Abstract
Plant-derived carbon (C) is considered fundamental to understand the interaction between rhizosphere microbes and plants in terrestrial ecosystems. Biochar soil amendment may enhance plant performance via changing soil properties or microbial diversity in the rhizosphere. However, our knowledge of how plant-microbiome associations respond to biochar amendment remains rather limited. Herein, 13CO2 steady-state labeling combined with DNA stable-isotope probing was used to characterize soil bacterial communities in the rhizosphere contributing to the utilization of plant-derived C. The diversity of bacteria active in the utilization of root exudates was determined after biochar amendment in a legume-based intercropping system (Vicia faba L., with Zea mays L.). The results showed the biochar application not only changed the bacterial community structure and diversity in the rhizosphere, but also altered bacterial members actively assimilating plant-derived C. There were more labeled species in the biochar-amended soils than the control soils. Compared with the control, the biochar amendment increased the relative abundances of Firmicutes and Bacteroidetes members (i.e., Bacillus, Clostridium, Sporomusa, Desulfosporosinus, and Alicyclobacillus) while decreasing the abundances of Proteobacteria members (e.g., Methylobacterium and Sphingomonas) utilizing plant-derived C. In contrast, slow-growing species of the phyla Acidobacteria, Planctomycetes, and Gemmatimonadetes were barely labeled. The bacteria found stimulated by the biochar amendment are known for their ability to fix nitrogen, solubilize phosphorus, or reduce iron and sulfur, which may potentially contribute to the “biochar effect” in the rhizosphere. This study is the first to provide empirical evidence that biochar amendment can alter the soil bacterial community assimilating plant-derived C; this may have consequences for nutrient cycling and improving plant performance in intercropping systems.
Keywords: biochar, plant-derived carbon, 13CO2 steady-state labeling, stable-isotope probing, intercropping, rhizosphere microbes
Introduction
Modern agricultural systems provide high crop yields, but they also generate serious impacts on the environmental. For example, intensive use of mineral fertilizers and pesticides leads to such soil acidification (Gollany et al., 2005), groundwater contamination (Gollany et al., 2004), and increasing greenhouse gas emissions (Dalal et al., 2003). Intercropping is an ancient and traditional agricultural practice, and especially legume-based intercropping systems have great potential for contributing to agricultural productivity through increasing nitrogen (N) inputs and phosphorous (P) bioavailability (Rose et al., 2015; Tang et al., 2016) in the terrestrial system. In this regard, an intercropping system provides a way to introduce soil available nutrients into the agro-ecosystem, thus avoiding the excessive use of fertilizers and pesticides relied upon during conventional cultivation. Furthermore, a recent meta-analysis indicated that intercropping can increase crop yields in Africa by an average of 23% compared with monocropping (Himmelstein et al., 2017). Hence, an improved understanding of nutrient cycling in intercropping systems may offer new insights into achieving long-term agricultural productivity.
Plants and microbiomes build and engage in a complex and varied molecular “dialogue” in the rhizosphere (Gkarmiri et al., 2017). It has been estimated that rhizodeposition accounts for 17% of total photoassimilated carbon (C), and belowground C allocation by grasses was higher than crops (Nguyen, 2003; Pausch and Kuzyakov, 2018). The beneficial effects of an intercropping system are closely related to the partitioning of C that occurs belowground and its utilization by different functional groups of microbes (Fan et al., 2008). However, this soil microbial community is often influenced by plant species due to differences in the quantity and quality of C resources produced by root exudates (Garland, 1996; Liu et al., 2017). Additional factors controlling plant C allocation belowground include drought events, water status, and fertilization regimes (Drigo et al., 2010; Yao et al., 2012; Fuchslueger et al., 2016; Wang et al., 2016). Nevertheless, plants can actively select beneficial microbes or disrupt the invasion of pathogenic microbes in the rhizosphere by secreting particular root exudates (Chapelle et al., 2016). Meanwhile, shifts in the microbial community consuming plant-derived C may have strong effects on plant development and soil nutrient cycling (Hannula et al., 2017).
Biochar is a C-rich product of biomass pyrolysis intended for use as a soil amendment. Biochar amendment has been found to increase both the soil water-holding capacity (Abel et al., 2013) and nutrient availability (Zheng et al., 2018), and to also increase the pH in acidic soil (Whitman et al., 2016). Some studies have shown that biochar-induced changes in soil properties (e.g., pH and nutrient availability) may have beneficial effects on crop productivity (Lehmann et al., 2011; Liu et al., 2017). However, it is worth noting that application of the biochar into soil would be expected to have wide-ranging effects, depending on the quality of the biochar in terms of feedstock and pyrolysis temperature (Jaiswal et al., 2014; Imparato et al., 2016). Recently, some studies have shown that biochar amendment may increase the diversity of soil microbes; this phenomenon, may be contributed to the release of non-labile biochar-associated organic compounds or the changes in soil properties induced by biochar amendment (Kolton et al., 2011; Lehmann et al., 2011; Zhang et al., 2017). Kolton et al. (2017) found that biochar-enhanced plant performance was related not only to greater microbial richness but also linked to higher metabolic potential in the rhizosphere. However, it is still an open question whether biochar applications can facilitate soil microbes assimilating plant-derived C, leading to a tight-knit plant-microbiome association in the rhizosphere. Moreover, numerous studies tend to focus on the overall microbial community and its diversity in the rhizosphere of monocropping systems. By contrast, how biochar amendment may affect soil microbial community composition and diversity in intercropping systems is understudied. Plants can release a variety of root exudates into the rhizosphere, and these biologically active compounds from root exudate are known to influence the rhizosphere microbiome (Baetz and Martinoia, 2014; Mendes et al., 2017). Salicylic acid and γ-aminobutyric acid concentrations have been shown to be correlated with specific taxa that are enriched in the rhizosphere (Badri et al., 2013; Zhalnina et al., 2017). Biochar amendment may change the influences of root exudates on the rhizosphere microbiome. For example, biochar may alter rhizosphere microbial communities, facilitating propagation of microbes via absorption of root exudates (Gu et al., 2017) or via physical attributes generated by the porous properties of biochar (Pietikäinen et al., 2000).
In the present study, we performed 13CO2 steady-state labeling coupled with DNA-stable isotope probing (SIP) and investigated soil bacterial diversity via high-throughput amplicon sequencing in an intercropping system. The objectives of the present study were: (1) to explore the effects of biochar amendment on soil bacterial communities actively utilizing plant-derived C, and (2) to understand the interactions between root exudates and soil bacterial communities after biochar amendment in an intercropping system. Our hypothesis was that biochar amendment not only stimulates bacterial species affiliated with the phyla Bacteroidetes and Firmicutes, but also alters a subset of bacterial communities consuming plant-derived C in the rhizosphere, leading to a distinct plant-microbe association in the intercropping system.
Materials and Methods
Biochar Preparation and Soil Sampling
The biochar used in this experiment was made from apple (Malus pumila Mill.) wood chip, a common agricultural solid waste in China. Air-dried apple wood chips were pyrolyzed at 500°C for 5 h in a closed container under oxygen-limited condition, with nitrogen gas as the medium gas in a muffled furnace (Isotemp, Fisher Scientific, United States). More details of biochar preparation are provided by Xu et al. (2014). The biochar had a pH value of 10.9 (1:2.5 water); the C content was 73.6%; and the N content was 0.6%. The ratio of mass loss upon biochar production was 77%.
