Invasive procedures are widely used to treat chronic pain, however no rigorous quantitative synthesis of the safety and efficacy of invasive procedures has been done using accepted methods for bias reduction. This review suggests that a moderate amount of evidence does not support the use of invasive procedures as compared to sham procedures for patients with chronic back or knee pain and that rigorous clinical research is needed.
Keywords: Surgery, Placebo, Sham, Pain, Systematic Review, Meta-analysis
Abstract
Objective
To assess the evidence for the safety and efficacy of invasive procedures for reducing chronic pain and improving function and health-related quality of life compared with sham (placebo) procedures.
Design
Systematic review with meta-analysis.
Methods
Studies were identified by searching multiple electronic databases, examining reference lists, and communicating with experts. Randomized controlled trials comparing invasive procedures with identical but otherwise sham procedures for chronic pain conditions were selected. Three authors independently extracted and described study characteristics and assessed Cochrane risk of bias. Two subsets of data on back and knee pain, respectively, were pooled using random-effects meta-analysis. Overall quality of the literature was assessed through Grading of Recommendations, Assessment, Development, and Evaluation.
Results
Twenty-five trials (2,000 participants) were included in the review assessing the effect of invasive procedures over sham. Conditions included low back (N = 7 trials), arthritis (4), angina (4), abdominal pain (3), endometriosis (3), biliary colic (2), and migraine (2). Thirteen trials (52%) reported an adequate concealment of allocation. Fourteen studies (56%) reported on adverse events. Of these, the risk of any adverse event was significantly higher for invasive procedures (12%) than sham procedures (4%; risk difference = 0.05, 95% confidence interval [CI] = 0.01 to 0.09, P = 0.01, I2 = 65%). In the two meta-analysis subsets, the standardized mean difference for reduction of low back pain in seven studies (N = 445) was 0.18 (95% CI = –0.14 to 0.51, P = 0.26, I2 = 62%), and for knee pain in three studies (N = 496) it was 0.04 (95% CI = –0.11 to 0.19, P = 0.63, I2 = 36%). The relative contribution of within-group improvement in sham treatments accounted for 87% of the effect compared with active treatment across all conditions.
Conclusions
There is little evidence for the specific efficacy beyond sham for invasive procedures in chronic pain. A moderate amount of evidence does not support the use of invasive procedures as compared with sham procedures for patients with chronic back or knee pain. Given their high cost and safety concerns, more rigorous studies are required before invasive procedures are routinely used for patients with chronic pain.
Introduction
Chronic pain is a major worldwide problem. In the United States, it is estimated that more than 100 million people suffer from chronic pain, with costs between $560 and $635 billion dollars per year [1]. The estimated prevalence of pain lasting at least three months is 14.6% [2]. The prevalence of chronic musculoskeletal pain conditions and frequent headaches is 43% [3]. Data from the 2012 National Health Interview Study estimated the prevalence of chronic daily pain to be 25.3 million people or 11.2% of the population [4]. These numbers do not describe the full impact that chronic pain has on productivity, quality of life, and human suffering.
To treat pain, the use of opioids has increased dramatically over the last several decades, with 9.6 to 11.5 million adults or approximately 3%–4% of the adult US population having been prescribed long-term opioid therapy [5]. Opioids have limited effectiveness for chronic pain and are accompanied by substantial risk of adverse outcomes including addiction, overdose, and deaths. Deaths from opioids now exceed deaths from motor vehicle accidents [6]. Thus, the need for nonpharmacological approaches for treating chronic pain has grown.
Invasive procedures (including surgery) might mitigate the need for chronic opioid and other pharmacological therapies and be viable options for chronic pain treatment. Procedures that completely replace damaged or arthritic joints or change major anatomical structures can produce long-term reduction in pain and improvement in function [7]. However, invasive procedures are increasingly being used for pain where the anatomical causes for the pain are not so clear.
The development of minimally invasive procedures has expanded the use of such interventions for treating a variety of chronic pain conditions such as low back pain [8], arthritis [9], and endometriosis [10]. In 2014, more than $45 billion was spent in the United States on surgical treatments for chronic low back pain (LBP). Arthroplasty costs for chronic knee pain topped $41 billion [11]. Invasive procedures are considered effective and are standard care for these two conditions. However, many types of invasive procedures are marketed, used, and paid for without evidence from rigorous study designs involving randomization, allocation concealment and blinding, or placebo controls. In the absence of these controls for common sources of bias, studies on invasive procedures may be giving a false impression of their true efficacy. Individual efficacy studies of invasive procedures have been published for LBP [12,13] and osteoarthritis of the knee [14], and a recent meta-analysis estimated the magnitude of the effects of sham surgery on subjective and objective outcomes [15]. However, no comprehensive systematic review of the current evidence on the safety and efficacy of invasive procedures compared with placebo treatment in chronic pain has been done.
It is the purpose of this study to identify and evaluate the current evidence for invasive procedures compared with their identical sham procedures in the treatment of chronic pain and assess the impact on reducing pain, medication use, disability, adverse events, and enhancing health-related quality of life for patients with various chronic pain conditions.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for reporting this systematic review with meta-analyses.
