Abstract
Inflammation and endoplasmic reticulum (ER) stress are common denominators for vision-threatening diseases such as diabetic retinopathy and age-related macular degeneration. Based on our previous study, supplementation with muscadine grape polyphenols (MGPs) alleviated systemic insulin resistance and proinflammatory responses. In this study, we hypothesized that MGPs would also be effective in attenuating ocular inflammation and ER stress. We tested this hypothesis using the human retinal pigmented epithelium (ARPE-19) cells and C57BL/6 mice. In ARPE-19 cells, tumor necrosis factor-α–induced proinflammatory gene expression of interleukin (IL)-1β, IL-6, and monocyte chemotactic protein-1 was decreased by 35.0%, 68.8%, and 62.5%, respectively, with MGP pretreatment, which was primarily due to the diminished mitogen-activated protein kinase activation and subsequent reduction of nuclear factor κ-B activation. Consistently, acute ocular inflammation and leukocyte infiltration were almost completely dampened (>95%) by MGP supplementation (100–200 mg/kg body weight) in C57BL/6 mice. Moreover, MGPs reduced inflammation-mediated loss of tight junctions and retinal permeability. To further investigate the protective roles of MGPs against ER stress, ARPE-19 cells were stimulated with thapsigargin. Pretreatment with MGPs significantly decreased the following: 1) ER stress-mediated vascular endothelial growth factor secretion (3.47 ± 0.06 vs. 1.58 ± 0.02 μg/L, P< 0.0001), 2) unfolded protein response, and 3) early apoptotic cell death (64.4 ± 6.85 vs. 33.7 ± 4.32%, P= 0.0003). Collectively, we have demonstrated that MGP is effective in attenuating ocular inflammation and ER stress. Our work also suggests that MGP may provide a novel dietary strategy to prevent vision-threatening retinal diseases.
Introduction
Indigenous to the southeastern region of the United States, muscadine grapes (Vitis rotundifolia) are 1 of the key agricultural products that have been widely cultivated and consumed in the United States. Muscadine grapes contain an array of health-promoting phytochemicals that improve symptoms of chronic diseases, such as insulin resistance (1, 2) and inflammation (3, 4), and even cancer (5, 6). Recently, our group has also reported that supplementation with muscadine grape polyphenols (MGPs) reduced high-fat diet–mediated metabolic complications and systemic markers of inflammation in C57BL/6 mice (7). A growing body of evidence suggests that MGPs exert protective roles against chronic metabolic diseases. However, few studies have been conducted to determine if dietary supplementation with MGPs could provide beneficial effects for vision-related retinal diseases such as diabetic retinopathy (DR) and age-related macular degeneration (AMD).
The pathologic development of retinal diseases and vision impairment is associated with compounding risk factors including oxidative stress (8–11) and inflammation (12, 13). Recently, endoplasmic reticulum (ER) stress has been recognized as another key risk factor that exacerbates pathogenic progression of DR (14–16) and AMD (17). Because of the asymptomatic and irreversible nature of these diseases, and their poor prognosis, there are limitations in drug therapy in treating DR (18) and AMD (19). Therefore, a nutritional intervention approach could be a reasonable and preferable strategy to prevent or delay the progression of these retinal diseases. Unexpectedly, the known antioxidant vitamins (i.e., vitamins C and E) have not been successful in preventing DR progression (20, 21). Dietary polyphenols are alternative candidates because many polyphenolic compounds possess anti-inflammatory and antioxidative properties that may provide defensive mechanisms through retinal microvasculature. Several polyphenols such as resveratrol (22–24), curcumin (25, 26), and ellagic acid (EA) (27, 28) have been investigated as candidates to test their effectiveness in retinal diseases. However, their efficacy has not been fully validated yet. This is possibly due to the fact that DR is caused by complex etiologies, and thus a single phytochemical may not be sufficient to block multiple risk factors. This led us to hypothesize that nutritional intervention through combinatory phytochemicals could be advantageous in preventing vision-threatening retinal diseases by blocking multiple risk factors simultaneously.
In this study, we investigated the effects of MGPs on the following: 1) inflammation in human retinal pigmented epithelium (ARPE-19) cells and C57BL/6 mice, and 2) ER stress in ARPE-19 cells. Here, we demonstrated that MGPs proficiently attenuate retinal inflammation and ER stress by interrupting the signal transduction to downstream targets.
Materials and Methods
Chemical reagents.
DMEM/F12, HBSS, sodium pyruvate and radioimmune precipitation assay buffer, NE-PER nuclear and cytoplasmic extraction reagents, and a human vascular endothelial growth factor (VEGF)–α ELISA kit were obtained from Thermo Scientific. FBS was purchased from Mediatech. The TriZol reagent and Fluo-4 NW calcium assay kit were obtained from Invitrogen. LPS (fromEscherichia coli055:B5), thapsigargin, ellagic acid (EA), kaempferol, myricetin, and quercetin were purchased from Sigma-Aldrich. Human recombinant TNF -α (210-TA-020) was purchased from R&D Systems.
Muscadine grape phytochemicals.
Previously, we have described the preparation of MGPs (the Nobel muscadine grapes were purchased from a local vineyard) (7). The composition of MGPs is also found in Table 2 in Gourineni et al. (7). MGP was further fractionated into an anthocyanin (Acy) fraction and a non-anthocyanin (NAcy) fraction using a published method (29). The Acy fraction contained anthocyanin 3,5-diflucosides. The NAcy fraction contained EA, kaempferol, myricetin, and quercetin.
Cell culture and MGP treatment.
ARPE-19 (American Type Culture Collection CRL-2302) cells were cultured in DMEM/F12 containing 10% FBS in 5% CO2? at 37°C. The stock solutions of MGP and individual phytochemicals were prepared in DMSO, kept at −20°C, and freshly diluted right before treatment.
Endotoxin-induced ocular inflammation.
C57BL/6 male mice (8 wk old) were purchased from the Jackson Laboratory and housed at the University of Florida Animal Care Services facilities with a 12-h light/12-h dark cycle. Mice were fed a standard rodent diet (7012 Teklad LM-485; 19.1% protein, 5.8% fat, 75.1% nitrogen-free extract; Harlan) without restriction to water. All protocols and procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Mice were randomly assigned into 4 groups based on MGP supplementation and LPS treatment: 1) vehicle control (5% DMSO) with ocular saline injection (n= 3); 2) vehicle control with ocular LPS injection (n= 5); 3) 100-mg/kg body weight MGP supplementation with LPS injection (n= 6); and 4) 200-mg/kg body weight MGP supplementation with LPS injection (n= 6). The mice were gavaged with either vehicle or MGPs for 7 d. Both eyes were injected with either saline or 25-ng LPS/eye, based on treatment group. The mice were killed by intraperitoneal injection of ketamine (75 mg/kg) and xylazine (5 mg/kg) followed by cervical dislocation 24 h after LPS (or saline) injection, and both eyes were enucleated immediately. For each mouse, one eye was processed for qPCR analysis and the other eye was processed for histologic examination.
