Abstract
Background
Autoimmunity associated with autoantibodies against the β1-adrenergic receptor (β1-AAB) is increasingly accepted as the driver of human dilated cardiomyopathy (DCM). Unfortunately, there is a lack of animal models to extend the knowledge about β1-AAB autoimmunity in DCM and to develop appropriate treatment strategies.
Objectives
To introduce an animal model, we investigated the β1-AAB associated autoimmunity in Doberman Pinscher (DP) with dilated cardiomyopathy, which has similarities to human DCM.
Materials and methods
Eighty-seven DP with cardiomyopathy in terms of pathological ECG and echocardiography (DoCM) and 31 dogs (at enrollment) without DoCM (controls) were analyzed for serum activity of β1-AAB with a bioassay that records the chronotropic response of spontaneously beating cultured neonatal rat cardiomyocytes to the DP’s IgG. To locate the receptor binding site of β1-AAB and the autoantibody’s sensitivity to inhibition, competing experiments with related blockers were performed with the bioassay. In controls that developed DoCM during follow-up, β1-AAB were analyzed during progress.
Results
Fifty-nine (67.8%) DoCM dogs and 19 (61.3%) controls were β1-AAB positive. Of the controls that developed DoCM, 8 were β1-AAB positive (p = 0.044 vs. dogs remaining in the control group); their β1-AAB activity increased with the cardiomyopathy progress (p<0.02). To supplement DoCM group with the 9 animals which developed cardiomyopathy in the follow up, a more pronounced β1-AAB positivity became visible in the DoCM group (p = 0.066).
Total and cardiac mortality were higher in β1-AAB positive DP (p = 0.002; p = 0037). The dogs’ β1-AAB recognized a specific epitope on the second extracellular receptor and were sensitive to inhibition by drugs already successfully tested to inhibit the corresponding human autoantibody.
Conclusions
Doberman Pinschers presented β1-AAB associated autoimmunity, similar as in the pathogenesis of human DCM. Consequently, DP could compensate the lack of animal models for the investigation of β1-AAB autoimmunity in human DCM.
Introduction
Autoimmunity is increasingly accepted as the origin or amplifier of heart failure [1]. For cardiomyopathies, preferably for idiopathic dilated cardiomyopathy (DCM) (recently recalculated prevalence of 1 in 250–400 individuals for the US [2]) and for those cardiomyopathies secondary to non-ischemic causes, such as myocarditis, autoimmunity associated with multiple autoantibodies to heart antigens was discussed to break the self-tolerance that causes or supports disease pathogenesis [3]. Among the autoantibodies, there are “classic” ones which induce immune responses, resulting in the destruction of affected tissues, whereby autoantibodies directed against contractile elements such as anti-myosin and anti-troponin autoantibodies are particularly important [4, 5].
Starting in the 1970s, the classic autoantibodies were supplemented by an additional class of autoantibodies that bind to G-protein coupled receptors (GPCR-AAB). After receptor binding, GPCR-AAB, in the majority of cases, agonistically activate their related receptors in a similar way to the physiological agonists; therefore GPCR-AAB were called “functional autoantibodies”. However, mechanisms for the prevention of over-boarding receptor stimulation such as receptor down-regulation and desensitization, which are well known with physiological agonists, are lacking for GPCR-AAB. Consequently, GPCR-AAB are discussed as disease drivers as repeatedly summarized in [6–9].
With the finding of GPCR-AAB such as directed against the β1-adrenergic receptor (β1-AAB) and muscarinic receptor 2 (M2-AAB) [10, 11] in patients with DCM of non-ischemic reasons, an autoimmune background specifically associated with GPCR-AAB emerged. In contrast, patients with ischemic cardiomyopathy and healthy individuals carry GPCR-AAB only in very small amounts or these are completely absent [9].
GPCR-AAB directed against the β1-adrenerdic receptor are seen in 70–80% of patients with non-ischemic DCM [6–9].
In a distinct group of these patients, preferentially those suffering from arrhythmia, M2-AAB were additionally found with a prevalence of up to 40% [6]. Among all heart specific classical and functional autoantibodies, however, the strongest pathogenicity has been demonstrated related to human DCM for β1-AAB, so that they are increasingly accepted as pathogenic drivers and treatment targets, as summarized in [6–9].
Three lines of investigation have established the concept of β1-AAB dependent autoimmunity in the pathogenesis of human DCM; first, experiments using myocardial cells to demonstrate the cardio-pathogenic effects of β1-AAB at the cellular and subcellular levels; second, animal experiments where rodents were immunized for β1-AAB generation to demonstrate their cardio-pathogenic effects; and third, and most impressive, clinical trials demonstrating the benefit to DCM patients when specifically their β1-AAB were removed by immunoadsorption (IA) [12, 13]. This treatment option is now increasingly accepted for DCM patients positive for β1-AAB. However, to overcome the problems of IA resulting from costs, logistics and patient burden, drug-associated treatment concepts for the in vivo neutralization of β1-AAB come increasingly to the fore [14, 15]. In order to manifest and extend the knowledge about functional autoantibodies in human DCM in general and specifically of β1-AAB and even more to prove related treatment concepts in pre-clinical studies, animal models more related to human DCM than the more artificial rodent immunization models are unfortunately lacking [16, 17].
Although, there are also rodent models with naturally occurring DCM and transgenic mouse lines that were engineered for cardiomyopathy development. There are even mice crossed from transgenic and knockout ones which develop a “so-called” autoimmune cardiomyopathy [18, 19]. However, there is currently no evidence that such rodents may be suitable to eliminate the lack of models for analyzing the pathogenic role of functional autoantibody associated autoimmunity in human DCM and specifically the role of β1-AAB and M2-AAB as a driver and treatment target. What’s more, due to several disadvantages between small animal models such as mice and rats as well as human, e.g. in genetic regulation and cardiac performance, listed in [19, 20], it was stated that “rodents are phylogenetically very distant from human and some pathophysiological features of diseases and their response to pharmacological treatment may not be reliable predictors” [18]. Consequently, “for research aimed at clinical translation, it is imperative that initial results from small rodent studies be confirmed in a large animal model that more closely resembles humans …” [18] and there is “… a simple rule, the closer the heart or body weight of the animal to human heart and body, the more similar are the hearts” [18]. Among the large animals that can be used as models for human DCM, Doberman Pinscher (DP) should be of great interest due to their frequent development of dilated cardiomyopathy [21], which has many similarities with human DCM [22–26].
