Abstract
Background
The ability to assess the hemostatic effect of the direct oral anticoagulant (DOACs) may be valuable in clinical situations such as bleeding or thrombosis, before urgent surgery, or reversal of anticoagulation. We sought to assess the anticoagulant effect of DOACs with the new‐generation fully automated thrombelastograph TEG 6s using resonance‐frequency viscoelasticity measurements and disposable multichannel microfluidic cartridges.
Methods
A single dose of oral dabigatran 150 mg, rivaroxaban 20 mg, or apixaban 5 mg was given to 9 healthy males. Phlebotomy was performed at 0, 1, and 3 hours after administration of DOAC. TEG parameters were measured using TEG _6s. Concentrations of DOACs were measured using chromogenic assays. The TEG parameters were correlated to the DOAC concentrations.
Results
The reaction time (R) demonstrated the strongest response to DOAC intake. There were no correlations between other TEG parameters and DOAC concentrations. Using the direct thrombin inhibitor (DTI) channel, R was significantly correlated with dabigatran levels (r = 0.94, P < 0.0001). Using the anti–factor Xa (AFXa) channel, R was significantly correlated with rivaroxaban and apixaban levels (r = 0.93 and r = 0.83, respectively; P < 0.0001 for both). R >2.5 minutes for dabigatran (DTI channel), >2.5 minutes for apixaban, and >1.8 minutes for rivaroxaban (AFXa channel) were associated with 100% sensitivity and ≥ 90% specificity to detect DOAC levels of ≥ 50 ng/mL.
Conclusion
We have demonstrated that TEG _6s R has significant correlation with DOAC blood concentrations and has potential for monitoring the DOAC's effect on hemostasis with reasonable sensitivity in the small sample analyzed. This novel technology is easy to use on a small volume of whole blood without requiring a specialized laboratory. Further study is warranted to correlate R with clinical outcomes.
Keywords: apixaban, dabigatran, direct oral anticoagulants, rivaroxaban, thromboelastography
Essentials.
The thromboelastograph TEG_6s is a new technology that applies resonance‐frequency for measurement of blood viscoelasticity and uses disposable multichannel microfluidic cartridges.
We studied the correlation of TEG_6s parameters to direct oral anticoagulant (DOAC) levels in 9 healthy volunteers after oral intake of DOACs.
TEG_6s reaction time demonstrated highly significant correlation with dabigatran, rivaroxaban, and apixaban blood concentrations.
The reaction time demonstrated high sensitivity and specificity to detect DOAC concentrations of ≥50 ng/mL.
1. INTRODUCTION
The direct oral anticoagulants (DOACs) are gaining ground among both patients and clinicians for their ease of use with fixed daily doses without the need for repeated testing of the coagulation. The ability to assess the hemostatic effect of the DOACs may be valuable in clinical situations such as bleeding or thrombosis, before urgent surgery, or reversal of anticoagulation.1, 2 At present, there are no assays approved for clinical use in the United States. DOAC‐specific assays are used for research purposes in the United States and for both clinical and research use in the other parts of the world.
Thromboelastography (TEG) is a point‐of‐care test for global evaluation of hemostasis. It measures the viscoelastic properties of clotting blood ex vivo. Changes in viscoelastic properties depend on the amount and interaction of platelets and fibrinogen and the fibrin formation rate.3 The previous generations of this technology, TEG_3000 and 5000, have been extensively used to monitor hemostasis in patients having orthotopic liver transplantation and open heart surgery, in addition to routine coagulation tests in management of peri‐ and postoperative bleeding, and to reduce infusion of blood products.4, 5
There are limited clinical data on the antithrombotic effect of DOACs. Most published data are from in vitro experiments testing the correlation of increasing drug concentrations and reaction time (R).6, 7, 8 The majority of these studies were performed on TEG_5000 using a kaolin assay. The TEG_5000 with kaolin assay had a number of limitations, including the need for experienced operators and manual mixing of citrated blood with kaolin or other reagents prior to analysis. The technology was inherently sensitive to vibration. Finally, the kaolin assay was less sensitive to the effect of apixaban as compared to dabigatran and rivaroxaban.9 The TEG_6s (Haemonetics Corporation, Braintree, MA) is a new, fully automated 4‐channel instrument. The system is less sensitive to vibration and employs multichannel microfluidic cartridges, eliminating manual mixing of any type prior to sample analysis. This new technology has been described in detail by Gurbel et al10. To date, there is only 1 study by Bliden et al11 on the application of this technology to assess the anticoagulant effects of the DOACs. The study used blood from 26 healthy volunteers to obtain a normal range and compared it to blood from patients on dabigatran, rivaroxaban, and apixaban at a single time point. The DOAC concentrations were not measured. The study was a technical validation study of the TEG_6s system and demonstrated ≥92% sensitivity and ≥95% specificity in identifying patients on DOAC therapy using TEG_6s R. The observations on other TEG parameters were not displayed.
