Abstract
Higher brain function relies on proper development of the cerebral cortex, including correct positioning of neurons and dendrite morphology. Disruptions in these processes may result in various neurocognitive disorders. Mutations in the CPE gene, which encodes carboxypeptidase E (CPE), have been linked to depression and intellectual disability. However, it remains unclear whether CPE is involved in early brain development and in turn contributes to the pathophysiology of neurocognitive disorders. Here, we investigate the effects of CPE knockdown on early brain development and explore the functional significance of the interaction between CPE and its binding partner p150Glued. We demonstrate that CPE is required for cortical neuron migration and dendrite arborization. Furthermore, we show that expression of CPE-C10 redistributes p150Glued from the centrosome and that disruption of CPE interaction with p150Glued leads to abnormal neuronal migration and dendrite morphology, suggesting that a complex between CPE and p150Glued is necessary for proper neurodevelopment.
Keywords: carboxypeptidase E, cortical neuron migration, dendritic arborization, dynactin, in utero electroporation
Introduction
Functional connectivity between neurons in the brain depends on where the neurons are positioned and how their dendrites are shaped during development (reviewed in Rakic (1988)). Abnormal neuronal migration and dendrite patterning may result in various neurocognitive disorders due to developmental defects (Mulvey and Dougherty 2018). Often, aberrant brain structure results from improper function or expression of a complex of proteins rather than of a single protein (reviewed in (de la Torre-Ubieta et al. 2018)). Thus, studying the interaction between multiple proteins that regulate neuronal development will aid in our understanding of processes that result in abnormal brain function.
The cortex is patterned from the inside out (as reviewed in (Bystron et al. 2008)). Proliferative cells derived from the neural plate form a ventricular zone (VZ), and these cells divide symmetrically and have radial extensions. At the onset of neurogenesis, which occurs at embryonic day (E) 33 in humans (Bystron et al. 2006) and at E10 in mice (Garcia-Moreno et al. 2007), cells in the VZ start to divide in an asymmetric fashion, where one daughter cell migrates along radial glia toward the pial surface. The daughter cells then accumulate in the cortical plate (CP), where the earliest born neurons differentiate and become layer 6 neurons, and subsequent neurons migrate beyond these neurons to the CP to become layer 5–2 neurons, successively. The intermediate zone (IZ) lies between the VZ and CP, and although future neurons migrate through the IZ, the IZ is eventually replaced by white matter. This process is referred to as a radial neuronal migration. In contrast, tangentially migrating cells eventually become inhibitory neurons. Any perturbation in this migration process results in aberrant neural connectivity and neuropsychiatric disorders, such as schizophrenia and autism spectrum disorder (Geschwind and Rakic 2013).
Carboxypeptidase E (CPE) is an enzyme that processes prohormones (Fricker and Snyder 1982; Hook et al. 1982). Recent studies have reported nonenzymatic functions of CPE protein, including prohormone sorting and vesicle transport, in the endocrine and nervous systems (reviewed in (Cawley et al. 2012)). One neuropeptide sorted and transported by CPE is brain-derived neurotrophic factor (BDNF). Disruption of CPE binding to BDNF-containing vesicles results in reduced BDNF localization to neurites of hippocampal neurons (Lou et al. 2005; Park et al. 2008a, 2008b). The importance of CPE function in the brain has been further supported by studies in CPE−/− mice and humans. Knockout mice exhibit aberrant dendritic architecture and spine morphology, deficits in learning and memory, and neurodegeneration under stress (Woronowicz et al. 2008, 2010). Studies on a spontaneous mutant mouse model, Cpefat/fat, a point mutation resulting in a lack of CPE enzymatic activity (Coleman and Eicher 1990; Fricker et al. 1996), revealed anxiety and depressive-like behaviors in these mice (Rodriguiz et al. 2013). Similarly, a truncation mutation of CPE was identified in a morbidly obese human female with intellectual disability (Alsters et al. 2015), suggesting that this protein plays a role in brain function in both species.
The mechanism by which CPE regulates neurodevelopment is still poorly understood, but it is known that one of its binding partners, p150Glued, may play an important role in CPE function. The cytoplasmic tail of CPE interacts with p150Glued (Park et al. 2008a, 2008b), the major subunit of the dynactin complex, which binds directly to microtubules and motor proteins (reviewed in (Schroer 2004)). Dynactin is involved in coordination of bidirectional cargo transport by regulating both dynein-mediated retrograde transport and kinesin-2-mediated anterograde transport (King and Schroer 2000; Berezuk and Schroer 2007). In addition to the regulation of motor activities, dynactin functions at the centrosome to anchor microtubules and recruit cell cycle regulators (Quintyne et al. 1999; Quintyne and Schroer 2002). Mutations in the dynactin gene (DCTN1), encoding p150Glued, are associated with Perry syndrome (Farrer et al. 2009; Wider et al. 2010). In addition, it was recently reported that full-length p150Glued is a neuron-specific anti-catastrophe factor of microtubules, and one of the causal mutations in p150Glued found in patients with Perry syndrome abolishes this anti-catastrophe activity (Lazarus et al. 2013). Thus, by regulating microtubule dynamics and molecular motors, p150Glued may play a role in shaping brain structure and function.
As such, understanding the roles of CPE and p150Glued and their interaction in regulating brain development can provide insight into mechanisms underlying neurocognitive disorders, such as major depressive disorder and Perry syndrome, and elucidate potential therapeutic targets for these disorders. We previously reported the involvement of CPE in mediating nitric oxide synthase 1 adaptor protein (NOS1AP)-promoted decreases in dendritic arborization (Carrel et al. 2009), but an understanding of the mechanism by which CPE itself regulates dendrite branching has not been fully elucidated. In addition, since CPE interacts with p150Glued, and members of the dynactin complex regulate cortical development (Smith et al. 2000; Tai et al. 2002; Dujardin et al. 2003), we asked whether CPE plays a role in cortical neuron migration. We also investigated the effects of CPE knockdown on dendrite branching and explored the functional significance of its carboxyl terminal interaction with p150Glued. We found that CPE is required for proper cortical neuron migration and dendrite morphology. Furthermore, we identify the carboxyl terminus of CPE as an important domain in mediating these CPE functions via its interaction with p150Glued. This work is the first to examine the role of CPE in cortical cell migration and in cell autonomous effects on dendrite morphology, providing novel roles for CPE and its interaction with p150Glued in cortical development. It underscores the importance of a role for CPE during brain development and suggests that disruption of the interaction between CPE and p150Glued may result in impaired brain function, such as that observed in patients with major depressive disorder and Perry syndrome.
Materials and Methods
DNA Constructs and RNA Interference
cDNA encoding the carboxyl terminal 10 amino acids of the rat full-length CPE protein was subcloned into pEGFP-C1 and pCAG-GFP vectors. siRNA against CPE and negative control siRNA (CPE siRNA, Mouse S64324; CPE siRNA Rat S220210; negative control #1, cat# 4 390 743) were purchased from Life Technologies (Carlsbad, CA). shRNAs targeting the CPE transcript (5′-GGTGGAATGCAAGACTTCA-3′) and an unrelated sequence (5′-GAGCATTTGTATGAGCGCG-3′) were designed and ligated into the pGE2hrGFPII vector (Agilent Technologies, Santa Clara, CA). The CPE shRNA target sequence that we designed and the purchased siRNA target both the membrane-associated and soluble forms of CPE. pCAG-IRES-EGFP plasmid (pCIG) was a gift from Dr Gabriella D’Arcangelo (Rutgers University, USA), and pCAG-IRES-TagRFP plasmid (pCIR) was a gift from Dr Marie-Catherine Tiveron (Institut de Biologie du Développement de Marseille, France). CPE rescue construct was obtained by subcloning the sequence encoding rat full-length CPE protein into pCAG-GFP vector and by inserting 5 silent mutations in the sequence targeted by CPE shRNA by site-directed mutagenesis.
Antibodies and Reagents
Mouse CPE (BD 610 758) and mouse MAP2 (BD 556 320) primary antibodies were purchased from BD Biosciences (San Jose, CA). Mouse dynactin p150Glued antibody (SC-135 890) was from Santa Cruz (Dallas, TX). Rabbit anti-pericentrin (ab4448) was from Abcam (Cambridge, UK). Chicken anti-GFP (PA-9533) was from Thermo Fisher (Waltham, MA). Mouse GAPDH (MAB374) and chicken β-III tubulin (AB9354) primary antibodies were from EMD Millipore (Billerica, MA). Rabbit anti-Tuj1 (MRB-435P) was from Covance Antibody Products (Dedham, MA). Rabbit anti-CPE (SAB2700907) was from MilliporeSigma (St. Louis, MO).