Study Eligibility Criteria
Randomized controlled trials (RCTs) that compared any invasive procedure, including classical surgery, with a parallel sham procedure for patients with chronic pain conditions were eligible. Invasive procedures were defined as when an instrument was inserted into the body (either endoscopically or percutaneously) for the purposes of manipulating tissue or changing anatomy. Procedures used only as a method to deliver another active treatment such as a drug (e.g., steroids), cells, implantation of an electrical device, or new joint were excluded. To be eligible, all procedures needed to be compared with an identical yet sham procedure that used the same invasive approach, instruments, and ritual but eliminated the hypothesized active component of tissue manipulation. Chronic pain conditions were defined as those conditions where pain lasted more than three months [7,16]. Other outcomes related to function and health-related quality of life were captured when reported. Only studies with observation periods of one or more months after treatment were eligible.
Identification and Selection of Studies
The search strategy was adapted from a previously published systematic review and meta-analysis conducted by the authors investigating nonspecific components in sham-controlled surgical trials for all conditions [17]. An updated search was conducted for the purposes of this paper through January 2018 across PubMed, EMBASE, CINAHL, Central (Cochrane Library), PILOTS, PsycInfo, DoD Biomedical Research, and clinicaltrials.gov to capture any recent relevant literature. Search terms included (“Diagnostic Techniques, Surgical” OR “Orthopedic Procedures” OR “Specialties, Surgical” OR “Surgical Procedures, Operative” OR “surgery” [Subheading] or surgery) AND (“Placebos” OR “Placebo Effect” or sham surg* or placebo surg* or mock surg* or simulated surg* or placebo proc* or sham proc* or mock proc* or simulated proc*). All searches were restricted to humans and RCT study design [17]. In addition, reference lists were examined, and experts in the field were contacted to ensure comprehensiveness of the included studies. Four investigators (CC, KM, KL, LC) screened titles and abstracts independently and in duplicate using a structured form, and studies on chronic pain conditions were selected for analysis. Any disagreements in selection or classification were resolved through discussion and consensus and approved by the first author (WJ).
Data Collection and Study Appraisal
We used the Mobius Analytics Systematic Review System (Mobius Analytics Inc., Ottawa, Ontario, Canada) for data entry and execution of the review. All studies meeting the predefined inclusion criteria were assessed for methodological quality independently and in duplicate using the Cochrane risk of bias tool [18]. Data were extracted to capture study characteristics and pain outcomes at all time points. Additional outcomes related to function, medication use, health-related quality of life, and adverse events were also extracted. Study appraisal and data extraction were performed by three investigators (CC, KM, LC). These investigators are all experienced in systematic review methods, including data extraction and extracted data in duplicate. In addition, 20% of the studies were checked by the primary author (WJ). All discrepancies were tracked and resolved by discussion, with final decisions made by the primary author.
Data Synthesis and Analysis
Studies were grouped according to chronic pain condition and the procedure reported in the study. Where data were available for a reduction in pain intensity, disability, health-related quality of life, adverse events, dropouts, and/or medication use, the sample size, mean, and standard deviation for each treatment group at each time point were extracted in duplicate (CC, KM). For continuous data, standardized mean differences (SMDs) were computed as the difference between groups in pre–post change scores by using Comprehensive Meta-Analysis, version 3.3.070 (Biostat, Englewood, NJ, USA). When standard deviations for change scores were not reported, they were calculated from pre and post standard deviations [19], using r = 0.5 for the product–moment correlation. For studies with dichotomous outcomes, either the relative risk between the percentage of responders in the sham and active treatment groups (responder ratio) or the risk difference between groups (for adverse events and study dropouts) was calculated with Cochrane Collaboration’s Review Manager (RevMan; version 5.2.7). This was done regardless of whether the studies were pooled for analysis or not.
A forest plot was created for each study that had data capable of supporting an effect size analysis to facilitate a visual comparison of results across studies and conditions.
Because of the variety of conditions and treatments, a meta-analysis was not done for the entire study sample. However, the authors judged meta-analysis feasible when 1) there were more than three studies within a single chronic pain condition with data available in the papers; 2) the interventions and outcomes were similar enough to allow for a clinically meaningful estimate of the reduction in pain intensity; and 3) when the comparison was made to the intervention’s own sham. This approach meets current standards for meta-analysis [20,21]. Low back pain and osteoarthritis of the knee met these criteria. Meta-analyses of SMDs, relative risks, and risk differences were then performed with the generic inverse model of RevMan for low back and knee pain using random effects models. Statistical heterogeneity was examined by Cochrane’s Q test and I2, with low, moderate, and high I2 values of 25%, 50%, and 75%, respectively. Egger’s test was used to assess funnel plot asymmetry as a measure of publication bias [22]. Pooled effect sizes for the pain-related outcome of primary interest in back and knee pain were translated into the visual analog scale (VAS; 0–100) for ease of clinical interpretation using a standard deviation of 25 points [23]. A P value of less than 0.05 was set as the level of significance.
The overall quality of the body of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation approach based on the following criteria: risk of bias, inconsistency, indirectness, imprecision, magnitude of the estimates of effects, and publication bias [24]. Two authors (CC, KM) independently performed this exercise and then met with the primary author (WJ) to review and come to consensus.