The aim of this study was to evaluate the anticoagulant effect of dabigatran, rivaroxaban, and apixaban on TEG_6s parameters by performing repeated measurements over time and to establish correlations with the drug concentrations used for treating atrial fibrillation or venous thromboembolism. The study furthermore intended to better define effects of administration of these agents on the TEG parameters and to demonstrate capability of TEG_6s parameters for measurement of DOACs.
2. MATERIALS AND METHODS
2.1. Study design
The study was approved by the local institutional review board and was performed in accordance with the Helsinki II declaration. We enrolled men between 18 and 70 years of age who had normal laboratory screening test results, no abnormalities on physical examination, and normal vital signs. All participants provided written informed consent. The rationale for including only men was to avoid unpredictable interaction between the hormonal cycle and coagulation status among female subjects of reproductive age. Laboratory screening included renal and hepatic function, hepatitis B virus, hepatitis C virus, HIV serology, a complete blood cell count, prothrombin time, and activated partial thromboplastin time. Exclusion criteria were as follows: use of aspirin or nonsteroidal anti‐inflammatory agents, history of allergy to blood products, a personal or family history of coagulation disorders, participation within 30 days in another research study, and any medication use within 7 days of the start of the study. Each subject received a single dose of 150 mg of dabigatran, 20 mg of rivaroxaban, or 5 mg of apixaban at different time points with at least 2 weeks between tests to allow for a washout period. Dose selection was based on standard doses recommended for treating atrial fibrillation and venous thromboembolic disease. Blood samples were obtained at time 0 before intake of the anticoagulant, and then 1 and 3 hours later. Phlebotomy was performed without a tourniquet. Samples for coagulation analysis were collected in a standard blue‐top citrated tube (buffered sodium citrate 0.109 mol/L 3.2%, BD Vacutainer, Franklin Lakes, NJ). Samples were centrifuged at 7200 rpm/4400 g for 3 minutes.12 Platelet‐poor plasma was kept at −80°C before being used in batch analysis for DOAC concentrations.
2.2. Thrombelastography
The principles of the thromboelastographic measurement were previously described.3 The basic TEG parameters include the R and coagulation time (K) in minutes, the angle of alpha in degrees, and the maximum amplitude (MA) in mm. Because both K and angle of alpha express the speed of clot formation, we chose to display only the angle of alpha and not the K in the present paper and as previously discussed.13 We used the thrombelastograph TEG_6s. The details of this new technology have been described previously by Gurbel et al10. The TEG_6s applies resonance‐frequency to vibrate a very thin layer of blood dispersed over a miniature ring inside the cartridge system. The degree of fluctuation of this film of blood is correlated to its viscoelasticity and is displayed over time as the blood clots. This technology, with the use of the premixed disposable multichannel microfluidic cartridges, is in contrast to the previous models’ torsion wire with pins and cups. A citrated whole blood sample (0.6‐0.7 mL) is pipetted into the entry port of the cartridge, which directs the blood into 4 separate analysis channels each containing different dried reagents based on the type of cartridge used. The global hemostasis cartridge that is currently commercially available contains kaolin in channel 1, kaolin with heparinase in channel 2, tissue factor and kaolin in channel 3 (RapidTEG channel), and abciximab and kaolin in channel 4 (functional fibrinogen channel). The cartridge used for the purpose of this test has kaolin in channel 1 (the citrated kaolin [CK] channel), ecarin in channel 2 (the direct thrombin inhibitor [DTI] channel), factor Xa in channel 3 (the antifactor‐Xa [AFXa] channel) and abciximab with kaolin in channel 4 (the functional fibrinogen channel). This particular cartridge is currently not commercially available and is under investigation for use on patients who are anticoagulated with DOACs. All TEG analyses were performed within 30 minutes of the phlebotomy.