Histology and analysis of infiltrated leukocytes into eyes.
The enucleated eyes were fixed in 4% paraformaldehyde overnight at 4°C. After cutting 500 μm off each eye, 8 serial sections (5-μm each) were prepared using 80-μm intervals, and then stained with hematoxylin and eosin (H&E). Using light microscopy (Zeiss Axiovert 200 equipped with AxioCam MRC5), infiltrated leukocytes were counted based on their distinguished appearance in H&E-stained slides.
Real-time qPCR.
Total RNA was isolated from the cell cultures or eyes using TriZol reagent. Reverse transcription was performed using 2-μg mRNA per sample, per the manufacturer's protocol (iScript cDNA synthesis kit; Bio-Rad). Each 20-μL PCR reaction contained cDNA template, SYBR green PCR master mix (Bio-Rad), and 1-μmol/L gene-specific primer. Gene expression was determined by real-time qPCR (CFX96; Bio-Rad), and relative gene expression was normalized by 36B4. See primer sequences in Supplemental Table 1.
Western blot analysis.
Total protein lysates from ARPE-19 cells were obtained using ice-cold radioimmune precipitation assay buffer with protease inhibitors and phosphatase inhibitors (2-mmol/L Na3?VO4?, 20-mmol/L β-glycerophosphate, and 10-mmol/L NaF). To determine NF-κB activation, nuclear and cytosolic cellular fractions were prepared using a commercial kit. The proteins were separated using 12% or 15% SDS-PAGE, transferred to polyvinylidene difluoride membranes using a semi-dry transfer unit (Hoefer), and incubated with relevant primary antibodies. Using Western Lightning Plus ECL (Perkin Elmer), chemiluminescence was detected from solution via a FluorChem E (Cell Biosciences) imaging system. Polyclonal antibodies targeted to phosphorylated c-Jun N-terminal kinase (p-JNK) (#2676), phosphorylated p38 MAPK (p-p38) (#4511), phosphorylated-extracellular-signal-regulated kinases (#4370), total extracellular-signal-regulated kinases (#4695), NF-κB subunit p65 (p65) (#8242), NF-κB inhibitor α (IκBα) (#4812), Lamin A/C (#4777), phosphorylated-eukaryotic translation initiation factor 2α (p-eIF2α) (#9721), activating transcription factor 4 (ATF4) (#11815), CCAAT/enhancer-binding protein homologous protein (CHOP) (# 2895), and binding of immunoglobulin protein (BiP) (#3183) were purchased from Cell Signaling Technology. Mouse monoclonal antibodies targeted to GAPDH (SC-137179) and β-Actin (A2228) were purchased from Santa Cruz Biotechnology and Sigma-Aldrich, respectively.
Measurement of transepithelial electrical resistance.
Approximately 0.6 × 105cells/well of ARPE-19 cells were seeded in the apical compartment of a 6-well transwell plate (pore size: 0.4 μm) and then differentiated for 21 d to develop tight junctions. To induce inflammation, ARPE-19 cells were stimulated with either by TNF -α (0.5 μg/L) alone or TNF -α plus MGPs. The transepithelial electrical resistance of the cultures on the transwell plates was measured with a volt-ohm meter (Millicell ERS-2; EMD Millipore). Final resistance-area products (Ωcm2) were obtained by multiplying the resistance with the surface area of the apical membrane insert.
VEGFα secretion.
For VEGFα protein determination, VEGFα secretion into culture medium was quantified using a commercial ELISA kit following the manufacturer's instructions.
Intracellular calcium release.
Intracellular calcium ([Ca2+]i?) was measured using a Fluo-4 NW calcium assay kit (F36206) according to the manufacturer's protocol. Briefly, cultures of ARPE-19 cells in 96-well plates were treated with either vehicle (DMSO) or MGPs (100 μg/mL) for 12 h. Then, cultures were preloaded with cell-permeable calcium indicator (Flow-4 AM) for 30 min before injection of either 50 μL of vehicle (HBSS) or thapsigargin (final concentration: 5 μmol/L). Fluorescence intensity (Ex = 485 nm, Em = 528 nm) was monitored over time using Synergy H1 (BioTek). [Ca2+]i? concentrations were monitored by a preloaded Flow-4 NW calcium indicator using a fluorometer. The ratio of calcium-specific fluorescence (F/F0?) was plotted over time.
Flow cytometric analysis of apoptosis.
Retinal apoptosis was assessed using an Annexin V-FITC apoptosis detection kit (Bender Med Systems) following the manufacturer's instructions and measured using the Accuri C6 flow cytometer (BD). ARPE-19 cells were pretreated with either vehicle (DMSO) or thapsigargin alone, or thapsigargin plus 100 μg/mL of MGPs before induction of apoptosis by adding 5 μmol/L of thapsigargin for 72 h. Propidium iodide was used for detecting necrotic cells, and Annexin V was used for detecting apoptotic cells.
Statistical analysis.
Results are presented as means ± SEMs. The data were analyzed statistically using 1-factor ANOVA followed by Tukey's post hoc analysis. For the analysis of percentage of infiltrated cells (unequal sample sizes), a nonparametric 1-factor ANOVA with Kruskal-Wallis test was conducted (do not assume a normal distribution of residual, but assume an identically shaped and scaled distribution for each group). For the measurement TEER value over time, a 1-factor ANOVA with repeated measures was conducted. For [Ca2+]i? determination, 2-factor ANOVA with repeated measures was used. All statistical analyses were performed with GraphPad Prism 5 (version 5.04).
Results
MGPs reduced NF-κB activation in ARPE-19 cells.
