Abstract
Emotion regulation is thought to involve communication between and within large-scale brain networks that underlie emotion reactivity and cognitive control. Aberrant network interaction might therefore be a key neural feature of mental disorders that involve emotion dysregulation. Here we tested whether connectivity hierarchies within and between emotion reactivity and cognitive reappraisal networks distinguishes social anxiety disorder (SAD) patients (n = 70) from healthy controls (HC) (n = 25). To investigate network organization, we implemented a graph-theory method called Dependency Network Analysis. Participants underwent fMRI while watching or reappraising video clips involving interpersonal verbal criticism. During reappraisal, the reappraisal network exerted less influence on the reactivity network in SAD participants. Specifically, the influence of the right inferior frontal gyrus on both reappraisal and reactivity networks was significantly reduced in SAD compared with HC, and correlated negatively with negative emotion ratings among SAD participants. Surprisingly, the amygdala exhibited reduced influence on the reappraisal network in SAD relative to HC. Yet, during the watch condition, the left amygdala’s influence on the reactivity network increased with greater social anxiety symptoms among SAD participants. These findings refine our understanding of network organization that contributes to efficient reappraisal or to disturbances in applying this strategy in SAD.
Keywords: cognitive reappraisal, emotional reactivity, fMRI, graph theory network analysis, social anxiety disorder
Introduction
A growing perspective in cognitive neuroscience views the communication within and between large-scale functional networks as the biological basis for the formation of human emotional experience (Menon 2011; Sylvester et al. 2012). However, the most informative metric of such communication has yet to be agreed upon. One candidate—the mathematical field of graph theory—recently emerged as a tool for characterizing fMRI driven brain network features that can distinguish between processes, as well as between healthy and pathological states (Bassett et al. 2008; Bullmore and Sporns 2009; Sporns 2011).
To date, most of the fMRI studies that have conducted graph theoretical analysis have done so during resting state, showing that graph analysis can identify specific brain network features as possible markers of psychopathology (Micheloyannis et al. 2006; Stam and Reijneveld 2007; Stam et al. 2007; Liu et al. 2008; van den Heuvel and Hulshoff Pol 2010; Menon 2011). However, resting state network organization studies have been limited in probing specific mental process, thus, hampering diagnostic specificity.
More recently, several studies have demonstrated functional brain network dynamics in response to experimental task demands. For example, Bassett et al. (2011, 2013) showed that network features such as modularity and flexibility changed during a motor learning task, and Raz et al. (2012, 2013) showed that interbrain- and intrabrain-network cohesion levels were associated with emotional experience during film viewing. These foundational efforts have paved the way for addressing an essential aspect of network organization that is related to its hierarchy as indicated by metrics of influence and dependency (Hutchison et al. 2013). In particular, it has been suggested that an examination of hierarchy of nodes in the network could help characterize its functional specificity (Sporns 2011) and accurately identify the nodes that are most critical for task performance. Importantly, in addition to advancing our knowledge about mechanisms underlying pathological processes, the ability to identify regions that are critical for specific mental processes can facilitate the development of more effective therapeutic interventions.
One transdiagnostic mental process that relies on relatively well-understood brain systems is emotion regulation: strategies used to decrease, maintain or increase emotional responses (Gross 1998; Phillips 2008; Gyurak et al. 2011; Etkin et al. 2015). One particularly important and well-studied form of emotion regulation is cognitive reappraisal, an explicit emotion regulation strategy that involves modulating an emotional response by reinterpreting the meaning of an emotional stimulus or event. Cognitive reappraisal is a fundamental skill incorporated in nearly all psychosocial treatments for mood and anxiety disorders (Clark and Wells 1995). Furthermore, successful implementation of emotional regulation contributes to treatment outcome and resilience among patients (Min et al. 2013).
Current neuroscientific views of emotion regulation maintain that reappraisal is achieved via the interaction between 2 distinct brain networks (Ochsner et al. 2012; Etkin et al. 2015): 1) an “explicit cognitive regulation” network, which is assumed to support effortful forms of emotion regulation such as reappraisal, and consists of frontoparietal and mid-dorsal frontal brain regions implicated in cognitive control (inferior frontal gyrus [IFG], dorsolateral prefrontal cortex [dlPFC], superior parietal lobule [SPL] and middle frontal gyrus [MFG] and presupplementary motor area [pre-SMA]), known to allocate attention and employ decision making (Buhle et al. 2014), and 2) an “emotional reactivity” network, which is involved in the perception and generation of an emotional response, comprised of limbic (e.g., amygdala), paralimbic (e.g., periaqueductal gray [PAG]), and cortical (e.g., insula and dorsal cingulate cortex) regions.
Generally, the explicit cognitive regulation network is assumed to support mental construction of reappraisals which can modulate emotional responses generated by the reactivity network (Buhle et al. 2014). In accordance with the functional network approach described above, it is assumed that uncoordinated interaction between emotional reactivity and regulation networks may be a critical aspect of the emotion dysregulation that characterizes individuals diagnosed with different anxiety and mood disorders (Etkin et al. 2015; Jazaieri et al. 2015; Morawetz, Bode, Derntl et al. 2016).
In the present study, we applied Dependency Network Analysis (DEPNA)—a newly developed graph based analysis method—to distinguish different patterns of network hierarchy and information flow within and between brain networks (Jacob et al. 2016, 2018). DEPNA evaluates a brain region’s importance within a given network according to its influence over the correlations between all other pairs of brain regions, henceforth, termed “Influencing Degree.” Influence hierarchy was evaluated while individuals implemented reappraisal or reacted naturally to interpersonal criticism delivered by others through short video clips (Ziv et al. 2013a, 2013b).
To more robustly investigate the role of network hierarchy during reappraisal of negative emotion in a specific clinical sample, we characterized the hierarchy and dynamics of fMRI connectivity in social anxiety disorder (SAD) patients compared with healthy controls (HC) in the context of ecologically valid and personally salient social criticism. With a lifetime prevalence of about 12% (Kessler et al. 2005), SAD is a psychiatric disturbance uniquely driven by heightened emotional reactivity to and distorted cognitive processing of social information (Stein and Stein 2008). While this maladaptive processing can be modified by practicing cognitive reappraisal in the context of cognitive behavioral therapy (CBT), individuals with SAD have deficits in implementing this strategy, suggesting abnormality in the reappraisal-related network (Werner et al. 2011; Jazaieri et al. 2015).
In regards to heightened emotional reactivity various neuroimaging studies of SAD have shown a link between social anxiety symptoms and amygdala hyperactivity (Etkin and Wager 2007) and hyperconnectivity (Brühl et al. 2014) in response to social information. Regarding emotional regulation, several studies of SAD have found differences in recruitment of reappraisal-related brain areas, including the IFG or dlPFC and their functional connectivity with regions implicated in emotional reactivity (Goldin, Manber et al. 2009; Goldin, Manber-Ball et al. 2009; Ziv et al. 2013a). More specifically, when reappraising an emotional response to negative self-beliefs, SAD patients showed reduced inverse functional connectivity between the amygdala and reappraisal-related prefrontal cortical brain regions relative to HC (Goldin, Manber-Ball et al. 2009). Correspondingly, greater inverse functional coupling of the amygdala with cognitive control regions, as well as positive functional connectivity within reappraisal-related regions in the prefrontal cortex, was evident following CBT in SAD patients, suggesting a causal role for effective internetwork relations when employing emotion regulation (Goldin et al. 2013). Additionally, people with SAD exhibit hyperactivity in emotional reactivity regions such as the amygdala; a key region involved in the generation of emotional reactions (Etkin and Wager 2007). SAD patients also exhibit ineffective coupling of the amygdala with the cognitive reappraisal network during different mental tasks, such as viewing socioemotional stimuli (Freitas-Ferrari et al. 2010; Brühl et al. 2014), regulating negative emotions (Goldin, Manber et al. 2009; Goldin, Manber-Ball et al. 2009) or merely resting in the fMRI scanner (Liao et al. 2010; Hahn et al. 2011).