2.3. Measurement of DOAC concentrations
Plasma was separated from each sample. We used chromogenic anti‐FXa assays for rivaroxaban and apixaban (BIOPHEN DiXaI, HYPHEN BioMed, Neuville‐sur‐Oise, France) and chromogenic anti–factor IIa assay (BIOPHEN™ DTI, HYPHEN BioMed, Neuville‐sur‐Oise, France) for dabigatran. The analysis was performed on Siemens BCS XP R470570 (Siemens, Munich, Germany) and in accordance with the manufacturer's instructions by experienced laboratory technicians. Low calibrators were applied on samples with concentrations ≤100 ng/mL and standard calibrators for samples with concentration of >100 ng/mL for all 3 DOACs.
2.4. Statistical analysis
We determined the correlation of TEG parameters (R, alpha, and MA) with DOAC concentrations using Pearson's correlation coefficient and linear regression models.
Alteration of each TEG parameters over time was analyzed for each DOAC agent using repeated‐measures analysis of variance. All data were presented as mean and standard deviation (SD), unless otherwise indicated. P < 0.05 (2‐tailed) was considered statistically significant.
The clinically relevant DOAC concentration cutoffs based on current available literature are 30 ng/mL for urgent invasive procedures with high bleeding risk,14 50 ng/mL for antidote administration15 and 100 ng/mL for thrombolysis in stroke.16 The receiver operating characteristic curve was calculated for sensitivity and specificity of R for these concentrations of DOAC agents. We used Prism software version 8 (GraphPad Software Inc., La Jolla, CA) and MedCalc version 14.12.0 (MedCalc Software bvba, Ostend, Belgium) for data analysis.
3. RESULTS
Nine healthy male volunteers (mean age, 41 ± 12 SD; median, 39; and range, 25‐59 years old) enrolled in the study. In the present study, the highest concentrations for dabigatran were achieved at 3 hours, with mean concentration of 102 ng/mL ± 48 SD (median, 92.1 ng/mL; total range, 40.7‐196.9). For rivaroxaban the highest concentrations were achieved at 3 hours, with mean concentration of 205.2 ng/mL ± 73.7 SD (median, 205.5 ng/mL; total range, 94.2‐317.9). For apixaban the highest concentrations were achieved at 3 hours, with mean concentration of 105 ng/mL ± 19.7 SD (median, 104.2 ng/mL; total range, 73.8‐144.5) (Figure 1A).
Figure 1.