COS-7 Cell Transfection and Immunocytochemistry
COS-7 cells were plated at 15 800 cells/cm2 on coverslips 0.1 mg/mL poly-d-lysine and transfected 24 h after plating with pEGFP-C1 or pEGFP-C1-CPE-C10 using Lipofectamine 2000 (Thermo Fisher, Waltham, MA) following the manufacturer’s protocol. Cells were fixed 2 days after transfection with incubation in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 min and immunostained for GFP, pericentrin, and dynactin p150Glued, followed by nuclear staining with Hoechst 33 225 dye. Coverslips were mounted onto glass slides with Fluoromount G (Southern Biotechnology, Birmingham, AL).
Immunofluorescent Microscopy and p150Glued Localization Analysis
Slides of COS-7 cells were prepared as described above. Images were taken at 600× using an Olympus Optical (Tokyo, Japan) IX50 microscope and fluorescent imaging system. For the assessment of p150Glued localization, a straight line was drawn from one side of the cell through the centrosome (positive for pericentrin immunostaining) and the nucleus (labeled by Hoechst 33 225 dye) to the other end of the cell. To quantify the percentage of p150Glued concentrated on the centrosome, the intensities of p150Glued and pericentrin were plotted along the line. p150Glued intensity within the peak of pericentrin was measured (value A), and the intensity within 1 micron away on each side was measured (value B). The ratio of A to B was calculated to compute the percentage of p150Glued localized to the centrosome.
Primary Neuronal Culture and Dendrite Branching Analysis
Hippocampal neuron cultures were prepared from rat embryos at 18 days of gestation as we reported (Firestein et al. 1999). Cells dissociated from the hippocampi were plated at 10 500 cells/cm2 onto coverslips previously coated with 0.1 mg/mL poly-d-lysine and maintained in neurobasal medium (Thermo Fisher, Waltham, MA) supplemented with B27 (Thermo Fisher, Waltham, MA), GlutaMAX (Thermo Fisher, Waltham, MA), penicillin, and streptomycin. Cultures were cotransfected with pCAG-mOrange and indicated CPE constructs or siRNA at day in vitro (DIV) 7 using Lipofectamine LTX with Plus (Thermo Fisher, Waltham, MA), fixed at DIV 10 with 4% PFA in PBS, and immunostained for GFP and MAP2. Neuron iamges were taken using Olympus Optical (Tokyo, Japan) IX50 microscope and fluorescent imaging system, and dendrite morphology was assessed as previously described using our Bonfire program (Kutzing et al. 2010; Langhammer et al. 2010).
Coimmunoprecipitation and Western Blot Analysis
Cortices from mice at embryonic day (E)16, postnatal day (P)0, P10, and adult (P60–90) were dissected and homogenized in RIPA buffer containing protease inhibitors (50 mM Tris, PH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate, 1 mM EDTA, 1 mM PMSF, cOmplete™ Mini Protease Inhibitor Cocktail (MilliporeSigma, St. Louis, MO)). Equal amounts of extracted proteins were pre-cleared with protein G agarose (Thermo Fisher Pierce™, Waltham, MA) for 1 h and then subjected to immunoprecipitation with monoclonal dynactin p150Glued antibody at 4°C overnight. Samples were then incubated with protein G agarose for 1 h, and immunoprecipitates were washed with TEE buffer (25 mM Tris, PH 7.4, 1 mM EDTA, 1 mM EGTA). Proteins were eluted with protein loading buffer (50 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 5% glycerol, 0.01% bromophenol blue, 5% β-Mercaptoethanol), resolved by SDS-PAGE, and transferred to PVDF membranes. Membranes were probed with rabbit anti-CPE and mouse anti-p150Glued. Immunoreactive bands were visualized with HyGlo quick spray (Denville Scientific; South Plainfield, NJ), and resulting films were quantified with ImageJ software (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, Maryland).
Neuro2A Cell Transfection and Lysis
Neuro2A (N2a) cells were plated at a density of 63 000 cells/cm2 in 6-well plates. N2a cells were transfected 12–16 h later with shRNA targeting CPE transcript, or control shRNA targeting an unrelated sequence, in combination with an shRNA-resistant CPE overexpression construct. Cells were lysed 48 h later in RIPA buffer containing protease inhibitors.
In utero Electroporation, Immunohistochemistry, and Microscopy
In utero electroporation (IUE) was performed as we previously described (Carrel et al. 2015). Pregnant Swiss mice at gestation day 14.5 (E14.5) were anesthetized with isoflurane. Briefly, the abdominal cavity was opened to expose the uterine horns. Plasmids (1–3 μL of 2–2.5 μg/μL) with Fast Green (1 mg/mL; Sigma) were microinjected through the uterus into the lateral ventricles of embryos by pulled glass capillaries (Drummond Scientific, Broomall, PA). The heads of the embryos were placed between tweezer-type electrodes. Square electric pulses (35 V, 50 ms) were passed 5 times at 1-s intervals using an NEPA21 electroporator (SONIDEL Limited, Dublin, Ireland). Embryos were allowed to develop in utero for 3 days after electroporation (until E17.5) or were kept until 7 days after birth (postnatal day (P) 7). Embryonic mouse brains were dissected and fixed for 48 h in 4% PFA in PBS at 4°C. P7 mouse brains were fixed by transcardial perfusion of 4% PFA in PBS and postfixed for 24 h. Brains were cryoprotected in 30% sucrose in PBS, frozen in PolyFreeze (Sigma), and sectioned coronally at 16 μm (E17.5 brains) and 30 and 80 μm (P7 brains) using a cryostat.
For the analysis of migration at E17.5 and P7 and cell morphology at E17.5, images of immunofluorescent mouse brain sections were taken on a Zeiss Axio Observer.Z1 microscope using a 20× numerical aperture (NA) 0.8 objective, with a Clara E CCD Camera (Andor Technology Limited, Belfast, UK).
For the analysis of CPE shRNA efficiency in embryonic neocortex at E17.5, images of transfected cells in brain sections were taken on a Zeiss LSM 510 confocal laser-scanning microscope (Zeiss) using a 63× NA 1.4 objective. In each experiment, unsaturated acquisitions of CPE signal were performed with the same exposure settings and laser gain for each condition.
Analysis of neuronal morphology at P7 was performed as follows. Z-stacks of 30–50 mm thickness were acquired on a Zeiss LSM 510 confocal laser-scanning microscope (Zeiss) using a 20× NA 0.5 objective, and z-series were projected to two-dimensional representations.
Analysis of Neuronal Migration and Dendritic Branching in Vivo
Using the image analysis software ImageJ (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, Maryland), we counted TagRFP-positive cells. Cortical regions of interest containing positive cells were manually selected using Hoechst 33 225 staining of the nuclei in each section analyzed. For each region, we enhanced the signal from fluorescent cell bodies and lowered the signal from fluorescent processes using a combination of ImageJ built-in minimum and unsharp mask filters. We automatically counted cells as local maxima, while keeping the same level of noise tolerance for a given set of experiments after validation of this level by manual counting of 3–4 sections. For normalization, we used median sections in a series containing transfected cells with 2 sections per brain at E20, separated by at least 48 mm. Brains that did not fulfill these 2 criteria were discarded.
For the analysis of neuronal morphology at P7, we used NeuronJ software (http://rsb.info.nih.gov/ij/) (Meijering et al. 2004) to trace and quantify neurites. We also measured total neurite length and number of branches for each individual neuron.
For the analysis of CPE shRNA efficiency in vivo, transfected cells were delimitated using ImageJ software, and CPE signal intensity in transfected cells was measured and normalized to CPE signal in the whole image to avoid variability due to immunostaining efficiency on each section.