Doberman cardiomyopathy is characterized by three stages. DP in stage one are presumed to have genetic mutations which lead to myocardial alteration on a subcellular level but the majority of cellular changes that occur is still unknown [27, 28]. However, affected heart mitochondrial protein expression, increased oxidative stress and apoptosis have been evidenced [29]. In this stage, approximately corresponding to NYHA class 1 of human heart failure, the heart is electrically and morphologically normal [24, 30]. Dogs in stage two, (NYHA class 2) have either ventricular premature complexes (VPC) or a systolic dysfunction, or both; overt clinical signs are, however, absent. Related to the dog owners’ point of view, the stage 2 is called “occult stage”. Dogs in stage three (NYHA class 3/4) present with clinical signs, very similar as found in human heart failure, such as congestive heart failure (CHF), arrhythmia, syncope and exercise intolerance [21]. This stage is referred to as the overt stage of Doberman cardiomyopathy.
Here, we demonstrate for the first time that DP frequently carry β1-AAB that could act as a pathogenic driver in the pathogenesis of cardiomyopathy in a similar way to β1-AAB in human DCM [6–9]. Therefore, we suggest that DP could be a suitable model for basic investigation to determine the relationship between β1-AAB-associated autoimmunity and cardiomyopathy, and even more importantly, to prove treatment concepts to counteract β1-AAB in vivo.
Materials and methods
The study was conducted in accordance with the German animal welfare law. The study protocol was approved by the “Regierung von Oberbayern—Sachgebiet 54, Verbraucherschutz und Veterinärwesen (approval number 55.2-1-54-2532-35-2016)”. DP were enrolled based on owner study agreement.
Animals
Client-owned purebred DP attending the Cardiology Department of “Medizinische Kleintierklinik, Ludwig-Maximilians-Universität München” for routine check-up, cardiomyopathy diagnostics or cardiomyopathy follow-up were analyzed for β1-AAB and M2-AAB.
Based on owner study agreement a total of 118 DP (male: n = 60; 50.8%, female: n = 58; 49.2%) between 1 and 13 years old (median 6 years) were enrolled. To identify Doberman cardiomyopathy, Holter-ECG was performed for the detection of arrhythmia and echocardiography to evidence cardiac dysfunction. In parallel, blood was sampled for the measurement of functional autoantibodies. Based on the guidelines of the European Society of Veterinary Cardiology [31], Stage 2 Doberman cardiomyopathy (subsequently referred to as DoCM) was diagnosed for dogs with >300 VPC/24h or two subsequent examinations within a year showing between 50 and 300 VPC/24h [32] and echocardiographic indicative for cardiac dysfunction. For that purpose, the left ventricular end-systolic (ESVI) and end-diastolic volume (EDVI) were measured and indexed to body surface area based on Simpson’s method. An ESVI of >55 ml/m2 or/and EDVI of >95 ml2 were considered to be indicative of DCM.
Measurement of autoantibodies directed against the β1-adrenergic receptor (β1-AAB) and muscarinic receptor 2 (M2-AAB)
To measure β-AAB and M2-AAB, a bioassay established by Wallukat and Wollenberger was used [11], which was modified and standardized as described in [33]. In this bioassay, the chronotropic response of spontaneously beating cultured neonatal rat cardiomyocytes to the IgG prepared from the dogs’ serum was recorded. For this purpose, six fields with synchronic and rhythmic beating cardiomyocytes on the culture flask were marked. The basal beating rate of the six fields were counted for 15 seconds and average. After addition of the IgG preparation to the culture flasks and incubation for 40 to 60 min at 37°C, the beating rate in the six fields were counted again for 15 s and average. (1 unit of β1-AAB activity = 1 beat/min frequency increase; lower limit of detection (LLD) = 4.0 U; cut off β 1-AAB positivity ≥ 8.0 U; 1 unit of M2-AAB activity = 1 beat/min frequency decrease; lower limit of detection (LLD) = -4.0 U; cut off M2 positivity ≥ -8.0 U).
Through the use of specific blockers of the β1-adrenergic (bisoprolol) and muscarinic receptor 2 (atropine), the cells’ chronotropic response can be attributed to β1-AAB or M2-AAB.
For comprehensive information about sample (IgG) preparation, bioassay test setup and measurement procedure of GPCR-AAB, see [6, 34].
Localization of the receptor binding site with their specific epitope targeted by the autoantibodies directed against the β1-adrenergic receptor (β1-AAB) and muscarinic receptor 2 (M2-AAB)
In human cardiomyopathies [35], β1-AAB targeting the first and second extracellular loops of their related receptor are described. We have therefore tested these two targets for the dogs’ β1-AAB. With regard to M2-AAB in cardiomyopathies, to the best of our knowledge there are only those that target the second extracellular loop of the muscarinic receptor 2 [8]. Due to limited samples, we have therefore only tested the second receptor loop for the M2-AAB of DP.