To date, several studies examined connectivity patterns of large-scale brain networks in SAD patients (Liao et al. 2010; Ding et al. 2011; Hahn et al. 2011; Liu et al. 2015). However, because these studies were conducted during fMRI resting state, they have not been able to directly probe specific information processing capacities or deficits in SAD patients. To test the directionality of effective connectivity during a specific mental task, Sladky et al. (2015) recently implemented a dynamic causal modeling (DCM) analysis on data obtained during facial emotion and object discrimination tasks. They found excitatory connections between the amygdala and the orbitofrontal cortex (OFC) alongside reduced amygdala influence over the dlPFC in a group of SAD patients. These findings suggest that in addition to the amygdala being influenced by higher cognitive areas during regulation of negative affect, its influence over prefrontal regions is not to be neglected. However, this direction of influence has not been tested in the context of reappraisal thus far. Moreover, while DCM (Friston et al. 2003) is currently the most powerful method for fMRI causality inference, it confines the a priori examination of effective connectivity to a small set of regions and requires a specific biophysical model (Friston 2009).
Based on findings to date, we first hypothesized that emotion dysregulation in SAD is related to deficient recruitment of a brain network associated with cognitive reappraisal, as well as to atypical interactions between reappraisal- and emotional reactivity-related networks (Hypothesis 1). We further assumed that such deficits would manifest in lesser influencing degree values (i.e., less impact on the reappraisal and reactivity networks connectivity) in the SAD group compared with HC as detected by the DEPNA. If true, we would expect to observe the following testable effects as support for our Hypothesis 1, namely, that when participants implement reappraisal of interpersonal criticism, reappraisal-related brain regions would exert less influence both within the reappraisal network (intranetwork analysis, Hypothesis 1a) and on the reactivity network (internetwork analysis, Hypothesis 1b) in SAD participants relative to HC. In addition, we also examined whether the influence of the emotional reactivity network on the reappraisal network differed between SAD and HC participants (Hypothesis 1c). Furthermore, we hypothesized that people with SAD would demonstrate hyperconnectivity within the emotional reactivity network compared with HC (Hypothesis 2). We would then expect to observe greater influencing degrees for SAD versus HC within the emotional reactivity network brain regions (intranetwork analysis) during the “watch” condition (Hypothesis 2a). We further hypothesized that the amygdala would demonstrate greater influence over other brain regions within the emotional reactivity network (i.e., more likely to generate the emotional process) in SAD participants relative to HC, and that this would correlate with individual differences in social anxiety symptoms and subjective ratings within the SAD group (Hypothesis 2b). Lastly, we examined whether there was an association between the influencing degrees and individual differences in self-reported emotion ratings, social anxiety symptoms, and trait reappraisal across all SAD participants.
Materials and Methods
Participants
Participants included 70 patients (32 females) who met DSM-IV (Association 2013) diagnostic criteria for generalized SAD and 25 (13 females) demographically matched HC with no history of DSM-IV psychiatric disorders. The demographic and clinical variables are presented in Table 1, see Ziv et al. (2013b) for more details. Both SAD and HC had a mean age of 33 years (range: SAD 21–53 years; HC 21–52 years, see Table 1) and mean years of education of 17 (range: SAD 12–23 years; HC 16–20 years, see Table 1). All participants provided informed consent prior to entering the study in accordance with institutional review board regulations.
Table 1.
Demographic and clinical variables
| HC (N) | SAD (N) | t (df) | P | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Female (n) | 11 (25) | 33 (70) | ||
| Age (mean years ± STD) | 32.8 ± 9.7 (25) | 33.5 ± 9.2 (70) | 0.3 (93) | 0.74 |
| Education (mean years ± STD) | 17.7 ± 1.4 (25) | 16.8 ± 2.4 (70) | 1.7 (88) | 0.08 |
| LSAS-SR (mean ± STD) | 15.6 ± 9.4 (25) | 82.0 ± 18.3 (70) | 17.3 (93) | 0.000* |
| ERQ reappraisal capability | 41.1 ± 8.4 (21) | 26.9 ± 10.5 (63) | −5.6 (82) | 0.000* |
| ERQ reappraisal frequency | 38.2 ± 7.9 (21) | 28.7 ± 11.3 (63) | −3.6 (82) | 0.001* |
LSAS-SR = Liebowitz Social Anxiety Scale-Self-Report. ERQ = emotion regulation Questionnaire. *P < 0.0001.
Exclusion Criteria
Patients were excluded if they reported current pharmacotherapy or psychotherapy, history of neurological disorders, and current psychiatric disorders (other than SAD, generalized anxiety disorder, agoraphobia without a history of panic attacks, dysthymia, or specific phobia).
Self-Report Questionnaires
Participants completed self-report measures of SAD clinical symptoms and individual differences in reappraisal tendencies. As shown in Table 1, patients reported significantly greater social anxiety symptoms (Liebowitz Social Anxiety Scale-Self-Report [LSAS-SR] (Liebowitz 1987), and lesser reappraisal frequency and self-efficacy [ERQ] (Gross and John 2003)) compared with HC.
Dynamic Interpersonal Criticism fMRI Task
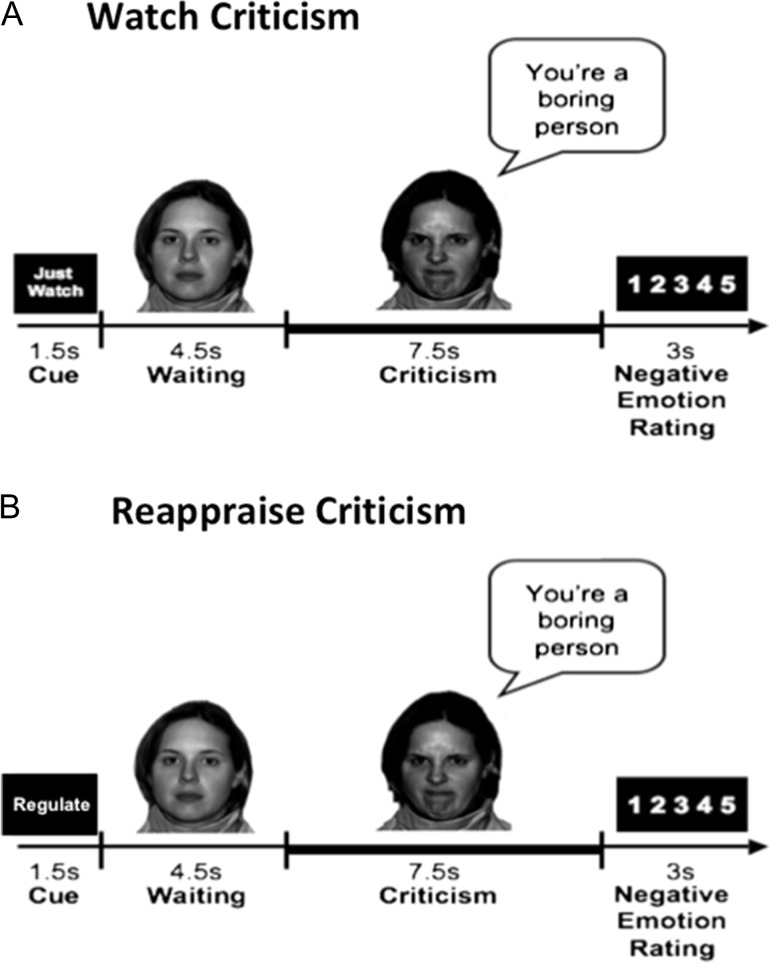
This task was originally described by Ziv et al. (2013b). In this task, videotaped actors displayed interpersonal criticism (Fig. 1) while participants were asked to either just watch or actively reappraise negative reactions to verbal and facial content displayed in short video clips. Each condition included 16 trials. Participants were trained prior to scanning to reappraise on a separate set of stimuli not used in the scanner. In the “Watch” condition, participants were instructed to view the video and evaluate whether the statement represents something true about themselves. The instructions for the “Reappraise” condition were to view the video and try to down-regulate their negative emotions evoked by the criticism, by actively reinterpreting the meaning of the emotion-inducing stimulus (Ochsner et al. 2004). After each condition, participants provided a negative emotion rating using a button response pad inside the scanner by responding to the question: “How negative do you feel?” (1 = not at all to 5 = very much).
Figure 1.
Dynamic interpersonal criticism task. Each block “Watch” or “Reappraise” consisted of: 1) a 1.5 s “Cue”; 2) a video-clip of an actor delivering interpersonal criticism; and 3) a negative emotion rating scale. Participants were instructed to either “Just Watch”—react normally to the stimuli without any attempt to control, modify or regulate their reactions—or to “Reappraise” by trying to down-regulate negative emotion reactions by actively reinterpreting the meaning of the emotion-inducing stimulus. The task consists of 2 conditions: (A) Watch Criticism and (B) Reappraise Criticism. This figure was taken from Ziv et al. (2013a,b).