Results
Study Selection
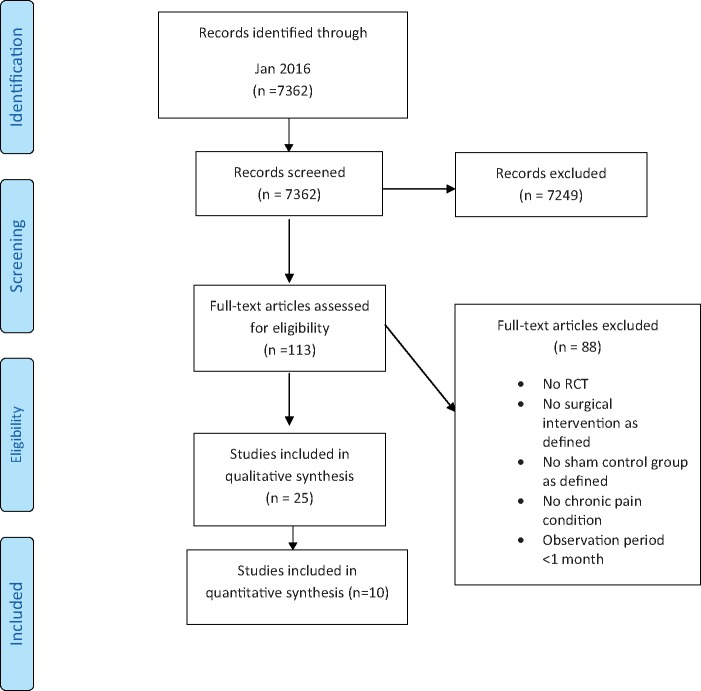
We identified 7,362 citations based on the original search executed [17] and updated through January 2018. Twenty-five studies (in 28 publications) published between 1959 and 2013, involving a total of 2,000 patients with specific chronic pain conditions, met eligibility criteria for the systematic review (Figure 1). No studies met the eligibility criteria from 2014 through January 2018.
Figure 1.
PRISMA flow diagram. RCT = randomized controlled trial.
Characteristics of Included Studies
Characteristics of included studies are summarized in Table 1. Of the 25 studies on chronic pain conditions, low back pain (N = 7 studies) was the most frequent diagnosis reported [12,13,25–29], followed by knee osteoarthritis (N = 4) [14,30–32], angina from coronary artery disease (N = 4) [33–37], abdominal pain (N = 3) [38–40], endometriosis (N = 3) [41–45], biliary pain (N = 2) [46,47], and migraine (N = 2) [48,49]. The total number of enrolled patients per study ranged from 10 [30] to 298 (Table 1) [37].
Table 1.
Characteristics of included studies
| Source | Condition | Total No., Treatment/Control | Active Treatment | Sham Treatment | Selected Cochrane ROB Items | Pain and Related Outcome(s) |
|---|---|---|---|---|---|---|
| Low back pain | ||||||
| van Kleef et al., 1999 [25] | Chronic low back pain | T 15/C 16 | Radiofrequency lumbar facet denervation | Electrodes were introduced, but no radiofrequency lesion was made |
|
|
| Leclaire et al., 2001 [26] | Low back pain (>3 mo) | T 36/C 34 | Percutaneous radiofrequency articular denervation | Same procedure without denervation |
|
|
| Freeman et al., 2005 [27] | Chronic discogenic low back pain | T 38/C 19 | Delivering of electrothermal energy via catheter | Identical positioning of catheter without delivering electrothermal energy |
|
|
| Nath et al., 2008 [28] | Chronic low back pain | T 20/C 20 | Percutaneous radiofrequency neurotomy | Identical procedure except no current was used |
|
|
| Buchbinder et al., 2009 [12] | Painful osteoporotic vertebral fractures | T 38/C 40 | Vertebroplasty | Simulated vertebroplasty with insertion of the needle until lamina and odor of cement |
|
|
| Kallmes et al., 2009 [13] | Painful osteoporotic vertebral fractures | T 68/C 63 | Vertebroplasty | Simulated procedure with odor of cement |
|
|
| Patel et al., 2012 [29] | Chronic sacroiliac joint pain | T 34/C 17 | Lateral branch neurotomy using cooled radiofrequency | Identical procedure without delivery of radiofrequency energy |
|
|
| Arthritis | ||||||
| Moseley et al., 1996 [30] | Arthritis | lavage: 3/debridement: 2/C 5 | Arthroscopic debridement or arthroscopic lavage | Skin incisions but no placement of instruments into the knee |
|
Patients who felt the operation was worthwhile |
| Moseley et al., 2002 [14] | Arthritis | Lavage: 61/Debridement: 59/C 60 | Arthroscopic debridement or arthroscopic lavage | Skin incisions but no placement of instruments into the knee |
|
Pain intensity 1–3 mo: SMD: –0.23 (–0.55, 0.09), 6 mo: –0.27 (–0.59, 0.05), 12 mo: 0.17 (–0.22, 0.56) |
| Bradley et al., 2002 [31] | Arthritis | T 89/C 91 | Arthroscopic knee irradiation | Needle was advanced to, but not through, the joint capsule |
|
|
| Sihvonen et al., 2013 [32] | Arthritis | T 70/C 76 | Arthroscopic partial meniscectomy | Simulation of arthroscopic meniscectomy |
|
|
| Angina | ||||||
| Cobb et al., 1959 [33] | Angina pectoris | T 8/C 9 | Ligation of the internal mammary arteries | Skin incision with exposure of the internal mammary arteries with no ligation |
|
|
| Dimond et al., 1960 [34] | Angina pectoris | not stated | Ligation of internal mammary arteries | Skin incision with exposure of the internal mammary arteries with no ligation |
|
|
| Salem et al., 2004 [36] Salem et al., 2005 [35] | Aangina pectoris | T 40/C 42 | Percutaneous myocardial laser revascularization plus optimal medical therapy | Sham procedure involving the laser catheter being inserted but not activated plus optimal medical therapy |
|
|
| Leon et al., 2005 [37] | Angina pectoris | T 196/C 102 | Diagnostic catheterization and direct myocardial revascularization via laser catheterization | Diagnostic catheterization, and the laser was turned on outside the body |
|
|
| Abdominal pain | ||||||
| Swank et al., 2003 [38] | Chronic abdominal pain and adhesions | T 52/C 48 | Laparoscopic adhesion lysis | Diagnostic laparoscopy only |
|
|
| Cote et al., 2012 [39] | Painful pancreatic sphincter dysfunction | T 11/C 9 | Endoscopic sphincterotomy | Sham endoscopy (not described) |