To localize the extracellular binding site (loops) of the GPCR-AAB, 50 μl of IgG positive for the respective autoantibody was pre-incubated for 30 min with 2 μl of solutions containing synthetic peptides (50 μmol/l) (Biosyntan GmbH, Berlin-Buch, Germany) which represent the first (L1: 122WGRWEYGSFFCEL134) and second extracellular loops (L2: 197HWWRAESDEARRCYNDPKCCDFVTNR222) of the β1-adrenergic receptor and the second extracellular loop (L2: 168VRTVEDGECYIQFFSNAAVTFG189) of the muscarinic receptor 2. 40 μl of this mixture was added to the bioassay for measurement of the autoantibodies’ chronotropic activities. To exclusively localize the extracellular binding site of β1-AAB, the bioassay was performed in the presence of 1μmol/l atropine to block the M2-AAB activity if present in the IgG preparation. To exclusively localize the target of M2-AAB, the bioassay was performed in the presence of 1μmol/l bisoprolol to block β1-AAB activity. A comparable procedure was used to map the specific epitope on the receptor loop targeted by the β1-AAB and M2-AAB. In this case, the bioassay was performed after the pre-treatment of IgG positive for the respective GPCR-AAB with an excess of synthetic peptides (Biosyntan GmbH, Berlin-Buch, Germany), which overlapped to represent the amino acid sequence of the receptor loop; first described for β1-AAB in [35]. For mapping of the β1-AAB targeted epitope on the second extracellular receptor, peptides were used, as follows: P1: HWWRAE, P2: RAESDE, P3: ARRCYND, P4: PKCCDF, and P5: DFVTNR; for M2-AAB epitope mapping: P1: VRTED, P2: EDGECY, P3: CYIQFF, P4: FFSNAA P5: AAVTFG. For this, 50 μl of IgG positive for β1-AAB was pre-incubated for 30 min with 2 μl of solutions containing the synthetic peptides (100 μmol/l) (Biosyntan GmbH, Berlin-Buch, Germany). Then, this mixture was added to the Bioassay for GPCR-AAB measurement. In the case of finding the β1-AAB epitope, the activity was measured as described above in the presence of atropine; for M2-AAB, the bioassay was performed in the presence of bisoprolol.
In vitro indication for the ability to neutralize Doberman pinscher autoantibodies directed against the β1-adrenergic receptor and muscarinic receptor 2 by drugs already successfully tested in animal and clinical studies for the neutralization of human autoantibodies directed against the β1-adrenergic receptor
For this purpose, the chronotropic activity of IgG positive for β1-AAB was monitored in the bioassay after pre-incubation of the IgG with drugs that have already been successfully tested in animal and clinical studies for β-AAB inhibition. We tested here a peptide which mimics the amino acid sequence of the second extracellular loop of the β1-adrenergic receptor (D1) and was synthesized at our request by Biosyntan GmbH, Berlin-Buch, Germany. This peptide acts comparable to the second loop mimics COR-1 which was already studied to counteract β1-AAB in patients with DCM [14]. The other two substances are aptamers [15, 36]: the first (aptamer 110; D2) is able to neutralize only β1-AAB due to specific β1-AAB binding, which was demonstrated in vitro and in an animal study [37, 38], while the second (BC 007; D3), as demonstrated in animal and human studies, is able to inhibit several GPCR-AAB, including β1-AAB and M2-AAB [39, 40]. After pre-incubation of IgG positive for the respective GPCR-AAB with the drugs (test concentration 1 μmol/l), the mixture was added to the bioassay for measurement of the chronotropic activity of IgG.
Statistics
Undetectable marker concentrations (<lower limit of detection, LLD) were numerically expressed as values representing one-half of the LLD. Statistical analysis was performed using the SPSS software package (SPSS Inc., Chicago, US) with Pearson chi-square test and Fisher’s exact tests for the comparison of binary variables. The Kruskal-Wallis H-test combined with the Mann-Whitney U-test for post-hoc analysis was employed for intergroup comparison of continuous data. The Friedman test combined with Wilcoxon test for post-hoc analysis was employed for the intra-individual comparison of continuous data.
For the graphical representation of continuous data of follow up, box plots indicate the median and interquartile range (IQR; 25th and 75th percentiles), while whiskers with ends represent the largest and smallest values inside 1.5 times the IQR, outliers (open circles) representing values between 1.5 and 3 times the IQR, and extremes (stars) placed more than 3 times the IQR. Data demonstrating the receptor binding site of β1-AAB and the autoantibody’s sensitivity to inhibition are expressed as x±SD and were analyzed using the Student`s paired t-test.
Results
Basic characteristics
Among the study cohort of 118 DP as presented in Table 1 and S1 Table, 87 (73.7%) dogs suffered from DoCM which was in age and gender composition comparable to the control group. The cardiomyopathy group consisted of dogs exclusively demonstrating arrhythmias (n = 17; 19.5%—VPC/24h: median 205, min 1, max 6465; twice VPC/24 within one year: 286/173/385; EDVI: 76.45/55/91, ESVI: 40.51/22/54) indicated as the DoCM-VPC group, with exclusively echocardiographic measures outside of the reference intervals (DoCM-ECHO, n = 27; 31.0%—VPC/24h: 5/0/1521; twice VPC/24 within one year: 211/0/1521; EDVI: 107.4/87/196; ESVI: 66.24/50/164) as well as those dogs presenting with arrhythmias and echocardiographic pathologies in combination (DoCM-VPC/ECHO, n = 43; 49.5%—VPC/24h: 700/0/15 000; twice VPC/24 within one year: 279/124/380; ESVI: 106.8/91/160; EDVI: 67.35/42/106). The groups did not differ significantly in age. In terms of gender composition, the groups DoCM-ECHO, DoCM-VPC/ECHO and the control group are comparable, whereas in the group DoCM-ECHO female animals dominated, especially compared to the group DoCM-ECHO (p>0.05). All dogs were in the pre-clinical, occult stage of the disease. Dogs presenting with severe systemic diseases, end-stage heart failure or non-DCM cardiac diseases were excluded.