Results
CPE is Expressed in Neurons of the Developing Rodent Cortex
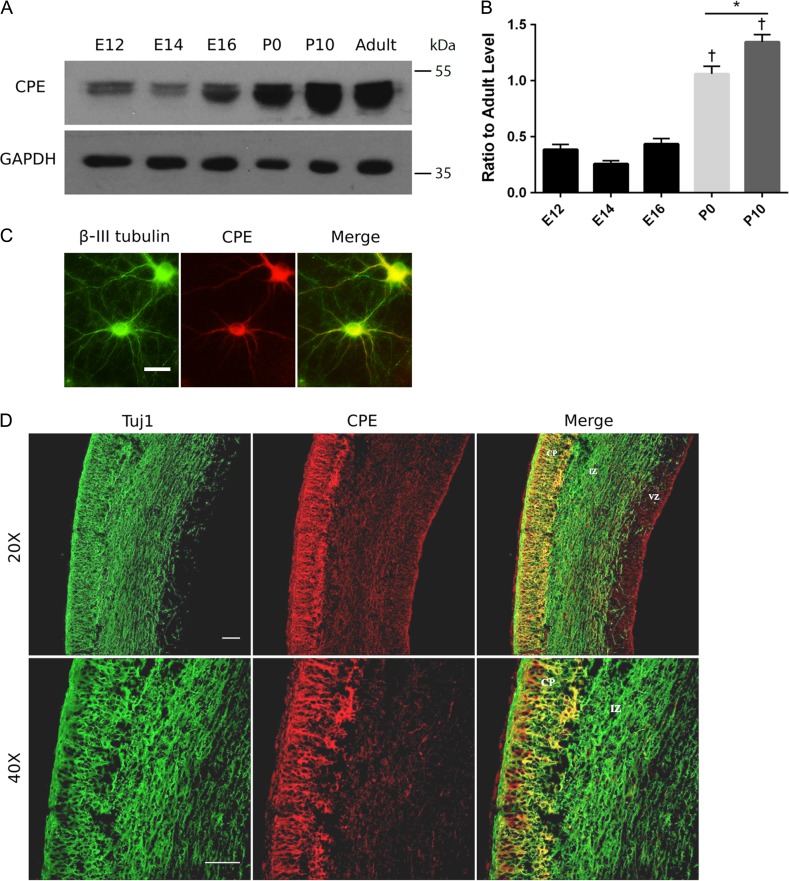
To address the role of CPE in brain development, we examined the expression profile of CPE protein at different developmental stages. We studied CPE expression in mouse brain from embryonic day (E)12 to postnatal day (P)10, which is a crucial time window for positioning of neurons in different layers of the neocortex and in single neuron development (Jiang and Nardelli 2016). Cortices from mice at E12, E14, E16, P0, P10, and P60–90 (adult) were isolated and examined by Western blot analysis. We detected the expression of both a membrane-associated form (molecular mass, ~53 kDa) (Hook 1985) and a soluble form (molecular mass, ~50 kDa) (Fricker and Devi 1993) of CPE as early as E12, and the expression levels remain stable from E12 to E16. CPE expression significantly increases from E16 to P0 and from P0 to P10. During adulthood, CPE protein levels drop and remain at a similar level as seen at P0 (Fig. 1A,B). These results suggest that CPE may play an important role during embryonic and postnatal brain development.
Figure 1.
CPE is expressed in neurons in the developing rodent brain. (A) CPE protein expression increases developmentally in mouse cortex. Proteins were extracted from cortices of mice at the indicated embryonic (E), postnatal (P), or adult ages and were resolved by SDS-PAGE. Proteins were transferred to membranes and immunoblotted for CPE and GAPDH. Representative blots are shown. (B) Quantitation of CPE expression levels. Intensities of CPE bands were quantitated and normalized to GAPDH intensities. Ratio of CPE levels at indicated developmental ages to adult levels is shown. Error bars indicate ± standard error of the mean (SEM). n = 3 for all conditions. †P < 0.0001 (E12, E14, and E16 vs. P0 or P10) as determined by one-way ANOVA followed by Tukey’s multiple comparisons test. *P < 0.05 as determined by one-way ANOVA followed by Tukey’s multiple comparisons test. (C) Representative images showing endogenous CPE protein distribution in rat hippocampal neurons at DIV 10. Neurons were immunostained for CPE (red) and β-III tubulin (green). Scale bar = 50 μm. (D) CPE is expressed predominantly in neurons in the CP. Coronal sections of E18 rat lateral neocortex were immunostained with anti-CPE (red) and anti-Tuj1 (neuron-specific Class III β-tubulin; green). Images were taken using confocal microscopy. Scale bar = 50 μm.
We next examined the subcellular distribution of CPE in cultured primary hippocampal neurons. CPE protein is evenly distributed throughout the dendrites and colocalizes with β-III tubulin (Fig. 1C), suggesting a function for CPE other than its previously reported role in vesicle transport (Park et al. 2008a, 2008b).
To further investigate CPE expression in specific cell types, we dissected brains from rats at E18 and immunostained for CPE and neuron-specific class III β-tubulin (Tuj1) in the lateral neocortex. We observed that cells expressing high levels of CPE protein are, for the most part, positioned in the CP, while cells in the IZ and VZ show much lower CPE expression levels (Fig. 1D). Moreover, cells that express CPE are Tuj1-positive (Fig. 1D), indicating that CPE is expressed in neurons of the neocortex.
CPE is Required for Proper Cortical Neuron Migration
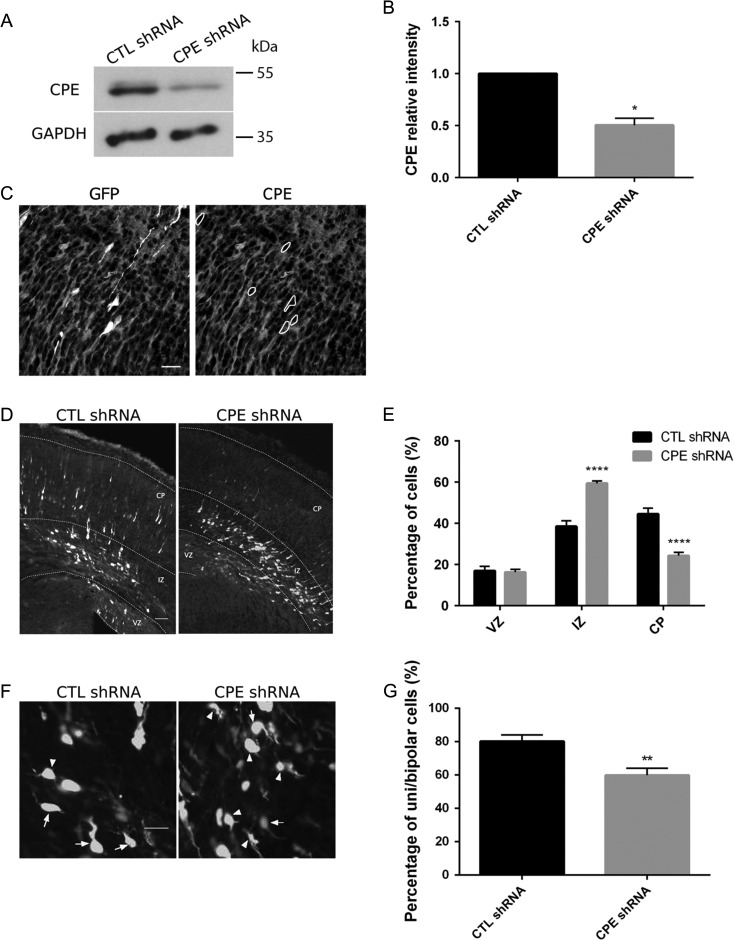
It has been reported that dynactin and associated proteins regulate cortical cell radial migration (Tsai and Gleeson 2005). Since CPE associates with p150Glued, a member of the dynactin complex, we investigated whether CPE deficiency alters neuronal migration. Thus, we designed a short-hairpin (sh)RNA against CPE to examine the effect of CPE knockdown in vivo. To test the specificity of the shRNA, we employed both Western blot analysis and immunohistochemistry. Rat cortical neurons expressing CPE shRNA demonstrate a 50% decrease in CPE levels compared with neurons expressing control shRNA (Fig. 2A,B). Immunostaining for CPE in coronal brain sections after in utero electroporation (IUE) further supports knockdown of CPE in single transfected neurons (Fig. 2C; Supplementary Figure 1).
Figure 2.
CPE is required for proper cortical neuron migration. (A) CPE shRNA knockdown efficiency in cortical neurons. E18 cortical neurons were electroporated with pGE-control shRNA (CTL shRNA) or pGE-CPE shRNA (CPE shRNA) before plating using Amaxa Rat Neuron transfection kit. After 96 h, cells were lysed, and extracted proteins were resolved using SDS-PAGE. Proteins were transferred to membrane and probed with antibodies to CPE and GAPDH. Representative blots are shown. (B) Quantitation of CPE protein levels relative to control (normalized to GAPDH). Error bars indicate ± SEM. n = 3. *P < 0.05 by Student’s t-test. (C) CPE shRNA knockdown specificity in embryonic neocortex. CPE shRNA construct including GFP marker was electroporated into the lateral ventricle of E14.5 mice and analyzed 3 days later. Transfected cells are GFP-positive (white); CPE protein (gray) is detected by immunostaining. Representative images of coronal sections of the lateral neocortex are shown. GFP-positive cells (marked by the dotted circles) in the left image show low levels of CPE protein as demonstrated on the right. Scale bar = 25 μm. (D) Mice at E14.5 were electroporated in utero with constructs as indicated, and brains were analyzed at E17.5. Representative images of coronal brain sections are shown for each condition. White, transfected cells; gray, Hoechst labeling of nuclei. Scale bar = 50 μm. (E) Quantitation of the percentage of transfected cells in each cortical area. Error bars indicate ± SEM. n = 7, CTL shRNA; n = 7, CPE shRNA. ****P < 0.0001 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test. (F) Representative images of transfected cells in the IZ are shown for each condition. Arrows point to unipolar or bipolar cells; Arrowheads point to multipolar cells. Scale bar = 20 μm. (G) Quantitation of percentage of unipolar and bipolar cells under each condition. Morphology of transfected cells across the entire IZ was analyzed. Error bars indicate ± SEM. n = 7, CTL shRNA; n = 7, CPE shRNA. **P < 0.01 as determined by unpaired Student’s t-test.