|
|
| Boelens et al., 2013 [40] | Painful anterior cutaneous nerve Entrapment syndrome | T 22/C 22 | Neurectomy of the intercostal nerve endings at the level of the abdominal wall | Sham surgery with exposure of intercostal nerve endings with no further surgical procedure |
|
Patients ≥50% improvement in pain 1–3 mo: RR: 4.0 (1.59, 10.06) |
| Endometriosis | ||||||
| Sutton et al., 1994 [42] Sutton et al., 1997 [41] | Endometriosis | T32/C 31 | Laparoscopic laser treatment | Diagnostic laparoscopy |
|
|
| Abbott et al., 2004 [43] | Endometriosis | T 20/C 19 | Laparoscopic excision | Diagnostic laparoscopy |
|
|
| Jarrell et al., 2005 [44] Jarrell et al., 2007 [45] | Endometriosis | T 8/C 7 | Laparoscopic biopsy and excision | Diagnostic laparoscopy |
|
(data not usable) |
| Cholia (biliary cholic/pain) | ||||||
| Geenen et al., 1989 [46] | Cholia | T 23/C 24 | Endoscopic sphincterotomy | Sham sphincterotomy was performed with activation of the electrocautery unit in the duodenal lumen |
|
Patients reporting good or fair clinical improvement at 12 mo: RR: 2.24 (1.12, 4.46) |
| Toouli et al., 2000 [47] | Cholia | T 37/C 42 | Endoscopic sphincterotomy | Endoscopy with noise simulation of sphincterotomy |
|
Patients with improvement in abdominal pain at 24 mo: RR: 1.45 (0.94, 1.91) |
| Migraine | ||||||
| Dowson et al., 2008 [48] | Migraine with aura | T 74/C 73 | Patent foramen ovale closure with the starflex septal repair implant | Skin incision in the groin only |
|
|
| Guyuron et al., 2009 [49] | Migraine headache | T 49/C 26 | Endoscopic removal of muscles and nerves in the predominant trigger sites | Exposure of the muscles and nerves through a similar incision, but the integrity of the structures was maintained |
|
|
Standardized mean differences were computed as the difference between groups in pre–post change scores by using Comprehensive Meta-Analysis, version 3.3.070 (Biostat, Englewood, NJ, USA). When standard deviations for change scores were not reported, they were calculated from pre and post standard deviations [19], using r = 0.5 for the product–moment correlation. For studies with dichotomous outcomes, either the relative risk between the percentage of responders in the sham and active treatment groups (responder ratio) or the risk difference between groups (for adverse events) was calculated with Cochrane Collaboration’s Review Manager (RevMan; version 5.2.7). A negative effect size favors sham treatment over active treatment in all cases, but for number adverse events risk difference, a positive effect indicates that there were fewer reported in the sham than the active.
Adapted and updated from: Jonas WB, Crawford C, Colloca L, et al. To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open 2015;5(12):e009655 [17].
C = sham control; CCS = Canadian Cardiovascular Society; QoL = quality of life; RD = risk difference; RR = responder ratio; SMD = standardized mean difference; T = real treatment.
The procedures used included arthroscopic surgery or irrigation [14,30–32], heart catheterization with laser treatment or septal repair [35–37,48], endoscopic sphincterectomy [39,46,47], percutaneous or open neurectomy (mechanical or via radiofrequency) [25,26,28,29,40,49], laparoscopic surgery or laser treatment [38,43–45], vertebroblasty [12,13], intradisc delivery of electrothermal energy [27], and surgical ligation of internal mammary arteries [33,34]. All control groups used a parallel sham procedure mimicking the active procedure. Sham percutaneous and endoscopic procedures typically involved skin incisions only or insertion and removal of a needle or a scope without further tissue manipulation (Table 1).
In addition to pain as an outcome, more than half of the studies reported at least one secondary outcome, including function-related outcomes (disability; N = 6), health-related quality of life parameters (global, physical, mental; N = 11), and medication use (N = 3). Seventeen studies reported a dichotomous (responder) outcome.
Risk of Bias in Included Studies
Overall, the risk of bias was moderate to low. Of the 25 studies included in the systematic review, 17 studies (68%) reported an adequate method for generating the allocation sequence; however, only 13 (52%) had adequate concealment of allocation. Blinding of patients and outcome assessors was adequate in 21 (84%) studies, and incomplete data were adequately addressed in 18 (72%). Blinding of surgeons could not be done. Seventeen (68%) were free from suggestion of selective outcome reporting, and 19 (76%) were judged to be free of other sources of bias.