Table 1. Doberman pinschers under study: Basic characteristics and serum activities of autoantibodies directed against the β1-adrenergic (β1-AAB) and muscarinic receptor 2 (M2-AAB).
| Basic characteristics | Autoantibody presence (n/%) | ||||
|---|---|---|---|---|---|
| β1-AAB (n/%) | M2-AAB (n/%) § | ||||
| (+) | (-) | (+) | (-) | ||
| Total study cohort (n) | 118 | 78/66.1 | 40/33.9 | 7/5.9 | 111/94.1 |
| Age (years; median/min/max | 6/1/13 | ||||
| Male (n/%) | 60/50.8 | ||||
| Female (n/%) | 58/49.2 | ||||
| Lost to follow up | 5/4.2 | ||||
| Survivors (n/%) | 59/50.0 | 31/52.5 | 28/47.5 | 5/8.5 | 54/91.5 |
| Non-survivors (n/%) | 54/45.8 | 43/79.6** | 11/20.4 | 2/3.7 | 52/96.3 |
| Non-Survivors due to cardiac reason (n/%) | 35/29.7 | 26/74.3* | 9/25.7 | 0/0 | 35/100 |
| Non-survivors due to non-cardiac reason (n/%) | 19/16.1 | 17/89.5* | 2/10.5 | 2/10.5 | 17/89.5 |
| Doberman cardiomyopathy total (n/%) | 87/73.7 | 59/67.8 | 28/32.2 | 5/5.7 | 83/94.3 |
| Age (years; median/min/max | 7/2/11 | ||||
| Male (n/%) | 46/52.6 | ||||
| Female (n/%) | 41/47.2 | ||||
| Lost to follow up (n/%) | 2/2.3 | ||||
| Survivors (n/%) | 43/49.4 | ||||
| Non-survivors (n/%) | 42/48.3 | ||||
| Non-survivors due to cardiac reason (n/%) | 30/34.5 | ||||
| Non-survivors due to non-cardiac reason (n/%) | 12/13.8 | ||||
| Arrhythmia exclusively (n/%) Diagnostic criteria VPC/24h > 300 or twice 50–300 VPC/24h within one year | 17/19.5 | 12/70.6 | 5/29.4 | 1/5.9 | 16/94.1 |
| Age (years; median/min/max) | 6/2/10 | ||||
| Male (n/%) | 13/76.5 | ||||
| Female (n/%) | 4/23.5 | ||||
| Medication (n/%) | |||||
| No treatment | 5/29.4 | ||||
| Beta-blocker | 2/11.8 | ||||
| Antiarrhythmic drug | 2/11.8 | ||||
| Antiarrhythmic drug/ACE inhibitor | 8/47.0 | ||||
| Echocardiographic pathologies (n/%) Diagnostic criteria ESVI >55 ml/m2 or EDVI >95 ml/m2 | 27/31.0 | 15/56.6 | 12/44.4 | 0/0 | 27/100 |
| Age (years; median/min/max) | 8/3/11 | ||||
| Male (n/%) | 8/29.6 | ||||
| Female (n/%) | 19/70.4 | ||||
| Medication (n/%) | |||||
| No treatment | 2/7.5 | ||||
| Calcium sensitizer/PDE3 inhibitor | 12/44.5 | ||||
| Calcium sensitizer/PDE3 inhibitor/ACE inhibitor | 13/48.0 | ||||
| Arrhythmia + echocardiographic pathologies Diagnostic criteria VPC/24 >300 or twice 50–300 VPC/24h within one year ESVI >55 ml/m2 or EDVI >95 ml/m2 | 43/49.5 | 32/74.4 | 11/25.6 | 4/9.3 | 39/90.7 |
| Age (years; median/min/max) | 7/2/10 | ||||
| Male (n/%) | 20/46.5 | ||||
| Female (n/%) | 23/53.5 | ||||
| Medication (n/%) | |||||
| No treatment | 1/2.5 | ||||
| Calcium sensitizer/PDE3 inhibitor | 1/2.5 | ||||
| Calcium sensitizer/PDE3 inhibitor/ACE inhibitor | 16/37.2 | ||||
| Calcium sensitizer/PDE3 inhibitor/ACE inhibitor/beta-blocker | 8/18.6 | ||||
| Calcium sensitizer/PDE3 inhibitor/ACE inhibitor/beta-blocker/antiarrhythmic drug | 13/30.2 | ||||
| ACE inhibitor/antiarrhythmic drug | 2/4.6 | ||||
| ACE inhibitor/beta-blocker | 2/4.6 | ||||
| Control group (n/%) Diagnostic criteria VPC/24 <300 or <twice 50–300 VPC/24h within one year ESVI <55 ml/m2 or EDVI <95 ml/m2 | 31/26.3 | 19/61.3 | 12/38.7 | 2/6.5 | 29/93.5 |
| Age (years; median/min/max) | 6/1/13 | ||||
| Male (n/%) | 17/54.8 | ||||
| Female (n/%) | 14/45.2 | ||||
| Lost to follow up (n/%) | 3/9.7 | ||||
| Survivors (n/%) | 16/51.6 | ||||
| Non-survivors | 12/38.7 | ||||
| Non-survivors due to cardiac reason (n/%) | 5/16.1 | ||||
| Non-survivors due to non-cardiac reason (n/%) | 7/22.6 | ||||
§ all M2-AAB positive dogs were also positive for β1-AAB; β1-AAB (+) vs. (-)
* p<0.05;
** p<0.01.
The group of dogs (n = 31; 26.3% of the total number—VPC/24h: 2/0/97; twice VPC/24 within one year: 184/0/279; EDVI: 77.8/53/95; ESVI: 39.3/25/55) which did not fulfill these criteria for DoCM were defined as the primary control group (C). Related to the diagnostic criteria of DoCM used in our study, the control group was composed of healthy animals and those DP at stage 1 of DoCM. At study enrolment, 9 dogs (T0: median age 3 years; min 2, max 3 years) classified into the control group developed cardiomyopathy during the follow-up, diagnosed primarily by VPC detection (T1: median age of 7 years; min 5, max 9 years); they progressed to a diagnosis by the detection of VPCs combined with pathological echocardiography (T2: median age 9 years; min 5, max 9 years).
Autoantibodies directed against the β1-adrenergic and muscarinic receptor 2 in Doberman pinschers at the time of enrolment
As indicated in Table 1 and S1 Table, 78 (66.1%) of the dogs in the total study cohort presented with β1-AAB values outside of the reference range ≥8 U/min, which means that the dogs were positive for β1-AAB. Seven of these dogs showed parallel pathological M2-AAB values. The rest (n = 40; 33.9%) presented with β1-AAB values in the reference range (< 8 U/min). None of these dogs was positive for M2-AAB. The dogs were sub-divided into those with DoCM and those without signs of DoCM (control group) at enrolment; however, 59 (67.8%) of the dogs with DoCM and 19 (61.3%) of the control group were positive for β1-AAB. Among the β1-AAB positive dogs, 5 of the DoCM group and 2 of the control group were also positive for M2-AAB. The remaining 28 (32.2%) in the DoCM group and 12 (38.7%) in the control group were β1-AAB negative and negative for M2-AAB. Both positivity for β1-AAB and negativity, respectively, were not significantly different between the groups. However, the median β1-AAB activity was 19.32 U/min in the DoCM group, which was slightly higher than the 16 U/min reported in the control group.