An asterisk-counting task was used as a low-level cognitive control condition. This condition included 16 trials (12 s each), during which participants pressed a button to indicate the number of asterisks on the screen which changed from 1 to 5 asterisks every 3 s.
To assess whether the interpersonal criticism task yielded differences in negative emotion ratings as a function of the group and task condition, we analyzed mean negative emotion ratings with a 2 condition (watch vs. reappraise criticism) × 2 group (HC vs. SAD) repeated-measures analysis of variance (ANOVA) using Statistica 10 (StatSoft).
fMRI Data Acquisition and Preprocessing
Image acquisition was performed on a General Electric 3-T Signa magnet (GE Healthcare, Milwaukee, WI). Head movements were minimized using a bite-bar and foam padding. A total of 684 functional volumes (2 scans × 342 TRs per scan) were obtained at a repetition time of 1500 ms. Preprocessing included volume registration, motion correction, spatial smoothing, high-pass filtering, linear detrending, and spatial normalization using Analysis of Functional Neuroimages (AFNI) software (Cox 1996). The functional data were corrected using global signal regression. The 2 functional runs were concatenated prior to statistical analysis. Further details of image acquisition and preprocessing are provided in Supplement 1.
Dependency Network Analysis
Functional analysis was performed on 2 networks. The cognitive reappraisal network was selected according to a meta-analysis of neuroimaging studies on cognitive reappraisal (Buhle et al. 2014) and comprised 7 brain regions: bilateral IFG, bilateral MFG, bilateral SPL, and pre-SMA. The emotional reactivity network was selected according to a meta-analysis of neuroimaging studies on emotion (Kober et al. 2008) and comprised 6 brain regions: bilateral amygdala, bilateral middle insula (midIns), PAG, and dACC. For each ROI we created a spherical mask (radius = 4 mm, volume = 268.1 mm3) centered on the peak coordinates (Tables 2 and 3). The averaged BOLD signal was extracted for each ROI mask for each subject for each condition (watch and reappraise criticism). For each condition, we computed the average time course (15 s, 10 TRs) across all 16 trials. These time-points are mathematically sufficient to conduct correlation tests, and in terms of fMRI data, they are also sufficient to obtain the hemodynamic response function (HRF), which typically peaks after 6 s (Liao et al. 2002). Each trial consisted of a 12 s movie clip followed by a 3 s period during which the participant was cued to make a negative emotion rating. However, due to the relatively slow BOLD signal and HRF delay, we did not expect to obtain an effect from the ratings from just 2 time-points. We also note that in contrast to other functional connectivity studies, the current study is conducted using fMRI data from a block design task. We calculated functional connectivity based on each condition’s averaged BOLD signal, thus inherently controlling for motion artifacts.
Table 2.
Reappraise Criticism intranetwork and internetwork influence
| Region | Coordinates TAL [x, y, z] | HC influencing degree | SAD influencing degree | t (df) | P |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Intracognitive reappraisal network influence | |||||
| Left IFG | −40, 41, 2 | 0.86 ± 0.88 | 0.88 ± 0.85 | −0.11 (93) | 0.92 |
| Right IFG | 49, 24, 9 | 1.49 ± 1.18 | 0.82 ± 0.69 | 3.42 (93) | 0.001* |
| Left MFG | −32, −4, 52 | 1.31 ± 1.01 | 1.12 ± 0.80 | 0.97 (93) | 0.34 |
| Right MFG | 46, 8, 46 | 0.57 ± 0.69 | 0.58 ± 0.51 | −0.02 (93) | 0.98 |
| Left SPL | −40, −67, 35 | 0.63 ± 0.80 | 0.60 ± 0.64 | 0.23 (93) | 0.82 |
| Right SPL | 54, −52, 35 | 1.57 ± 1.07 | 1.43 ± 1.09 | 0.54 (93) | 0.59 |
| Pre-SMA | −2, 7, 62 | 1.02 ± 1.07 | 0.92 ± 0.96 | 0.44 (93) | 0.66 |
| Cognitive reappraisal regions’ influence on the emotional reactivity network | |||||
| Left IFG | −40, 41, 2 | 0.11 ± 0.08 | 0.10 ± 0.08 | 0.82 (93) | 0.41 |
| Right IFG | 49, 24, 9 | 0.17 ± 0.10 | 0.11 ± 0.08 | 3.33 (93) | 0.001* |
| Left MFG | −32, −4, 52 | 0.15 ± 0.09 | 0.12 ± 0.08 | 1.44 (93) | 0.15 |
| Right MFG | 46, 8, 46 | 0.10 ± 0.09 | 0.08 ± 0.07 | 1.43 (93) | 0.16 |
| Left SPL | −40, −67, 35 | 0.08 ± 0.09 | 0.07 ± 0.05 | 0.42 (93) | 0.68 |
| Right SPL | 54, −52, 35 | 0.16 ± 0.09 | 0.13 ± 0.08 | 1.57 (93) | 0.12 |
| Pre-SMA | −2, 7, 62 | 0.12 ± 0.08 | 0.11 ± 0.08 | 0.43 (93) | 0.67 |
| Emotional reactivity regions’ influence on the cognitive reappraisal network | |||||
| Left Amy | −19, −6, −13 | 0.14 ± 0.11 | 0.08 ± 0.06 | 3.94 (93) | 0.0002* |
| Right Amy | 18, −4, −14 | 0.14 ± 0.10 | 0.09 ± 0.07 | 3.19 (93) | 0.002* |
| Left midIns | −38, −1, 2 | 0.12 ± 0.10 | 0.09 ± 0.06 | 1.76 (93) | 0.08 |
| Right midIns | 40, −6, 4 | 0.10 ± 0.08 | 0.08 ± 0.06 | 1.07 (93) | 0.29 |
| PAG | 1, −29, −4 | 0.09 ± 0.08 | 0.06 ± 0.06 | 1.82 (93) | 0.07 |
| dACC | −1, 18, 35 | 0.12 ± 0.11 | 0.10 ± 0.08 | 1.25 (93) | 0.22 |
Amy, amygdala; dACC, dorsal anterior cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; midIns, middle insula; PAG, periaqueductal gray; pre-SMA, presupplementary motor area; SPL, superior parietal lobule. *P < 0.05 FDR corrected.
Table 3.
Watch Criticism intraemotional reactivity network influence
| Region | Coordinates TAL[x, y, z] | HC influencing degree | SAD influencing degree | t (df) | P |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Left Amy | −19, −6, −13 | 0.94 ± 0.77 | 0.77 ± 0.69 | 1.05 (93) | 0.36 |
| Right Amy | 18, −4, −14 | 0.85 ± 0.86 | 0.85 ± 0.85 | −0.004 (93) | 0.68 |
| Left midIns | −38, −1, 2 | 0.73 ± 0.79 | 0.73 ± 0.88 | −0.01 (93) | 0.90 |
| Right midIns | 40, −6, 4 | 0.54 ± 0.51 | 0.52 ± 0.49 | 0.23 (93) | 0.96 |
| PAG | 1, −29, −4 | 0.54 ± 0.48 | 0.55 ± 0.64 | −0.10 (93) | 0.56 |
| dACC | −1, 18, 35 | 0.62 ± 0.89 | 0.42 ± 0.44 | 1.43 (93) | 0.66 |
Amy, amygdala; dACC, dorsal anterior cingulate cortex; midIns, middle insula; PAG, periaqueductal gray.
