERT changes decision making. There is already some indirect evidence of this. Individual clinicians acting alone may be reluctant to make decisions regarding advanced therapies, whereas multidisciplinary discussion may provide reassurance that other clinicians agree with a given therapeutic approach, especially when that approach is associated with a higher risk of bleeding. Thus, the fact that more patients undergo advanced therapy when a PERT is available suggests that a PERT does change clinical decision making.65 However, as above, use of advanced therapies is likely influenced by the resources available and other differences between institutions, and whether the decision to employ advanced therapies improves patient outcomes requires more research.
6.2.2. Practice variation and quality care
There is no standard approach to creating a PERT, and there is variability in the structure of PERTs across institutions.9, 10 There also appears to be substantial variation in the use of specific therapies across institutions with PERTs.9, 10 Unpublished data from the PERT Consortium show that the use of CDT varies from 0% to 20% across institutions, and the use of advanced therapies varies from 16% to 46%. Whether this variation is related to different patient demographics and risk profiles or due to potential biases that may be inherent in the PERT process such as resources available in an institution or experiences or treatment preferences by the selected PERT members is not known. However, the presence of such practice variation may represent a call to action to optimize clinical decision making.83 Thus, future research should explore whether practice variation across PERTs belies variation in quality care, and whether standardization of practice would improve outcomes.
6.2.3. Cost‐effectiveness
As above, implementation of a PERT requires investments in infrastructure and clinician time. Currently, there is no mechanism to provide reimbursement for multidisciplinary care, so much of the multidisciplinary approach endorsed by PERT relies on physician volunteerism. In addition to costs associated with creating a PERT program, future research must consider costs associated with the use of advanced therapies, patient length of stay, and disposition (eg, ICU admission). In one placebo‐controlled clinical trial (TOPCOAT [Tenecteplase or Placebo: Cardiopulmonary Outcomes at Three Months]), intravenous thrombolysis therapy was associated with shorter hospital length of stay and decreased duration of ICU admission for patients with intermediate‐risk PE.84 It is possible that the PERT approach may have a similar beneficial effect, but research is required to demonstrate these outcomes.
6.2.4. Prevention, follow‐up, and education
While the primary focus of most PERT programs is acute inpatient care, integrating multiple specialists into a single “PERT follow‐up clinic” may improve long‐term treatment and secondary prevention.12 For example, rates of IVC filter removal are low, so research demonstrating that follow‐up in a PERT clinic improves these rates would be inviting.85, 86 Similarly, future research should explore whether involving hematologists/oncologists in outpatient PE care improves compliance with age‐appropriate cancer screening. Several studies have demonstrated that medication adherence decreases significantly over time, especially with anticoagulants.87 Having patients participate in a dedicated PE clinic may help encourage medication adherence as well as ensure appropriate dosing, as incorrect dosing is associated with an increase in adverse events.88 Numerous studies have also shown that PE is associated with decreased quality of life and physical function,86, 89, 90, 91 but identifying post‐PE complications is difficult.92 Established PE follow‐up programs may help identify patients who should be screened for post‐PE syndrome, postthrombotic syndrome, or chronic thrombotic pulmonary hypertension. Finally, the presence of a PERT team may represent an opportunity to improve education for patients and trainees. Multidisciplinary discussions of a PERT are, by their nature, inclusive and interactive. One published survey found that trainees participating in PERT discussions considered them beneficial to their education and understanding of PE care.60 Some PERTs involve patients or family members in shared decision making during case discussions as well. Whether this practice aids in patient and family understanding and satisfaction should also be explored.
6.3. Current and future PERT endeavors
To date, there are 89 institutions that have a PERT registered with the PERT Consortium. Importantly, there are hundreds of other hospitals and institutions around the world that treat patients with PE and may not know about PERT or have the resources or infrastructure to create such a team. To address this need, many operational PERTs are reaching out to their neighboring communities to let them know about their multidisciplinary approach to PE care and to provide their services if needed. The PERT Consortium has also become a venue for institutions to collaborate on many aspects related to patient care. Currently, the PERT Consortium is working on a consensus practice document including PERT algorithms aimed at providing a comprehensive review of diagnosis, management, and follow‐up care of PE patients. This document is being created by a group of experts from a multitude of disciplines who are carefully reviewing the available literature along with practice surveys and algorithms from many institutions. The PERT Consortium hopes that these decision‐making tools will be helpful to clinicians when deciding which patients are candidates for various treatment options. Moreover, as more institutions join the PERT Consortium, there is opportunity to collect a substantial amount of information on PE patients. Indeed, the PERT Consortium has recently created a centralized database that will allow institutions to benchmark their performance and quality of care with other institutions. This quality‐of‐care information will be especially important in establishing the effectiveness of PERTs.