In August 2016, soil was collected from a depth of 0–5 cm in a fully managed field in Ningbo (29°56′21″N, 121°35′23″E), Zhejiang Province, China. The soil is a Plinthosol (FAO/UNESCO) with clay loam texture. The soil pH, total C, and total N were 6.89 (1:2.5 water), 15.3 g kg–1, and 2.1 g kg–1, respectively. After removing plant residues and stones, the fresh soils were passed through a 2-mm sieve and stored in a 300-L plastic bucket at the room temperature for 7 days until use.
Experimental Design
In order to explore the effect of biochar amendment on bacterial communities in the rhizosphere, a relatively high ratio of biochar amendment (2%, w/w) was selected. This biochar rate was also used in a field experiment in an intercropping system (Liu et al., 2017). Soil was amended with biochar and 250 g amount of soil (dry weight basis) was added to each plastic pot (5.3 cm diameter, 13.2 cm height). Soil water-holding capacities of control and biochar treatments were measured by gravimetrically following saturation and free-draining, and then different mass of water was added in control and biochar treatment separately to reach approximately 40% water-holding capacity. A legume plant, faba bean (Vicia faba L.), and a cereal crop, maize (Zea mays L.), were selected to represent the intercropping system. All the seeds were immersed in a 3% H2O2 solution for 10 min and washed with distilled water, then germinated in Petri dishes covered with wet filter papers for 3 days at 28°C in the dark before sowing. One faba bean and one maize seedling were planted in horizontal orientation per pot. These seedling pairs were cultivated in a growth chamber under 14-h light photoperiod at 28°C (day) and 20°C (night) temperatures.
After 2 weeks of growth (i.e., at day 14), a continuous steady-state labeling approach was implemented according to Wang et al. (2016). Briefly, 13CO2 at 99.8 atom % excess (Sigma-Aldrich, United States) and at ambient CO2 concentrations was introduced, with a total 13CO2 and 12CO2 concentration of 350 ppm. To achieve this CO2 concentration, the flow rates of CO2-free air and 13CO2 into the plant growth chamber (Zhicheng Technology Co., Ltd., Ningbo, China) were set to 9.0 L min–1 and 3.15 mL min–1, respectively. For the unlabeled chamber, which served as the control, the same gas flow was used except that 12CO2 was used instead of 13CO2. There were four treatments in the experiment (unlabeled-non-biochar, unlabeled-biochar, 13CO2-labeled-biochar, and 13CO2- labeled-biochar), with three replicates per treatment. Three pots for each treatment were randomly placed into the unlabeled and 13CO2 labeled chambers, respectively. During this labeling, plants were kept in the growth chamber under the same light and temperature conditions as they had before. Soil water conditions were maintained by adding deionized water every 2 days by weight for both control and biochar treatments during the 35-day labeling period.
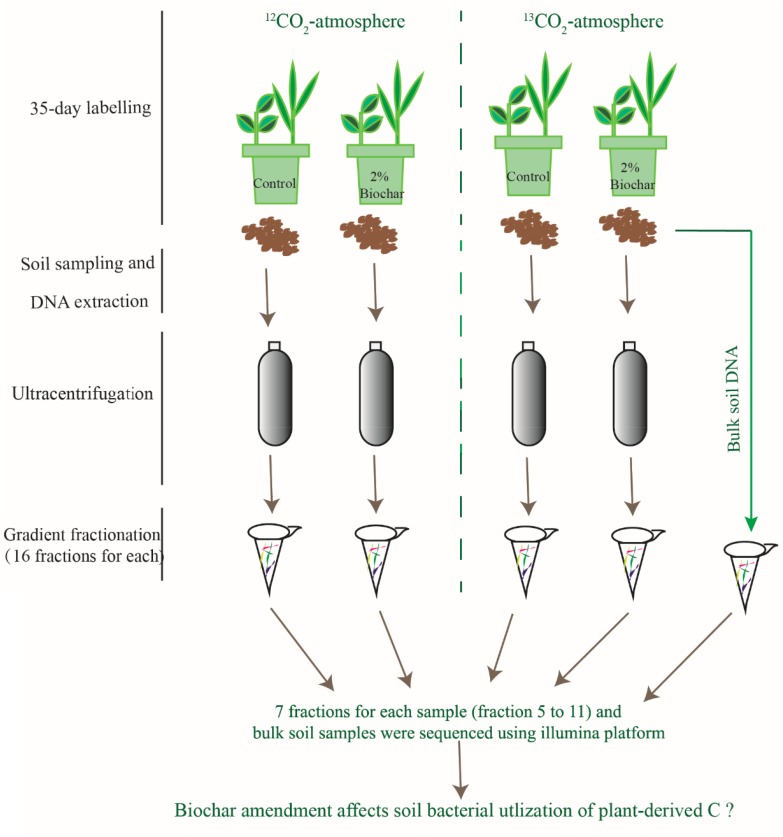
The unlabeled and 13CO2-labeled plants and soils were destructively sampled at day 49 (i.e., after 35-day of continuous labeling). The image of plants in the pots is shown in the Supplementary Figure S1. The roots and shoots were washed with water, then dried for 45 min at 105°C and again at 65°C for 48 h to measure their biomass. Since the pot was small in size the root growth in each pot was extremely extensive, all soil in a given pot was considered as rhizosphere soil. Soil samples from each pot were immediately freeze-dried for later DNA extraction. An overall conceptual diagram for the study design is shown in Figure 1.
FIGURE 1.
A conceptual diagram for the study design, methods and objective.
DNA Extraction
Total genomic DNA was extracted from 0.50 g of freeze-dried rhizosphere soil samples using the Fast DNA SPIN Kit for soil (MP Biomedicals, Santa Ana, CA, United States) following the manufacturer’s protocols. The DNA concentration was measured using a NanoDropTM 2000 spectrophotometer (Thermo Scientific, Waltham, MA, United States).
Enrichment Analysis Using 13C
Two micrograms of DNA was placed into a tin capsule (Thermo Scientific), and dried for approximately 2.5 h at 50°C. Freeze-dried rhizosphere soil samples from the unlabeled and 13CO2-labeled chambers were milled to pass through a 200-mesh sieve. Between 2 and 4 mg of soil from each sample was weighed, and both the DNA and soil samples were examined with an elemental analyzer (Thermo Scientific FLASH 2000, Germany) coupled online to an isotope ratio mass spectrometer (Delta V advantage, Thermo Finnigan, Germany). The δ13C (‰) was calculated as δ13C (‰) = [(Rsample – Rstandard)/Rstandard] × 1000, where R is the molar ratio of 13C to 12C. DNA concentration in the 13C-labeled sample was calculated as 13C-DNA (ng g–1 soil) = 13C atom% excess × DNA concentration (ng g–1 soil), where 13C atom% excess = 13C atom% of the samples from 13CO2-labeled chamber –unlabeled chamber.
Soil Physicochemical Analysis
The pH was measured in a soil-to-water ratio of 1:2.5 (w/v) with a pH-meter (Bao, 1999). Soil organic C and total N were measured with an element analyzer (vario MACRO cube, Elemental, Germany). Soil Olsen-P was extracted with 0.5 M of NaHCO3 in a soil-to-water ratio of 1:10 (w/v) for 30 min and measured following the methods described by Olsen (1954). Soil nitrate (NO3–) and ammonium (NH4+) were extracted with 2 M of KCl in a soil-to-water ratio of 1:10 (w/v) for 30 min, and then determined by using an automated continuous flow analyzer (AutoAnalyzer3, SEAL, Germany).