Among the control dogs, there were 9 dogs, 8 of these with positivity for β1-AAB, who developed DoCM in the follow-up. Excluding these dogs from the control group, the statistical evaluation presented slight significance (p = 0.097) for more β1-AAB positivity in the DoCM group compared with the control group. When reassembling the DoCM group by supplementing it with the 9 animals developing cardiomyopathy in the follow-up, a more pronounced β1-AAB positivity in the DoCM group became marked (p = 0.066).
In the different DoCM groups, there were no significant differences with respect to the positivity of β1-AAB (DoCM-VPC: n = 12, 70.6%; DoCM-Echo: n = 15, 55.6%; DoCM-VPC /Echo: n = 32, 74.4%).
Follow-up of autoantibodies directed against the β1-adrenergic receptor in primary healthy dogs who progress to cardiomyopathy
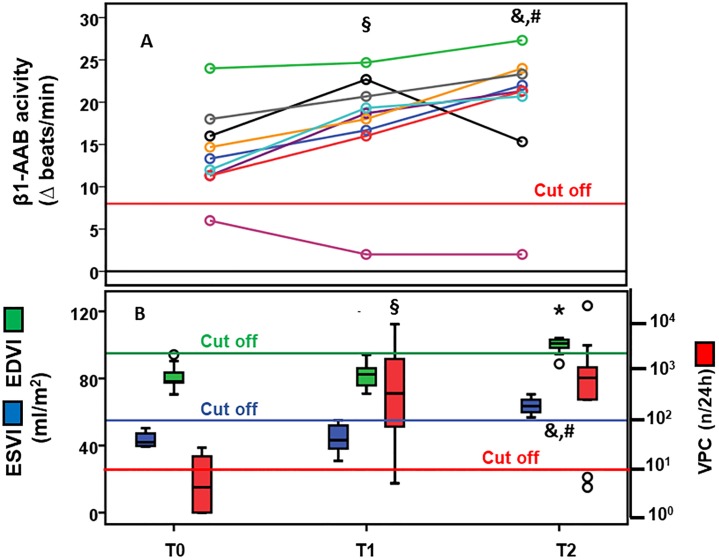
Among the dogs in the control group at the time of enrolment (T0), 9 animals subsequently developed a significant DoCM, indicated first by arrhythmia (T1) and later by arrhythmia in combination with pathological echocardiography (T2). One of these animals was negative for β1-AAB at the time of enrollment and remained negative despite the development of DoCM. The other 8 dogs were β1-AAB positive at study enrolment. Including all 9 dogs in the statistical analysis, the β1-AAB values increased from T0 to T1 (p<0.05) and from T0 to T2 (p<0.05). With regard to the 8 dogs with β1-AAB positivity at enrolment, their β1-AAB increased from T0 to T1 (p<0.02) and from T1 to T2 (p<0.02). The increase in β1-AAB from T1 to T2 was demonstrated for seven DP. However, in the one dog that showed a decrease of β1-AAB from T1 to T2, the β1-AAB value remained well within the pathological range (Fig 1 and S2 Table). In the comparison of dogs who were free of DoCM for the whole study period with those without the signs of DoCM at enrolment but who progressed to DoCM, a significantly higher proportion of β1-AAB positivity (p = 0.044) was calculated for the last animals.
Fig 1. (A) Activity of autoantibodies directed against the β1-adrenergic receptor and (B) left ventricular end-systolic (ESVI), and end-diastolic volume (EDVI) indexed to body surface area and ventricular premature contractions per 24 hours (VPC/24h) in Doberman pinschers (DP) (n = 9) primarily belonging to the control dogs during the development of cardiomyopathy.
(T0 = control group; T1 = cardiomyopathy indicated by arrhythmia; T2 = cardiomyopathy indicated by arrhythmia combined with pathological echocardiography); (A) § T1 vs. T0: p<0.05 in all DP, p<0.02 in β1-AAB positive DP at study enrolment; & T2 vs. T1: p<0 02 in β1-AAB positive DP at study enrolment; # T2 vs. T0: p<0.05 in all DP, p<0.02 in β1-AAB positive DP at study enrolment; (B) § T1 vs. T0: p<0.01 (VPC724h), & T2 vs. T1: p<0.02 (VPC/24h), p<0.01 (EDVI, ESVI), # T2 vs. T0: p<0.02 (VPC724h, EDVI), p<0.01 (ESVI).
Mortality of Doberman pinschers related to autoantibodies directed against the β1-adrenergic receptor
Of the 118 dogs enrolled, 59 (50%) survived the study period, 35 (29.7%) died due to cardiac reason such as sudden death (n = 30; 85.7%) or heart failure (n = 5; 14.3%) and 19 (16.1%) died from non-cardiac reasons (Table 1, S1 Table). The median survival time of dogs in relation to the time of enrolment was 1 year (min 0, max 9 years). Five (4.2%) dogs were lost to follow-up.
Of the surviving dogs, 28 (47.5%) were β1-AAB negative at study enrolment and 31 (52.5%) were β1-AAB positive. In contrast, only 11 (20.4%) of the non-survivors were β1-AAB negative while 43 (79.6%) were positive for β1-AAB, which documents a significantly higher prevalence (p<0.01; odd ratio 3.61 (1.57–8.33) of β1-AAB positivity in the non-survivors.