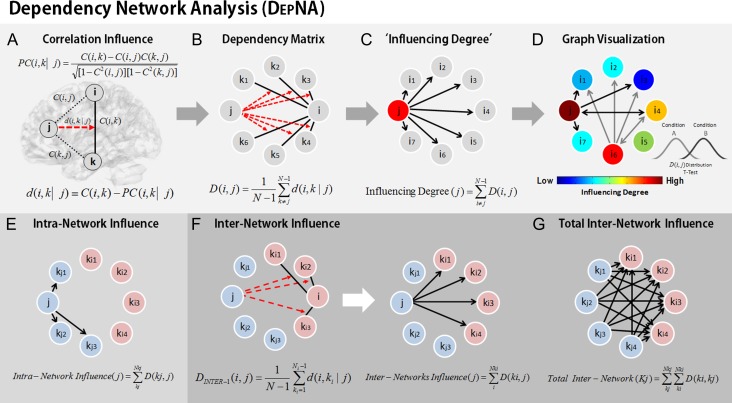
During watch and reappraise criticism conditions, we applied the DEPNA relationships of influence between the nodes of the emotional reactivity and cognitive reappraisal networks. DEPNA and its implementation are described in detail in (Jacob et al. 2016). The steps needed to calculate the network’s ROI DEPNA influence are described in Figure 2. DEPNA provides causal information by employing partial correlations. The correlation influence index (d) value stands for the statistical measure indicating how a third variable affects the correlation between 2 other variables. Thus, a large correlation influence measure (d) means that a significant fraction of the correlation between nodes i and k can be explained in terms of the effect of node j on this correlation. Briefly, the influence of ROI j on the pair of ROIs i and k is defined as the difference between the correlation between i and k and the partial correlation between them given j (Fig. 2A). We then compute the dependency matrix D, which is the averaged influence effect for each ROI (i.e., node) on all other pairwise correlations in the network (Fig. 2B). Each ROI’s Influencing Degree is then defined as the sum of the influences of ROI j on all other ROIs i (Fig. 2C). The higher this measure, the more this ROI influenced all other connections in the network. Finally, we created graph visualization by connecting only influences that were found significantly different between the 2 groups (Fig. 2D). The characteristics and interpretations of the DEPNA features are described in Table S1 in Supplement 1. The specific algorithm procedure is described in the following paragraphs.
Figure 2.
Dependency network analysis (DEPNA). (A) The correlation influence of node j on the pair of nodes i and k is defined as the difference between their correlation C(i,k) and their partial correlation with respect to the node j − PC(i,k|j). The partial correlation coefficient is a statistical measure indicating how a third variable affects the correlation between 2 other variables. Thus, the correlation influence measure d is large only when a significant fraction of the correlation between nodes i and k can be explained by the influence of node j. (B) Dependency matrix—next, we calculate the partial correlation effect for each ROI on all other pairwise correlations in the network. We define the total influence of node j on node i, D(i,j) as the average influence of node j on the correlations C(i,k), over all nodes k. The node dependencies define a dependency matrix D, whose (i,j) element is the influence of node j on node i. (C) “Influencing Degree”—we then define the influences of node j as the sum of the influence D(i,j) of j on all other nodes i. The higher this measure the more this node influenced all other connections in the network. (D) Graph Visualization—each ROI is color-coded according to its influencing or influenced degrees. All pairwise ROIs with dependency elements D that are significantly different between 2 conditions (or groups) at the P < 0.001 level are plotted as edges. Each edge is color-coded according to the t-test sign as light or dark gray. The arrows represent the direction of influence. (E) The intranetwork influence was computed for each node as the sum of its influences on the nodes within its network. (F) The internetwork influence was calculated as the sum of the influences D(ki,j) of a node j from one network only on the nodes ki in the second network. Each region gets a measure of its influence on the connections of the second network. (G) The total internetwork influence was computed as the sum of all internetwork influences from one network on the nodes within the second network.
The ROI–ROI correlations were calculated by Pearson’s formula (Rodgers and Nicewander 1988). First the correlation coefficients were normalized using a Fisher r-to-Z transformation. We then define the correlation influence of ROI j on the correlation between the pair of ROIs i and k as the difference between the normalized correlation and the partial correlation (Kenett et al. 2010) (Fig. 2A), given by the following equation:
| (1) |
where , , and are the normalized ROI–ROI correlations. The partial correlation coefficient is a statistical measure indicating how a third variable affects the correlation between 2 other variables (Shapira et al. 2009). To avoid cases where we sum over positive and negative influences, we reset all negative values to zero.
We then define the total influence of node j on node i, or the dependency of node i on node j to be (Fig. 2B):
| (3) |
As defined, is a measure of the average influence of node j on the correlations , over all nodes k. N is the number of nodes in the network. The node activity dependencies define a dependency matrix D whose element is the influence of node j on node i.
Next we sorted the nodes according to the system level influence of each node on the correlations between all other node pairs (Fig. 2C). The system level Influencing Degree of node j is simply defined as the sum of the influence of node j on all other nodes i, that is:
| (4) |
The DEPNA ‘Influencing Degree’ measure indicates the hierarchy of efferent (out-degree) influence of the node on the entire network. The higher this measure, the greater its impact on all other connections in the network and the more likely it is to be generating the information flow in the network.
To create network graph visualization we used the pairwise dependency connectivity matrix. A 2-tailed t statistic was computed to compare the 2 groups. We then connected only pairwise ROIs with dependencies that were significantly different between the 2 conditions (P < 0.05 level) creating a simple graph visualization of the differences between the groups. The brain visualization of the graph was conducted with the BrainNet Viewer (Xia et al. 2013, http://www.nitrc.org/projects/bnv/).
DEPNA was computed for each network (i.e., emotional reactivity, cognitive reappraisal), subject, and condition (i.e., reappraise criticism, watch criticism) resulting in an Influencing Degree for each region (Fig. 2C,D). In addition, we further investigated the internetwork influences of the emotional reactivity and cognitive reappraisal networks. The internetwork influence was computed for each network node’s influence on the connections within the second network (Fig. 2F). Finally, the total network influence on the second network was calculated as the sum of the internetwork influences (Fig. 2G). Next, we conducted a between-group t-test for each region’s degree of influence. All results in each hypothesis were corrected for multiple comparisons using false discovery rate (FDR) correction with P < 0.05 threshold (Benjamini and Hochberg 1995). We note that as the internetwork influence degree is the sum of the specific region’s influences on all regions within a second network, the number of regions in the second network may impact the level of influence. Therefore, we examined the influence of the reappraisal network regions on the reactivity network, and the influence of the reactivity network regions on the reappraisal network separately in 2 different analyses. In addition, we only compared the level of influence of each region (which is calculated for the same number of network regions) between the 2 groups (SAD vs. HC).
More specifically, during reappraisal, we expected that the reappraisal network would exert less influence both within itself (intranetwork analysis) and on the reactivity network (internetwork analysis) in SAD participants relative to HC. In addition, we examined whether the influence of the emotional reactivity network on the reappraisal network differed between SAD and HC (Hypothesis 1).
Our first hypothesis that during the reappraise condition the reappraisal network would exert less influence both within itself and on the reactivity network in SAD participants relative to HC, was tested on several network levels: 1) the intranetwork Influencing Degree measures within the reappraisal network; 2) regional internetwork influence of each reappraisal network regions on the connections within the emotional reactivity network regions and vice versa; and 3) total internetwork influence of the cognitive reappraisal network on the emotional reactivity network (i.e., the sum of all the reappraisal brain regions influences on the entire reactivity network) and vice versa. Our second hypothesis that during the “watch” condition SAD participants would exhibit greater influencing degree within the emotional reactivity network relative to HC, was tested by the intranetwork Influencing Degree within the emotional reactivity network. We further tested the SAD participants’ amygdala’s influence correlation with social anxiety symptoms and subjective ratings using Pearson correlations.
Next, we conducted Pearson correlations to assess the association between regions that were found to be significantly different between the groups and social anxiety symptoms, subjective mean negative emotional ratings, and reappraisal traits among SAD patients. A partial correlation was used to control for age and gender as covariates. We then corrected for multiple comparisons using FDR.
Control DEPNA Analysis
To further validate our results, we conducted intranetwork and internetwork DEPNA on the third asterisks control condition. We expected no differences between the groups. We then conducted for each network a between-group t-test for each region’s Influencing Degree and corrected for multiple comparisons using the FDR.
In addition, we conducted the identical DEPNA analysis on the reappraise and watch conditions, in a nontask related network. We chose to look at a motor network, as it is not task related in any manner.
We extracted the motor network, which included a set of regions that are consistently activated during hand or foot movements (Biswal et al. 1995; Shirer et al. 2012). Overall the motor network consisted of 6 regions of interest (ROIs), and these ROIs were defined according to the Wake Forest University (WFU) PickAtlas (Maldjian et al. 2003). The averaged BOLD signal (time series) was then extracted for each ROI mask image and each subject.
Results
Negative Emotion Ratings During Watch and Reappraisal
We found that, compared with HC, SAD patients reported significantly greater negative emotion across both watch and reappraise criticism conditions (main effect of group: F[1,93] = 34.88, P < 0.001, = 0.27). All participants rated their emotional experience less negatively during the “Reappraise Criticism” condition compared with the “Watch Criticism” condition (main effect of condition: F[1,93] = 70.17, P < 0.001, = 0.43). The condition × group interaction was not significant (interaction effect: F[1,93] = 0.032, P > 0.8, = 0.00). Although the interaction was not significant, we conducted pairwise comparisons between groups per condition to assure that the SAD group experienced more negative emotion during both watch and reappraise conditions (t[93] = 5.11, P < 0.0001 and t = 5.91, P < 0.0001, respectively).