Adverse Events
Of the 25 studies included in this analysis, five (20.0%) reported that no adverse events or complications occurred, nine (36.0%) described the adverse events and in which study arm they occurred, five (20.0%) described the adverse events but did not distinguish in which study arm they occurred, two (8.0%) described the adverse events insufficiently, and four (16.0%) did not report on or mention adverse events. In the 14 studies providing sufficient data, the risk of any adverse event was significantly higher in the active groups (12%) than in the sham groups (4%; risk difference = 0.05, 95% confidence interval [CI] = 0.01 to 0.09, P = 0.01, I2 = 65%). On average, the number of study dropouts did not differ between the active and sham groups (risk difference = 0.01, 95% CI = –0.01 to 0.03, P = 0.38, I2 = 9%) (Supplementary Data).
Study Results and Analysis
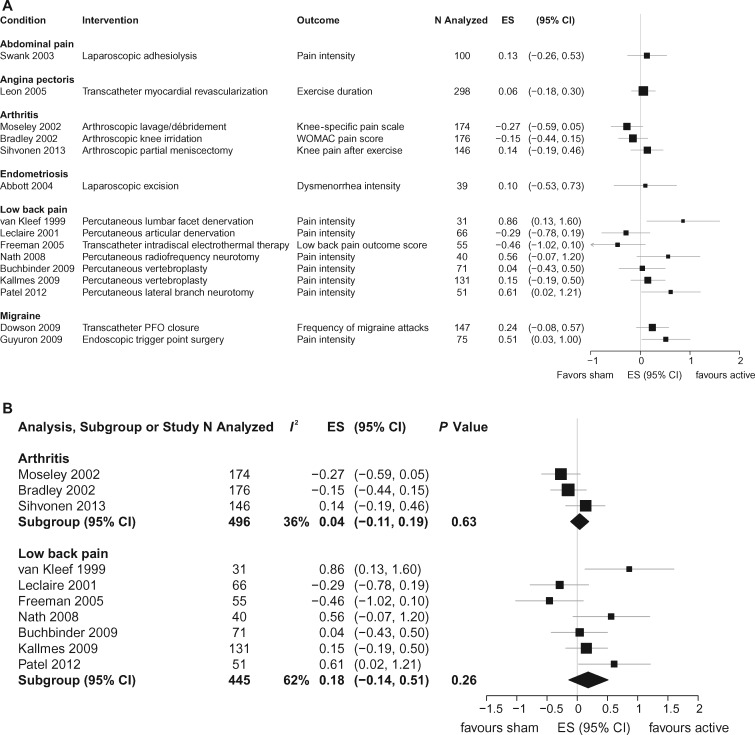
The findings for all studies are summarized in Table 1, and calculated SMDs are shown in Figure 2A. Four studies were published on patients with angina pectoris where the procedure consisted of laser revascularization or internal mammary artery ligation compared with an identical yet sham control procedure. Outcomes varied across studies and mostly consisted of subjective improvement as stated by the patient or physician. Concealment was unclear in three of these studies. Three studies were included on abdominal pain where patients underwent various procedures. These studies had small sample sizes, and results were inconsistent. Three studies were on patients with endometriosis treated with laparoscopic excision (N = 2) or laser ablation (N = 1) and compared with the same but sham treatment. Sample sizes were small, and outcomes varied from study to study, making interpretation unclear. Two studies involved biliary pain treated with endoscopic sphincterotomy or a sham treatment. Patient improvement was noted after 12–24 months post-treatment. Two migraine studies used either patent foramen closure or muscle removal compared with sham procedures and recorded frequency of migraine attacks or pain intensity after six months. Both reported improvement over sham treatment for the active treatments.
Figure 2.
A) Individual between-group effects of invasive treatments compared with sham procedures. B) Meta-analysis for arthritis and low back pain. CI = confidence interval; ES = effect size.
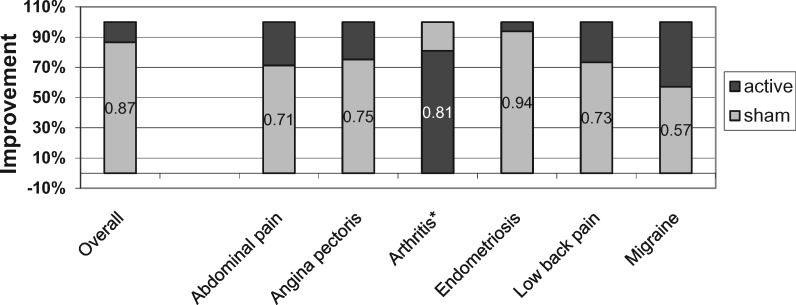
There were at least three studies sufficient for pooling with meta-analysis in both low back pain and knee pain from osteoarthritis. Seven studies with 445 participants were included in the meta-analysis for back pain. The overall pooled SMD for reduction of low back pain was 0.18 (95% CI = –0.14 to 0.51, P = 0.26, I2 = 62%). Translated into the VAS pain score using a 0–100 point scale, this equates to a 4.5-point reduction in pain. Three studies involving 496 participants were included for knee pain due to osteoarthritis. The SMD was 0.04 (95% CI = –0.11 to 0.19, P = 0.63, I2 = 36%) for this condition, equating to a one-point VAS score reduction (Figure 2B). The proportion of improvement due to sham treatment in low back pain was 73%. In osteoarthritis, the average improvement in the sham surgery group was greater than after real surgery. On average, when compared with their own identical controls across all studies, pain reduction in the sham groups accounted for 87% of the improvement seen with active treatments (Figure 3). There did not appear to be evidence for substantial publication bias (Egger’s test P = 0.17).