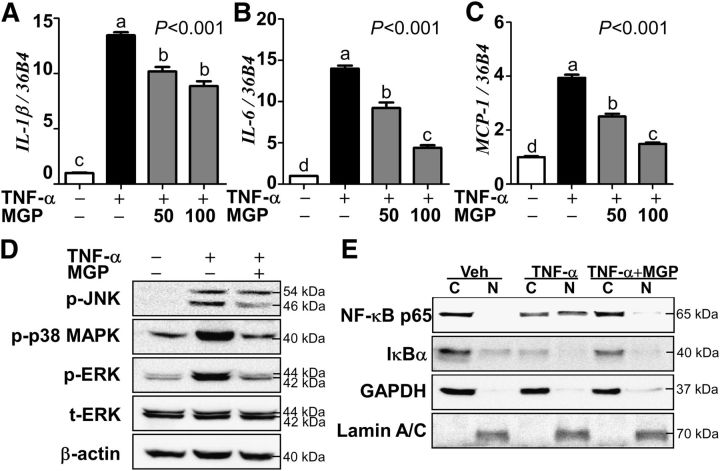
We have previously reported that MGP supplementation reduces systemic inflammation in vivo (7). However, it is unknown whether MGPs exert an anti-inflammatory role in the eyes. To test the hypothesis that MGPs will attenuate retinal inflammation, ARPE-19 cells were stimulated with TNF -α (0.5 μg/L) in the presence or absence of MGPs. TNF -α treatment markedly increased the proinflammatory cytokine gene expressions of interleukin(IL)-1β, IL-6, and monocyte chemo-attractive protein 1 (MCP-1). As we expected, pretreatment with MGPs (50–100 μg/mL) significantly reduced proinflammatory gene expression (Fig. 1A–C).
FIGURE 1.
TNF -α–induced proinflammatory gene expression, MAPK, and NF-κB activation in MGP-treated ARPE-19 cells. The panels show proinflammatory gene expression of IL-1β(A), IL-6(B), and MCP-1(C). Panel (D) shows phosphorylation of MAPK JNK, p38, and ERK. Panel (E) shows nuclear translocation of NF-κB p65 and cytosolic degradation of IκBα. Values are means ± SEMs, n= 9 (A–C). Means without a common letter differ, P< 0.05. In panel (D), t-ERK and β-actin were used as references. In panel (E), GAPDH and Lamin A/C were used to validate cytosolic (C) and nuclear (N) fractionation. + and − indicate the presence or absence of TNF -α and/or MGP treatment. ARPE-19, human retinal pigmented epithelium; ERK, extracellular-signal-regulated kinase; IκBα, nuclear factor κ-B inhibitor α IL-1β, interleukin-1β IL-6, interleukin-6; JNK, Jun N-terminal kinase; MCP-1,monocyte chemotactic protein-1; MGP, muscadine grape polyphenol; NF-κB p65, NF-κB p65 subunit; p-ERK, phosphorylated-extracellular-signal-regulated kinase; p-JNK, c-Jun N-terminal kinase; p-p38 MAPK, phosphorylated-p38 MAPK; t-ERK, total extracellular-signal-regulated kinase; Veh, vehicle-injected eyes without supplementation.
NF-κB activation plays a pivotal role in retinal dysfunction (30). Next, we examined whether MGPs reduce MAPK activation, the upstream targets of NF-κB activation. Upon TNF -α stimulation, phosphorylation levels of the 3 MAPKs (i.e., p-JNK, p-p38, and phosphorylated-extracellular-signal-regulated kinase 1/2) were significantly increased compared with the vehicle control, which was significantly blocked by pretreatment with MGPs (Fig. 1D). To determine the extent to which MGPs reduce NF-κB activation, ARPE-19 cells were pre-exposed to MGPs before TNF -α stimulation, and then total cell lysates were separated into nuclear and cytosolic fractions. In response to TNF -α stimulation, inhibitory IκBα protein is rapidly degraded and NF-κB p65 is translocated into the nucleus in ARPE-19 cells. In contrast, the majority of IκBα and NF-κB p65 proteins remained in the cytosol with the pretreatment of MGPs, which was comparable with the vehicle control (Fig. 1E). These data indicate that MGP treatment could effectively attenuate MAPK/NF-κB axis activation resulting in a decreased expression of its proinflammatory target genes in ARPE-19 cells.
MGPs attenuated acute ocular inflammation in vivo.
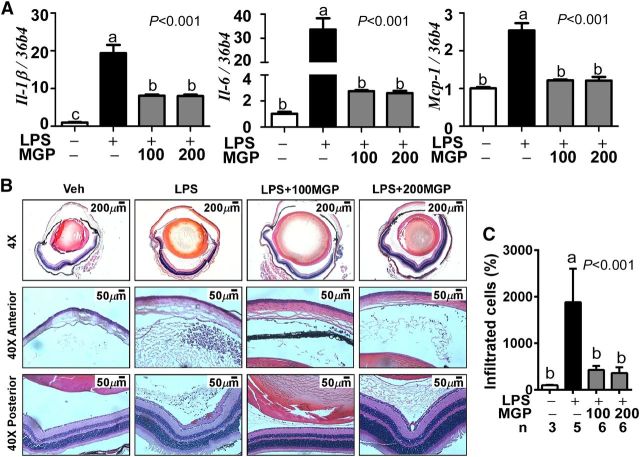
Based on its anti-inflammatory properties on ARPE-19 cells (Fig. 1), we reasoned that MGP supplementation should be effective in reducing ocular inflammation in vivo. Similarly, although acute ocular inflammation was induced in mice by intravitreal injection of LPS, proinflammatory target genes of Il-1β, Il-6, and Mcp-1were remarkably reduced in the eyes of MGP-fed mice compared with control mice (Fig. 2A). The recruitment of immune cells into inflamed regions is a hallmark of ocular inflammation (31, 32). The number of infiltrated leukocytes into eyes was quantified from the H&E-stained serial sections. LPS injection caused a massive immune cell infiltration, both in the anterior chamber and posterior chamber compared with vehicle control (Fig. 2B). In accordance with the reduced chemokine Mcp-1expression (Fig. 2A), leukocyte infiltration was significantly decreased with MGP supplementation in both the anterior and posterior chamber (Fig. 2B, C). Notably, there were no additional advantages in supplementation of 200-mg/kg versus 100-mg/kg body weight of MGPs in terms of reducing inflammation or immune cell recruitment.
FIGURE 2.
Ocular inflammation and leukocyte infiltration in control and MGP-supplemented C57BL/6 mice. Panel (A) shows proinflammatory gene expression of Il-1β, Il-6, and Mcp-1from enucleated eyeballs. Leukocyte infiltration was visualized by hematoxylin and eosin staining (B). Representative images of the entire eyeball section (top), anterior chamber (middle), and posterior chamber (bottom) are shown. Panel (C) shows the relative leukocyte recruitment (%) into the inflamed eyeball. Values are means ± SEMs (A, C). Values without a common letter differ, P< 0.05; n= 4 for panel (A), and the number of eyes for panel (C) is denoted under the figure. + and − indicate the presence or absence of LPS and/or MGP treatment. IL-1β, interleukin-1β IL-10, interleukin-10; LPS, LPS-injected eyes without supplementation; LPS+100MGP, LPS-injected eyes after 100-mg/kg body weight muscadine grape polyphenol supplementation; LPS+200MGP, LPS-injected eyes after 200-mg/kg body weight muscadine grape polyphenol supplementation; Mcp-1,monocyte chemotactic protein-1; MGP, muscadine grape polyphenol; Veh, vehicle-injected eyes without supplementation.