Caesium Chloride Ultracentrifugation
From each sample, 3 μg of DNA was fractionated by caesium chloride (CsCl) equilibrium density gradient ultracentrifugation, as described by Long et al. (2018). Briefly, the CsCl solution was added to a 5.1-mL Quick-Seal polyallomer centrifuge tube (Beckman Coulter, Brea, CA, United States) that contained 1.6914 g mL–1 of CsCl verified by an AR200 refractometer (Reichert, Depew, NY, United States). Centrifugation was performed at 180,300 rcf for 44 h at 20°C using a Vti 65.2 vertical rotor (Beckman Coulter). After completing this centrifugation, DNA was collected from the tube bottom to its top in 16 equal fractions (300 μL per fraction) by replacing the gradient medium with sterile water using a single-channel syringe pump. The density of each fraction was then measured using an AR200 refractometer (Reichert). DNA in each fraction was purified with PEG 6000 and 70% ethanol, and then dissolved in 30 μL of double-distilled H2O for the downstream analysis.
PCR Amplification and Illumina Sequencing
Seven DNA fractions for each sample with a buoyant density of 1.67–1.72 g mL–1 were analyzed. These DNA fractions were selected because they encompassed most of the extracted DNA in both labeled and unlabeled samples. In addition, six non-fractionated DNA samples (three control and three biochar samples for each) from the 13CO2-labeled chamber were analyzed. Sequencing of the 16S rRNA gene was done via multiplexed barcoded amplicon sequencing. Primers 515f/907r targeting approximately 392 bp in the V4–V5 hypervariable region of the bacterial 16S rRNA gene were used for PCR amplification (Zhou et al., 2011). Both forward and reverse primers were barcoded with a unique error-correcting eight-base barcode (Hamady et al., 2008). The PCR reaction contained 10 μL of 5 × GoTaq® Green Master Mix (Promega, Fitchburg, WI, United States), 10 μM of each primer, 1 μL of template DNA, and ultraclean water to a volume of 50 μL (done three times). Each sample was amplified in triplicate with the following conditions: 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s, with a final extension at 72°C for 10 min. The triplicate amplicons were pooled and purified using a Universal DNA Purification Kit (Tiangen, Beijing, China). Purified PCR products were quantified with a NanoDropTM 2000 spectrophotometer (Thermo Scientific), and then sent to Novogene (Beijing, China) for sequencing, Paired-end sequencing (2 × 250 bp) was performed on an Illumina HiSeq2500 platform (Illumina, San Diego, CA, United States).
Sequence Processing and Analysis
Bacterial sequences were processed using QIIME v1.9.1 (Caporaso et al., 2010). Briefly, forward and reverse reads were merged using FLASH (Fast Length Adjustment of SHort reads) (Magoč and Salzberg, 2011) before downstream processing. Reads with a low average quality score (<20) and short length (<100 nt) were removed (Kong, 2011). Sequences were checked for any chimeras and filtered by the UCHIME algorithm in the USEARCH tool (Edgar, 2010) using the rRNA Gold fasta file of chimer-checked reference sequences. High-quality sequences were assigned to operational taxonomic units (OTUs) with a similarity threshold of 97%, through an open reference OTU-picking strategy using UCLUST. The most abundant sequence in the cluster for each OTU unit was selected as a representative sequence for that OTU, and it was then assigned to taxonomy against the latest version of the Greengenes database (v13_8) (McDonald et al., 2012). Any OTUs affiliated to “chloroplast,” “Archaea,” “mitochondria,” or “unassigned” were discarded from the final OTU table. Quality control removed 1,15,3,09,1 reads, leaving 9,50,0,10,1 high-quality sequences. Individual samples contained from 37,4,06 to 189,218 total sequences each, with a mean number of 105,557 ± 37,159. The 16S rRNA gene sequencing data have been deposited at the National Center for Biotechnology Information short Reads Archive under study PRJNA528377.
Statistical Analysis
In addition to stable isotope incorporation, buoyant density of a macromolecule is affected by various factors such as the G+C content of DNA (Youngblut and Buckley, 2014). 13C-DNA may have the same buoyant density as 12C-DNA in the same centrifuge tube (Pepe-Ranney et al., 2016). Hence, it is important to use the unlabeled substrates to gain reliable isotopic labeling results for robust analysis and inference. By comparing heavy gradient fractions in the labeled samples to their corresponding fractions in the unlabeled samples, those active microbes with labeled DNA can be obtained. Here, we applied the DESeq2 package, an RNA-Seq differential expression statistical framework (Love et al., 2014), to gain insight into the bacterial OTUs enriched in the heavy fractions of 13C-labeled gradients vs. unlabeled samples. The DESeq2 package contains several features for establishing a statistically robust framework that can account for reading differences in the sequence libraries (Angel et al., 2018). We divided the CsCl gradient fractions into “light” or “heavy” fractions. Values > 1.70 g mL–1 were defined as the cut-off concentration for heavy category fractions, since we were mainly interested in OTU enrichment in the heavy fractions. OTUs were filtered independently prior to conducting multiple comparisons. To ensure sufficient data was available for comparisons, a sparsity threshold of 0.35 was selected for the heavy fractions of both unlabeled and 13C-labeled gradients (Love et al., 2014). In this way, an OTU not found in at least 35% of the heavy fractions (13C-labeled and unlabeled gradients) was removed as statistically uninformative. Removing those OTUs lacking sufficient sequence counts to provide statistically significant results can reduce the multiple redundant comparisons performed (Pepe-Ranney et al., 2016). In addition, a false discovery rate of 0.10 was applied, with the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) applied for the P-value corrections. OTUs statistically enriched in the heavy fractions from 13C-labeled samples to corresponding unlabeled samples were interpreted as being the labeled bacteria (Pepe-Ranney et al., 2016).
Statistical tests were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, United States). The differences in soil properties, bacterial community composition, and plant biomass between treatments were determined by one-way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) test, and P < 0.05 was considered statistically significant. For the 16S rRNA sequencing data, non-metric-multidimensional scaling (NMDS) and principal coordinate ordinations (PCoA) based on Bray-Curtis dissimilarities were applied in R (R Core Team, 2013) with the “phyloseq” package (McMurdie and Holmes, 2013); the results were visualized with the “ggplot2” package (Wickham, 2010). The differences in bacterial community composition between unlabeled and 13CO2-labeled samples in various fractions were tested using Adonis tests with 999 permutations in the “vegan” package (Oksanen et al., 2016). Additionally, species richness and its associated error were estimated for each sample using the “breakaway” package in R (Willis et al., 2017).
Results
13C Enrichment and Soil Properties
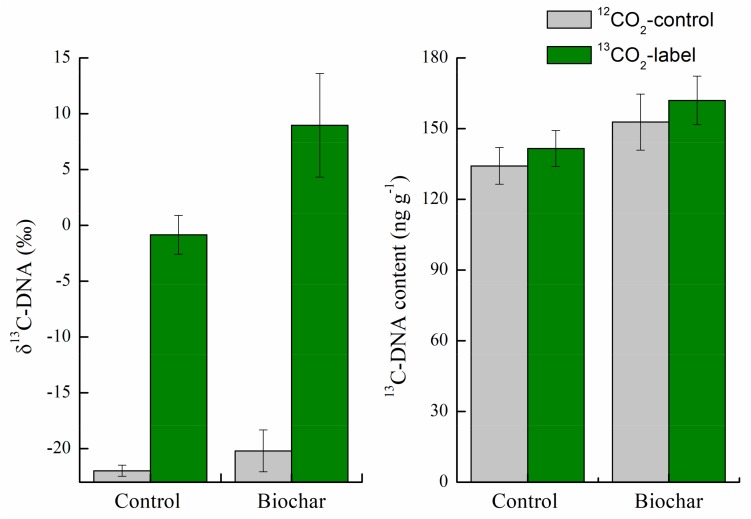
After 35-day of continuous labeling, the overall isotopic signatures of δ13C were −20.1‰ ± 1.87‰ and 398‰ ± 32.2‰ in the unlabeled and 13CO2-labeled soils of the control treatment. For the biochar treatment, the corresponding values were −21.9‰ ± 0.49‰ and 335‰ ± 15.8‰ (Supplementary Figure S2). Additionally, relatively high δ13C (8.97‰) in the DNA and13C-DNA content (162 ng g–1) were found in the biochar treatment; the corresponding values were −0.84‰ and 141 ng g–1 in the control treatment (Figure 2). These results indicated the successful incorporation of 13C-labeled rhizodeposits into soil microbes.