These results confirm that when cued, participants with SAD can reappraise emotional reactivity to the same degree as HC. However, as expected, SAD patients experienced the interpersonal criticism stimuli more negatively than the HC group for both of the experimental conditions, suggesting greater overall emotional reactivity in SAD compared with HC.
Network Hierarchy During “Reappraise Criticism”
To address our first hypothesis and to assess whether the reappraisal network exhibited a lesser influence among SAD patients relative to HCs during reappraisal, we applied the DEPNA influencing index on 3 different network configurations: 1) intranetwork influence; 2) internetwork influence; and 3) total internetwork influence. Each of these metrics results were also correlated to subjective and clinical measures in the SAD group.
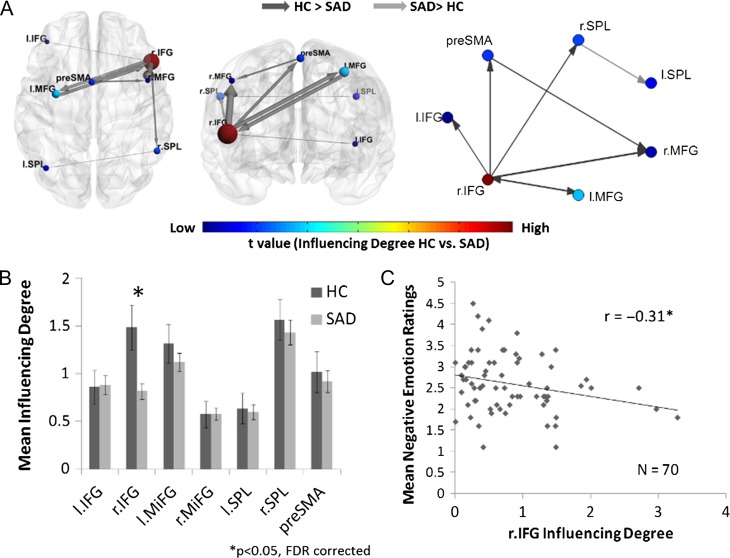
Confirming Hypothesis 1a, analysis of the influence hierarchy within the cognitive reappraisal network (i.e., intranetwork analysis) during the reappraise condition, revealed that the SAD group exhibited reduced influence of the right IFG on other reappraisal regions, as compared with HC (t[93] = 3.42, P < 0.001, qFDR < 0.05) (Table 2 and Fig. 3). Additionally, we addressed the correlation of right IFG Influencing Degree with the subjective mean negative emotion ratings during the reappraise condition specifically within SAD patients and found a negative relation between the 2 (r = −0.31, P < 0.012) (Fig. 3C). As the right IFG’s influence on the cognitive reappraisal network regions increased, the negative emotions ratings during the reappraisal condition decreased among SAD patients.
Figure 3.
Intracognitive regulation network influence during the reappraise condition. (A) The cognitive reappraisal brain network illustration and graph visualization. Each region is color-coded according to the t statistic value from the t-test between the “Influencing Degree” of the 2 groups. All pairwise ROIs with connections, significant at the P < 0.05 level, are plotted as edges. (B) The regions’ Influencing Degree averaged over all 70 SAD patients and 25 healthy controls. The intranetwork analysis found that the SAD group had significantly lower right IFG influence within the cognitive reappraisal network. (C) In addition, the right IFG’s intranetwork Influencing Degree of SAD patients was negatively correlated with the subjective mean negative emotion ratings during the reappraisal condition. Therefore, as the SAD patients’ right IFG influence on the cognitive reappraisal network regions was higher, the less they reported negative emotions.
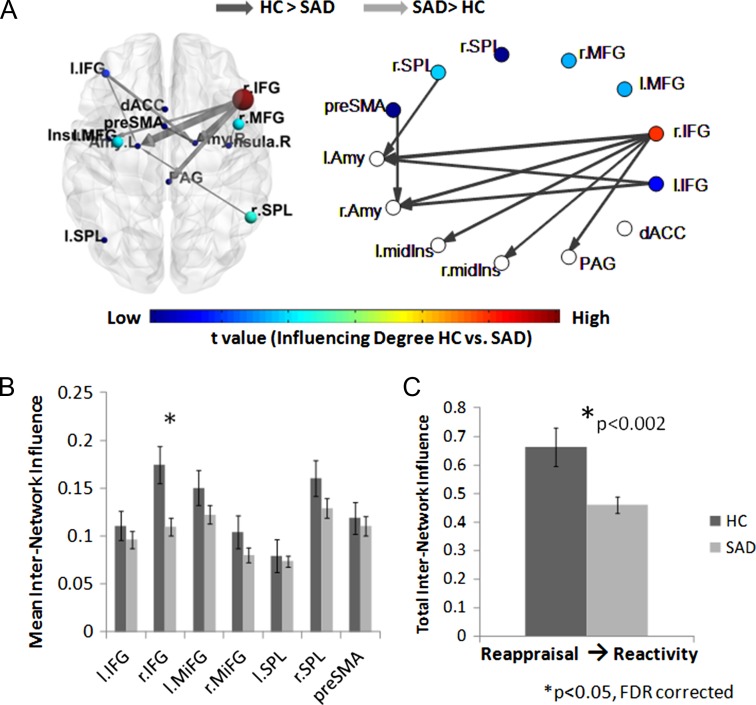
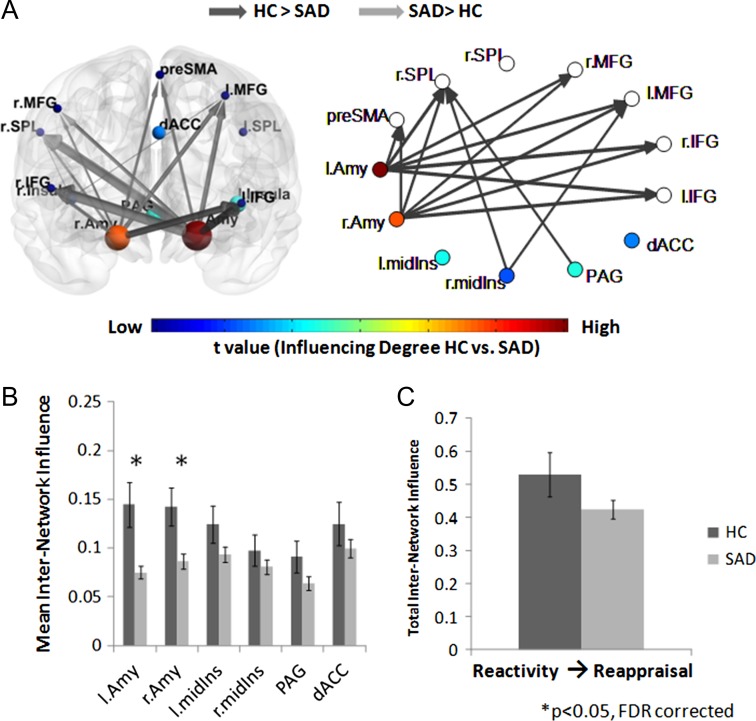
To assess the influence of the reappraisal network regions on the emotional reactivity network regions (Hypothesis 1b), we calculated the DEPNA Influencing Degree measure on the internetwork level during reappraisal. The internetwork influence analysis revealed that the SAD group once again exhibited a decreased influence of the right IFG on the emotional reactivity network, relative to HC (t[93] = 3.33, P < 0.002, qFDR < 0.05) (Table 2 and Fig. 4). Furthermore, the total interinfluence analysis revealed that compared with HC, SAD patients exhibited decreased overall influence of the cognitive reappraisal network on the emotional reactivity network (t[93] = 3.25, P < 0.002, qFDR < 0.05) (Fig. 4C).
Figure 4.
Cognitive reappraisal network influence on the emotional reactivity network during the reappraise condition. (A) The significant group differences illustrated on the brain network illustration and graph visualization. Each region is color-coded according to the t statistic value from the t-test assessing group differences in internetwork influence All pairwise ROIs with connections, significant at the P < 0.05 level, are plotted as edges. (B) The internetwork influence of the cognitive reappraisal network regions on the emotional reactivity regions averaged over all 70 SAD patients and 25 healthy controls. The SAD group showed significantly lower right IFG influence on the emotional reactivity network. (C) In addition, the SAD group exhibited a significantly lower total influence of the reappraisal network on the emotional reactivity network compared with HC.