The increased prevalence of β1-AAB in the non-survivors concerned the dogs that specifically died due to cardiac reasons (n = 9; 25.7% β1-AAB negative vs. n = 26 (74.3%) β1-AAB positive; p<0.05; odds ratio 2.61 (1.05–6.51) but also those died due to non-cardiac reasons (p<0.05; odds ratio 7.93 (1.68–37.49). With respect to M2-AAB, 54 (91.5%) of the surviving dogs were negative at study enrolment and 5 (5.7%) were positive. However, M2-AAB positivity did significantly increase the risk for death.
Characteristic features of autoantibodies directed against β1-adrenergic and muscarinic receptor 2 present in Doberman pinschers
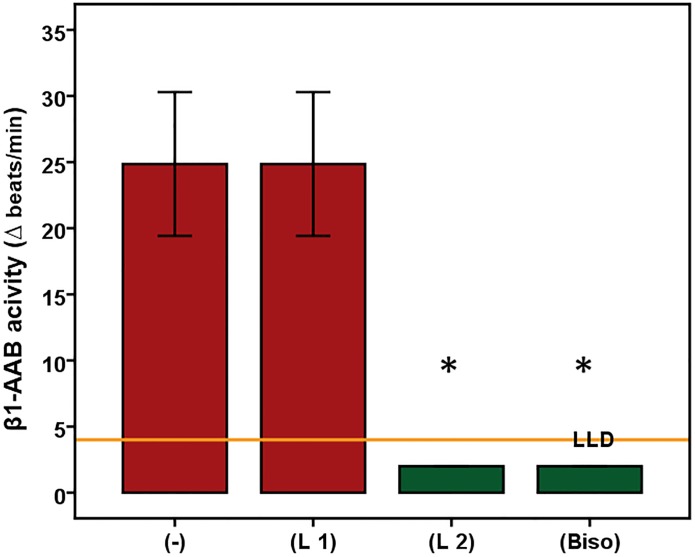
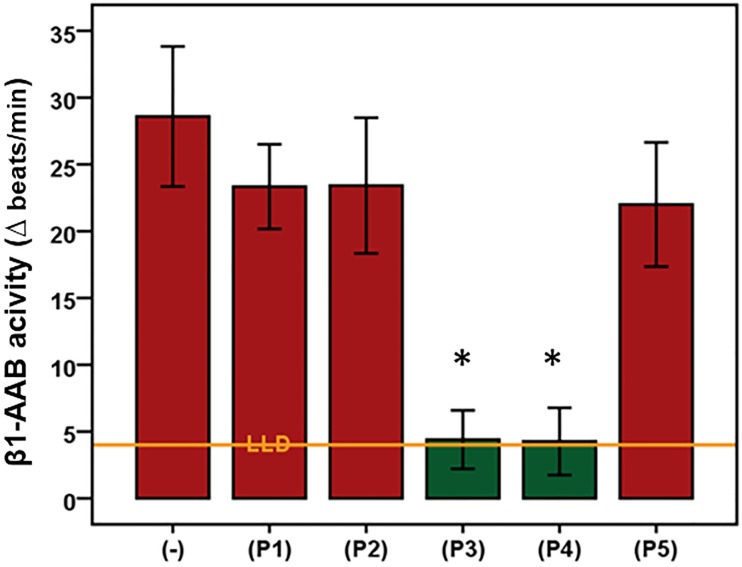
As demonstrated for β1-AAB in Figs 2 and 3 and S3 Table, both β1-AAB and M2-AAB (S3 Table) found in DP targeted the second extracellular loops of the corresponding receptors. In epitope mapping, peptides P3 and P4 blocked β1-AAB activity, which located the epitope for β1-AAB between the amino acids 205 and 218. M2-AAB activity was blocked by the peptide P2 which located the M2-AAB epitope between the amino acids 172 to 176.
Fig 2. Autoantibodies directed against the β1-adrenergic receptor (β1-AAB) of Doberman pinschers (n = 6) target the second extracellular receptor loop.
Using the bioassay of spontaneously beating cultured neonatal rat cardiomyocytes, the chronotropic activities of the Doberman pinschers’ β1-AAB is demonstrated by the absence (-) or presence (L1/L2) of the peptides (L1 = first loop; L2 = second loop) competing with the first and second extracellular receptor loops. To rule out any interference with M2-AAB, if present in the sample, the bioassay was performed in the presence of atropine to block the M2-AAB activity. The control experiment was performed in the presence of bisoprolol (Biso). Values are expressed as x±SD; values below the low limit of detection (LLD) were displayed as half range values. LLD = 4 beats/min. * p<0.001.
Fig 3. Mapping of the second extracellular loop of the β1-adrenergic receptor for epitope localization targeted by the related autoantibodies (β1-AAB) of Doberman pinschers.
Using the bioassay of spontaneously beating cultured neonatal rat cardiomyocytes, the β1-AAB (n = 5) were measured in the absence (-) or presence (P1-P5) of competing peptides that overlapped to represent the second extracellular receptor (P1: HWWRAE, P2: RAESDE, P3: ARRCYND, P4: PKCCDF, and P5: DFVTNR). To rule out any interference with M2-AAB, if present in the sample, the bioassay was performed in the presence of atropine to block the M2-AAB activity. Values are expressed as x±SD; values below the low limit of detection (LLD) were displayed as half range values. LLD = 4 beats/min. * p<0.002.