MGPs protected inflammation-induced retinal permeability.
Retinal inflammation and its accompanied loss of tight junctions are key culprits for retinal dysfunction (33–36). To determine whether MGPs play a protective role in retinal integrity, we examined the effects of MGPs on tight-junction expression. The LPS treatment reduced ∼50% mRNA expression of occludin (Ocln), a tight-junction protein, in mice. In contrast, MGP supplementation prior to LPS treatment prevented the loss of OclnmRNA (Fig. 3A). To evaluate the protective role of MGPs on retinal permeability, we mimicked an epithelium monolayer in ARPE-19 cells by employing a transwell system. Consistent with the in vivo experiment, TNF -α treatment decreased ∼40% of OCLNgene expression in ARPE-19 monolayers. However, MGP pretreatment completely blocked the TNF -α–mediated loss of OCLNgene expression (Fig. 3B). In addition, the transepithelial electrical resistance value was significantly higher in MGP-pretreated cells than cells with TNF -α simulation alone (Fig. 3C). Taken together, these data suggest that MGP treatment would be effective in maintaining the integrity of the retinal monolayer by attenuating the inflammation-mediated loss of tight junctions.
FIGURE 3.
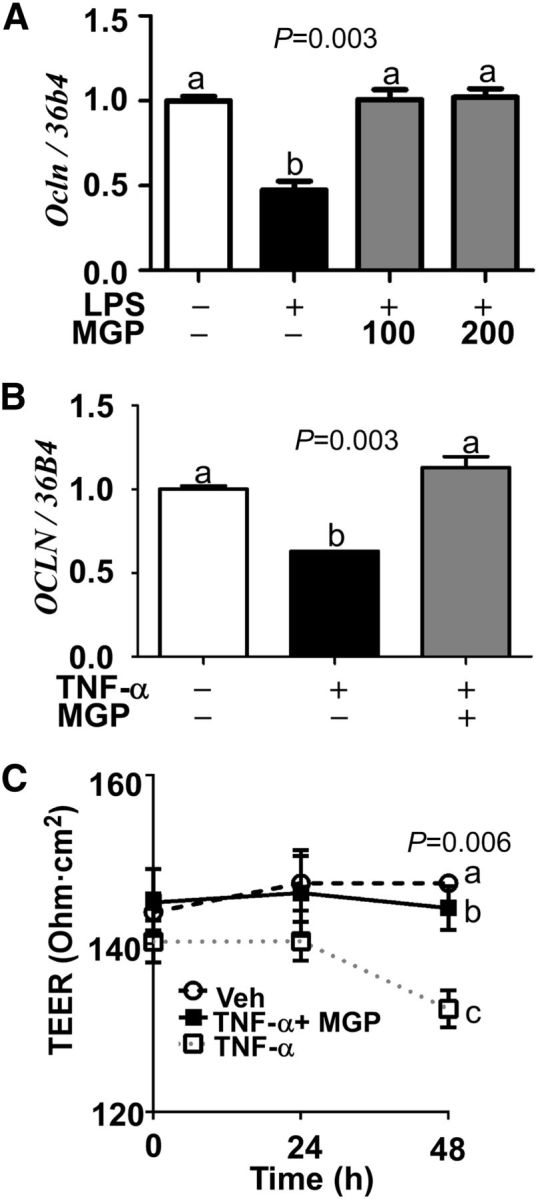
Ocular tight junction expression and retinal permeability in MGP-supplemented C57BL/6 mice and ARPE-19 cells. Panel (A) shows relative Oclnexpression in LPS-injected C57BL/6 mice. Panel (B) shows relative OCLNexpression and panel (C) shows changes in TEER value analyzed from the ARPE-19 cells grown in transwell. + and − indicate the presence or absence of prior treatment, either TNF -α or LPS, in conjunction with given MGP concentration (100- or 200-mg/kg body weight) (A) or 100-mg/kg body weight MGP (B, C). Values are means ± SEMs; n= 6 eyes from different mice (A), and n= 3 (B, C). Means without a common letter differ, P< 0.05. ARPE-19, human retinal pigmented epithelium; MGP, muscadine grape polyphenol; OCLN, occludin; TEER, transepithelial electrical resistance; Veh, vehicle-injected eyes without supplementation.
MGPs decreased ER stress in ARPE-19 cells.
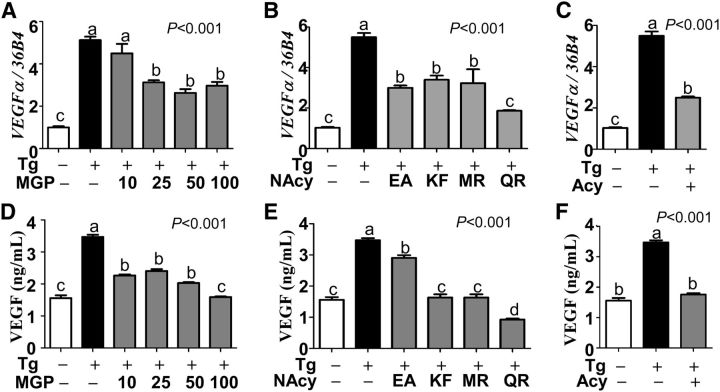
A growing body of evidence from animal and clinical investigations suggests that ER stress in eyes is involved in various pathologic conditions such as retinopathy (14–16), AMD (17), and abnormal angiogenesis (37, 38). Given the potent anti-inflammatory properties of MGPs (Figs. 1–3), we raised the question whether MGPs would be effective in attenuating ER stress and VEGFα secretion. To address this question, ER stress was induced in ARPE-19 cells by stimulating with thapsigargin (5 μmol/L) in the presence or absence of MGPs. Although there was approximately a 5-fold increase of VEGFαgene expression in response to thapsigargin stimulation, it was significantly reduced in the presence of 25 to 100 μg/mL of MGP treatment (Fig. 4A). In an attempt to identify whether any single compound in MGPs was responsible for antagonizing VEGFαexpression, ARPE-19 cells were treated with either NAcy constituents (i.e., EA, kaempferol, myricetin, and quercetin) or total Acy fraction. Intriguingly, 10 μmol/L of EA and 3 other flavonols of kaempferol, myricetin, and quercetin were similarly effective in reducing VEGFαexpression (Fig. 4B). Additionally surprising, 25 μg/mL of the Acy fraction was effective in reducing VEGFαgene expression (Fig. 4C). Consistent with VEGFαgene expression, MGPs, NAcy constituents (i.e., EA, kaempferol, myricetin, and quercetin), and Acy fraction effectively blocked thapsigargin-inducible VEGFα secretion into the medium (Fig. 4D–F).