To examine the cortical neuron migration, we performed IUE in mice at E14.5, introducing either CPE shRNA or scramble-control shRNA together with cDNA encoding red fluorescent protein (RFP) and analyzed the distribution of RFP-positive cells in each cortical area at E17.5. In brain sections from mice expressing control shRNA, ~45% of transfected cells migrate to the CP and ~40% of transfected cells are located in the IZ. In contrast, only ~25% of transfected cells reach the CP when CPE is knocked down, and a significant number of transfected cells stall in the IZ (Fig. 2D,E).
Multipolar neurons transition to a bipolar morphology when localized to the IZ so that they can continue locomotion to the CP (Rakic 2006; Nishimura et al. 2010; Ohtaka-Maruyama and Okado 2015). Thus, we asked whether CPE regulates this transition, which may explain the observed defects in radial migration when CPE is knocked down. We analyzed the morphology of transfected cells in the IZ of coronal brain sections after IUE and found that knockdown of CPE significantly decreases the percentage of unipolar and bipolar cells in the IZ (Fig. 2F,G). Thus, the migration defects observed may be explained by the failure to transition from multipolar to bipolar morphology.
CPE Regulates Dendrite Morphology in Vivo and in Cultured Hippocampal Neurons
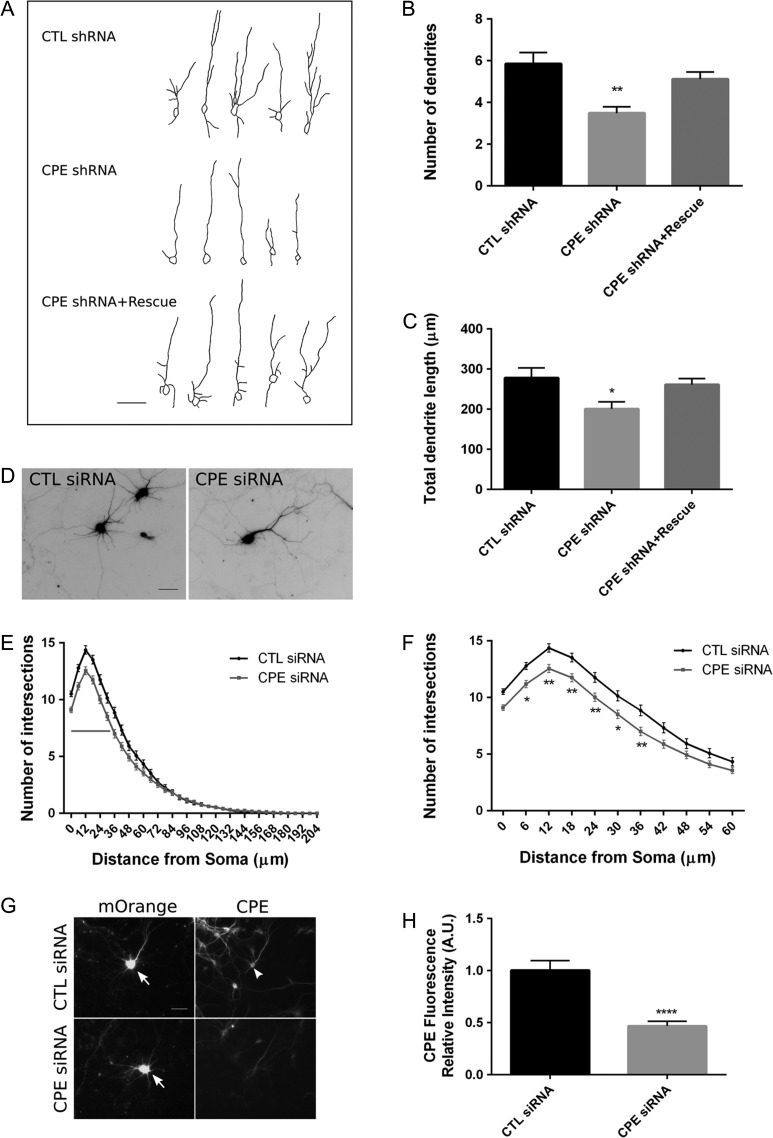
CPE-knockout mice show aberrant dendritic architecture in cortical and hippocampal neurons at 14 weeks of age (Woronowicz et al. 2010). We find that expression of CPE increases significantly from E16 to P10 in mice and that CPE is expressed in differentiating neurons in the CP, suggesting a role for CPE in postmigratory neuronal development. Thus, we examined the potential involvement of CPE in regulating dendrite morphology in vivo from E14.5 to P7. We observed that in cortical layer II/III, neurons electroporated with CPE shRNA at E14.5 exhibit a lower number of dendrites and shorter total dendrite length than neurons under control condition at P7 (Fig. 3A–C). To demonstrate the specificity of CPE shRNA, we generated an shRNA-resistant CPE construct and coelectroporated this construct with CPE shRNA. As expected, the shRNA-resistant CPE construct, which restores CPE expression (Supplementary Figure 1), rescues the decreases in the number and total length of dendrites caused by CPE knockdown (Fig. 3A–C), supporting specificity of the shRNA for CPE knockdown.
Figure 3.
Knockdown of CPE decreases dendritic branching in cortical neurons in vivo and in cultured hippocampal neurons in vitro. (A) Mice at E14.5 were electroporated in utero with constructs as indicated, and neurons within cortical layer II/III were analyzed at P7. Representative tracings of dendrites are shown for each condition. Scale bar = 50 μm. (B, C) Quantitation of the number of dendrites (B) and total dendrite length (C) of neurons under each condition. Error bars indicate ± SEM. n (neurons) = 26, CTL shRNA; n = 29, CPE shRNA; n = 50, CPE shRNA+rescue. *P < 0.05, **P < 0.01 as determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. (D) Hippocampal neurons were cotransfected with pCAG-mOrange and negative control siRNA (CTL siRNA) or CPE siRNA as indicated at DIV 7. Neurons were fixed and immunostained for GFP and MAP2 at DIV 10. Neurons positive for both GFP and mOrange were assessed for Sholl analysis. Representative mOrange fluorescent images of neurons are shown as inverted black images. Scale bar = 50 μm. (E) Sholl analysis of neurons transfected with the indicated siRNA. Gray line indicates P value is at least less than 0.001 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test. (F) Sholl analysis within 60 μm from soma. n (neurons) = 84, CTL siRNA; n = 95, CPE siRNA. *P < 0.05, **P < 0.01 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test. (G) Representative images showing CPE siRNA knockdown efficiency in transfected neurons. Hippocampal neurons were cotransfected with pCAG-mOrange (indicated by arrows) and either CTL siRNA or CPE siRNA at DIV 7, fixed at DIV 10, and immunostained for CPE (indicated by arrowhead in transfected cell). Scale bar = 50 μm. (H) Quantitation of CPE fluorescence intensity in the cell body of transfected neurons. Error bars indicate ± SEM. n (neurons) = 12, CTL siRNA; n = 19, CPE siRNA. ****P < 0.0001 as determined by Student’s t-test.
To support the idea that CPE knockdown disrupts dendritogenesis, we performed similar experiments in hippocampal neuronal cultures between day in vitro (DIV) 7 and DIV 10, as both primary dendrite extension and higher order dendrite formation actively take place during this time window (Banker and Goslin 1988; Dotti et al. 1988). To knock down CPE in culture, we used a commercially purchased siRNA against rat CPE, and we demonstrated its specificity by immunocytochemistry (Fig. 3G). Transfected neurons show ~50% reduction in CPE protein levels (Fig. 3H). Results of Sholl analysis show that knockdown of CPE results in decreased dendrite branching at a distance of 0–42μm from the soma (Fig. 3D–F). These results are in agreement with our in vivo data, indicating that CPE is required for dendritic arborization.