FIGURE 2.
Isotopic signatures of δ13C (‰) and 13C-DNA concentration (ng g–1) in the control and biochar treatments after 35-day of continuous labeling. Bars are means ± SE. Significant differences between treatments were tested by one-way ANOVA with Fisher’s LSD test.
Compared with the control treatment, significantly higher (P < 0.05) C/N ratio was found in the biochar amendment soils (Table 1). In addition, relatively high soil pH, Olsen-P, NH4+, and NO3– contents were detected in the biochar treatment, although none of these values were significantly different compared with the control treatment. Similarly, there were no significant differences (P > 0.05) in total plant biomass between the biochar (4.65 g dry biomass) and control (4.05 g dry biomass).
TABLE 1.
Effect of the biochar amendment on soil properties in a legume-based intercropping system.
| Treatment | pH (1:2.5 H2O) | SOC (g kg–1) | TN (g kg–1) | C/N | Olsen-P (mg kg–1) | NH4+-N (mg kg–1) | NO3–-N (mg kg–1) |
| Control | 5.35 (0.05) | 19.3 (0.26)b | 1.97 (0.05) | 9.82 (0.16)b | 42.2 (1.1) | 4.49 (0.10) | 0.38 (0.08) |
| Biochar | 5.48 (0.03) | 24.8 (0.73)a | 2.11 (0.03) | 11.8 (0.39)a | 44.6 (0.53) | 4.74 (0.13) | 1.10 (0.35) |
SOC, soil organic carbon; TN, total nitrogen; C/N, carbon to nitrogen ratio; Olsen-P, available phosphorous; NH4+-N, ammonium nitrogen; and NO3–-N, nitrate nitrogen. Values are the means of the three replicates ± SE; Volumes within a column followed by the same lower case letters are a significant difference at P < 0.05 level.
Overall Bacterial Community Structure in the Rhizosphere
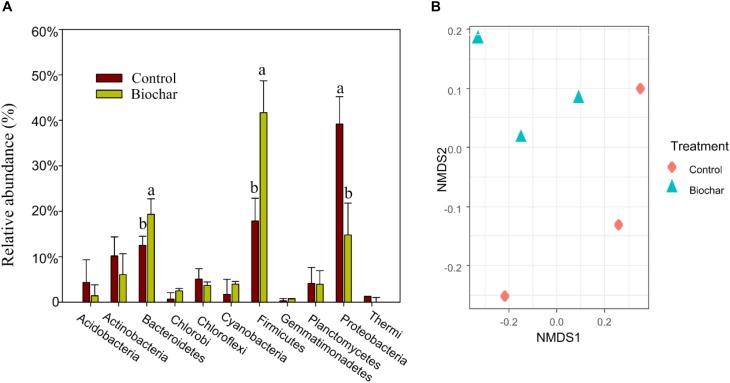
In bulk DNA samples, the phyla Firmicutes and Bacteroidetes were the predominant bacterial taxa found in both the biochar and control treatments. However, the biochar application to soil changed the relative abundance of taxa at the phylum level (Figure 3A) as well as their community structure (Figure 3B). Specifically, applying biochar increased (P < 0.05) the relative abundances of Firmicutes and Bacteroidetes, whereas the relative abundance of Proteobacteria decreased (P < 0.05). At the family level, the biochar treatment increased the relative abundances of Alicyclobacillaceae, Bacillaceae, Clostridiaceae, Peptococcaceae, Ruminococcaceae, and Ignavibacteriaceae, while it significantly decreased the relative abundance of Oxalobacteraceae (P < 0.05; Supplementary Figure S3).
FIGURE 3.
Effect of the biochar amendment on soil bacterial communities in the rhizosphere. (A) Taxonomic composition. Significant differences between treatments were tested by one-way ANOVA with Fisher’s LSD test, different letters indicate that significance at P < 0.05 level. (B) Ordination of Bray-Curtis dissimilarities (k = 4; stress = 0.04).
According to the Chao 1 estimator, the bacterial community of the biochar treatment had significantly higher (P < 0.01) species richness compared with the control treatment. However, the observed OTUs (Supplementary Figure S4) and richness estimated by the “breakaway” package (Supplementary Figure S5) were not significantly different between treatments (P > 0.05).
Soil Bacterial Community Compositions Between 13CO2-Labeled and Unlabeled Fractions
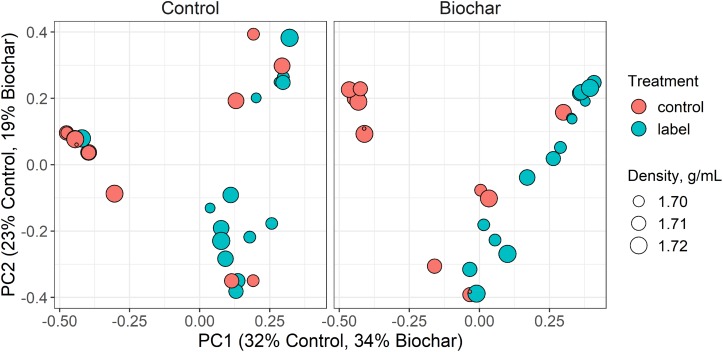
Incorporation of 13C into microbial DNA was evaluated by investigating the 16S rRNA gene sequence structure between the 13C-labeled and unlabeled samples from corresponding density fractions (Figure 4). Sequence results from the 13C-labeled samples and their corresponding unlabeled controls diverged specifically in the heavy gradient fraction (Adonis test, P < 0.001, Figure 4). This result confirmed the successful incorporation of 13C-labeling into the DNA of rhizosphere microbes.
FIGURE 4.
Principal coordinate ordinations (PCoA) ordination based on Bray-Curtis dissimilarities of operational taxonomic units (OTUs) in the heavy (>1.70 g ml–1) gradient fractions of the control and biochar treatments. The size of the density legend represents the range of gradient fraction OTU profiles. The size of a point corresponds to its fraction density, while the distance between points represents the similarity in microbial community composition.