FIGURE 4.
ER stress-induced VEGFα gene expression and protein secretion in MGP-treated ARPE-19 cells. The relative gene expression of VEGFαby qPCR (A-C) and VEGFα protein secretion into medium by ELISA (D-F) are shown. ARPE-19 cells were pretreated with 10 to 100 μg/mL of total MGP (A, D), 10 μg/mL of NAcy components of MGP (B, E), or 25 μg/mL Acy fraction of MGP (C, F) with (+) or without (−) the addition of ER stressor Tg. Values are means ± SEMs, n= 9. Means without a common letter differ, P< 0.05. Acy, anthocyanin; ARPE-19, human retinal pigmented epithelium; EA, ellagic acid; ER, endoplasmic reticulum; KF, kaempferol; MGP, muscadine grape polyphenol; MR, myricetin; NAcy, non-anthcyanin; QR, quercetin; Tg, thapsigargin; VEGF, vascular endothelial growth factor; VEGFα, vascular endothelial growth factor α.
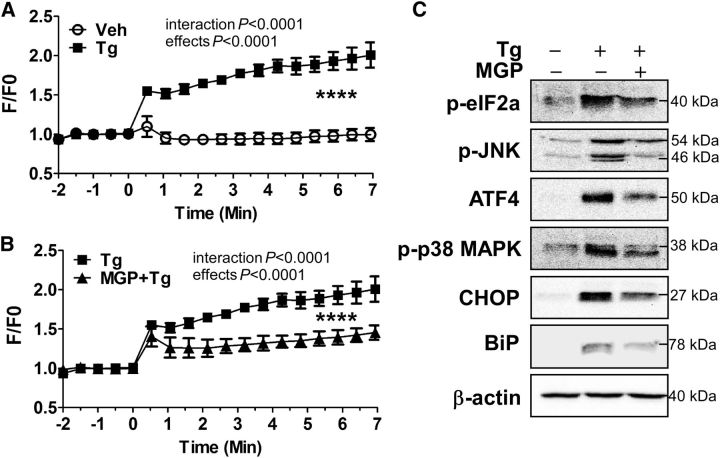
It is well understood that thapsigargin causes ER stress by depleting calcium from ER reservoirs (39). Based on the fact that MGPs decreased thapsigargin-mediated VEGFα secretion, we questioned whether MGPs would lower [Ca2+]i? concentrations. Thapsigargin treatment remarkably increased [Ca2+]i? in ARPE-19 cells (Fig. 5A). In contrast, MGP treatment attenuated ∼50% of [Ca2+]i? release from ARPE-19 cells compared with thapsigargin stimulation alone (Fig. 5B). Next, we examined the impact of MGP treatment on ER stress signaling pathways. Thapsigargin treatment significantly increased p-JNK/p-p38 and p-eIF2α/ATF4 axis activation as well as CHOP and ER chaperone BiP expression. Intriguingly, MGP treatment attenuated p-JNK/p-p38, p-eIF2α/ATF4, and BiP/CHOP activation (Fig. 5C).
FIGURE 5.
Tg-induced [Ca2+]i? and ER stress markers expression in MGP-treated ARPE-19 cells. Changes are shown in [Ca2+]i?-sensitive florescence concentrations over time in response to vehicle or Tg alone (A) and Tg with MGP treatment (B) in ARPE-19 cells. ER stress-related protein expression of p-eIF2α, ATF4, p-JNK, p-p38 MAPK, BiP, and CHOP by western blot analysis is shown in panel (C). In panels (A) and (B), values are means ± SEMs; n= 5, ****time effect, P< 0.0001; treatment effect, P< 0.0001. In panel (C), β-actin was used as a loading control. + and − indicate the presence or absence of prior treatment of Tg or MGP (100 μg/mL). ARPE-19, human retinal pigmented epithelium; ATF4, activating transcription factor 4; BiP, binding of immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein; ER, endoplasmic reticulum; F/F0, ratio of calcium-specific fluorescence; MGP, muscadine grape polyphenol; p-eIF2α, phosphorylated-eukaryotic translation initiation factor 2α p-JNK, phosphorylated c-Jun N-terminal kinase; p-p38 MAPK, phosphorylated-p38 MAPK; Tg, thapsigargin; Veh, vehicle-injected eyes without supplementation; [Ca2+]i?, intracellular calcium.
To determine whether reduction of ER stress by MGPs would decrease ER stress–mediated retinal apoptosis (40), an early apoptosis marker was assessed in the presence and absence of MGPs by flow cytometry. After 72 h of thapsigargin treatment, ∼64% of ARPE-19 cells underwent early apoptosis (analyzed by annexin V positive live cells). As we expected, 100 μg/mL of MGPs significantly reduced apoptotic cell populations to ∼35% (Table 1). Collectively, our data show that MGP supplementation attenuates ER stress–inducible apoptotic cell death in ARPE-19 cells.
TABLE 1.
Flow cytometric determination of apoptotic cells' death in the presence and absence of MGP in ARPE-19 cells1
| Distribution | ||||
|---|---|---|---|---|
| ARPE-19 population | Vehicle | Tg | Tg + MGP (100 μg/mL) | P |
| % | ||||
| AV− PI− | 96.2 ± 0.61a | 33.0 ± 5.15c | 63.3 ± 5.55b | 0.0002 |
| AV+ PI− | 3.53 ± 0.58c | 64.4 ± 6.85a | 33.6 ± 4.32b | 0.0003 |
| AV− PI+ | 0.1 ± 0.06 | 0.23 ± 0.15 | 0.17 ± 0.09 | 0.67 |
| AV+ PI+ | 0.17 ± 0.07 | 2.3 ± 1.63 | 2.8 ± 1.25 | 0.31 |
| Total | 100 | 100 | 100 | — |
Values are means ± SEMs, n= 3. Labeled means in a row without a common letter differ, P< 0.05. ARPE-19, human retinal pigmented epithelium; AV− PI−, annexin V negative and propidium iodide negative population (live); AV− PI+, annexin V negative and propidium iodide positive population (necrotic); AV+ PI−, annexin V positive and propidium iodide negative population (apoptotic); AV+ PI+, annexin V positive and propidium iodide positive population (both necrotic and apoptotic); MGP, muscadine grape polyphenol; Tg, thapsigargin.