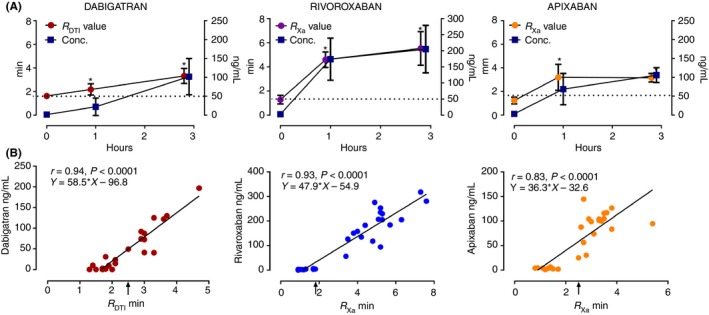
(A) The DOAC concentrations and the effect on TEG 6s R. *Indicates P < 0.05 compared to baseline level at time 0. The bars represent mean ± SD. (B) The scatter diagram of DOAC concentrations against R. The dotted line represents the 50 ng/mL threshold of the DOAC concentrations. The arrow represents the R value above which the subject is considered to have DOAC concentrations at or above 50 ng/mL for the 3 DOACs examined. RDTI indicates using the DTI channel; RX a indicates using the AFXa channel
The alteration of TEG_6s parameters R, alpha, and MA over 3 hours after intake of a single dose of DOAC is displayed in Table 1. R was significantly increased at 1 and 3 hours after administration (Table 1 and Figure 1A). The scatter diagrams of DOAC concentrations against R are displayed in Figure 1B. The R was strongly correlated with DOAC concentrations (r = 0.94 for dabigatran using DTI channel, r = 0.93 for rivaroxaban, r = 0.83 for apixaban using AFXa channel; P < 0.0001 for all). The deviation of alpha from baseline was minimal but statistically significant at 3 hours for both dabigatran using the DTI channel and apixaban using the AFXa channel. The MA had no significant deviation from baseline. There were no significant correlations between DOAC concentrations and K, alpha, or MA. To demonstrate the advantage of the DOAC‐specific cartridge as compared to standard CK assay, R from the CK channels (RCK) were displayed in Table 1. The RCK times for dabigatran and rivaroxaban were significantly increased as compared to baseline but not to the same extent as the DOAC‐specific channels. The RCK times for apixaban were not significantly increased at peak apixaban concentrations.
Table 1.
Alteration of TEG 6s parameters over time after oral intake of single dose of DOAC
| Agent | Parameter | Hours after intake | P‐value | Max change from baseline in % ± SD | ||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | ||||
| Dabigatrana | Conc. (ng/mL) | <5c | 22.3 ± 23.3 | 102 ± 47.9 | 0.0001 | |
| RDTI (min) | 1.6 ± 0.2 | 2.2 ± 0.5 | 3.3 ± 0.6 | <0.0001 | 106.3 ± 35 | |
| Alpha (degrees) | 72.4 ± 1.2 | 72.9 ± 1.2 | 73.8 ± 1.0 | 0.004 | 2.1 ± 1.2 | |
| MA (mm) | 63.0 ± 2.5 | 63.3 ± 2.3 | 63.5 ± 1.8 | ns | 0.9 ± 1.6 | |
| RCK (min) | 6.8 ± 1.0 | 8.8 ± 1.7 | 12.7 ± 2.4 | <0.0001 | 89 ± 37.6 | |
| Rivaroxabanb | Conc. | <5c | 174.1 ± 65.5 | 205.2 ± 73.7 | <0.0001 | |
| RXa | 1.3 ± 0.4 | 4.5 ± 0.7 | 5.6 ± 1.5 | <0.0001 | 354.5 ± 108 | |
| Alpha | 72.4 ± 1.0 | 74.0 ± 0.8 | 73.6 ± 1.8 | ns | 2.1 ± 2.7 | |
| MA | 61.2 ± 1.3 | 62.0 ± 1.7 | 61.7 ± 1.4 | ns | 1.1 ± 1.3 | |
| RCK | 6.5 ± 1.1 | 10.0 ± 1.2 | 11.6 ± 2.0 | <0.0001 | 83.7 ± 49.6 | |
| Apixabanb | Conc. | <5c | 68.8 ± 41.4 | 105.9 ± 19.7 | <0.0001 | |
| RXa | 1.2 ± 0.3 | 3.2 ± 1.1 | 3.2 ± 0.4 | <0.0001 | 209.3 ± 97 | |
| Alpha | 71.8 ± 1.8 | 73.2 ± 1.5 | 73.6 ± 1.0 | 0.004 | 2.7 ± 2.1 | |
| MA | 60.3 ± 2.2 | 60.3 ± 1.9 | 60.8 ± 1.9 | ns | 0.4 ± 3.0 | |
| RCK | 6.9 ± 0.8 | 8.4 ± 1.7 | 7.9 ± 1.0 | ns | 15.7 ± 18.4 | |
AFXa, antifactor‐Xa; DTI, direct thrombin inhibitor; MA, maximum amplitude; R, reaction time; SD, standard deviation.