Bacterial Utilization of Plant-Derived C Stimulated by Biochar Amendment
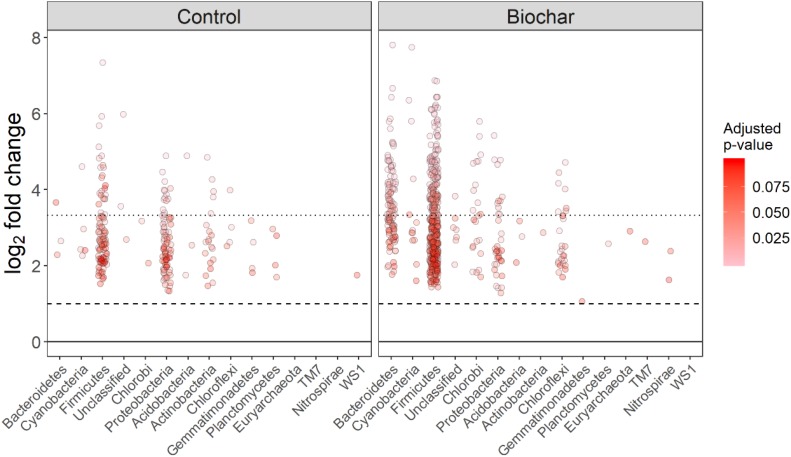
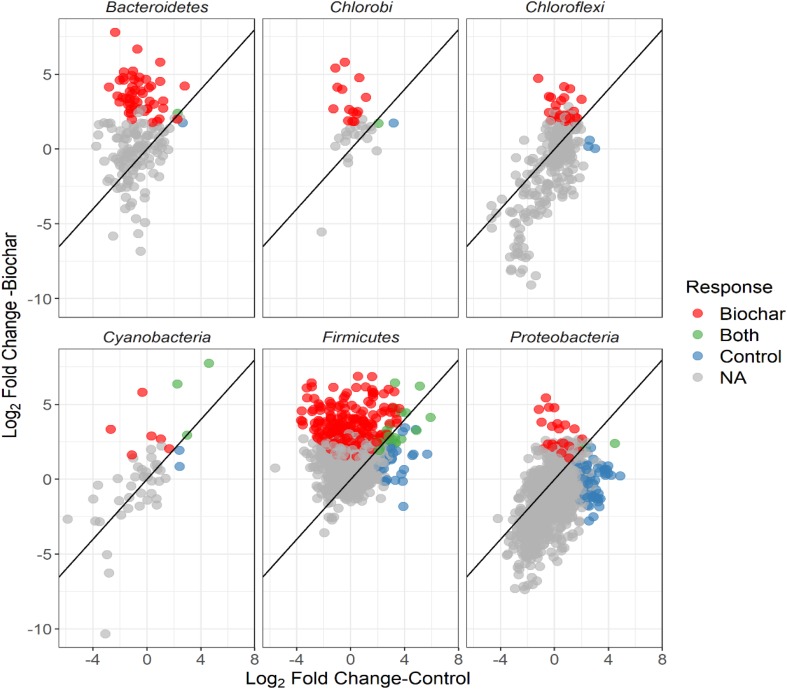
The non-normalized OTU abundance matrix taking 13C into their DNA was identified by a differential change in their abundance within the heavy gradient fractions when comparing the 13C-labeled and unlabeled samples (Figure 5). A total of 6812 and 5589 OTUs passed the 0.35-sparsity threshold filter in the control and biochar treatment, respectively. We observed a wide range of taxa actively utilizing root exudates at the phylum level: namely Firmicutes, Bacteroidetes, Proteobacteria, Chlorobi, and Actinobacteria (Figures 5, 6). In contrast, members of Acidobacteria, Planctomycetes, and Gemmatimonadetes barely used plant-derived C in both control and biochar treatments during 35-day continuous labeling. A total of 223 and 626 labeled OTUs were observed in the control and biochar treatments, which accounted for 3.27 and 11.2% of total OTUs, respectively. Of these labeled bacteria, Firmicutes (96 OTUs) and Proteobacteria (78 OTUs) were the most common taxa utilizing plant-derived C in the control treatment, which accounted for 43 and 35% of total labeled bacteria, respectively. Compared with the control, the biochar application greatly stimulated Firmicutes (403 OTUs) and Bacteroidetes (111 OTUs), while it decreased members of Proteobacteria (35 OTUs) utilizing root exudates; these taxa accounted for 64, 18, and 5.5% of the total labeled bacteria, respectively. The OTUs identified as root-exudate consumers were highly diverse in both treatments (Figure 7). There were 51 shared OTUs with 578 and 172 OTUs were unique to the biochar and control treatments, respectively. The shared OTUs were mainly distributed within the phylum Firmicutes (Figure 7 and Supplementary Figure S6). With regard to the overall taxonomic composition of the bacterial community, the biochar amendment increased the relative abundances of Firmicutes and Bacteroidetes, while decreasing the abundance of Proteobacteria in the > 1.70 g mL–1 heavy fractions (Supplementary Figure S7).
FIGURE 5.
Log2-fold changes in the relative abundance of OTUs (13C-labeled vs. unlabeled) in the heavy (>1.70 g mL–1) gradients for the control and biochar treatments. All the OTUs passed the 35%-sparsity threshold (i.e., OTUs were found in at least 35% fractions) of the heavy fractions. Each circle shows a single OTU, and circles denote proportion fold-changes that had an adjusted P-value below a false discovery rate of 10%.
FIGURE 6.
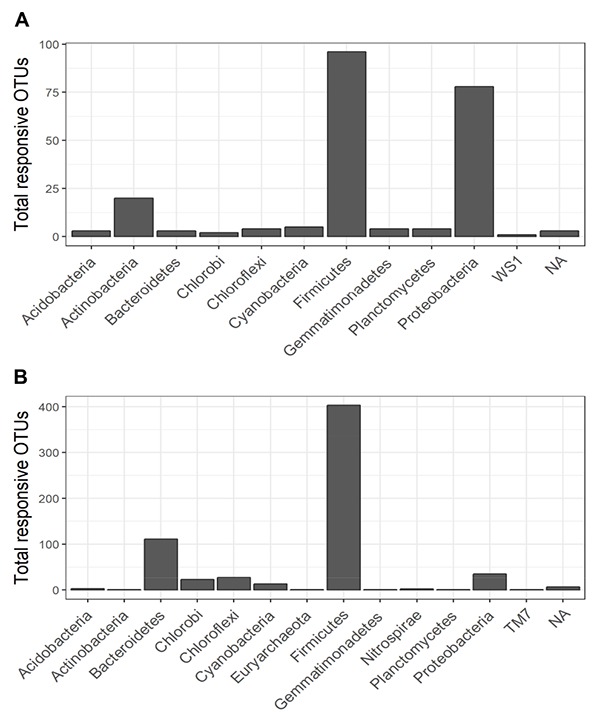
Total responsive OTUs in each bacterial phylum that responded significantly (i.e., with BH-adjusted P-values < 0.1) in the heavy gradient fraction of the control (A) and biochar (B) treatments.
FIGURE 7.
Log2-fold changes of the relative abundance of OTUs in response to biochar (13C-labeled vs. unlabeled) or control (13C-labeled vs. unlabeled) for the top six abundant bacterial phyla. Color of circles indicate unique, shared and non-reposed OTUs between the control and biochar treatments.