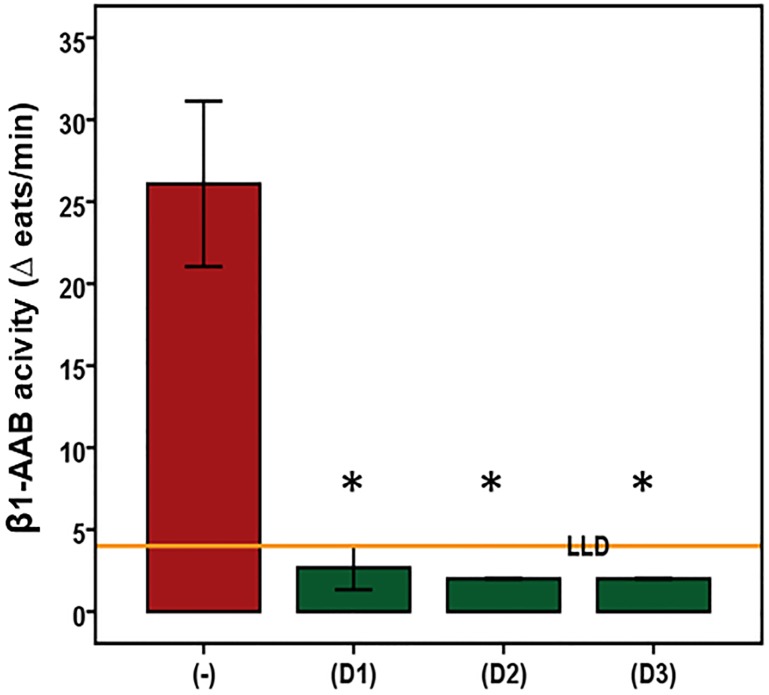
Based on bioassay measurements, Fig 4 and shows that all three drugs that were successful in neutralizing β1-AAB in humans with DCM were also able to neutralize β1-AAB of DP. Compared to the chronotropic activity of the untreated β1-AAB positive IgG of DP, the same IgG preincubated with the drugs showed no chronotropic activity in spontaneously beating neonatal cardiomyocytes. As indicated in S3 Table, BC007 neutralized also M2-AAB.
Fig 4. In vitro indication for the ability to neutralize Doberman pinscher autoantibodies directed against the β1-adrenergic receptor by drugs documented to neutralize human autoantibodies directed against the β1-adrenergic receptor.
Using the bioassay of spontaneously beating cultured neonatal rat cardiomyocytes, the β1-AAB were measured in the absence (-) or presence of the drugs (D1 = second loop peptide (n = 4), D2 = aptamer 110 (n = 4), D3 = aptamer BC 007 (n = 4)). To rule out any interference with M2-AAB, if present in the sample, the bioassay was performed in the presence of atropine to block the M2-AAB activity. Values are expressed as x±SD; values below the low limit of detection (LLD) were displayed as half range values. LLD = 4 beats/min. * p<0.005.
Discussion
Dilated cardiomyopathy with a cumulative prevalence of 58% [21, 31, 41, 42] is the most common form of cardiomyopathy in Doberman pinschers which “… closely resembles the human form of the disease” [25] and therefore repeatedly suggested for modeling human DCM [18, 31]. In the final state, as mentioned already in above, DP with cardiomyopathy present with typical signs such as congestive heart failure (CHF), arrhythmia, syncope and exercise intolerance very similar as found in human heart failure. From an anatomical and morphological points of view, left ventricular chamber dilatation and fibrotic cardiac rearrangement were seen [21].
The majority of dogs (93.5%) do not survive 2 years after the diagnosis of cardiomyopathy [43], which is in agreement with our results. Despite optimal treatment, the survival of the dogs is about 130 days (median) after entering the overt stage [44].
Almost 30 years ago, Smucker et al. [26] suggested that DP should be used as a model for human DCM. Nevertheless, the Doberman pinscher in our eyes has remained a largely unused model for human DCM to this day. DP as a DCM model, although expressly emphasized for its use as a model in investigations of arrhythmia mechanisms such as the pharmacodynamics of antiarrhythmics [22] did not gain widespread acceptance in basic research and also not in pre-clinical studies for the testing of human drugs.
For the pathogenesis of Doberman cardiomyopathy, a genetic background is discussed and an autosomal dominant inheritance was proposed [42]. However, it has been stated that the absence of a specific genetic mutation does not ensure that the dog will never develop cardiomyopathy and a relevant genetic mutation does not guarantee the development of cardiomyopathy [31]. Comparable to the genetic background discussed for Doberman cardiomyopathy, genetic reasons are also assumed to be prominent in human DCM [45]. A familial disease history was found in 25% of the human DCM patients [46] detected by a highly diverse genetic background with several gene mutations that, nevertheless, produces a relatively unique DCM phenotype [2].
For this complexity in the pathogenesis of Doberman and human DCM, consequently, further causes must be considered such as the phenomenon of autoimmunity against the heart. However, it is currently beyond any doubt that autoimmunity is in tight relation to the individuals’ genetic backgrounds [47]. Today, it is increasingly accepted that autoimmunity is an important pathogenic driver of human DCM [48] and that functional autoantibodies such as β1-AAB and M2-AAB diseases have come to the fore [6–9]. Finding a comparable autoimmunity in the Doberman cardiomyopathy, additionally to all the other similarities with human DCM summarized [22–26], would Doberman pinschers predestinate as model to investigate the autoimmune background of human DCM in general and specifically to develop treatment strategies directed to the functional autoantibodies. We present here for the first time data suggesting that the autoimmune background of Doberman cardiomyopathy, as already demonstrated for human DCM, is preferably associated with β1-AAB. Consequently, β1-AAB could control DoCM in a way already discussed for human DCM [6–9].
In our study cohort, DP with DoCM showed a prevalence of β1-AAB of about 70%, comparable as is in human DCM [6]. Furthermore, non-surviving dogs were significantly more often positive for β1-AAB than survivors, which clearly agrees the findings with patients with human DCM studies [49–51].
Interestingly, the dogs of our control group also carried β1-AAB (about 60%). Whether the β-AAB positive dogs are those dogs being genetically compromised for cardiomyopathy and having already subcellular myocardial pathologies and therefore presenting with stage one Doberman cardiomyopathy remains speculative. It remains also speculative whether these dogs are those who progress to stage 2 cardiomyopathy characterized by only arrhythmia and later then additionally by pathological echocardiography. However, 60% of β1-AAB positivity in the control group corresponds to the documented cardiomyopathy prevalence in DP [21]. Additionally, there was a significantly higher frequency of β1-AAB positivity in the control dogs who developed DoCM during the study period which could support the assumption of β1-AAB dependent driving to relevant cardiomyopathy in Doberman pinschers. Related to humans, we discussed the role of β1-AAB autoimmunity in progressing to cardiomyopathy for Chagas’ patients, where 30% of asymptomatic patients were positive for β1-AAB and, based on epidemiologic data, nearly 30% of asymptomatic Chagas’ patients also progress to Chagas’ cardiomyopathy [33]. Taking all of this together, we assume a prominent driving role for β1-AAB associated autoimmunity in the pathogenesis of Doberman cardiomyopathy, as is increasingly being accepted for human DCM. The resembled role of β1-AAB associated autoimmunity in the pathogenesis of Doberman cardiomyopathy and human DCM can also be demonstrated in our eyes by the fact that the β1-AAB level increased in the course of cardiomyopathy progression both in DP and in human patients, the latter being explicitly observed in patients with Chagas’ disease [33]. Consequently, measurement of β1-AAB could be potentially used for monitoring and prognosis of Doberman and Chagas’ cardiomyopathy. Unfortunately, corresponding longitudinal studies focused directly on patients developing DCM are still lacking, but we hope that bio-banking concepts will facilitate the access of such data in the near future.