To further explore the reciprocal relationships between both networks, we additionally calculated the internetwork influence of the reactivity network on the cognitive reappraisal network (Hypothesis 1c). This analysis revealed that the SAD group exhibited a decreased influence of right and left amygdala on the cognitive reappraisal network (t[93] = 3.19, P < 0.002 and t[93] = 3.94, P < 0.0002, respectively, qFDR < 0.05) (Table 2 and Fig. 5). Finally, the total influence of the emotional reactivity network on the cognitive reappraisal network (i.e., total internetwork analysis) was not significantly different between SAD and HCs (P > 0.1) (Fig. 5C).
Figure 5.
Emotional reactivity network influence on the cognitive reappraisal network during the reappraise condition. (A) The significant group differences illustrated on the brain network illustration and graph visualization. Each region is color-coded according to the t statistic value from the t-test assessing groups difference in internetwork influence. All pairwise ROIs with connections, significant at the P < 0.05 level, are plotted as edges. (B) The internetwork influence of the emotional reactivity network regions on the cognitive reappraisal regions averaged over all 70 SAD patients and 25 healthy controls. The SAD group showed significantly lower right and left amygdala influence on the cognitive reappraisal network. (C) As opposed to significantly decreased influence of the reappraisal network on the reactivity network, the total influence of the emotional reactivity network on the cognitive reappraisal network was not significantly different between the SAD and HC groups.
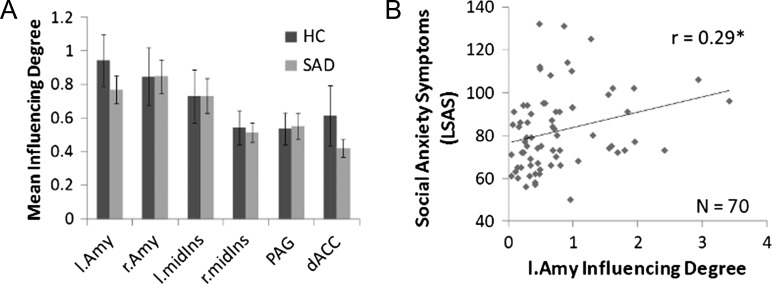
Network Hierarchy During “Watch Criticism”
To address Hypothesis 2a and to evaluate if the influence within the emotional reactivity network was higher during the watch condition among SAD patients compared with HCs, we calculated the DEPNA intranetwork Influencing Degree measures. At odds with our expectations, we found that these measures did not differ significantly between SAD patients and HC (Table 3 and Fig. 6A). While the amygdala’s influence within the reactivity network did not differ between SAD and HC, in accordance with Hypothesis 2b, the left amygdala’s Influencing Degree was found to be positively correlated with social anxiety symptoms (LSAS-SR) (Liebowitz 1987) among the SAD group (r = 0.29, P < 0.016) (Fig. 6B).
Figure 6.
Intraemotional reactivity network influence during the watch condition. (A) The emotional reactivity network regions’ “Influencing Degree” averaged over all 70 SAD patients and 25 HCs. The intraemotional reactivity analysis did not found significant differences between the groups. (B) As expected, among the SAD group the left amygdala’s influence on the emotional reactivity regions was found in positive correlation with social anxiety symptoms scores.
Of note, no significant differences in the internetwork DEPNA were found between SAD and HC during the watch condition (see Fig. S1 in Supplementary 1).
Control Analyses
In order to control for overall main effects differences between the SAD and HC groups, we conducted 2 control analyses. First, to control for a main effect in the paradigm conditions, we conducted the DEPNA analysis of the cognitive reappraisal and emotional reactivity network during a low-level cognitive task that served as a control condition, namely, counting the number of asterisks (1–5 asterisks) that appeared on the screen. As expected, DEPNA found no significant differences between the 2 groups during the asterisk-counting condition either in the intranetwork or internetwork analyses (see Fig. S2 in Supplement 1). The lack of group differences in the asterisks condition highlights that modulation of the influencing degrees in the network of interest, the cognitive reappraisal network, is context-specific. Also, the lack of differential right IFG Influencing Degree in the asterisks condition rules out the possibility of general poorer cognitive control in SAD relative to HC.
In order to control for main effect differences on the network level, we conducted an identical DEPNA analysis, only on a motor brain network, which is not task related and in which we do not expect to observe differences between the groups. As expected, DEPNA found no significant differences within the motor network (see Fig. S3 in Supplement 1). Thus, testing differences between the groups in the relevant task conditions but in irrelevant networks (i.e., motor) indicates that our results of group differences are also network-specific.
Discussion
The goal of the current study was to examine the effect of reappraisal on the organization and hierarchy of emotional reactivity and cognitive reappraisal networks. We applied our newly developed graph based network hierarchy analysis—DEPNA—to a task in which participants with or without SAD were reacting to or reappraising interpersonal criticism, a clinically relevant context in which SAD patients show exaggerated emotional reactivity and deficits in emotion regulation.
During the reappraise condition, as hypothesized, SAD participants exhibited significantly less influence of the entire cognitive reappraisal network on the emotional reactivity network compared with HCs. Moreover, we found that the right IFG, a cognitive reappraisal-related region, showed decreased influence within the reappraisal network and on the emotional reactivity network in SAD participants compared with HCs. Also, in SAD participants only, higher right IFG influence within the cognitive reappraisal network was associated with lower negative emotion ratings during the reappraise condition. Our analysis of the emotional reactivity network’s influence on the cognitive reappraisal network found that both the right and left amygdala had a decreased internetwork influence in SAD relative to HCs. Contrary to our expectations, during the watch condition there was no difference in influence within the emotional reactivity network in SAD participants compared with HCs. Nonetheless, in accordance with our hypothesis greater influence of the left amygdala on the reactivity network correlated with more social anxiety symptoms in SAD participants.
Our results identified that influencing degree of the right IFG during reappraisal distinguished SAD from HC (Figs 3 and 4). Importantly, this measure of network influence did not differ between the groups in the watch condition or in a control task (the asterisk-counting condition; see Figs S1 and S2 in Supplement 1). Intriguingly, in a previous analysis of this dataset the right IFG activity did not differ between groups during reappraisal (Ziv et al. 2013a), suggesting that network influence provides different information than just BOLD signal intensity differences. Cognitive reappraisal of emotions demands the implementation of domain-general cognitive control processes, such as attention and working memory, which are supported by a frontoparietal network including the IFG (Ochsner and Gross 2005; Ochsner et al. 2012). In fact, the IFG is known to play a key role in cognitive control operations such as response inhibition, task switching, selective attention and manipulation of information in working memory (Hampshire et al. 2009, 2010; Aron et al. 2014)—and is thus likely to support selection of goal-appropriate (and inhibition of inappropriate) reappraisals (Buhle et al. 2014; Morawetz, Bode, Baudewig et al. 2016). Several studies and meta-analyses on the neural bases of emotion regulation corroborate the IFG’s assumed role in supporting explicit cognitive strategies for emotion regulation, specifically reappraisal (Wager et al. 2008; Diekhof et al. 2011; Buhle et al. 2014; Frank et al. 2014; Morawetz, Bode, Derntl et al. 2016). Specifically, this region was found to be consistently under-recruited in people diagnosed with different mood and anxiety disorders relative to HC during reappraisal (Picó-Pérez et al. 2017; Zilverstand et al. 2017).
Regarding the IFG’s functional connectivity patterns, previous neuroimaging findings showed decreased functional connectivity of the right IFG with frontal, parietal, and occipital regions during resting-state in SAD participants, as compared with HCs (Ding et al. 2011). The current study extends these findings by demonstrating a decreased right IFG influence within the frontoparietal cognitive reappraisal network in the context of down-regulating emotional reactivity to social interpersonal criticism (Fig. 3). Importantly, this influencing degree was associated with the SAD participants’ subjective negative emotion ratings (Fig. 3C). In a study in which healthy participants were asked to reappraise negative interpersonal stimuli, the IFG connectivity with other prefrontal regulation-related areas was associated with individual differences in negative affect reduction (Morawetz, Bode, Derntl et al. 2016). In addition, a strong inhibitory influence of the IFG on dlPFC during reappraisal was recently demonstrated by a DCM analysis in healthy participants (Morawetz, Bode, Baudewig, Kirilina et al. 2016). The latter result supports the assumed role of the IFG in selecting goal-appropriate reappraisals and inhibiting working memory-related dlPFC activation once the selection process is completed (Ochsner et al. 2012). Our results correspond with these findings, as they suggest an influencing role for the right IFG in assembling frontoparietal functional couplings during reappraisal. Moreover, we relate this influencing degree of the IFG to individual differences in successful reappraisal of negative emotion in a clinical sample of SAD participants. Taken together with the recent findings discussed above, the IFG emerges as a region that plays an important role in assembling frontoparietal functional couplings during reappraisal. This extends the classical finding that the association of the IFG with successful reappraisal is mediated via subcortical regions (Wager et al. 2008).