Figure 3.
Relative contribution of within-group improvement in sham treatments to improvement in active treatment. *For arthritis, the improvement was larger for the sham treatments than for the active treatments.
Secondary Outcomes
Several studies reported on secondary outcomes such as disability, medication use, and quality of life. Six studies measured disability-related outcomes from three to six months postsurgery. Looking at all studies, the reduction in disability postprocedure did not differ between the two groups at three months or at six months in the majority of studies (SMD range = –0.21 to 0.20) (Table 1). Only three studies measured medication use from three to six months postprocedure. They reported conflicting results for reduction in medication use between groups at either time point (SMD range = –0.47 to 0.68). Eleven studies reported on health-related quality of life using either a global score, physical or mental, or a combination, primarily measured with the SF-36 or SF-12. Of those reporting on global health, the studies appear to favor active treatment over sham fairly consistently; over time, however, the majority reported small SMDs. When assessing physical quality of life, the SMDs seem to favor the sham over the active treatments overall, whiched showed small SMDs. Mental quality of life showed no to small effects across studies (Table 1).
Discussion
There is currently insufficient evidence to support the specific efficacy of invasive procedures for the treatment of chronic pain. Very few studies have been done on any one condition, treatments and pain measures differed, and outcomes were inconsistent between studies. Quantitative pooling of outcomes for seven studies on low back pain and three on knee osteoarthritis showed no difference in pain at six months compared with sham procedures. At least for back pain and knee pain, sham surgical procedures explain the majority of the benefit, with confidence in these estimates being strong.
This study has several limitations. First, there are few studies in any one pain condition, resulting in substantial clinical heterogeneity across populations and interventions. A sufficient number of studies with reasonably low heterogeneity were present only for back and knee pain. Second, many types of invasive procedures for pain have not been subjected to sham-controlled studies, so our results may not apply to those procedures and conditions. Finally, none of the studies were double-blind, precluding full rigor in the evaluation of these procedures for chronic pain.
Our findings raise several questions for clinicians, researchers, and policy makers. First, can we justify widespread use of these procedures without rigorous testing? Without such testing, the true efficacy of invasive procedures for chronic pain will remain unknown [50,51]. The risks of surgical and invasive procedures are not minor and appear to be higher with real compared with sham procedures. Risks in both groups include anesthesia, permanent injury to the body, psychologic stress, and time, cost, and productivity losses [52]. Without more rigorous examination, large numbers of patients are exposed to risky and possibly unnecessary procedures. Furthermore, new procedures will be invented and applied with the belief that they are specific and necessary without knowing whether this is true [53]. It is currently felt to be unethical to deliver new drug treatments without testing them for their specific effects against placebo comparison arms [54]. Why should it be different with invasive procedures?
However, is it even possible to properly test invasive procedures against sham comparisons? Blinding of patients, who are both recipients of the interventions and assessors of subjective outcomes, is challenging. Mimicking a complex, invasive procedure such as surgery or insertion of a scope or a needle requires an elaborate sham procedure. Double-blinding is not possible as the surgeon knows which procedure is applied. In addition, there is significant controversy over the ethics of using sham procedures, even with carefully informed patients, further restricting the number of such studies being done [55,56]. Placebo controls are controversial in general, and recommending sham surgery procedures even more so [57]. As patients report between 60% and 70% reduction in pain after invasive procedures, why not just compare them with proven treatments?
Would doing sham surgical studies change practice? The answer seems to be “sometimes.” When sham internal mammary studies of angina were published in the 1960s, the use of this procedure rapidly dropped off and was replaced by coronary bypass grafting, which has never been tested against sham bypass. However, only marginal changes have occurred in the use of vertebroplasty for low back pain after two studies reported no benefit of real over sham procedures [58]. When these studies were published, the accompanying editorial rationalized their continued use under the guise of “patient-centered” care and “informed choice” [59]. However, passing choice for interventions over to patients, especially when the evidence is controversial, should not be used as a substitute for evidence-based professional recommendations. A recent study of PCI stenting for angina showed no difference in pain or function compared with sham PCI, but the impact on this practice has yet to be determined [60].
The medical profession needs more nonpharmacological approaches for chronic pain, so it is unfortunate that the current evidence does not support the efficacy of invasive procedures for this problem. The implications of continuing to use these procedures without knowing whether they provide specific benefit are in urgent need of further research and discussion. In the meantime, it seems prudent that invasive procedures for chronic pain be avoided unless done as part of a clinical research study.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Ms. Lexie Robinson, Ms. Viviane Enslein, Ms. Cindy Lentino, and Ms. Charmagne Paat for their administrative support throughout the project. This project is based on a larger project recently published in BMJ Open: Jonas WB, Crawford C, Colloca L, et al. To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomized, sham controlled trials. BMJ Open 2015;5:e009655 [17].