CPE Interacts with p150Glued and Overexpression of the Carboxyl Terminal 10 Amino Acids of CPE (CPE-10) Redistributes p150Glued from the Centrosome
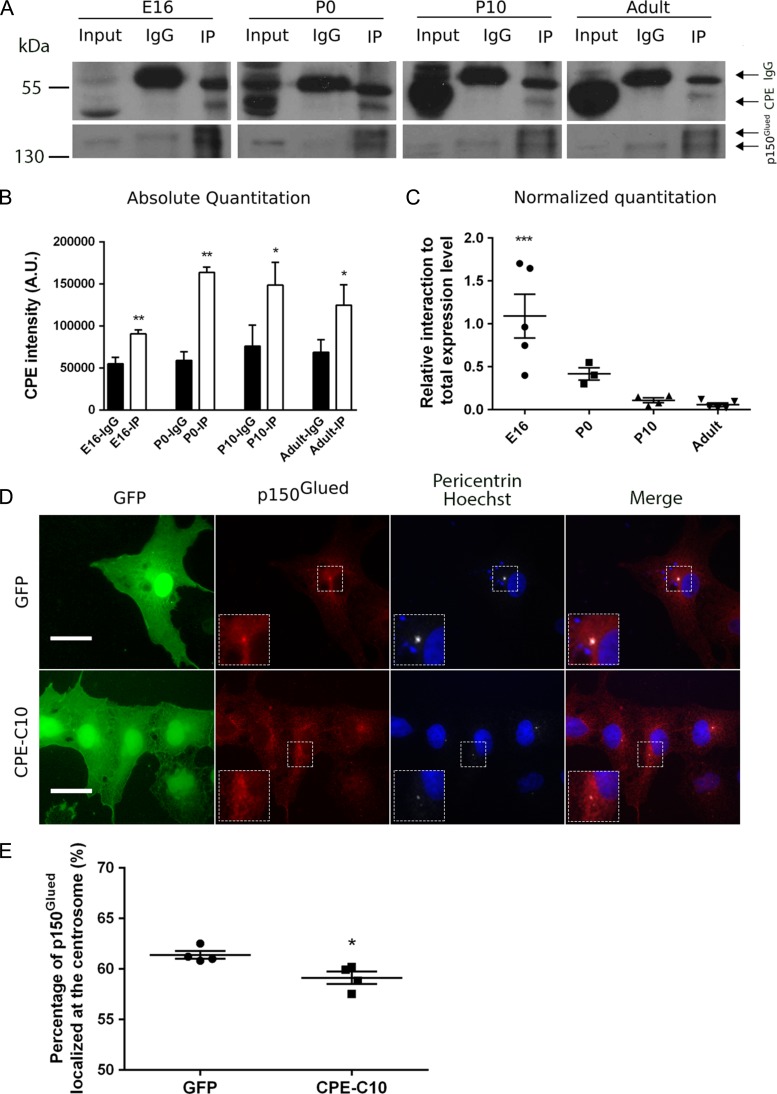
It has been reported that in pituitary corticotrope tumor (AtT20) cells, the carboxyl terminal 10 amino acids of CPE interact with p150Glued (Park et al. 2008a, 2008b), the largest subunit in the dynactin protein complex. To confirm whether this interaction between CPE and p150Glued exists in brain and whether this interaction changes during development, we performed a coimmunoprecipitation assay using mouse brain lysates from different developmental stages. As expected, p150Glued coimmunoprecipitates with CPE both in adult and developing cortices (Fig. 4A,B). Both isoforms of p150Glued are detected in the immunoprecipitates, consistent with the literature (Zhapparova et al. 2009). Since CPE expression levels increase during development (Fig. 4A), we quantitated the interaction beween CPE and p150Glued relative to total CPE and p150Glued levels (% CPE coimmunoprecipitated with p150Glued) at different developmental stages. Our data suggest that the percentage of CPE protein that interacts with p150Glued peaks at E16 and decreases throughout development (Fig. 4C). These results support a role for the interaction between CPE and p150Glued in early brain development, and in specific, during the time window of development for neuronal migration and dendrite patterning.
Figure 4.
CPE interacts with p150Glued and overexpression of the carboxyl terminus of CPE redistributes p150Glued from the centrosome. (A) CPE and p150Glued interact in mouse cortex. Mouse cortices were dissected at indicated embryonic (E), postnatal (P), and adult ages. Proteins were extracted and subjected to immunoprecipitation with anti-p150Glued or mouse-IgG. Cortical protein extract (input), p150Glued immunoprecipitates (IP), and mouse-IgG immunoprecipitates (IgG) were resolved by SDS-PAGE, transferred to membrane, and immunoblotted for CPE and p150Glued. Input represents 5% of extracted proteins. Representative blots are shown. In top blot, arrows point to 2 bands in IP and IgG lanes detected by anti-CPE; upper bands are IgG and lower bands are CPE. In bottom blot, arrows point to the 2 isoforms of p150Glued found in brain. (B) Quantitation of absolute CPE band intensities, represented in panel A. Error bars indicate ± SEM. n = 5, E16; n = 4, P0; n = 4, P10; n = 5, adult. CPE is present in coimmunoprecipitates at a significantly higher level than control (IgG). *P < 0.05, **P < 0.01 as determined by paired Student’s t-test. (C) Quantitation of relative CPE and p150Glued interaction by developmental ages. At each age, percentage of coimmunoprecipitated CPE (IP, CPE band) out of total CPE expression level (Input, CPE band) was quantitated as defined as X; percentage of immunoprecipitated p150Glued (IP, p150Glued band) out of total p150Glued expression level (input, p150Glued band) was quantitated as defined as Y; Ratio of X to Y was calculated and graphed. Error bars indicate ± SEM. n = 5, E16; n = 3, P0; n = 4, P10; n = 5, adult. Outlier was excluded by Grubbs’ test. E16, P0, and P10 were compared with adult. ***P < 0.001 as determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. (D) Representative images showing p150Glued localization in COS-7 cells. COS-7 cells were transfected with pEGFP (GFP) or a construct encoding the carboxyl terminal 10 amino acids of CPE (pEGFP-CPE-C10 referred to as CPE-C10) as indicated. Cells were fixed after 48 h and immunostained with anti-GFP (green), anti-p150Glued (red), anti-pericentrin (white), and nuclei were labeled with Hoechst dye (blue). Inset is area magnified two-fold. Scale bar = 25 μm. (E) Quantitation of the percentage of p150Glued protein (pixels) localized at the centrosome. For each independent experiment, 15–25 individual transfected cells of each condition were analyzed as described in Materials and Methods. Error bars indicate ± SEM. n = 4 for both conditions. *P < 0.05 as determined by Student’s t-test.
p150Glued predominantly localizes and anchors microtubules to the centrosome and recruits cell cycle regulators (Quintyne et al. 1999; Quintyne and Schroer 2002). Given that CPE interacts with p150Glued (Park et al. 2008a, 2008b), it is possible that CPE affects the subcellular localization of p150Glued, which in turn, alters the cytoskeleton and other downstream processes. Excess CPE-C10, a peptide containing the carboxyl terminal 10 amino acids of CPE, competes endogenous CPE binding to p150Glued (Park et al. 2008a, 2008b). Therefore, we overexpressed CPE-C10 in COS-7 cells to disrupt the normal interaction between CPE and p150Glued and analyzed the subcellular distribution of p150Glued. Centrosomes were identified by immunolabeling with an antibody to pericentrin, an integral component of the pericentriolar material (Doxsey et al. 1994). In cells expressing GFP, there was abundant colocalization of p150Glued with pericentrin at the centrosome, while overexpression of CPE-C10 resulted in a significant decrease in the percentage of p150Glued localized at the centrosome (Fig. 4D,E), suggesting a dominant negative effect of CPE-C10 on p150Glued localization. Taken together, these results support our hypothesis that the interaction between CPE and p150Glued is important for proper localization of p150Glued, which may in turn influence p150Glued function in regulating the microtubule cytoskeleton.