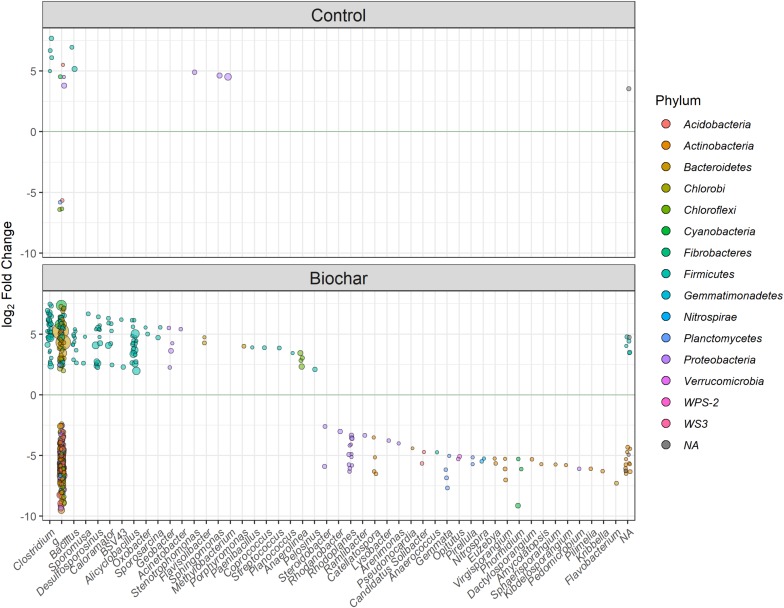
At the genus level, we detected a total of 19 genera in the biochar treatment, mainly belonging to Clostridium, Desulfosporosinus, Alicyclobacillus, Caloramator, and Bacillus (within the phylum Firmicutes), Flavisolibacter and Porphyromonas (phylum Bacteroidetes), and Geobacter (within the phylum Proteobacteria). All of these taxa were significantly enriched (P < 0.01) in the heavy fractions of labeled samples relative to the corresponding unlabeled samples (Figure 8). By contrast, in the control treatment, we only detected five genera that were significantly (P < 0.01) enriched in its heavy fractions, which belonged to the phyla Firmicutes and Proteobacteria.
FIGURE 8.
Differentially abundant OTUs between the labeled vs. unlabeled heavy (>1.70 g ml–1) gradient fractions for the control and biochar treatments at the genus level. Highly enriched OTUs with a significance of P < 0.01 are shown. The g_ indicates unclassified microbe at the genus level; the circle size represents the mean normalized counts of the OTUs across all samples.
Discussion
Recent studies have shown that using a system of legume-based intercropping can enhance N fixation and phosphorous P availability in the rhizosphere over that of a monoculture (Tang et al., 2014; Rose et al., 2015). In the present study, we found no significant differences in soil available nutrients or plant biomass in the legume-based intercropping system between the biochar and control treatments. The minor differences in soil nutrient availability and plant biomass between treatments can be attributed to multiple factors. For example, the original soil organic matter content in the intercropping system was relatively high, while the biochar rate (2%) used in the study could not high enough to markedly improve soil nutrient conditions or plant grawth. Other, studies have shown that biochar amendment may have the potential to improve soil nutrient status and alleviate soil nutrient stress (Ding et al., 2016; Pandit et al., 2018). Furthermore, Graber et al. (2015) found that substances extracted from biochar have a strong effect on root hair development under abiotic growing conditions.
Phylogenetic affiliation of the 16S rRNA gene sequences revealed that biochar-induced shifts in soil microbial community composition were mainly ascribed to a remarkable increase in the relative abundances of the phyla Firmicutes and Bacteroidetes, as well as a significant decrease in the relative abundance of the phylum Proteobacteria. These results are consistent with those reported by Hu et al. (2014), who found that Firmicutes and Bacteroidetes occurred distinctively in a red-oxidized loam soil after short-term biochar amendment compared with the control samples. Members of Bacteroidetes, widely found in soils and animal-centric canals, have the ability to degrade polysaccharides and cellulose (Fierer et al., 2007; Hu et al., 2014; Wolińska et al., 2017). A study also found that the relative abundance of Bacteroidetes was substantially higher in the root-associated community of biochar-amended soil, while the abundance of Proteobacteria was much higher in the control treatment (Kolton et al., 2011). Firmicutes are recognized as copiotrophs (or r-strategists), which explains their predominance in the soil after biochar amendment. A large proportion of members from the family Clostridiaceae in the phylum Firmicutes are capable of N fixation (Ding et al., 2017). The Chao 1 estimator of the bacterial community greatly increased after biochar amendment, although whereas we did not find significant differences in the observed species. The similarity in observed species between treatments may be attributed to the fact that identifying diversity in complex soil environments is very challenging. Furthermore, the richness estimated by the “breakaway” package did not significantly differ between treatments. The discrepancies between Chao 1 estimator and estimated richness may be attributed to the results of Chao 1 problematic in environments where many rare OTUs are never detected (Willis et al., 2017).
Pulse-labeling plants with 13CO2 emphasized the current photosynthate, which may not label all plant C pools to the same degree (De Visser et al., 1997; Yao et al., 2012). In the current study, we applied a steady-labeling approach instead of pulse-labeling to ensure that all the plant-derived C was labeled to the same degree. During the 35-day period of continuous labeling with 13CO2, the biomass of faba bean and maize seedlings was synthesized. At the end of this labeling period, the δ13C values of rhizosphere soil were labeled up to 300‰ for both the control and biochar treatments (Supplementary Figure S2). This labeled CO2 in soil would have participated in a fair number of processes, including microbial incorporation and the respiration of root exudates. Hence, the experimental set-up used in this study allowed us to explore, in a more direct way, the effects of soil biochar amendment on the interaction between plant and microbes in the rhizosphere. The “cross-feeding” is a common limitation for DNA-SIP approach. Ideally a short sampling time should be selected to reduce the cross-feeding effect, but this is difficult to optimize in practice. If sampling is too late, the rate of label incorporation by primary autotrophic assimilation may be slower than secondary heterotrophic incorporation; if sampling is too early there may be insufficient13C label in DNA for accurate detection. Herein the 35-day continuous labeling was performed to generate enough amount of labeled DNA. Despite the above-mentioned limitations and complications, the continuous labeling technique combined with DNA-SIP is the most powerful tool available for identifying the microbial communities primarily utilizing plant-derived C.
For both the unlabeled 13CO2-labeled and labeled samples, fraction density played a major role in 16S rRNA gene composition. This result was expected because genome G+C content is positively related to DNA buoyant density and therefore influence the 16S rRNA gene sequence structure. Additionally, we observed that the biochar application changed the soil bacterial community composition and diversity in the rhizosphere, while altering the members involved in actively assimilating the plant-derived C. Recent studies have documented that despite the high diversity of microbes occurring in the rhizosphere, only a few microbial groups (less than 4% of the total proportion) effectively utilize the plant-derived C (Gschwendtner et al., 2016; Hannula et al., 2017). In the current study, we found a wide range of bacterial taxa involved in the utilization of plant-derived C, in both control and biochar treatments (Figure 4). These results may suggest that bacterial species from these taxa grew more slowly, and this phenomenon may be attributed to different lifestyle strategies of these taxa. For example, Acidobacteria are recognized as slow-growing bacteria, or “oligotrophs,” which have high C use efficiency and are abundant in soil with high recalcitrant organic matter concentration (Fierer et al., 2007; Trivedi et al., 2017). Similarly, Gemmatimonadetes have been found to decrease their relative abundance after fresh organic matter input, and they are more likely to decompose existing soil organic C rather than fresh organic matter (Pascault et al., 2013; Whitman et al., 2016).