From a whole network perspective, in agreement with the general hypothesis regarding emotion dysregulation, the SAD group exhibited reduced total internetwork influence of the cognitive reappraisal network on the entire emotional reactivity network (Fig. 4C). This extends current findings showing hypoactivation of frontoparietal regions alongside hyperactivation of some emotional reactivity regions in anxious and depressed individuals during emotional reappraisal tasks (Picó-Pérez et al. 2017), by delineating network interactions more precisely. Specifically, the higher influence of the right IFG at the internetwork level found among HCs relative to SAD participants may indicate its functioning as a major node that modulates effective connectivity in emotional reactivity regions.
This notion is in agreement with studies demonstrating the contribution of IFG-centered frontolimbic communication to adaptive emotional regulation. For instance, when SAD participants were asked to reappraise negative self-beliefs, Goldin and colleagues found an earlier onset of IFG recruitment and an inverse functional connectivity of this region with the amygdala in HCs compared with SAD (Goldin, Manber-Ball et al. 2009). Our results extend these studies of specific IFG-amygdala connectivity, by demonstrating that through a large-scale network perspective the IFG is depicted as a region with high impact on multiple functional couplings that underlie multiple processes: the IFG involvement in influencing neural activity within the frontoparietal reappraisal network might reflect its role in selecting suitable reappraisals and inhibiting competing ones, as suggested by Morawetz, Bode, Baudewig, Kirilina et al. (2016). In addition, its influence over emotional reactivity regions may serve the inhibition of prepotent responses. Deficits in both processes may be evident in people with mood and anxiety disorders such as SAD, yet whether one of the processes is more dominant in maintaining emotional dysregulation remains an open question.
Furthermore, analysis of the internetwork influence of the reactivity network on the cognitive reappraisal network during the reappraisal condition found a decreased internetwork influence of the SAD groups’ right and left amygdala as compared with HCs (Fig. 5). This finding is surprising, as various neuroimaging studies typically associate SAD with amygdala hyperactivity during task or rest (Birbaumer et al. 1998; Schneider et al. 1999; Stein et al. 2002; Phan et al. 2006) and enhanced coupling with cognitive control regions (Brühl et al. 2014).
Yet, when investigating the amygdala during emotional reappraisal, its activation does not differ between SAD and HCs (Goldin, Manber et al. 2009; Goldin, Manber-Ball et al. 2009; Ziv et al. 2013a; Gaebler et al. 2014). We note that previous investigations of these data are in agreement with these studies showing no significant difference between groups in amygdala activity during reappraisal compared with just watch (Ziv et al. 2013a). Rather, the amygdala’s functional connectivity with prefrontal cognitive control regions during reappraisal was linked to adaptive reappraisal in HCs and to CBT-induced brain alterations in SAD (Manber-Ball et al. 2009; Goldin et al. 2013). In addition, our results are also in agreement with structural diffusion tensor imaging (DTI) studies on anxiety that demonstrated weaker structural connections of the amygdala with prefrontal areas associated with emotion regulation among people with higher trait anxiety (Kim and Whalen 2009; Greening and Mitchell 2015). Relatedly, a recent optogenetic study in mice showed that the amygdala’s influence over a regulatory prefrontal region (the medial prefrontal cortex) is crucial for formation and preservation of fear memories (Klavir et al. 2017).
Our results are also in line with a recent effective connectivity study, which by conducting a DCM analysis found decreased amygdala influence on the regulation-related dlPFC region among SAD participants compared with HC (Sladky et al. 2015). In addition, increased connectivity of the amygdala with regulation-related regions was found to correlate with reductions in negative affect following reappraisal among healthy participants (Banks et al. 2007). Hence, effective coupling between the amygdala and regulation-related areas, and potentially also the amygdala’s influence over these areas, seem critical for efficient emotion regulation processing. Our finding extends this notion to SAD participants and their ability to implement reappraisal in a specific social context, demonstrating a lower overall influence of the amygdala on the entire reappraisal network.
Overall, these findings challenge the concept of the amygdala as just a target region “to-be down regulated” (Buhle et al. 2014; Morawetz, Bode, Derntl et al. 2016), and raise a question about its function and role in shaping network organization that supports effective cognitive reappraisal of emotion. One possibility is that at least for some participants, there is a threshold of amygdala influence on frontoparietal networks necessary to kick-start or activate reappraisal processes. Another possibility is that the association between the amygdala’s influence and reappraisal success may reflect a shift in emotional engagement and attention allocation towards the more benign mental representations constructed during the reappraisal process. These interpretations are in line with the amygdala’s well-established role in detection and encoding of affective stimuli (Zald 2003), but remain to be more thoroughly tested.
As might be expected from previous imaging studies on social anxiety showing heightened activation and connectivity within brain networks involved in negative affect generation (Etkin and Wager 2007; Brühl et al. 2014; Yang et al. 2017), the SAD group did not demonstrate higher influence during the watch condition in emotional reactivity network regions compared with HCs. Indeed, previous studies did not find differential effects in emotional reactivity regions such as the amygdala and insula between SAD and HC (Ziv et al. 2013a; 2013b). Additionally several studies suggest that activation of emotional reactivity regions does not necessarily differ between HC and SAD in tasks where participants watched passively emotional stimuli (Furmark et al. 2009; Schmidt et al. 2010). Instead, variation in neural activation of such regions was explained by the severity of social anxiety symptoms only within the SAD group. Likewise, here a specific inspection of the amygdala revealed that greater influence of the left amygdala on the reactivity network was positively correlated with social anxiety symptoms in the SAD group (Fig. 6B). This finding enhances the characterization of the relation between different amygdala features and social anxiety severity, by highlighting its central role in influencing other emotional reactivity regions in negative social contexts.
Several limitations of this study should be taken into account. First, SAD participants were asked to down-regulate negative emotion in a social context. Further studies are needed to examine whether the same aberrant brain network influence hierarchy appears with different emotional stimuli and in other populations diagnosed with emotional disorders. Second, the main limitation of the DEPNA method is that it models the dependencies among the data itself based on partial correlations. Hence, while DEPNA can be used to make inferences regarding the influence hierarchy within and between a network, it does not permit true causal influences, because “data cannot cause data; data are caused by underlying brain states” (Friston 2009, p. 0223). Third, coordinates of nodes comprising the networks of interest were derived from meta-analyses on HC rather than from SAD-specific data. This choice for selection of ROIs may have potentially biased the results of the network analysis. However, our goal was to test how SAD differ from HC in “normative” emotion regulation-related networks, as this could better demarcate a brain therapeutic target. In any case, the coordinates of ROIs in which we found significant differences between SAD and HC (i.e., right IFG and bilateral amygdala) are adjacent to those reported in meta-analyses on reappraisal (Picó-Pérez et al. 2017) and emotional reactivity (Brühl et al. 2014) in SAD patients.
To conclude, our study demonstrates the added value of graph based analysis to elucidate brain network connectivity in psychopathology. Our results indicate that the hierarchical organization of emotional reactivity and cognitive reappraisal networks may underlie behavioral deficits in cognitive reappraisal which characterize mood and anxiety disorders such as SAD. These findings add to existing brain research on reappraisal and emotional regulation in general, by inspecting the operation of relatively large-scale brain networks and examining specific influences of certain nodes within these networks. From a system-level perspective, the cognitive reappraisal network exerted less influence on the entire emotional reactivity network in SAD participants when participants were asked to reappraise interpersonal criticism. More specifically the DEPNA identified 2 critical hubs for adaptive cognitive reappraisal of emotion, the right IFG and amygdala, both exerting substantially more influence in HCs over the cognitive reappraisal network. The influencing degrees of these critical regions were also linked with behavioral measures of both reappraisal success and social anxiety symptoms. These findings emphasize that bidirectional influences of major nodes in both networks upon each other may underlie efficient regulation of emotion via cognitive reappraisal. Hence, we propose that the influencing degree of regions may serve as a target for future connectivity-based diagnosis and neuromodulation therapies.