Supplementary Data
Supplementary data may be found online at http://painmedicine.oxfordjournals.org.
Funding sources: Funding was provided through the support of Samueli Institute. Karin Meissner received support from the Theophrastus Foundation and the Schweizer-Arau Foundation, Germany.
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work. The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
References
- 1. Institute of Medicine Committee on Advancing Pain Research. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press, National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 2. Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D.. Prevalence of chronic pain in a representative sample in the United States. Pain Med 2008;9(7):803–12. [DOI] [PubMed] [Google Scholar]
- 3. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008;9(10):883–91. [DOI] [PubMed] [Google Scholar]
- 4. Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015;16(8):769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anastassopoulos KP, Chow W, Tapia CI, et al. Reported side effects, bother, satisfaction, and adherence in patients taking hydrocodone for non-cancer pain. J Opioid Manag 2013;9(2):97–109. [DOI] [PubMed] [Google Scholar]
- 6. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. Phys Ther 2015;95(2):e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedly J, Standaert C, Chan L.. Epidemiology of spine care: The back pain dilemma. Phys Med Rehabil Clin N Am 2010;21(4):659–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanna A, Gougoulias N, Longo UG, Maffulli N.. Minimally invasive total knee arthroplasty: A systematic review. Orthop Clin North Am 2009;40(4):479–89, viii. [DOI] [PubMed] [Google Scholar]
- 10. Donnez J, Squifflet J, Donnez O.. Minimally invasive gynecologic procedures. Curr Opin Obstet Gynecol 2011;23(4):289–95. [DOI] [PubMed] [Google Scholar]
- 11. Agency for Healthcare Research and Quality. 2014 National Statistics. Washington, DC: Department of Health and Human Services; 2014. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=H4sIAAAAAAAAACtL8_MMNjRJzcsxTEtLdEnKTCpOKghJSkxKSgOD1EwYlQoAIP7_XSsAAAA2B3291FD61B52E5F061C46BD0964691A90CEC744.
- 12. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361(6):557–68. [DOI] [PubMed] [Google Scholar]
- 13. Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361(6):569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moseley JB Jr, O'Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347(2):81–8. [DOI] [PubMed] [Google Scholar]
- 15. Gu A, Gu C, Ahmed A, et al. Sham surgical procedures for pain intervention resulting in significant improvement in pain: Systematic review and meta-analysis. J Clin Epidemiol 2017;16:1–4. [DOI] [PubMed] [Google Scholar]
- 16. Pain Management Task Force. Final Report May 2010. Washington, DC: Defense and Veterans Center for Integrative Pain Management; 2010. Available at: http://www.dvcipm.org/files/reports/pain-task-force-final-report-may-2010.pdf.
- 17. Jonas WB, Crawford C, Colloca L, et al. To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open 2015;5(12):e009655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Cochrane Collaboration. The Cochrane Collaboration’s tool for assessing risk of bias. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 510. 2011. Available at: http://handbook.cochrane.org/chapter_8/8_5_the_cochrane_collaborations_tool_for_assessing_risk_of_bias.htm; wwwhandbookcochraneorg2011. (Accessed January 13, 2018).
- 19. Follmann D, Elliott P, Suh I, Cutler J.. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45(7):769–73. [DOI] [PubMed] [Google Scholar]
- 20. Gøtzsche P. Why we need a broad perspective on meta-analysis. BMJ 2000;321(7261):585.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kriston L. Dealing with clinical heterogeneity in meta-analysis. Assumptions, methods, interpretation. Int J Meth Psych Res 2013;22(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egger M, Smith GD, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL.. Defining the clinically important difference in pain outcome measures. Pain 2000;88(3):287–94. [DOI] [PubMed] [Google Scholar]
- 24. GRADE Working Group. The Grading of Recommendations Assessment, Development and Evaluation. 2000. Available at: http://www.gradeworkinggroup.org/.