In the control treatment, Firmicutes and Proteobacteria were two predominant bacterial phyla utilizing the plant-derived C. By comparison, the biochar treatment clearly stimulated more bacterial members to utilize the plant-derived C (Figure 4). Specifically, the biochar amendment significantly enhanced the utilization of plant-derived C by Firmicutes and Bacteroidetes members (e.g., Clostridium, Sporomusa, Desulfosporosinus, Alicyclobacillus, Flavisolibacter, Geobacter, and Bacillus), however, in the control treatment, Proteobacteria members (e.g., Methylobacterium and Sphingomonas) tended to increase in their relative abundance to consume the plant-derived C. Bacillus and Clostridium were found in both the control and biochar treatments, and these two taxa are known, respectively, for their N-fixing and P-solubilizing abilities (Long et al., 2018). Our results imply that Bacillus and Clostridium may play an important role in N and P cycling in a faba bean-maize intercropping system. Interestingly, Desulfosporosinus, Alicyclobacillus, and Geobacter are known for their iron and sulfur oxidation abilities (Ding et al., 2015). Therefore, the participation of these taxa in nutrient cycling (e.g., by fixing N or solubilizing P) should be addressed in the intercropping system in future research. Generally, the pronounced shifts in the soil bacterial community utilizing the plant-derived C provide strong evidence that biochar amendment induces a distinct plant-microbiome interaction in the rhizosphere.
Our results indicate that biochar amendment not only changed the overall bacterial community in the rhizosphere but also favored the presence and abundance of bacteria with N-fixing and P-solubilizing abilities in the legume-based intercropping system (V. faba L., with Z. mays L.). Their utilization of plant-derived C may thus have crucial consequences for C storage and dynamics, as well as nutrient cycling and plant performance more generally, in agro-ecosystems that use intercropping.
Author Contributions
HY designed the study. YL and HL performed the experiments. YL and HY analyzed the data. HL and HY wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (41525002, 41471206, 41601249, and 41877051), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15020301), the Talents of Guizhou Science and Technology Cooperation Platform [(2017)5726-52], and the Ningbo Municipal Science and Technology Bureau (2015C10031).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01361/full#supplementary-material
References
- Abel S., Peters A., Trinks S., Schonsky H., Facklam M., Wessolek G. (2013). Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202 183–191. 10.1016/j.geoderma.2013.03.003 [DOI] [Google Scholar]
- Angel R., Panholzl C., Gabriel R., Herbold C., Wanek W., Richter A., et al. (2018). Application of stable-isotope labelling techniques for the detection of active diazotrophs. Environ. Microbiol. 20 44–61. 10.1111/1462-2920.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri D. V., Chaparro J. M., Zhang R., Shen Q., Vivanco J. M. (2013). Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 288 4502–4512. 10.1074/jbc.M112.433300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz U., Martinoia E. (2014). Root exudates: the hidden part of palnt defense. Trends Plant Sci. 19 90–98. 10.1016/j.tplants.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Bao S. D. (1999). Analytical Methods of Soil Agrochemistry (in Chinese). Beijing: China Agricultural Press. [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. stat. Soc. Ser. B Stat. Methodol. 57 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelle E., Mendes R., Bakker P. A., Raaijmakers J. M. (2016). Fungal invasion of the rhizosphere microbiome. ISME J. 10 265–268. 10.1038/ismej.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal R. C., Wang W., Robertson G. P., Parton W. J. (2003). Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Soil Res. 41 165–195. [Google Scholar]
- De Visser R., Vianden H., Schnyder H. (1997). Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation: assessment by 13C and 15N steady-state labelling. Plant Cell. Environ. 20 37–46. 10.1046/j.1365-3040.1997.d01-9.x [DOI] [Google Scholar]
- Ding L. J., Su J. Q., Li H., Zhu Y. G., Cao Z. H. (2017). Bacterial succession along a long-term chronosequence of paddy soil in the Yangtze River Delta. China Soil Biol. Biochem. 104 59–67. 10.1016/j.soilbio.2016.10.013 [DOI] [Google Scholar]
- Ding L. J., Su J. Q., Xu H. J., Jia Z. J., Zhu Y. G. (2015). Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. ISME J. 9 721–734. 10.1038/ismej.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Liu Y., Liu S., Li Z., Tan X., Huang X., et al. (2016). Biochar to improve soil fertility. a review. Agron. Sustain. Dev. 36:36. [Google Scholar]
- Drigo B., Pijl A. S., Duyts H., Kielak A. M., Gamper H. A., Houtekamer M. J., et al. (2010). Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc. Natl. Acad. Sci. U.S.A. 107 10938–10942. 10.1073/pnas.0912421107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Fan F., Zhang F., Qu Z., Lu Y. (2008). Plant carbon partitioning below ground in the presence of different neighboring species. Soil Biol. Biochem. 40 2266–2272. 10.1016/j.soilbio.2008.05.003 [DOI] [Google Scholar]
- Fierer N., Bradford M. A., Jackson R. B. (2007). Toward an ecological classification of soil bacteria. Ecology 88 1354–1364. 10.1890/05-1839 [DOI] [PubMed] [Google Scholar]
- Fuchslueger L., Bahn M., Hasibeder R., Kienzl S., Fritz K., Schmitt M., et al. (2016). Drought history affects grassland plant and microbial carbon turnover during and after a subsequent drought event. J. Ecol. 104 1453–1465. 10.1111/1365-2745.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland J. L. (1996). Patterns of potential C source utilization by rhizosphere communities. Soil Biol. Biochem. 28 223–230. 10.1016/0038-0717(95)00113-1 [DOI] [Google Scholar]
- Gkarmiri K., Mahmood S., Ekblad A., Alström S., Högberg N., Finlay R. (2017). Identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Appl. Environ. Microbiol. 83:e01938-17. 10.1128/AEM.01938-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollany H., Allmaras R., Copeland S., Albrecht S., Douglas C. (2005). Tillage and nitrogen fertilizer influence on carbon and soluble silica relations in a Pacific Northwest Mollisol. Soil Sci. Soc. Am. J. 69 1102–1109. [Google Scholar]
- Gollany H. T., Molina J.-A. E., Clapp C. E., Allmaras R. R., Layese M. F., Baker J. M., et al. (2004). Nitrogen leaching and denitrification in continuous corn as related to residue management and nitrogen fertilization. Environ. Manage. 33 S289–S298. [Google Scholar]
- Graber E., Tsechansky L., Mayzlish-Gati E., Shema R., Koltai H. (2015). A humic substances product extracted from biochar reduces Arabidopsis root hair density and length under P-sufficient and P-starvation conditions. Plant Soil 395 21–30. 10.1007/s11104-015-2524-3 [DOI] [Google Scholar]
- Gschwendtner S., Engel M., Lueders T., Buegger F., Schloter M. (2016). Nitrogen fertilization affects bacteria utilizing plant-derived carbon in the rhizosphere of beech seedlings. Plant Soil 407 203–215. 10.1007/s11104-016-2888-z [DOI] [Google Scholar]
- Gu Y., Hou Y., Huang D., Hao Z., Wang X., Wei Z., et al. (2017). Application of biochar reduces Ralstonia solanacearum infection via effecs on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 415 269–281. 10.1007/s11104-016-3159-8 [DOI] [Google Scholar]
- Hamady M., Walker J. J., Harris J. K., Gold N. J., Knight R. (2008). Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 5 235–237. 10.1038/nmeth.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula S. E., Morrien E., de Hollander M., van der Putten W. H., van Veen J. A., de Boer W. (2017). Shifts in rhizosphere fungal community during secondary succession following abandonment from agriculture. ISME J. 11 2294–2304. 10.1038/ismej.2017.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelstein J., Ares A., Gallagher D., Myers J. (2017). A meta-analysis of intercropping in Africa: impacts on crop yield, farmer income, and integrated pest management effects. Int. J. Agr. Sustain. 15 1–10. 10.1080/14735903.2016.1242332 [DOI] [Google Scholar]