7. CONCLUSION
The PERT is a novel team‐approach program with the purpose of providing better and more coordinated care to acute PE patients by facilitating rapid consultation and expert consensus with a multitude of experienced specialists. PERT streamlines interdisciplinary communication, obtains consensus in patient care, and organizes and mobilizes resources to execute the agreed‐upon plan. Thus far, numerous descriptive and retrospective studies demonstrate that it is feasible to create such a multidisciplinary team and that there are many ways to establish a PERT. The formation of the PERT Consortium is a venue through which these teams together can collect data that can help inform treatment decisions, influence guidelines and algorithms, guide hospital policy, and shape future research in PE care. This additional evidence will help evaluate the value of this innovative model and determine if this collaborative approach improves PE outcomes, changes clinical care in a positive way, is cost‐effective, enriches patients’ quality of life, and advances the science of PE treatment.
RELATIONSHIP DISCLOSURE
RR is an institutional research grant recipient from Janssen Pharmaceuticals and Bristol Meyer Squibb and is a consultant to Portola and Bristol Meyer Squibb and Janssen. BRL is an institutional grant recipient from Actelion and a consultant to Bayer and Gilead. CK is an institutional grant recipient from Diagnostica Stago, Siemens Healthcare, and Janssen Pharmaceuticals and is a consultant to Bristol Meyer Squibb. KZ and AS report nothing to disclose.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing and editing of the manuscript. RR takes responsibility for the integrity and accuracy of the data. Concept and design: RR; acquisition, analysis, or interpretation of data: RR, KZ, AS, BRL, and CK; drafting of the manuscript: RR, KZ, AS, BRL, and CK; critical revision of the manuscript for important intellectual content: RR, KZ, AS, BRL, and CK; final approval of the manuscript: RR, KZ, AS, BRL, and CK.
ACKNOWLEDGMENTS
For more information about PERT, please go to: www.pertconsortium.org
Rosovsky R, Zhao K, Sista A, Rivera‐Lebron B, Kabrhel C. Pulmonary embolism response teams: Purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3:315–330. 10.1002/rth2.12216
REFERENCES
- 1. Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–7. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 3. Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O'Neil BJ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011;57:700–6. [DOI] [PubMed] [Google Scholar]
- 4. Blood Clots: A Serious but Preventable Medical Condition cdc.gov. Updated May 4, 2016. http://www.cdc.gov/ncbddd/dvt/documents/blood-clots-fact-sheet.pdf. Accessed May 6, 2016.
- 5. Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011;86:217–20. [DOI] [PubMed] [Google Scholar]
- 6. Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–82. [DOI] [PubMed] [Google Scholar]
- 7. Secemsky E, Chang Y, Jain CC, Beckman JA, Giri J, Jaff MR, et al. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med. 2018;131:1506–14.e0. [DOI] [PubMed] [Google Scholar]
- 8. Kabrhel C, Rosovsky R, Channick R, Jaff MR, Weinberg I, Sundt T, et al. A multidisciplinary pulmonary embolism response team: initial 30‐month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150:384–93. [DOI] [PubMed] [Google Scholar]
- 9. Barnes G, Giri J, Courtney DM, Naydenov S, Wood T, Rosovsky R, et al. Nuts and bolts of running a pulmonary embolism response team: results from an organizational survey of the National PERT Consortium members. Hosp Pract. 1995;45:76–80. [DOI] [PubMed] [Google Scholar]
- 10. Barnes GD, Kabrhel C, Courtney DM, Naydenov S, Wood T, Rosovsky R, et al. Diversity in the pulmonary embolism response team model: an organizational Survey of the National PERT Consortium members. Chest. 2016;150:1414–7. [DOI] [PubMed] [Google Scholar]
- 11. Provias T, Dudzinski DM, Jaff MR, Rosenfield K, Channick R, Baker J, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract. 2014;42:31–7. [DOI] [PubMed] [Google Scholar]