- 25. van Kleef M, Barendse GA, Kessels A, et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976) 1999;24(18):1937–42. [DOI] [PubMed] [Google Scholar]
- 26. Leclaire R, Fortin L, Lambert R, Bergeron YM, Rossignol M.. Radiofrequency facet joint denervation in the treatment of low back pain: A placebo-controlled clinical trial to assess efficacy. Spine 2001;26(13):1411–6; discussion 7. [DOI] [PubMed] [Google Scholar]
- 27. Freeman BJ, Fraser RD, Cain CM, Hall DJ, Chapple DC.. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976) 2005;30(21):2369–77; discussion 78. [DOI] [PubMed] [Google Scholar]
- 28. Nath S, Nath CA, Pettersson K.. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: A randomized double-blind trial. Spine 2008;33(12):1291–7. [DOI] [PubMed] [Google Scholar]
- 29. Patel N, Gross A, Brown L, Gekht G.. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med 2012;13(3):383–98. [DOI] [PubMed] [Google Scholar]
- 30. Moseley JB Jr, Wray NP, Kuykendall D, Willis K, Landon G.. Arthroscopic treatment of osteoarthritis of the knee: A prospective, randomized, placebo-controlled trial: Results of a pilot study. Am J Sports Med 1996;24(1):28–34. [DOI] [PubMed] [Google Scholar]
- 31. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: A sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum 2002;46(1):100–8. [DOI] [PubMed] [Google Scholar]
- 32. Sihvonen R, Paavola M, Malmivaara A, et al. ; Group FDMLSF. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013;369(26):2515–24. [DOI] [PubMed] [Google Scholar]
- 33. Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA.. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med 1959;260(22):1115–8. [DOI] [PubMed] [Google Scholar]
- 34. Dimond EG, Kittle CF, Crockett JE.. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol 1960;5:483–6. [DOI] [PubMed] [Google Scholar]
- 35. Salem M, Rotevatn S, Stavnes S, et al. Release of cardiac biochemical markers after percutaneous myocardial laser or sham procedures. Int J Cardiol 2005;104(2):144–51. [DOI] [PubMed] [Google Scholar]
- 36. Salem M, Rotevatn S, Stavnes S, et al. Usefulness and safety of percutaneous myocardial laser revascularization for refractory angina pectoris. Am J Cardiol 2004;93(9):1086–91. [DOI] [PubMed] [Google Scholar]
- 37. Leon MB, Kornowski R, Downey WE, et al. A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005;46(10):1812–9. [DOI] [PubMed] [Google Scholar]
- 38. Swank D, Swank-Bordewijk S, Hop W, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: A blinded randomised controlled multi-centre trial. Lancet 2003;361(9365):1247–51. [DOI] [PubMed] [Google Scholar]
- 39. Cote G, Imperiale T, Schmidt S, et al. Similar efficacies of biliary, with or without pancreatic, sphincterotomy in treatment of idiopathic recurrent acute pancreatitis. Gastroenterology 2012;143(6):1502–9.e1. [DOI] [PubMed] [Google Scholar]
- 40. Boelens O, Assen T, Houterman S, Scheltinga M, Roumen R.. A double-blind, randomized, controlled trial on surgery for chronic abdominal pain due to anterior cutaneous nerve entrapment syndrome. Ann Surg 2013;257(5):845–9. [DOI] [PubMed] [Google Scholar]
- 41. Sutton CJ, Pooley AS, Ewen SP, Haines P.. Follow-up report on a randomized controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal to moderate endometriosis. Fertil Steril 1997;68(6):1070–4. [DOI] [PubMed] [Google Scholar]
- 42. Sutton CJ, Ewen SP, Whitelaw N, Haines P.. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril 1994;62(4):696–700. [DOI] [PubMed] [Google Scholar]
- 43. Abbott J, Hawe J, Hunter D, et al. Laparoscopic excision of endometriosis: A randomized, placebo-controlled trial. Fertil Steril 2004;82(4):878–84. [DOI] [PubMed] [Google Scholar]
- 44. Jarrell J, Mohindra R, Ross S, Taenzer P, Brant R.. Laparoscopy and reported pain among patients with endometriosis. J Obstet Gynaecol Can 2005;27(5):477–85. [DOI] [PubMed] [Google Scholar]
- 45. Jarrell J, Brant R, Leung W, Taenzer P.. Women’s pain experience predicts future surgery for pain associated with endometriosis. J Obstet Gynaecol Can 2007;29(12):988–91. [DOI] [PubMed] [Google Scholar]
- 46. Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP.. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989;320(2):82–7. [DOI] [PubMed] [Google Scholar]
- 47. Toouli J, Roberts-Thomson IC, Kellow J, et al. Manometry based randomised trial of endoscopic sphincterotomy for sphincter of Oddi dysfunction. Gut 2000;46(1):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dowson A, Mullen MJ, Peatfield R, et al. Migraine Intervention With STARFlex Technology (MIST) trial: A prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008;117(11):1397–404. Erratum appears in Circulation 2009;120(9):E71–2. [DOI] [PubMed] [Google Scholar]
- 49. Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg 2009;124(2):461–8. [DOI] [PubMed] [Google Scholar]
- 50. Mitka M. Bariatric surgery continues to show benefits for patients with diabetes. JAMA 2012;307(18):1901–2. [DOI] [PubMed] [Google Scholar]
- 51. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366(17):1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo X, Pietrobon R, Sun SX, Liu GG, Hey L.. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976) 2004;29(1):79–86. [DOI] [PubMed] [Google Scholar]
- 53. Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG.. Prescribing “placebo treatments”: Results of national survey of US internists and rheumatologists. BMJ 2008;337:a1938.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Temple R, Ellenberg SS.. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: Ethical and scientific issues. Ann Intern Med 2000;133(6):455–63. [DOI] [PubMed] [Google Scholar]
- 55. Miller FG, Kaptchuk TJ.. Sham procedures and the ethics of clinical trials. J R Soc Med 2004;97(12):576–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miller FG, Wendler D.. The ethics of sham invasive intervention trials. Clin Trials 2009;6(5):401–2. [DOI] [PubMed] [Google Scholar]
- 57. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ 2001;79(4):373. [PMC free article] [PubMed] [Google Scholar]
- 58. Kroon F, Staples M, Ebeling PR, et al. Two-year results of a randomized placebo-controlled trial of vertebroplasty for acute osteoporotic vertebral fractures. J Bone Miner Res 2014;29(6):1346–55. [DOI] [PubMed] [Google Scholar]
- 59. Buchbinder R, Osborne R, Staples M.. Trials of vertebroplasty for vertebral fractures-reply. N Engl J Med 2009;361(21):2097–100. [DOI] [PubMed] [Google Scholar]
- 60. Wise J. Heart stents for stable angina show no benefit over placebo, study finds. BMJ 2017;359:j5076. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.