Discussion
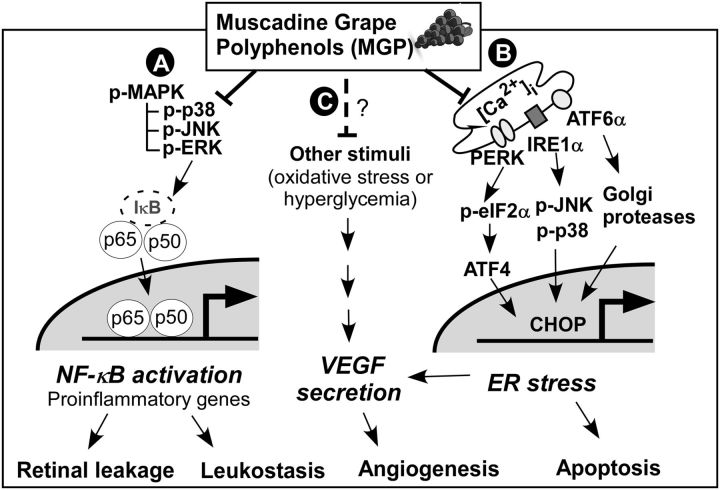
Responsible for vision, the eyes are delicate and highly nutrition-sensitive organs. Chronic stresses in retinal epithelium can cause irreversible damage mediating aberrant angiogenesis, cell death, and eventually loss of eyesight (17). The goal of this study was to investigate the protective effects of MGPs against vision-threatening risk factors, especially inflammation and ER stress. In this work, we demonstrated that MGPs were effective in reducing inflammation-mediated cytokine expression, leukocyte infiltration, and retinal vascular leakage, likely because of an attenuation of NF-κB activation. In addition, MGPs reduced ER stress–mediated VEGFα secretion, ER stress, and early apoptosis by decreasing [Ca2+]i? and subsequent signal transduction downstream. Based on these results, we proposed that MGPs might attenuate ocular inflammation and ER stress by interrupting upstream signaling pathways (summarized in Fig. 6).
FIGURE 6.
A proposed mechanism by which MGPs attenuate ocular inflammation and ER stress. Supplementation of MGP will reduce MAPK and NF-κB activation, which in turn decreases inflammation-mediated retinal permeability and leukocyte recruitment into eyes (A). MGPs decrease intracellular calcium release from ER and subsequent signal transduction for ER stress, including p-eIF2α, ATF4, p-JNK, p-p38, and CHOP, which in turn alleviates ER stress-mediated apoptotic cell death and VEGFα secretion (B). It remains to be identified whether MGPs could reduce other angiogenic signals such as oxidative stress or hyperglycemia, and thus diminish VEGFα secretion and angiogenesis (C). In addition, it is yet to be revealed whether MGP also mitigates signaling crosstalk among these risk factors. ATF4, activating transcription factor 4; ATF6α, activating transcription factor 6α CHOP, CCAAT/enhancer-binding protein homologous protein; ER, endoplasmic reticulum; IκB, nuclear factor κ-B inhibitor; IRE1α, inositol-requiring enzyme-1α MGP, muscadine grape polyphenol; p-eIF2α, phosphorylated-eukaryotic translation initiation factor 2α p-ERK, phosphorylated-extracellular-signal-regulated kinase; p-JNK, phosphorylated c-Jun N-terminal kinase; p-MAPK, phosphorylated MAPK; p-p38, phosphorylated p38 MAPK; p50, NF-κB subunit p50; p65, NF-κB subunit p65; PERK, protein kinase R–like ER-localized eIF2α kinase; VEGF, vascular endothelial growth factor; [Ca2+]i?, intracellular calcium.
Inflammation is the critical etiology this causally associated with vision-threatening retinal diseases such as DR (34, 41, 42), AMD (43), and uveitis (44). There is evidence that inflammatory cytokines are elevated in the vitreous humor in proliferative DR (45), AMD (46), and uveitis (47). Additionally, aberrant expression of proinflammatory cytokines within the neural retina and up-regulation of adhesion molecules on the microvasculature lead to leukostatic responses and vascular leakage; this will eventually lead to acellular capillary formation and neurovascular dysfunction (48). Key underlining mechanisms that trigger damages to ocular tissues are likely to include cascades of intracellular signaling for NF-κB activation (49, 50). Inhibition of NF-κB serves as a useful therapeutic target to treat inflammatory retinal diseases. Indeed, blockage of NF-κB by corticosteroids has been the most frequently prescribed medication for the treatment of severe uveitis (51). However, considering the limitation of pharmacologic strategies and the adverse effects of steroidal anti-inflammatory drugs (52), nutritional intervention for the treatment and/or prevention of ocular inflammation would be a safe and effective approach. To investigate the anti-inflammatory potential of MGPs in retinal inflammation, we used well-accepted experimental models: TNF -α–induced acute inflammation in ARPE-19 cells and an endotoxin (LPS)-induced uveitis model in mice. MGPs significantly reduced cytokine and chemokine production in vivo and in vitro (Figs. 1, 2). In parallel, MGPs were competent to inhibit MAPK and NF-κB activation as well as endotoxin-mediated leukocyte infiltration (Fig. 2B, C). These results clearly suggest the likelihood that supplementation with MGPs could be effective in intervening against the prevalence of inflammatory diseases in retinal epithelium. Retinal-pigmented epithelium constitutes the outer blood-retinal barrier, which plays pivotal roles in the transport of nutrients and water, light absorption, phagocytosis, and immune responses, and thereby serves as the gatekeeper for the maintenance of the retina integrity (53). Escalated concentrations of cytokines are 1 of the fundamental causes that weaken the retinal epithelium by disrupting tight junctions (33, 35). Shirasawa et al. (54) have demonstrated that TNF -α treatment increases retinal permeability by losing tight-junction proteins. MGP supplementation could inhibit the inflammation mediated-mediated loss of tight-junction expression and sustain epithelium integrity against inflammation (Fig. 2). This implies that MGP supplementation may be a beneficial and economical source for intervention in the progression of retinopathy by assisting current pharmaceutical approaches.