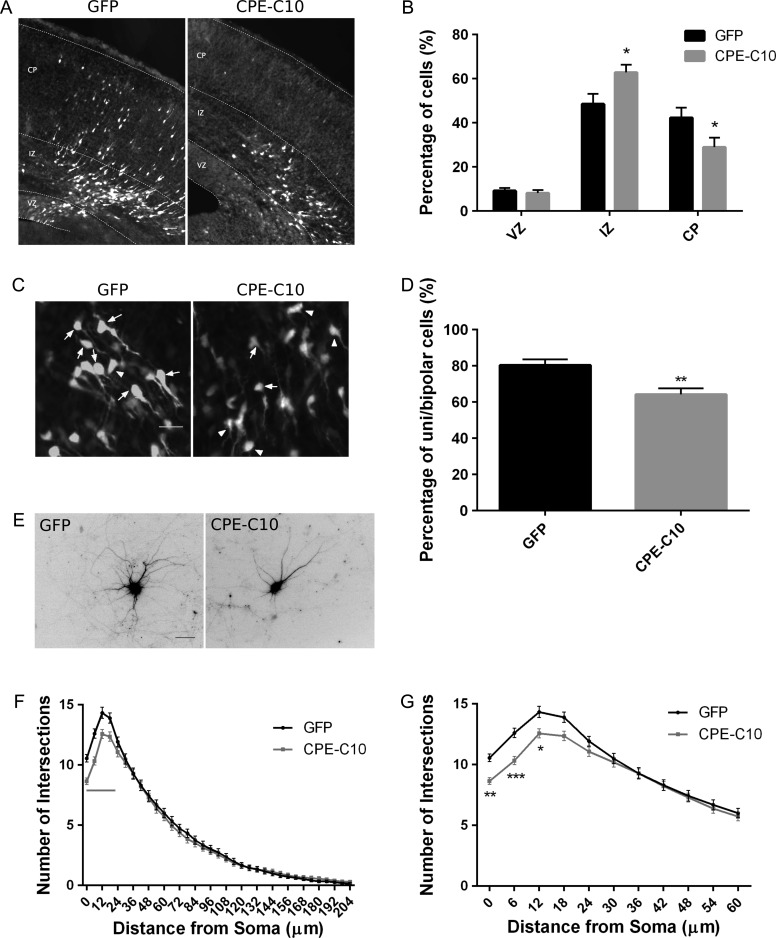
Overexpression of CPE-C10 Disrupts Neuronal Migration and Dendritic Arborization
We next tested the role of the interaction between the carboxyl terminus of CPE and p150Glued in regulating neuronal migration and dendritic arborization. GFP-tagged CPE-C10 or GFP (control) was expressed in neural progenitor cells using IUE at E14.5, and neuronal migration was examined at E17.5. We observed that the expression of CPE-C10 results in a significant decrease in the percentage of cells reaching the CP (Fig. 5A,B), suggesting that binding of a CPE-interacting protein, such as p150Glued, may be necessary for proper cortical migration and that disruption of this interaction attenuates the function of CPE in neurodevelopment. Together, our data suggest that CPE is required for proper cortical neuron migration and that its carboxyl terminus, and likely interaction with p150Glued, may be involved in this process.
Figure 5.
Overexpression of the carboxyl terminus of CPE disrupts neuronal migration and dendritic arborization. (A) Mice at E14.5 were electroporated in utero with constructs as indicated, and brains were analyzed at E17.5. Representative images of coronal brain sections are shown for each condition. White, transfected cells; gray, Hoechst labeling of nuclei. Scale bar = 50 μm. (B) Quantitation of the percentage of transfected cells in each cortical area. Error bars indicate ± SEM. n = 8, GFP; n = 6, CPE-C10 (carboxyl terminal 10 amino acids of CPE). **P < 0.01 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test. (C) Representative images of transfected cells in the IZ are shown for each condition. Arrows point to unipolar or bipolar cells; arrowheads point to multipolar cells. Scale bar = 20 μm. (D) Quantitation of percentage of unipolar and bipolar cells under each condition. Morphology of transfected cells across the entire IZ was analyzed. Error bars indicate ± SEM. n = 10, GFP; n = 6, CPE-C10. **P < 0.01 as determined by Student’s t-test. (E) Hippocampal neurons were cotransfected with pCAG-mOrange and pEGFP (GFP) or pEGFP-CPE-C10 (CPE-C10) as indicated at DIV 7. Neurons were fixed and immunostained for GFP and MAP2 at DIV 10. Neurons positive for both GFP and mOrange were assessed for Sholl analysis. Representative mOrange fluorescent images of neurons are shown as inverted black images. Scale bar = 50 μm. (F) Sholl analysis of neurons expressing GFP or CPE-C10 on dendrite branching. Gray line indicates P value is at least less than 0.01 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test. (G) Sholl analysis within 60 μm from soma. Error bars indicate ± SEM. n (neurons) = 95, GFP; n = 90, CPE-C10. *P < 0.05, **P< 0.01, ***P < 0.001 as determined by 2-way ANOVA followed by Sidak’s multiple comparisons test.
We also analyzed the morphology of transfected neurons in the IZ to determine the mechanism underlying the failure of neurons to reach the CP. We observed that, similar to CPE knockdown, expression of CPE-C10 disrupts neuronal multipolar to bipolar transition (Fig. 5C,D), which may explain the migration defects mediated by CPE-C10 expression.
To further support the idea that the interaction between CPE and p150Glued regulates dendritic arborization, cultured hippocampal neurons were cotransfected with cDNA encoding mOrange and GFP or GFP-CPE-C10 at DIV 7. Neurons positive for both mOrange and GFP were analyzed for dendrite branching at DIV 10. Overexpression of CPE-C10 results in significantly decreased dendrite branching close to the soma (0–18 μm; Fig. 5E–G), suggesting that the effects of CPE on dendrite morphology are dependent on its carboxyl terminal interaction with p150Glued.
Discussion
We have identified a novel role for CPE and its interaction with p150Glued in cortical neuron migration and dendritogenesis. Knockdown of CPE or disruption of the CPE-p150Glued interaction results in decreased migration of neurons to the CP. We show that this is due to disrupted neuronal multipolar to bipolar transition. Furthermore, we show that both in vivo and in vitro, expression of CPE and its interaction with p150Glued are necessary for proper development and branching of proximal dendrites in cortical and hippocampal neurons. We also found that overexpression of the carboxyl terminal 10 amino acids of CPE decreases localization of p150Glued at the centrosome, providing a potential mechanism for the role of the CPE-p150Glued interaction in proper brain development. Our results suggest for the first time that in addition to vesicle sorting and transport, CPE acts via p150Glued, and hence microtubule organization, to regulate neuronal placement and shape.
Expression of CPE During Embryonic Brain Development
CPE protein is detected in all major areas of the adult rat brain (Birch et al. 1990), and its roles in prohormone processing and sorting and vesicle transport have been well studied (reviewed in (Cawley et al. 2012)). In addition to its expression in the adult nervous system, CPE has been reported to be present in mammalian embryos (Zheng et al. 1994; Selvaraj et al. 2017). Studies of CPE expression during mouse brain development show that CPE is expressed in neural tissues earlier than other prohormone convertases, such as PC1 and PC2, suggesting a distinct role for CPE during embryonic neurodevelopment. Our observations that CPE protein expression increases during embryonic and early postnatal development and is higher in neurons in the CP than in the IZ or VZ suggest that CPE may also regulate the development of later-born neurons or later processes in neuronal development, such as spine formation and synaptogenesis.
We did not detect expression of CPEΔN (molecular mass, ~40 KDa), a CPE isoform lacking the signaling peptide within the amino terminus transiently expressed during mouse embryonic development (Lee et al. 2011; Qin et al. 2014). It was previously reported that overexpressed CPE in hippocampal neurons localizes in punctate vesicles and mediates vesicle transport (Park et al. 2008a, 2008b). Our findings that endogenous CPE is evenly distributed throughout the soma and neurites suggest that CPE may have other functions besides sorting and transport of vesicles in neurons. Taken together, we propose that CPE contributes to early brain development, and impairments in neuronal positioning and morphology resulting from CPE gene mutations and decreased CPE protein levels may underlie neurocognitive deficits exhibited by CPE−/− and Cpefat/fat mice models (Woronowicz et al. 2008; Rodriguiz et al. 2013).
Roles of CPE in Cortical Development and Dendritic Arborization
It has been well established that abnormalities in cortical neuron migration and dendrite morphology disrupt higher brain functions, such as learning, memory, emotion, and cognition, and may cause neurocognitive disorders, including depression, bipolar disorder, and schizophrenia (Valiente and Marin 2010; Barkovich et al. 2012; Brennand et al. 2012; Kulkarni and Firestein 2012). It is of importance to understand how our data fit into the current understanding of neurodevelopmental disorders. As the cerebral cortex develops, pyramidal neurons are generated by neural progenitor cells in the VZ and migrate toward the pial surface (reviewed in (Bystron et al. 2008)). Neurons electroporated with a CPE shRNA construct fail to migrate to the CP, suggesting that CPE is required for proper cortical neuron migration. Normally in the IZ, newly differentiated neurons possess multipolar morphology and undergo transition to bipolar morphology, which is critical for subsequent glial-guided radial migration (reviewed in (Bystron et al. 2008)), and this transition relies heavily on microtubule dynamics (Ohtaka-Maruyama and Okado 2015). Knockdown of CPE protein increases the percentage of multipolar cells in the IZ, indicating the involvement of CPE in mediating neuronal multipolar to bipolar transition.