- 12. Rosovsky R, Borges J, Kabrhel C, Rosenfield K. Pulmonary embolism response team: inpatient structure, outpatient follow‐up, and is it the current standard of care? Clin Chest Med. 2018;39:621–30. [DOI] [PubMed] [Google Scholar]
- 13. Corrigan D, Prucnal C, Kabrhel C. Pulmonary embolism: the diagnosis, risk‐stratification, treatment and disposition of emergency department patients. Clin Exp Emerg Med. 2016;3:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017;10:pii:e004345. [DOI] [PubMed] [Google Scholar]
- 15. Dudzinski DM, Horowitz JM. Start‐up, organization and performance of a multidisciplinary pulmonary embolism response team for the diagnosis and treatment of acute pulmonary embolism. Rev Esp Cardiol (Engl Ed). 2017;70:9–13. [DOI] [PubMed] [Google Scholar]
- 16. Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation. 2016;133:98–103. [DOI] [PubMed] [Google Scholar]
- 17. Friedman T, Winokur RS, Quencer KB, Madoff DC. Patient assessment: clinical presentation, imaging diagnosis, risk stratification, and the role of pulmonary embolism response team. Semin Intervent Radiol. 2018;35:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galmer A, Weinberg I, Giri J, Jaff M, Weinberg M. The role of the pulmonary embolism response team: how to build one, who to include, scenarios, organization, and algorithms. Tech Vasc Interv Radiol. 2017;20:216–23. [DOI] [PubMed] [Google Scholar]
- 19. Giri JS, Piazza G. A midterm report card for pulmonary embolism response teams. Vasc Med. 2018;23:72–4. [DOI] [PubMed] [Google Scholar]
- 20. Huisman MV, Montero Cabezas JM, Klok FA. Pulmonary embolism response teams: what is the added value for patients with acute pulmonary embolism? Ned Tijdschr Geneeskd. 2017;161:D1570. [PubMed] [Google Scholar]
- 21. Kabrhel C. Achieving multidisciplinary collaboration for the creation of a pulmonary embolism response team: creating a “Team of Rivals.” Semin Intervent Radiol. 2017;34:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDaniel MC, Jaber WA, Ross CB. Pulmonary embolism response teams. J Med Assoc Ga. 2016; (suppl):10. [PubMed] [Google Scholar]
- 23. Merli GJ. Pulmonary embolism in 2017: how we got here and where are we going? Tech Vasc Interv Radiol. 2017;20:128–34. [DOI] [PubMed] [Google Scholar]
- 24. Monteleone PP, Rosenfield K, Rosovsky RP. Multidisciplinary pulmonary embolism response teams and systems. Cardiovasc Diagn Ther. 2016;6:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nosher JL, Patel A, Jagpal S, Gribbin C, Gendel V. Endovascular treatment of pulmonary embolism: Selective review of available techniques. World J Radiol. 2017;9:426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ozcinar E, Erol S, Aliyev A, Cakici M, Baran C, Bermede O. Could surgical pulmonary embolectomy be performed with acceptable outcomes without a pulmonary embolism response team? Ann Thorac Surg. 2017;104:1432. [DOI] [PubMed] [Google Scholar]
- 27. Reza N, Dudzinski DM. Pulmonary embolism response teams. Curr Treat Options Cardiovasc Med. 2015;17:387. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez Chiaradia DA, Cuttica MJ, Jimenez D. The role of the pulmonologist in a pulmonary embolism response team (PERT): a time to come on stage. Arch Bronconeumol. 2019;55:1–2. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez‐Lopez J, Channick R. The pulmonary embolism response team: what is the ideal model? Semin Respir Crit Care Med. 2017;38:51–5. [DOI] [PubMed] [Google Scholar]
- 30. Root CW, Dudzinski DM, Zakhary B, Friedman OA, Sista AK, Horowitz JM. Multidisciplinary approach to the management of pulmonary embolism patients: the pulmonary embolism response team (PERT). J Multidiscip Healthc. 2018;11:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serhal M, Haddadin IS, Heresi GA, Hornacek DA, Shishehbor MH, Bartholomew JR. Pulmonary embolism response teams. J Thromb Thrombolysis. 2017;44:19–29. [DOI] [PubMed] [Google Scholar]