It is important to identify the individual component(s) responsible for the anti-inflammatory effects of MGPs. The most well-studied polyphenol in retinal disease is resveratrol (3,5,4-trihydroxystilbene). Resveratrol is apparently efficacious in suppressing retinal inflammation by reducing leukocyte adhesion to retinal vessels and retinal neovascularization (22–24). Despite the proposed potency of resveratrol, we excluded the possibility that resveratrol is 1 of the active components of MGPs. The contents of resveratrol were under the lower limit of quantification. Primarily, MGPs are composed of Acy and NAcy (7). The Acy fraction did not exhibit any substantial anti-inflammatory properties. Among the major NAcy components tested in ARPE-19 cells, quercetin was most potent (quercetin>>EA>myricetin≈KM) in reducing TNF -α–induced proinflammatory gene expression. More notably, treatment with individual polyphenols was not as effective as using combinations of MGPs. For example, 100 μg/mL of MGPs, containing 0.2 μmol/L of quercetin and 6 μmol/L of EA, was more potent than a single treatment of 10 μmol/L of quercetin or EA in decreasing proinflammatory cytokine expression. Acy may provide synergistic roles by facilitating the NAcy uptake, protecting polyphenols from catabolic degradation, or boosting cellular defensive enzyme systems. Another important factor in the cause of retinal diseases, other than inflammation, seems to be ER stress. Unlike the inflammatory inhibition pattern, total MGP (>25 μg/mL), the 4 major individual flavonols, and Acy (25 μg/mL) were similarly effective in decreasing VEGF expression and secretion (Fig. 4). It is noteworthy that the Acy fraction of MGPs contributed to the alleviation of ER stress, although it was not potent in decreasing inflammation per se. Although mechanistic uncertainties exist, our data suggest that combinatory polyphenols of MGPs were more effective than individual polyphenolic compounds in attenuating multiple risk factors in ARPE-19 cells, and probably in vivo as well.
The decrease of VEGFα secretion by MGPs appears to be the consequence of reduced unfolded protein responses (UPRs) corresponding to the reduced calcium release from ER (Fig. 5B). There are at least 3 major UPR pathways (17). In our work, MGP treatment evidently decreased ER stress–mediated activation and downstream signaling targets. The MGP supplementation seems to be effective in attenuating at least 2 other UPR signaling pathways (i.e., protein kinase R–like ER-localized eIF2α kinase and inositol-requiring enzyme-1α) that are linked to CHOP activation (Fig. 5C). In agreement with decreased [Ca2+]i?, ER stress marker proteins, and CHOP activation, MGP treatment significantly attenuated early apoptosis (Table 1). Based on data, these results showed that MGPs sufficiently diminish ER stress signal transduction, UPRs, and apoptosis in human ARPE-19 cells, and inflammation.
Although our data are promising in that an MGP-containing diet may prevent or delay retinal vascular leakage and accompanying pathologic processes of vision loss, there are some limitations in our study design for immediate clinical application. We used 100 to 200 mg/kg body weight of MGPs, which is difficult to be achieved by regular dietary interventions. It needs to be determined whether supplementation with a physiologically attainable MGP concentration (probably within the range of 10–50 mg/kg body weight) would produce the same effect. In a different approach, a direct delivery system (e.g., eye drops) could be considered to increase MGP concentration within the retinal microenvironment. Our current study also lacks information regarding MGP metabolism in vivo. We are now analyzing the physiologically active forms of MGPs and their metabolites in plasma. Likewise, additional efforts will be made to define the compositional variations of polyphenols among different batches of grapes and cultivars, which will establish the nutritional significance of MGPs in ocular health and facilitate the development of new intervention strategies using MGPs.
Here we demonstrated that MGPs effectively attenuated at least 2 important variables related to the development of vision-threatening retinal diseases: inflammation and ER stress. MGPs attenuated NF-κB activation, which resulted in reduced proinflammatory cytokine expression, leukocyte recruitment, and retinal leakage. MGPs also effectively reduced ER stress signal transduction and apoptotic cell death. Additional studies are required to determine signaling crosstalk among ER stress, inflammation, and other oxidative insults and to establish optimal concentration ranges for the translation into humans. In conclusion, this study provides new insight that supplementation with MGPs may be beneficial to eye health by protecting retinal epithelium from inflammation and ER stress.
Supplementary Material
Primer sequences for qPCR
Acknowledgments
The authors thank Mee Ae Lee for technical assistance and Meri P. Nantz for editing the manuscript. S.C., Q.L., and J.-H.H. designed the research; J.-H.H., P.K.S., and P.Z. conducted the research; L.G. prepared the phytochemical fractionation; J.-H.H., Q.L., and S.C. analyzed the data; J.-H.H. and S.C. wrote the manuscript; and S.C. had primary responsibility for the final content. All authors read and approved the final manuscript.
Abbreviations
- Acy
anthocyanin
- AMD
age-related macular degeneration
- ARPE-19
human retinal pigmented epithelium
- ATF4
activating transcription factor 4
- BiP
binding of immunoglobulin protein
- CHOP
CCAAT/enhancer-binding protein homologous protein
- DR
diabetic retinopathy
- EA
ellagic acid
- H&E
hematoxylin and eosin
- IκBα
NF- κB inhibitor α
- IL-1β
interleukin-1β
- IL-10
interleukin-10
- MCP-1
monocyte chemo-attractive protein 1
- MGP
muscadine grape polyphenol
- NAcy
non-anthocyanin
- OCLN
occludin
- p-eIF2α
phosphorylated-eukaryotic translation initiation factor 2α
- p-JNK
phosphorylated c-Jun N-terminal kinase
- p-p38
phosphorylated p38 MAPK
- p65
NF-κB subunit p65
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
- [Ca2+]i?
intracellular calcium
Footnotes
Supported by the Institute of Food and Agricultural Science (IFAS) at the University of Florida (USDA-Hatch) and partly by the Florida Department of Agriculture and Consumer Service, Viticulture Research Program.