As neurons reach their final destination, they differentiate into a multipolar morphology with defined axons and dendritic arbors (Takano et al. 2015). Proper dendrite morphology, including shape, size, and patterning of the dendritic arbor, is critical for many aspects of neuronal function, such as the number and type of synapses that will be formed along the dendrites, electrical properties of a neuron, and the positioning of particular signaling molecules (London and Hausser 2005; Spruston 2008). CPE knockdown results in decreased number and total length of dendrites of pyramidal neurons in P7 mouse cortex, and these phenotypes are rescued by shRNA-resistant CPE expression, indicating a cell autonomous role for CPE in proper dendritogenesis. Although a previous study in 14-week-old CPE−/−mice reported increased dendritic branching proximal to the soma and decreased branching in distal dendritic arbor (Woronowicz et al. 2010), it was unclear in this study whether the effects of CPE deficiency on the arbor were cell autonomous or cell nonautonomous. Additionally, our study is the first to identify a role for CPE in an earlier developmental process, cortical cell migration. Together, our findings further demonstrate the importance of CPE in dendritogenesis and indicate that loss of CPE at different developmental stages may have distinct impacts on dendrite morphology.
Mechanism by Which CPE Regulates Cortical Development and Dendrite Morphology
Neuronal migration and dendrite morphogenesis are dependent on continuous morphological changes mediated by the cytoskeleton, and in specific, the centrosome and microtubule network (reviewed in (Dent 2017; Fukuda and Yanagi 2017)). A number of risk genes have been identified for psychiatric disorders, and many are involved in cytoskeletal regulation (Copf 2016; Fukuda and Yanagi 2017). p150Glued plays an important role in regulating the microtubule network (King and Schroer 2000; Askham et al. 2002; Deacon et al. 2003; Schroer 2004; Ligon et al. 2006; Berezuk and Schroer 2007; Lloyd et al. 2012; Lazarus et al. 2013). The observation that CPE-C10, which blocks the normal interaction between CPE and p150Glued in a dominant negative manner, alters p150Glued localization, neuronal migration, and dendrite branching suggests that the carboxyl terminus is the major domain responsible for CPE-mediated effects in neurodevelopment. Multiple lines of evidence from previous studies support the idea that p150Glued regulates cortical development and dendrite branching, giving further evidence that CPE may act in a p150Glued-dependent manner. First, accumulation of p150Glued at the centrosome and microtubule plus-end is critical for maintaining microtubule stability and dynamics (Quintyne et al. 1999; Lazarus et al. 2013). Therefore, disrupted p150Glued localization may affect microtubule organization, and in turn, alter neuronal migration and dendrite branching. Mutations within the glycine-rich (CAP-Gly) domain of the DCTN1 gene, encoding p150Glued, affect the affinity of dynactin for microtubules and cause Perry syndrome, further supporting a role for the CPE-p150Glued interaction in neurodevelopmental processes (Farrer et al. 2009). Second, several cytoskeletal regulators, such as disrupted in schizophrenia-1 (DISC1), regulate cortical migration and are associated with psychiatric disorders (Fukuda and Yanagi 2017). Finally, CPE interacts with p150Glued to recruit motor proteins and transport BDNF-containing vesicles, which increases the number of proximal dendrites in pyramidal neurons (McAllister et al. 1995; Horch et al. 1999; Kwinter et al. 2009). The involvement of BDNF and its receptor TrkB in neuronal migration has also been reported in different brain regions, and overexpression of BNDF causes aberrant cortical lamination (Ringstedt et al. 1998; Borghesani et al. 2002; Medina et al. 2004; Chiaramello et al. 2007).
In addition, the fact that excess CPE-C10 competes endogenous CPE but not motor proteins that bind to p150Glued (Park et al. 2008a, 2008b) strongly suggests that CPE directly interacts with p150Glued via its carboxyl terminus. However, current evidence cannot rule out the possibility of an indirect interaction with other proteins that exist in a complex with p150Glued. In addition, it is possible that there may be additional proteins that bind to the carboxyl terminus of CPE that are responsible for the action of CPE-C10. Thus, CPE may regulate the localization of p150Glued indirectly by influencing other components of the dynactin and motor protein complex.
Model of the Role for CPE and p150Glued Interaction in Neuronal Development
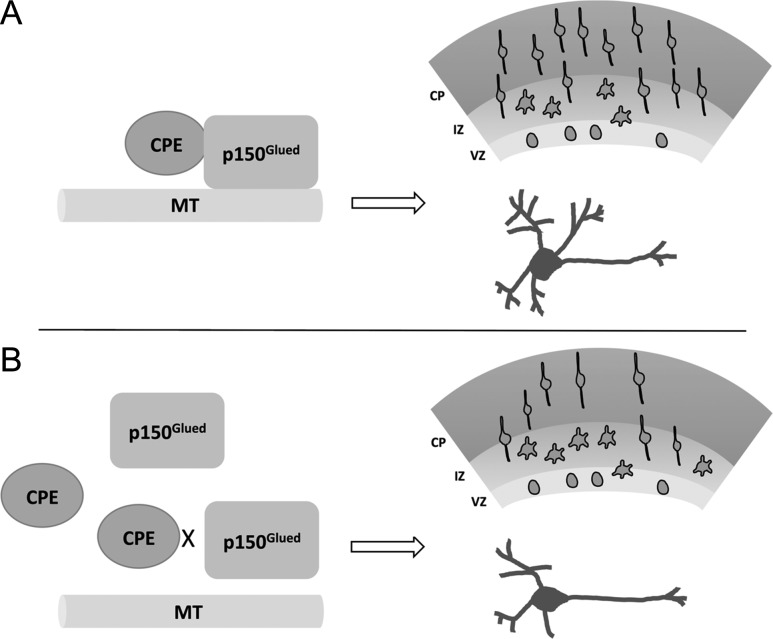
Taken together, we propose a model (Fig. 6) for understanding the mechanism underlying CPE-mediated effects on neuronal migration and dendrite branching. A balance of CPE-p150Glued protein complex and free CPE protein is required to maintain microtubule stability and dynamics, and in turn, to promote proper neuronal migration and dendrite branching. Decreased CPE protein or expression of CPE-C10 disrupts the normal CPE-p150Glued interaction and alters proper subcellular distribution of p150Glued to the centrosome or microtubules. This results in abnormal cytoskeletal function and disrupted neurodevelopment. In summary, our findings provide new insight for the functions of CPE during early brain development and potential involvement in neurocognitive disorders and demonstrate the importance of the interaction between the carboxyl terminus of CPE and p150Glued in mediating proper cortical neuron migration and dendrite morphology.
Figure 6.
Model of CPE and p150Glued interaction in regulating cortical neuron migration and dendrite morphology. (A) Under normal conditions, CPE regulates p150Glued localization via its carboxyl terminus to maintain necessary stability and dynamics of microtubules (MTs), thus ensuring proper cortical migration and dendrite morphology. (B) When CPE protein is knocked down or when CPE-C10 is overexpressed and competes endogenous CPE for binding to p150Glued, the normal interaction between CPE and p150Glued is disrupted, resulting in abnormal neuronal migration and decreased dendrite branching.
Supplementary Material
Authors’ Contributions
C.L., D.C., A.O., H.K., A.P., and I.F. carried out the experimental work. C.L., D.C., and B.L.F. analyzed the data and wrote the manuscript.