- 32. Todoran TM, Giri J, Barnes GD, Rosovsky RP, Chang Y, Jaff MR, et al. Treatment of submassive and massive pulmonary embolism: a clinical practice survey from the second annual meeting of the Pulmonary Embolism Response Team Consortium. J Thromb Thrombolysis. 2018;46:39–49. [DOI] [PubMed] [Google Scholar]
- 33. Witkin AS. Acute and chronic pulmonary embolism: the role of the pulmonary embolism response team. Curr Opin Cardiol. 2017;32:672–8. [DOI] [PubMed] [Google Scholar]
- 34. Witkin AS, Harshbarger S, Kabrhel C. Pulmonary Embolism Response Teams. Semin Thromb Hemost. 2016;42:857–64. [DOI] [PubMed] [Google Scholar]
- 35. Zern EK, Young MN, Rosenfield K, Kabrhel C. A pulmonary embolism response team: initial experiences and future directions. Expert Rev Cardiovasc Ther. 2017;15:481–9. [DOI] [PubMed] [Google Scholar]
- 36. Fasanya A, Silvas K, Alhassan S, Patel K, Singh AC, Malik K. Venous thromboembolism and pulmonary embolism response teams: an overview. Crit Care Nurs Q. 2017;40:237–50. [DOI] [PubMed] [Google Scholar]
- 37. Jaber WA, Fong PP, Weisz G, Lattouf O, Jenkins J, Rosenfield K, et al. Acute pulmonary embolism: with an emphasis on an interventional approach. J Am Coll Cardiol. 2016;67:991–1002. [DOI] [PubMed] [Google Scholar]
- 38. Porres‐Aguilar M, Anaya‐Ayala JE, Heresi GA, Rivera‐Lebron BN. Pulmonary embolism response teams: a novel approach for the care of complex patients with pulmonary embolism. Clin Appl Thromb Hemost. 2018;24:48S–55S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rali PM, Criner GJ. Submassive pulmonary embolism. Am J Respir Crit Care Med. 2018;198:588–98. [DOI] [PubMed] [Google Scholar]
- 40. Huber TC, Sharma A, Wilkins L. Pulmonary embolism response teams: overview and impact of a multidisciplinary treatment algorithm. J Radiol Nurs. 2017;36:206–10. [Google Scholar]
- 41. Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer‐Westendorf J, et al. Impact of thrombolytic therapy on the long‐term outcome of intermediate‐risk pulmonary embolism. J Am Coll Cardiol. 2017;69:1536–44. [DOI] [PubMed] [Google Scholar]
- 42. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 43. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. [DOI] [PubMed] [Google Scholar]
- 44. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69, 69a‐69k. [DOI] [PubMed] [Google Scholar]
- 45. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–9. [DOI] [PubMed] [Google Scholar]
- 46. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer‐Westendorf J, et al. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med. 2014;370:1402–11. [DOI] [PubMed] [Google Scholar]
- 47. Sista AK, Miller LE, Kahn SR, Kline JA. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta‐analysis. Vasc Med. 2017;22:37–43. [DOI] [PubMed] [Google Scholar]
- 48. Sista AK, Klok FA. Late outcomes of pulmonary embolism: the post‐PE syndrome. Thromb Res. 2017;164:157-62. [DOI] [PubMed] [Google Scholar]
- 49. Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA, et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med. 2017;130:990 e9–e21. [DOI] [PubMed] [Google Scholar]
- 50. Kahn SR, Hirsch AM, Akaberi A, Hernandez P, Anderson DR, Wells PS, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest. 2017;151:1058–68. [DOI] [PubMed] [Google Scholar]
- 51. Stevinson BG, Hernandez‐Nino J, Rose G, Kline JA. Echocardiographic and functional cardiopulmonary problems 6 months after first‐time pulmonary embolism in previously healthy patients. Eur Heart J. 2007;28:2517–24. [DOI] [PubMed] [Google Scholar]
- 52. Kline JA, Steuerwald MT, Marchick MR, Hernandez‐Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV. Prevalence and potential determinants of exertional dyspnea after acute pulmonary embolism. Respir Med. 2010;104:1744–9. [DOI] [PubMed] [Google Scholar]
- 54. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, Investigators M. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013;111:273–7. [DOI] [PubMed] [Google Scholar]
- 55. PERT Consortium . Homepage ‐ The PERT Consortium 2018. https://pertconsortium.org/. Accessed 2018 September 11.