- Hu L., Cao L., Zhang R. (2014). Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 30 1085–1092. 10.1007/s11274-013-1528-5 [DOI] [PubMed] [Google Scholar]
- Imparato V., Hansen V., Santos S. S., Nielsen T. K., Giagnoni L., Hauggaard-Nielsen H., et al. (2016). Gasification biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroecosystem. Soil Biol. Biochem. 99 128–136. 10.1016/j.soilbio.2016.05.004 [DOI] [Google Scholar]
- Jaiswal A. K., Elad Y., Graber E. R., Frenkel O. (2014). Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concerntration. Soil Biol. Biochem. 69 110–118. 10.1016/j.soilbio.2013.10.051 [DOI] [Google Scholar]
- Kolton M., Graber E. R., Tsehansky L., Elad Y., Cytryn E. (2017). Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 213 1393–1404. 10.1111/nph.14253 [DOI] [PubMed] [Google Scholar]
- Kolton M., Harel Y. M., Pasternak Z., Graber E. R., Elad Y., Cytryn E. (2011). Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 77 4924–4930. 10.1128/AEM.00148-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. (2011). Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98 152–153. 10.1016/j.ygeno.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Lehmann J., Rillig M. C., Thies J., Masiello C. A., Hockaday W. C., Crowley D. (2011). Biochar effects on soil biota–a review. Soil Biol. Biochem. 43 1812–1836. 10.1016/j.soilbio.2011.04.022 [DOI] [Google Scholar]
- Liu L., Wang Y., Yan X., Li J., Jiao N., Hu S. (2017). Biochar amendments increase the yield advantage of legume-based intercropping systems over monoculture. Agric. Ecosyst. Environ. 237 16–23. 10.1016/j.agee.2016.12.026 [DOI] [Google Scholar]
- Long X. E., Yao H., Huang Y., Wei W., Zhu Y. G. (2018). Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 118 103–114. 10.1016/j.soilbio.2017.12.014 [DOI] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč T., Salzberg S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D., Price M. N., Goodrich J., Nawrocki E. P., DeSantis T. Z., Probst A., et al. (2012). An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6 610–618. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P. J., Holmes S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes L. W., Raaijmakers J. M., de Hollander M., Mendes R., Tsai S. M. (2017). Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 12 212–214. 10.1038/ismej.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C. (2003). Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23 375–396. 10.1051/agro:2003011 [DOI] [Google Scholar]
- Oksanen J., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O’Hara R. B., et al. (2016). Vegan: Community Ecology Package. Available at: http://CRAN.R-project.org/package=vegan (accessed May 12, 2019). [Google Scholar]
- Olsen S. R. (1954). Estimation of Available Phosphorus in Soils by Extraction With Sodium Bicarbonate. Washington, DC: United States Department Of Agriculture. [Google Scholar]
- Pandit N. R., Mulder J., Hale S. E., Martinsen V., Schmidt H. P., Cornelissen G. (2018). Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 625 1380–1389. 10.1016/j.scitotenv.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Pascault N., Ranjard L., Kaisermann A., Bachar D., Christen R., Terrat S., et al. (2013). Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 16 810–822. 10.1007/s10021-013-9650-7 [DOI] [Google Scholar]
- Pausch J., Kuzyakov Y. (2018). Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 24 1–12. 10.1111/gcb.13850 [DOI] [PubMed] [Google Scholar]
- Pepe-Ranney C., Koechli C., Potrafka R., Andam C., Eggleston E., Garcia-Pichel F., et al. (2016). Non-cyanobacterial diazotrophs mediate dinitrogen fixation in biological soil crusts during early crust formation. ISME J. 10 287–298. 10.1038/ismej.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietikäinen J., Kiikkilä O., Fritze H. (2000). Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89 231–242. 10.1034/j.1600-0706.2000.890203.x [DOI] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foudation for Statistical Computing. [Google Scholar]
- Rose T. J., Julia C. C., Shepherd M., Rose M. T., Van Zwieten L. (2015). Faba bean is less susceptible to fertiliser N impacts on biological N2 fixation than chickpea in monoculture and intercropping systems. Biol. Fert. Soils 52 271–276. 10.1007/s00374-015-1062-8 [DOI] [Google Scholar]
- Tang X., Bernard L., Brauman A., Daufresne T., Deleporte P., Desclaux D., et al. (2014). Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol. Biochem. 75 86–93. 10.1016/j.soilbio.2014.04.001 [DOI] [Google Scholar]
- Tang X., Placella S. A., Daydé F., Bernard L., Robin A., Journet E.-P., et al. (2016). Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 407 119–134. 10.1007/s11104-016-2949-3 [DOI] [Google Scholar]
- Trivedi P., Delgado-Baquerizo M., Jeffries T. C., Trivedi C., Anderson I. C., Lai K., et al. (2017). Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 19 3070–3086. 10.1111/1462-2920.13779 [DOI] [PubMed] [Google Scholar]
- Wang J., Chapman S. J., Yao H. (2016). Incorporation of 13C-labelled rice rhizodeposition into soil microbial communities under different fertilizer applications. Appl. Soil Ecol. 101 11–19. 10.1016/j.apsoil.2016.01.010 [DOI] [Google Scholar]
- Whitman T., Pepe-Ranney C., Enders A., Koechli C., Campbell A., Buckley D. H., et al. (2016). Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J. 10 2918–2930. 10.1038/ismej.2016.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2010). ggplot2: elegant graphics for data analysis. J.Stat. Softw. 35 65–88. [Google Scholar]
- Willis A., Bunge J., Whitman T. (2017). Improved detection of changes in species richness in high diversity microbial communities. J. R. Stat. Soc. Ser. C. Appl. Stat. 66 963–977. 10.1111/rssc.12206 [DOI] [Google Scholar]
- Wolińska A., Kuźniar A., Zielenkiewicz U., Izak D., Nakonieczna A. S., Banach A., et al. (2017). Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 119 128–137. 10.1016/j.apsoil.2017.06.009 [DOI] [Google Scholar]
- Xu H. J., Wang X. H., Li H., Yao H. Y., Su J. Q., Zhu Y. G. (2014). Biochar impacts soil microbial community compostion and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 48 9391–9399. 10.1021/es5021058 [DOI] [PubMed] [Google Scholar]
- Yao H., Thornton B., Paterson E. (2012). Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol. Biochem. 53 72–77. 10.1016/j.soilbio.2012.05.006 [DOI] [Google Scholar]
- Youngblut N. D., Buckley D. H. (2014). Intra-genomic variation in G+ C content and its implications for DNA stable isotope probing. Environ. Microbiol. Rep. 6 767–775. 10.1111/1758-2229.12201 [DOI] [PubMed] [Google Scholar]
- Zhalnina K., Louie K. B., Hao Z., Mansoori N., da Rocha U. N., Shi S., et al. (2017). Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3 470–480. 10.1038/s41564-018-0129-3 [DOI] [PubMed] [Google Scholar]
- Zhang C., Lin Y., Tian X., Xu Q., Chen Z., Lin W. (2017). Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 112 90–96. 10.1016/j.apsoil.2016.12.005 [DOI] [Google Scholar]
- Zheng H., Wang X., Chen L., Wang Z., Xia Y., Zhang Y., et al. (2018). Enhanced growth of halophyte plants in biochar-amended coastal soil: roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 41 517–532. 10.1111/pce.12944 [DOI] [PubMed] [Google Scholar]
- Zhou J., Wu L., Deng Y., Zhi X., Jiang Y. H., Tu Q., et al. (2011). Reproducibility and quantitation of amplicon sequencing-based detection. ISME J. 5 1303–1313. 10.1038/ismej.2011.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.