Supplementary Material
Funding
Israeli Centers of Research Excellence (Grant number 51/11 to T.H.) and the Israeli Ministry of Science, Technology and Space (Grant number 211580 to T.H.), as well as the National Institute of Mental Health (Grant number MH092416 to J.G.).
Notes
We are very grateful to Dr. Neomi Singerת Dr. Tal Gonen, Dr. Gadi Gilam and Dr. Gal Raz for helpful discussions, and Vicki Myers for assistance in manuscript preparation. TH would like to thank the Sagol Foundation. Conflict of Interest: none declared.
References
- Aron AR, Robbins TW, Poldrack RA. 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 18(4):177–185. [DOI] [PubMed] [Google Scholar]
- Association AP 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Pub. [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. 2007. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2(4):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 28(37):9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. 2011. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA. 108(18):7641–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ, Grafton ST. 2013. Task-based core-periphery organization of human brain dynamics. PLoS Comput Biol. 9(9):e1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol). 57(1):289–300. [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. 1998. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 9(6):1223–1226. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S. 2014. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 47:260–280. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. 2014. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 24(11):2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 10(3):186–198. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. 1995. A cognitive model of social phobia. Soc Phobia: Diagn Assess Treat. 41(68):00022–00023. [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29(3):162–173. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. 2011. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 58(1):275–285. [DOI] [PubMed] [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, Zhang W, Gong Q. 2011. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging. 29(5):701–711. [DOI] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. 2015. The neural bases of emotion regulation. Nat Rev Neurosci. 16(11):693–700. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 164(10):1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. 2014. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 45:202–211. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Santos Filho A, Machado-de-Sousa JP, Chagas MHN, Nardi AE, Crippa JAS. 2010. Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog Neuro-Psychopharmacol Biol Psychiatry. 34(4):565–580. [DOI] [PubMed] [Google Scholar]
- Friston K. 2009. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 7(2):e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. 2003. Dynamic causal modelling. Neuroimage. 19(4):1273–1302. [DOI] [PubMed] [Google Scholar]
- Furmark T, Henningsson S, Appel L, Ahs F, Linnman C, Pissiota A, Faria V, Oreland L, Bani M, Pich EM, et al. 2009. Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci. 34(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- Gaebler M, Daniels JK, Lamke JP, Fydrich T, Walter H. 2014. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J Psychiatry Neurosci. 39(4):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. 2009. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 66(2):170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. 2009. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 66(12):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. 2013. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 70(10):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening SG, Mitchell DG. 2015. A network of amygdala connections predict individual differences in trait anxiety. Hum Brain Mapp. 36:4819–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. 1998. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 74(1):224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. 2003. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 85(2):348–362. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. 2011. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 25(3):400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. 2011. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 56(3):881–889. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 50:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. 2009. Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Neurosci. 9:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, et al. 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 80(0):360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Winetraub Y, Raz G, Ben-Simon E, Okon-Singer H, Rosenberg-Katz K, Hendler T, Ben-Jacob E. 2016. Dependency network analysis (DEPNA) reveals context related influence of brain network nodes. Sci Rep. 6:27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Gilam G, Lin T, Raz G, Hendler T. 2018. Anger modulates influence hierarchies within and between emotional reactivity and regulation networks. Front Behav Neurosci. 12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H, Morrison AS, Goldin PR, Gross JJ. 2015. The role of emotion and emotion regulation in social anxiety disorder. Curr Psychiatry Rep. 17(1):1–9. [DOI] [PubMed] [Google Scholar]
- Kenett DY, Tumminello M, Madi A, Gur-Gershgoren G, Mantegna RN, Ben-Jacob E. 2010. Dominating clasp of the financial sector revealed by partial correlation analysis of the stock market. PLoS One. 5(12):e15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. LIfetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 62(6):593–602. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. 2009. The structural integrity of an amygdala–prefrontal pathway predicts trait anxiety. J Neurosci. 29(37):11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. 2017. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 20(6):836–844. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. 2008. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 42(2):998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, Zhang W, Gong Q, Chen H. 2010. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state fMRI study. PLoS One. 5(12):e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CH, Worsley KJ, Poline JB, Aston JA, Duncan GH, Evans AC. 2002. Estimating the delay of the fMRI response. Neuroimage. 16(3):593–606. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. 1987. Social phobia. Mod Probl Pharmacopsychiatry. 22:141–173. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo W, Fouche J-P, Wang Y, Wang W, Ding J, Zeng L, Qiu C, Gong Q, Zhang W, et al. 2015. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct Funct. 220(1):101–115. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. 2008. Disrupted small-world networks in schizophrenia. Brain. 131(Pt 4):945–961. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19(3):1233–1239. [DOI] [PubMed] [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15(10):483–506. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M. 2006. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 87(1–3):60–66. [DOI] [PubMed] [Google Scholar]
- Min J-A, Yu JJ, Lee C-U, Chae J-H. 2013. Cognitive emotion regulation strategies contributing to resilience in patients with depression and/or anxiety disorders. Compr Psychiatry. 54(8):1190–1197. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Heekeren HR. 2016. Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci. 12(4):569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Kirilina E, Heekeren HR. 2016. Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cereb Cortex. 26(5):1923–1937. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, Heekeren HR. 2016. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 72:111–128. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. 2005. The cognitive control of emotion. Trends Cogn Sci. 9(5):242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 23(2):483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 1251(1):E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. 2006. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 59(5):424–429. [DOI] [PubMed] [Google Scholar]
- Phillips M. 2008. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 13(9):833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó-Pérez M, Radua J, Steward T, Menchón JM, Soriano-Mas C. 2017. Emotion regulation in mood and anxiety disorders: a meta-analysis of fMRI cognitive reappraisal studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 79(Part B):96–104. [DOI] [PubMed] [Google Scholar]
- Raz G, Jacob Y, Gonen T, Winetraub Y, Flash T, Soreq E, Hendler T. 2013. Cry for her or cry with her: context-dependent dissociation of two modes of cinematic empathy reflected in network cohesion dynamics. Soc Cogn Affect Neurosci. 9(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G, Winetraub Y, Jacob Y, Kinreich S, Maron-Katz A, Shaham G, Podlipsky I, Gilam G, Soreq E, Hendler T. 2012. Portraying emotions at their unfolding: a multilayered approach for probing dynamics of neural networks. Neuroimage. 60(2):1448–1461. [DOI] [PubMed] [Google Scholar]
- Rodgers JL, Nicewander WA. 1988. Thirteen ways to look at the correlation coefficient. Am Stat. 42(1):59–66. [Google Scholar]
- Schmidt S, Mohr A, Miltner WH, Straube T. 2010. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biol Psychol. 84(2):304–312. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Muller-Gartner HW, Posse S, Salloum JB, Grodd W, Himmelmann F, Gaebel W, Birbaumer N. 1999. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 45(7):863–871. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Kenett DY, Ben-Jacob E. 2009. The index cohesive effect on stock market correlations. Eur Phys J B. 72(4):657–669. [Google Scholar]
- Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius M. 2012. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 22(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Höflich A, Küblböck M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C. 2015. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cereb Cortex. 25(4):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. 2011. Networks of the Brain. Cambridge, MA: MIT Press. [Google Scholar]
- Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. 2007. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 17(1):92–99. [DOI] [PubMed] [Google Scholar]
- Stam C, Reijneveld J. 2007. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. 2002. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 59(11):1027–1034. [DOI] [PubMed] [Google Scholar]
- Stein MB, Stein DJ. 2008. Social anxiety disorder. Lancet. 371(9618):1115–1125. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. 2012. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35(9):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 20(8):519–534. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 59(6):1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KH, Goldin PR, Ball TM, Heimberg RG, Gross JJ. 2011. Assessing emotion regulation in social anxiety disorder: the emotion regulation interview. J Psychopathol Behav Assess. 33(3):346–354. [Google Scholar]
- Xia M, Wang J, He Y. 2013. BrainNet viewer: a network visualization tool for human brain connectomics. PloS one. 8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liu J, Meng Y, Xia M, Cui Z, Wu X, Hu X, Zhang W, Gong G, Gong Q, et al. 2017. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 10.1016/j.neuroimage.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Zald DH. 2003. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 41(1):88–123. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ. 2017. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 151:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. 2013. a. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. 2013. b. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.