- 56. Al‐Bawardy R, Rosenfield K, Borges J, Young MN, Albaghdadi M, Rosovsky R, et al. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a case series and review of the literature. Perfusion. 2018;34:22-8. [DOI] [PubMed] [Google Scholar]
- 57. Bloomer TL, Thomassee EJ, Fong PP. Acute pulmonary embolism network and multidisciplinary response team approach to treatment. Crit Pathw Cardiol. 2015;14:90–6. [DOI] [PubMed] [Google Scholar]
- 58. Carroll BJ, Pemberton H, Bauer KA, Chu LM, Weinstein JL, Levarge BL, et al. Initiation of a multidisciplinary, rapid response team to massive and submassive pulmonary embolism. Am J Cardiol. 2017;120:1393–8. [DOI] [PubMed] [Google Scholar]
- 59. Deadmon EK, Giordano NJ, Rosenfield K, Rosovsky R, Parry BA, Al‐Bawardy RF, et al. Comparison of emergency department patients to inpatients receiving a pulmonary embolism response team (PERT) activation. Acad Emerg Med. 2017;24:814–21. [DOI] [PubMed] [Google Scholar]
- 60. Elbadawi A, Wright C, Patel D, Chen YL, Mazzillo J, Cameron P, et al. The impact of a multi‐specialty team for high risk pulmonary embolism on resident and fellow education. Vasc Med. 2018;23:372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kabrhel C, Jaff MR, Channick RN, Baker JN, Rosenfield K. A multidisciplinary pulmonary embolism response team. Chest. 2013;144:1738–9. [DOI] [PubMed] [Google Scholar]
- 62. Kolkailah AA, Hirji S, Piazza G, Ejiofor JI, Ramirez Del Val F, Lee J, et al. Surgical pulmonary embolectomy and catheter‐directed thrombolysis for treatment of submassive pulmonary embolism. J Card Surg. 2018;33:252–9. [DOI] [PubMed] [Google Scholar]
- 63. Mahar JH, Haddadin I, Sadana D, Gadre A, Evans N, Hornacek D, et al. A pulmonary embolism response team (PERT) approach: initial experience from the Cleveland Clinic. J Thromb Thrombolysis. 2018;46:186–92. [DOI] [PubMed] [Google Scholar]
- 64. McNeill JN, Witkin AS, Chang Y, Kabrhel C, Channick RN. Does the time of day a pulmonary embolism response team is activated affect time to intervention or outcome? Chest. 2017;152:1353–4. [DOI] [PubMed] [Google Scholar]
- 65. Rosovsky R, Chang Y, Rosenfield K, Channick R, Jaff MR, Weinberg I, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10‐year analysis. J Thromb Thrombolysis. 2018;47:31-40. [DOI] [PubMed] [Google Scholar]
- 66. Sista AK, Friedman OA, Dou E, Denvir B, Askin G, Stern J, et al. A pulmonary embolism response team's initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med. 2018;23:65–71. [DOI] [PubMed] [Google Scholar]
- 67. Taslakian B, Chawala D, Sista AK. A survey of submassive pulmonary embolism treatment preferences among medical and endovascular physicians. J Vasc Interv Radiol. 2017;28:1693–9 e2. [DOI] [PubMed] [Google Scholar]
- 68. Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer‐Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound‐assisted catheter‐directed thrombolysis for acute intermediate‐risk pulmonary embolism. Circulation. 2014;129:479–86. [DOI] [PubMed] [Google Scholar]
- 69. Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate‐risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11:1401–10. [DOI] [PubMed] [Google Scholar]
- 70. Kuo WT, Banerjee A, Kim PS, DeMarco FJ Jr, Levy JR, Facchini FR, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148:667–73. [DOI] [PubMed] [Google Scholar]
- 71. Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A prospective, single‐arm, multicenter trial of ultrasound‐facilitated, catheter‐directed, low‐dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8:1382–92. [DOI] [PubMed] [Google Scholar]
- 72. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Donaldson CW, Baker JN, Narayan RL, Provias TS, Rassi AN, Giri JS, et al. Thrombectomy using suction filtration and veno‐venous bypass: single center experience with a novel device. Catheter Cardiovasc Interv. 2015;86:E81–7. [DOI] [PubMed] [Google Scholar]