Indicates using the DTI channel.
Indicates using the AFXa channel. The values indicated mean ± SD.
Mean levels were below the lower limits of detection defined by the manufacturer.
The total analysis time to obtain all TEG parameters including R, alpha, and MA once the sample had entered the TEG device was 30 to 50 minutes. If the focus was to obtain the isolated R value, the analysis time was as indicated in Table 1 within 3 to 7 minutes of the analysis start.
The sensitivity and specificity of the R to detect dabigatran concentrations ≥30, ≥50, and ≥100 ng/mL using DTI channel, rivaroxaban and apixaban concentrations ≥30, ≥50, and ≥100 ng/mL using the AFXa channel are displayed in Table 2.
Table 2.
Sensitivity and specificity of R to detect DOAC concentrations above thresholds mentioned
| DOAC | Threshold (ng/mL) | R (min) | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| Dabigatrana | 30 | >2.1 | 92 (61.5‐99.8) | 100 (78.2‐100) | 0.96 |
| 50 | >2.5 | 100 (63.1‐100) | 90 (66.9‐98.7) | 0.95 | |
| 100 | >3 | 100 (39.8‐100) | 96 (78.1‐99.9) | 0.99 | |
| Rivaroxabanb | 30 | c | |||
| 50 | >1.8 | 100 (81.5‐100) | 100 (66.4‐100) | 1.00 | |
| 100 | >3.4 | 100 (79.4‐100) | 91 (58.7‐99.8) | 0.95 | |
| Apixabanb | 30 | >2.5 | 100 (79.4‐100) | 100 (71.5‐100) | 1.00 |
| 50 | >2.5 | 100 (78.2‐100) | 92 (61.5‐99.8) | 0.98 | |
| 100 | >2.6 | 100 (63.1‐100) | 63 (38.4‐83.7) | 0.84 |
AFXa, antifactor‐Xa; AUC, area under the curve; DOAC, direct oral anticoagulant; DTI, direct thrombin inhibitor; R, reaction time.
The values in parentheses indicate 95% confidence interval.
Indicates using the DTI channel.
Indicates using the AFXa channel.
Due to no acquired concentrations between 30 and 50 ng/mL, no calculations could be obtained.
4. DISCUSSION
To our knowledge, this is the first study to evaluate the utility of TEG_6s in assessment of the anticoagulant effect of the most commonly used DOACs, dabigatran, rivaroxaban, and apixaban, through serial measurements over time. The TEG parameters were correlated with DOAC concentrations using cartridges specifically designed for this purpose containing ecarin and factor Xa in order to increase its sensitivity to DTI and AFXa inhibitors, respectively. We have demonstrated that R is the most sensitive TEG parameter to alterations in hemostasis by the DOACs. R demonstrated significant correlation with DOAC concentrations. We have furthermore demonstrated that R has useful sensitivity and specificity to detect low DOAC concentrations in this small sample of healthy volunteers. This function is potentially useful beyond situations mentioned in the Introduction section such as presurgical patients on DOAC with uncertainty about their hemostatic function, or prior to direct‐current cardioversion or radiofrequency ablation procedures in patients with atrial fibrillation with uncertainty about the adequacy of anticoagulation. As the “therapeutic ranges” for these agents are not yet defined, increased R above a certain threshold using the DTI channel for patients on dabigatran and AFXa channel for patients on AFXa inhibitors may provide useful information on the concentrations of these agents being below or within the range observed in the major clinical trials with the DOACs.