Funding
This work is funded in part by National Science Foundation (grant IOS-1353724 to B.L.F.). A.O. is supported by the National Institutes of Health T32 GM008339-28 from the NIGMS.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Alsters SI, Goldstone AP, Buxton JL, Zekavati A, Sosinsky A, Yiorkas AM, Holder S, Klaber RE, Bridges N, van Haelst MM, et al. 2015. Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS One. 10:e0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askham JM, Vaughan KT, Goodson HV, Morrison EE. 2002. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell. 13:3627–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Goslin K. 1988. Developments in neuronal cell culture. Nature. 336:185–186. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. 2012. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 135:1348–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezuk MA, Schroer TA. 2007. Dynactin enhances the processivity of kinesin-2. Traffic. 8:124–129. [DOI] [PubMed] [Google Scholar]
- Birch NP, Rodriguez C, Dixon JE, Mezey E. 1990. Distribution of carboxypeptidase H messenger RNA in rat brain using in situ hybridization histochemistry: implications for neuropeptide biosynthesis. Brain Res Mol Brain Res. 7:53–59. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. 2002. BDNF stimulates migration of cerebellar granule cells. Development. 129:1435–1442. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Tran N, Gage FH. 2012. Modeling psychiatric disorders at the cellular and network levels. Mol Psychiatry. 17:1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 9:110. [DOI] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnar Z, Blakemore C. 2006. The first neurons of the human cerebral cortex. Nat Neurosci. 9:880–886. [DOI] [PubMed] [Google Scholar]
- Carrel D, Du Y, Komlos D, Hadzimichalis NM, Kwon M, Wang B, Brzustowicz LM, Firestein BL. 2009. NOS1AP regulates dendrite patterning of hippocampal neurons through a carboxypeptidase E-mediated pathway. J Neurosci. 29:8248–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel D, Hernandez K, Kwon M, Mau C, Trivedi MP, Brzustowicz LM, Firestein BL. 2015. Nitric oxide synthase 1 adaptor protein, a protein implicated in schizophrenia, controls radial migration of cortical neurons. Biol Psychiatry. 77:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. 2012. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr Rev. 33:216–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. 2007. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 26:1780–1790. [DOI] [PubMed] [Google Scholar]
- Coleman DL, Eicher EM. 1990. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 81:424–427. [DOI] [PubMed] [Google Scholar]
- Copf T. 2016. Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci Biobehav Rev. 68:946–978. [DOI] [PubMed] [Google Scholar]
- de la Torre-Ubieta L, Stein JL, Won H, Opland CK, Liang D, Lu D, Geschwind DH. 2018. The dynamic landscape of open chromatin during human cortical neurogenesis. Cell. 172:289–304.e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI. 2003. Dynactin is required for bidirectional organelle transport. J Cell Biol. 160:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW. 2017. Of microtubules and memory: implications for microtubule dynamics in dendrites and spines. Mol Biol Cell. 28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. 1988. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 8:1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. 1994. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 76:639–650. [DOI] [PubMed] [Google Scholar]
- Dujardin DL, Barnhart LE, Stehman SA, Gomes ER, Gundersen GG, Vallee RB. 2003. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 163:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, et al. 2009. DCTN1 mutations in Perry syndrome. Nat Genet. 41:163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein BL, Firestein BL, Brenman JE, Aoki C, Sanchez-Perez AM, El-Husseini AE, Bredt DS. 1999. Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron. 24:659–672. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Berman YL, Leiter EH, Devi LA. 1996. Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J Biol Chem. 271:30619–30624. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Devi L. 1993. Posttranslational processing of carboxypeptidase E, a neuropeptide-processing enzyme, in AtT-20 cells and bovine pituitary secretory granules. J Neurochem. 61:1404–1415. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. 1982. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci USA. 79:3886–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Yanagi S. 2017. Psychiatric behaviors associated with cytoskeletal defects in radial neuronal migration. Cell Mol Life Sci. 74:3533–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moreno F, Lopez-Mascaraque L, De Carlos JA. 2007. Origins and migratory routes of murine Cajal–Retzius cells. J Comp Neurol. 500:419–432. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. 2013. Cortical evolution: judge the brain by its cover. Neuron. 80:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VY. 1985. Differential distribution of carboxypeptidase-processing enzyme activity and immunoreactivity in membrane and soluble components of chromaffin granules. J Neurochem. 45:987–989. [DOI] [PubMed] [Google Scholar]
- Hook VY, Eiden LE, Brownstein MJ. 1982. A carboxypeptidase processing enzyme for enkephalin precursors. Nature. 295:341–342. [DOI] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. 1999. Destabilization of cortical dendrites and spines by BDNF. Neuron. 23:353–364. [DOI] [PubMed] [Google Scholar]
- Jiang X, Nardelli J. 2016. Cellular and molecular introduction to brain development. Neurobiol Dis. 92:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA. 2000. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2:20–24. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Firestein BL. 2012. The dendritic tree and brain disorders. Mol Cell Neurosci. 50:10–20. [DOI] [PubMed] [Google Scholar]
- Kutzing MK, Langhammer CG, Luo V, Lakdawala H, Firestein BL. 2010. Automated Sholl analysis of digitized neuronal morphology at multiple scales. J Vis Exp. pii: 2354. doi: 10.3791/2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwinter DM, Lo K, Mafi P, Silverman MA. 2009. Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience. 162:1001–1010. [DOI] [PubMed] [Google Scholar]
- Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL. 2010. Automated Sholl analysis of digitized neuronal morphology at multiple scales: whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A. 77:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. 2013. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 11:e1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, et al. 2011. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. J Clin Invest. 121:880–892. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ligon LA, Shelly SS, Tokito MK, Holzbaur EL. 2006. Microtubule binding proteins CLIP-170, EB1, and p150Glued form distinct plus-end complexes. FEBS Lett. 580:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd TE, Machamer J, O’Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan ES, et al. 2012. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 74:344–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Hausser M. 2005. Dendritic computation. Annu Rev Neurosci. 28:503–532. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. 2005. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 45:245–255. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. 1995. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 15:791–803. [DOI] [PubMed] [Google Scholar]
- Medina DL, Sciarretta C, Calella AM, Von Bohlen Und Halbach O, Unsicker K, Minichiello L. 2004. TrkB regulates neocortex formation through the Shc/PLCgamma-mediated control of neuronal migration. EMBO J. 23:3803–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. 2004. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 58:167–176. [DOI] [PubMed] [Google Scholar]
- Mulvey B, Dougherty JD. 2018. Weaving new insights for the genetic regulation of human cognitive phenotypes. Cell. 172:10–13. [DOI] [PubMed] [Google Scholar]
- Nishimura YV, Sekine K, Chihama K, Nakajima K, Hoshino M, Nabeshima Y, Kawauchi T. 2010. Dissecting the factors involved in the locomotion mode of neuronal migration in the developing cerebral cortex. J Biol Chem. 285:5878–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaka-Maruyama C, Okado H. 2015. Molecular pathways underlying projection neuron production and migration during cerebral cortical development. Front Neurosci. 9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. 2008. a. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci. 39:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. 2008. b. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol. 22:989–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Cheng Y, Murthy SR, Selvaraj P, Loh YP. 2014. Carboxypeptidase E-DeltaN, a neuroprotein transiently expressed during development protects embryonic neurons against glutamate neurotoxicity. PLoS One. 9:e112996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. 1999. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 147:321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Schroer TA. 2002. Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J Cell Biol. 159:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1988. Specification of cerebral cortical areas. Science (New York, NY). 241:170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P. 2006. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 16(Suppl 1):i3–i17. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibanez CF. 1998. BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron. 21:305–315. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Wilkins JJ, Creson TK, Biswas R, Berezniuk I, Fricker AD, Fricker LD, Wetsel WC. 2013. Emergence of anxiety-like behaviours in depressive-like Cpe(fat/fat) mice. Int J Neuropsychopharmacol. 16:1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA. 2004. Dynactin. Annu Rev Cell Dev Biol. 20:759–779. [DOI] [PubMed] [Google Scholar]
- Selvaraj P, Xiao L, Lee C, Murthy SR, Cawley NX, Lane M, Merchenthaler I, Ahn S, Loh YP. 2017. Neurotrophic factor-alpha1: a key Wnt-beta-catenin dependent anti-proliferation factor and ERK-Sox9 activated inducer of embryonic neural stem cell differentiation to astrocytes in neurodevelopment. Stem Cells. 35:557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. 2000. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2:767–775. [DOI] [PubMed] [Google Scholar]
- Spruston N. 2008. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 9:206–221. [DOI] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB. 2002. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 156:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Xu C, Funahashi Y, Namba T, Kaibuchi K. 2015. Neuronal polarization. Development. 142:2088–2093. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. 2005. Nucleokinesis in neuronal migration. Neuron. 46:383–388. [DOI] [PubMed] [Google Scholar]
- Valiente M, Marin O. 2010. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 20:68–78. [DOI] [PubMed] [Google Scholar]
- Wider C, Dachsel JC, Farrer MJ, Dickson DW, Tsuboi Y, Wszolek ZK. 2010. Elucidating the genetics and pathology of Perry syndrome. J Neurol Sci. 289:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronowicz A, Cawley NX, Chang SY, Koshimizu H, Phillips AW, Xiong ZG, Loh YP. 2010. Carboxypeptidase E knockout mice exhibit abnormal dendritic arborization and spine morphology in central nervous system neurons. J Neurosci Res. 88:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronowicz A, Koshimizu H, Chang SY, Cawley NX, Hill JM, Rodriguiz RM, Abebe D, Dorfman C, Senatorov V, Zhou A, et al. 2008. Absence of carboxypeptidase E leads to adult hippocampal neuronal degeneration and memory deficits. Hippocampus. 18:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhapparova ON, Bryantseva SA, Dergunova LV, Raevskaya NM, Burakov AV, Bantysh OB, Shanina NA, Nadezhdina ES. 2009. Dynactin subunit p150Glued isoforms notable for differential interaction with microtubules. Traffic. 10:1635–1646. [DOI] [PubMed] [Google Scholar]
- Zheng M, Streck RD, Scott RE, Seidah NG, Pintar JE. 1994. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci. 14:4656–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.