- 74. Neely RC, Byrne JG, Gosev I, Cohn LH, Javed Q, Rawn JD, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg. 2015;100:1245–51; discussion 51‐2. [DOI] [PubMed] [Google Scholar]
- 75. Alberts MJ, Hademenos G, Latchaw RE, Jagoda A, Marler JR, Mayberg MR, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102–9. [DOI] [PubMed] [Google Scholar]
- 76. Deane SA, Gaudry PL, Pearson I, Misra S, McNeil RJ, Read C. The hospital trauma team: a model for trauma management. J Trauma. 1990;30:806–12. [DOI] [PubMed] [Google Scholar]
- 77. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34:2463–78. [DOI] [PubMed] [Google Scholar]
- 78. Grossman MD, Yelon JA, Szydiak L. Effect of American College of Surgeons trauma center designation on outcomes: measurable benefit at the extremes of age and injury. J Am Coll Surg. 2017;225:194–9. [DOI] [PubMed] [Google Scholar]
- 79. Jones DA, DeVita MA, Bellomo R. Rapid‐response teams. N Engl J Med. 2011;365:139–46. [DOI] [PubMed] [Google Scholar]
- 80. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta‐analysis. Crit Care. 2015;19:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ranji SR, Auerbach AD, Hurd CJ, O'Rourke K, Shojania KG. Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis. J Hosp Med. 2007;2:422–32. [DOI] [PubMed] [Google Scholar]
- 82. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: A systematic review and meta‐analysis. J Hosp Med. 2016;11:438–45. [DOI] [PubMed] [Google Scholar]
- 83. Krumholz HM. Variations in health care, patient preferences, and high‐quality decision making. JAMA. 2013;310:151–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double‐blind, placebo‐controlled randomized trial. J Thromb Haemost. 2014;12:459–68. [DOI] [PubMed] [Google Scholar]
- 85. Kang J, Ko HK, Shin JH, Ko GY, Jo KW, Huh JW, et al. Practice patterns of retrievable inferior vena cava filters and predictors of filter retrieval in patients with pulmonary embolism. Vasc Med. 2017;22:512–7. [DOI] [PubMed] [Google Scholar]
- 86. Yoon DY, Vavra AK, Eifler AC, Teter K, Eskandari MK, Ryu RK, et al. Why temporary filters are not removed: clinical predictors in 1,000 consecutive cases. Ann Vasc Surg. 2017;42:64–70. [DOI] [PubMed] [Google Scholar]
- 87. Manzoor BS, Lee TA, Sharp LK, Walton SM, Galanter WL, Nutescu EA. Real‐world adherence and persistence with direct oral anticoagulants in adults with atrial fibrillation. Pharmacotherapy. 2017;37:1221–30. [DOI] [PubMed] [Google Scholar]
- 88. Trujillo‐Santos J, Di Micco P, Dentali F, Douketis J, Diaz‐Peromingo JA, Nunez MJ, et al. Real‐life treatment of venous thromboembolism with direct oral anticoagulants: The influence of recommended dosing and regimens. Thromb Haemost. 2017;117:382–9. [DOI] [PubMed] [Google Scholar]
- 89. Hagan KA, Harrington LB, Kim J, Zeleznik O, Rimm EB, Grodstein F, et al. Reduction in physical function in women after venous thromboembolism. J Thromb Haemost. 2018;16:1564-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klok FA, Cohn DM, Middeldorp S, Scharloo M, Buller HR, van Kralingen KW, et al. Quality of life after pulmonary embolism: validation of the PEmb‐QoL Questionnaire. J Thromb Haemost. 2010;8:523–32. [DOI] [PubMed] [Google Scholar]
- 91. Lubberts B, Paulino Pereira NR, Kabrhel C, Kuter DJ, DiGiovanni CW. What is the effect of venous thromboembolism and related complications on patient reported health‐related quality of life? A meta‐analysis Thromb Haemost. 2016;116:417–31. [DOI] [PubMed] [Google Scholar]
- 92. Ende‐Verhaar YM, Huisman MV, Klok FA. To screen or not to screen for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2017;151:1–7. [DOI] [PubMed] [Google Scholar]