The TEG_6s applies resonance‐frequency viscoelasticity measurements and premixed disposable multichannel microfluidic cartridges to bypass the limitations of its prior models. This point‐of‐care device can provide the measurements on whole blood and eliminates the need for centrifugation. The analyses in the present study were performed within 30 minutes of the phlebotomy, as the subjects examined were not acutely ill. In practice, when dealing with acutely ill patients, the analysis can be performed immediately after phlebotomy. Once the analysis was started, the R times were resulted within 3 to 7 minutes as indicated in Table 1. The turnaround times, or time from receiving the blood sample in the lab to first result published, were not calculated, as the subjects were already present in the laboratory and the workflow was not representative of the clinical situation. In acute stroke patients, Seiffge et al16 were able to measure DOAC concentrations with median turnaround time of 35 minutes (29‐75 minutes) using the same chromogenic assays that were used in the present study. To achieve these numbers, their laboratory had these assays available 24 hours a day. Thrombin generation assay in its current format takes up to 2 hours for analysis of DOAC specimens in specialized laboratories.17 The TEG_6s analysis time can therefore be considered competitive in regards to the time efficiency as compared to the other methods currently under investigation.
The baseline values for the reaction time in the present study (1.6 ± 0.2 minutes for the DTI channel, 1.3 ± 0.3 minutes for the AFXa channel) are compatible with the preliminary reference range established by Bliden et al11 (1.5 ± 0.2 minutes for the DTI channel and 1.3 ± 0.3 minutes for the AFXa channel) suggestive of consistency in the measurements. At this point, no final reference range has been established by the manufacturer but is in process and will be published soon. The other TEG parameters, alpha, K, and MA, were not substantially altered irrespective of the type of TEG channel applied.8 This observation is in contrast to the effect of the heparins and warfarin on TEG parameters. The latter agents not only prolong R but also reduce alpha and MA.2, 13 This observation reflects the effect of the DOACs on primarily prolonging the whole blood clotting time as opposed to speed of clot formation or the interaction between platelets and fibrinogen.
Limitations of this study include the small number of younger healthy volunteers, rather than patients with atrial fibrillation or thromboembolic disease. The study was furthermore limited due to use of only 3 time points over 3 hours rather than more time points over 12 to 24 hours. For the TEG_6s to demonstrate sensitivity and specificity for the presence of DOACs in blood at very low concentrations, future studies need to emphasize measurements at true trough levels. We evaluated only a single dose of the anticoagulants rather than repeated doses at steady‐state drug concentrations. Peak concentrations achieved in the present study were within the range of concentrations documented in clinical studies with patients receiving DOACs for atrial fibrillation or thromboembolism, indicating that the single‐dose regimen in the present study was sufficient to obtain adequate blood DOAC concentrations.18, 19 The thresholds for R to detect the lower range of DOAC concentrations, sensitivities, and specificities displayed in the present study need to be viewed with caution in this feasibility study on 9 healthy volunteers and were primarily to demonstrate proof of concept. It is expected that these thresholds may change with a larger pool of healthy subject and patients on DOACs in larger studies under progress.
In conclusion, TEG_6s R has the ability to measure the DOACs’ effect on hemostasis with significant correlation with DOAC concentrations among the agents examined in this study on healthy volunteers. This novel technology is easy to use with a small amount of whole blood and without the need for a specialized laboratory or expertise. Larger clinical studies on patients receiving therapeutic doses of DOACs are warranted to correlate R and adverse clinical outcome in order for this test to be clinically useful.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
AUTHOR CONTRIBUTIONS
RA was responsible for the design of the study, part of the laboratory analysis, statistical analysis, and writing the manuscript. MA was responsible for the data collection and interpretation of the data and revising the manuscript. JDN was responsible for design of the study and revising the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Rebecca Starr for assistance with the manuscript language and Dr. Gregg Galloway for his assistance with the laboratory analysis.