Literature Cited
- 1. Park SH, Park TS, Cha YS. Grape seed extract (Vitis vinifera) partially reverses high fat diet-induced obesity in C57BL/6J mice. Nutr Res Pract. 2008;2:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition. 2006;22:1137–45. [DOI] [PubMed] [Google Scholar]
- 3. Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, Arola L, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–8. [DOI] [PubMed] [Google Scholar]
- 4. Terra X, Pallares V, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, Arola L, Blay M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem. 2011;22:380–7. [DOI] [PubMed] [Google Scholar]
- 5. Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, Young HA, Arany P, Green JE. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–405. [DOI] [PubMed] [Google Scholar]
- 6. Yi W, Fischer J, Akoh CC. Study of anticancer activities of muscadine grape phenolics in vitro. J Agric Food Chem. 2005;53:8804–12. [DOI] [PubMed] [Google Scholar]
- 7. Gourineni V, Shay NF, Chung S, Sandhu AK, Gu L. Muscadine grape (Vitis rotundifolia) and wine phytochemicals prevented obesity-associated metabolic complications in C57BL/6J mice. J Agric Food Chem. 2012;60:7674–81. [DOI] [PubMed] [Google Scholar]
- 8. Bharathselvi M, Biswas J, Selvi R, Coral K, Narayanasamy A, Ramakrishnan S, Sulochana KN. Increased homocysteine, homocysteine-thiolactone, protein homocysteinylation and oxidative stress in the circulation of patients with Eales' disease. Ann Clin Biochem. 2013;50:330–8. [DOI] [PubMed] [Google Scholar]
- 9. El-Remessy AB, Franklin T, Ghaley N, Yang J, Brands MW, Caldwell RB, Behzadian MA. Diabetes-induced superoxide anion and breakdown of the blood-retinal barrier: role of the VEGF/uPAR pathway. PLoS ONE. 2013;8:e71868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 10. Brantley MA, Jr, Osborn MP, Sanders BJ, Rezaei KA, Lu P, Li C, Milne GL, Cai J, Sternberg P., Jr Plasma biomarkers of oxidative stress and genetic variants in age-related macular degeneration. Am J Ophthalmol. 2012;153:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khurana RN, Parikh JG, Saraswathy S, Wu GS, Rao NA. Mitochondrial oxidative DNA damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49:3299–304. [DOI] [PubMed] [Google Scholar]
- 12. Tomić M, Ljubic S, Kastelan S. The role of inflammation and endothelial dysfunction in the pathogenesis of diabetic retinopathy. Coll Antropol. 2013;37(Suppl 1):51–7. [PubMed] [Google Scholar]
- 13. Yao J, Liu X, Yang Q, Zhuang M, Wang F, Chen X, Hang H, Zhang W, Liu Q. Proteomic analysis of the aqueous humor in patients with wet age-related macular degeneration. Proteomics Clin Appl. 2013;7:550–60. [DOI] [PubMed] [Google Scholar]
- 14. Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, Zhao L, Chen AY, Zhang X, Zhu J, Zhao J, Ouyang H, Luo H, Song Y, Lee J, et al. TCF7L2 variation and proliferative diabetic retinopathy. Diabetes. 2013;62:2613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fong DS, Aiello LP, Ferris FL, III, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–53. [DOI] [PubMed] [Google Scholar]
- 19. Sin HP, Liu DT, Lam DS. Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration. Acta Ophthalmol. 2013;91:6–11. [DOI] [PubMed] [Google Scholar]
- 20. Lee CT, Gayton EL, Beulens JW, Flanagan DW, Adler AI. Micronutrients and diabetic retinopathy a systematic review. Ophthalmology. 2010;117:71–8. [DOI] [PubMed] [Google Scholar]
- 21. Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2004;79:865–73. [DOI] [PubMed] [Google Scholar]
- 22. Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012;90:e31–7. [DOI] [PubMed] [Google Scholar]
- 23. Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–9. [DOI] [PubMed] [Google Scholar]
- 24. Yar AS, Menevse S, Dogan I, Alp E, Ergin V, Cumaoglu A, Aricioglu A, Ekmekci A, Menevse A. Investigation of ocular neovascularization-related genes and oxidative stress in diabetic rat eye tissues after resveratrol treatment. J Med Food. 2012;15:391–8. [DOI] [PubMed] [Google Scholar]
- 25. Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–9. [DOI] [PubMed] [Google Scholar]
- 26. Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–30. [DOI] [PubMed] [Google Scholar]
- 27. Larrosa M, Garcia-Conesa MT, Espin JC, Tomas-Barberan FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010;31:513–39. [DOI] [PubMed] [Google Scholar]
- 28. Muthenna P, Akileshwari C, Reddy GB. Ellagic acid, a new antiglycating agent: its inhibition of N-(carboxymethyl)lysine. Biochem J. 2012;442:221–30. [DOI] [PubMed] [Google Scholar]
- 29. Sandhu AK, Gu L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-MS(n). J Agric Food Chem. 2010;58:4681–92. [DOI] [PubMed] [Google Scholar]
- 30. Kaarniranta K, Salminen A. NF-kappaB signaling as a putative target for omega-3 metabolites in the prevention of age-related macular degeneration (AMD). Exp Gerontol. 2009;44:685–8. [DOI] [PubMed] [Google Scholar]
- 31. Trinh L, Brignole-Baudouin F, Labbe A, Raphael M, Bourges JL, Baudouin C. The corneal endothelium in an endotoxin-induced uveitis model: correlation between in vivo confocal microscopy and immunohistochemistry. Mol Vis. 2008;14:1149–56. [PMC free article] [PubMed] [Google Scholar]
- 32. Xu H, Forrester JV, Liversidge J, Crane IJ. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44:226–34. [DOI] [PubMed] [Google Scholar]
- 33. Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. [DOI] [PubMed] [Google Scholar]
- 35. Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53:5016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–30. [DOI] [PubMed] [Google Scholar]
- 37. Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci. 2002;43:2791–8. [PubMed] [Google Scholar]
- 38. Yoshikawa T, Ogata N, Izuta H, Shimazawa M, Hara H, Takahashi K. Increased expression of tight junctions in ARPE-19 cells under endoplasmic reticulum stress. Curr Eye Res. 2011;36:1153–63. [DOI] [PubMed] [Google Scholar]
- 39. Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19:131–5. [DOI] [PubMed] [Google Scholar]
- 40. Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30:16938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vagaja NN, Binz N, McLenachan S, Rakoczy EP, McMenamin PG. Influence of endotoxin-mediated retinal inflammation on phenotype of diabetic retinopathy in Ins2Akita mice. Br J Ophthalmol. 2013;97:1343–50. [DOI] [PubMed] [Google Scholar]
- 43. Cao S, Ko A, Partanen M, Pakzad-Vaezi K, Merkur AB, Albiani DA, Kirker AW, Wang A, Cui JZ, Forooghian F, et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am J Ophthalmol. 2013;156:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forrester JV, Klaska IP, Yu T, Kuffova L. Uveitis in mouse and man. Int Rev Immunol. 2013;32:76–96. [DOI] [PubMed] [Google Scholar]
- 45. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006;20:1366–9. [DOI] [PubMed] [Google Scholar]
- 46. Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–8. [DOI] [PubMed] [Google Scholar]
- 47. Takase H, Futagami Y, Yoshida T, Kamoi K, Sugita S, Imai Y, Mochizuki M. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–61. [DOI] [PubMed] [Google Scholar]
- 48. Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in diabetic retinopathy. Curr Diabetes Rev. 2007;3:3–14. [DOI] [PubMed] [Google Scholar]
- 49. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. [DOI] [PubMed] [Google Scholar]
- 50. Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl). 2004;82:434–48. [DOI] [PubMed] [Google Scholar]
- 51. Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54:2483–92. [DOI] [PubMed] [Google Scholar]
- 52. Denniston AK, Dick AD. Systemic therapies for inflammatory eye disease: past, present and future. BMC Ophthalmol. 2013;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier–implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shirasawa M, Sonoda S, Terasaki H, Arimura N, Otsuka H, Yamashita T, Uchino E, Hisatomi T, Ishibashi T, Sakamoto T. TNF-alpha disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Exp Eye Res. 2013;110:59–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for qPCR