Artang R, Anderson M, Nielsen JD. Fully automated thromboelastograph TEG 6s to measure anticoagulant effects of direct oral anticoagulants in healthy male volunteers. Res Pract Thromb Haemost. 2019;3:391–396. 10.1002/rth2.12206
REFERENCES
- 1. Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring Oral Direct Inhibitors (ODIs) of thrombin and factor Xa: A recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2013;11:756–60. [DOI] [PubMed] [Google Scholar]
- 2. Douxfils J, Ageno W, Samama CM, Lessire S, Ten Cate H, Verhamme P, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209–19. [DOI] [PubMed] [Google Scholar]
- 3. Chandler WL. The thromboelastography and the thromboelastograph technique. Semin Thromb Hemost. 1995;21(suppl 4):1–6. [PubMed] [Google Scholar]
- 4. Zuckerman L, Cohen E, Vagher JP, Woodward E, Caprini JA. Comparison of thrombelastography with common coagulation tests. Thromb Haemost. 1981;46:752–396. [PubMed] [Google Scholar]
- 5. Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW Jr, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–96. [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Y, Wu W, Wang L, Chintala M, Plump AS, Ogletree ML, et al. Differential profiles of thrombin inhibitors (heparin, hirudin, bivalirudin, and dabigatran) in the thrombin generation assay and thromboelastography in vitro. Blood Coagul Fibrinolysis. 2013;24:332–8. [DOI] [PubMed] [Google Scholar]
- 7. Dinkelaar J, Patiwael S, Harenberg J, Leyte A, Brinkman HJ. Global coagulation tests: their applicability for measuring direct factor Xa‐ and thrombin inhibition and reversal of anticoagulation by prothrombin complex concentrate. Clin Chem Lab Med. 2014;52:1615–23. [DOI] [PubMed] [Google Scholar]
- 8. Adelmann D, Wiegele M, Wohlgemuth RK, Koch S, Frantal S, Quehenberger P, et al. Measuring the activity of apixaban and rivaroxaban with rotational thrombelastometry. Thromb Res. 2014;134:918–23. [DOI] [PubMed] [Google Scholar]
- 9. Artang R, Galloway G, Dalsgaard Nielsen J. Monitoring novel anticoagulants dabigatran, rivroxaban and apixaban using thrombelastography. J Am Coll Cardiol. 2014;63(12 Supplement):A439. [Google Scholar]
- 10. Gurbel PA, Bliden KP, Tantry US, Monroe AL, Muresan AA, Brunner NE, et al. First report of the point‐of‐care TEG: a technical validation study of the TEG‐6S system. Platelets. 2016;27:642–9. [DOI] [PubMed] [Google Scholar]
- 11. Bliden KP, Chaudhary R, Mohammed N, Muresan AA, Lopez‐Espina CG, Cohen E, et al. Determination of non‐vitamin K oral anticoagulant (NOAC) effects using a new‐generation thrombelastography TEG 6s system. J Thromb Thrombolysis. 2017;43:437–45. [DOI] [PubMed] [Google Scholar]
- 12. Dincq AS, Lessire S, Pirard G, Siriez R, Guldenpfennig M, Baudar J, et al. Reduction of the turn‐around time for the measurement of rivaroxaban and apixaban: assessment of the performance of a rapid centrifugation method. Int J Lab Hematol. 2018;40:e105–8. [DOI] [PubMed] [Google Scholar]
- 13. Artang R, Frandsen NJ, Nielsen JD. Application of basic and composite thrombelastography parameters in monitoring of the antithrombotic effect of the low molecular weight heparin dalteparin: an in vivo study. Thromb J. 2009;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godier A, Dincq AS, Martin AC, Radu A, Leblanc I, Antona M, et al. Predictors of pre‐procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. 2017;38:2431–9. [DOI] [PubMed] [Google Scholar]
- 15. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; The Subcommittee on Control of Anticoagulation . When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623–7. [DOI] [PubMed] [Google Scholar]
- 16. Seiffge DJ, Kägi G, Michel P, Fischer U, Béjot Y, Wegener S, et al. Rivaroxaban plasma levels in acute ischemic stroke and intracerebral hemorrhage. Ann Neurol. 2018;83:451–9. [DOI] [PubMed] [Google Scholar]
- 17. Artang R, Anderson M, Riley P, Nielsen JD. Assessment of the effect of direct oral anticoagulants dabigatran, rivaroxaban, and apixaban in healthy male volunteers using a thrombin generation assay. Res Pract Thromb Haemost. 2017;1:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al.; RE‐LY Investigators . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial. J Am Coll Cardiol. 2014;63:321–8. [DOI] [PubMed] [Google Scholar]
- 19. Testa S, Tripodi A, Legnani C, Pengo V, Abbate R, Dellanoce C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