If we take a look at the characteristics of DP and human β1-AAB, some further analogies were obvious. β1-AAB of DP target the second extracellular receptor loop, where there is an epitope localized centrally and containing a cysteine residues. This epitope localization is comparable with the epitope targeted by β1-AAB found in DCM patients [35, 52]; however, it must be noted that in human DCM there is an additional β1-AAB that targets the first extracellular receptor loop [35]. We did not find such β1-AAB in Doberman pinschers. However, β1-AAB directed against the second extracellular receptor loop were sometimes [53] but not always [35] accused of being the determining cause of DCM.
As for β1-AAB of DCM patients described in [14, 38, 40], the β1-AAB activity of Doberman pinschers could be inhibited by peptides which mimic the second extracellular receptor loop or by aptamers binding the autoantibodies. In our view, this is a further indicator that the β1-AAB associated autoimmunity in Doberman pinschers and human is closely related. Although M2-AAB were found with a clearly lower prevalence in Doberman cardiomyopathy than published for human DCM, the characteristics are comparable. M2-AAB targeted the second extracellular receptor loop which was also published for M2-AAB of human cardiomyopathies, such as DCM and Chagas’ cardiomyopathy [54, 55]. For the Doberman M2-AAB, we have localized the specific epitope in the same region of the second receptor loop that was already described for M2-AAB of patients with DCM (unpublished data) or Chagas’ cardiomyopathy [55]. As for β1-AAB of human DCM patients shown [14, 15, 36], the β1-AAB activity of Doberman pinschers was inhibited by second loop peptide (D1), aptamer 110 (D2) and aptamer BC 007 (D3). The last was also able to neutralize M2-AAB which is in agree with the BC007 as so-called “broad-band neutralizer” of GPCR-AAB [40, 56].
Consequently, we suggest, to take the information about functional autoantibody associated autoimmunity in Doberman cardiomyopathy, together with all the other similarities of Doberman and human DCM as summarized in [22–26], to re-activate Doberman pinschers as a model of human DCM. We suggest to use the Doberman pinscher as model specifically for basic investigation of the functional autoantibody associated autoimmunity and still more importantly for pre-clinical studies in the development of treatment strategies directed against the functional autoantibody associated autoimmunity.
In this context, Doberman pinschers without pathologies in terms of ECG and echocardiography but with β1-AAB positivity (stage 1 cardiomyopathy) should be used as model for the development of strategies to prevent the β1-AAB-associated cardiomyopathy, whereas the β1-AAB positive dogs with stage 2 cardiomyopathy should be used as model for autoimmunity (specifically for β1-AAB) directed treatment studies such as suggested already for human DCM and summarized in [7–9].
From our point of view, this is all the more important since, firstly, none of the small animal models used for the modelling of human DCM to date develop functional autoantibodies and, secondly, the functional autoantibodies found in the immunization models seem, based on ELISA experiments [57] to differ from the human autoantibodies in quality and quantity.
We agree that a large animal model such as the DP is a priori cost-intensive and there are strong requirements based on “World Medical Statement on Animal Use in Biomedical Research” to respect the welfare of animals in general and specifically of large animals such as DP if used for research. However, a study design such as used for the present study with enrolment of client-owned purebred DP attending a veterinary-medical institution for routine check-up, disease diagnostics or follow-up would strongly minimize the cost and guarantee the DP’s welfare. In addition, the DP of our study came from different breeding populations throughout Europe and therefore has a greater genetic diversity than the DP from one litter or only a few and can therefore better reflect the pathogenic situation in human DCM with its already mentioned very different genetic background.
Last but not least, we have learned that dog owners have a great willingness to participate with their dogs in studies that test new treatment options, especially if it is expected that the treatments can also be beneficial to their dog; always provided that a study design is chosen that guarantees a minimal physiological and psychological impairment of the animals, which was ensured in our study by non-invasive heart examination and only blood analysis.
Study limitations
Based on the diagnostic criteria for DoCM used in our study, the control group consisted of healthy animals and DP at stage 1 of DoCM. It is assumed that the dogs at this stage exhibit genetic mutations that already lead to myocardial alteration at the subcellular level without becoming electrically or echocardiographically visible. We suspected that the β1-AAB-positive DP of the control group were those at level 1 of the Doberman cardiomyopathy. In the future, a detailed characterization of the DP with respect to a genetic predisposition is necessary to verify this speculation.
Conclusions
Doberman pinschers with cardiomyopathy presented with typical signs for autoimmunity, preferentially with autoimmunity associated with autoantibodies directed against G-protein coupled receptors, which is closely related to the autoimmunity found in patients with DCM. This, together with the higher prevalence of cardiomyopathy in Doberman pinschers, the fast disease progression and reaching the primary end point of death within around 4 months of entering the severe stage of cardiomyopathy, presents an excellent basis to re-activate Doberman pinschers as a model to study the basics of human DCM. This is specifically the case for GPCR-AAB associated autoimmunity as a disease cause and as a model for pre-clinical studies in drug development aimed at counteracting this form of autoimmunity and consequently DCM.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Satta N, Vuilleumier N. Auto-antibodies as possible markers and mediators of ischemic, dilated, and rhythmic cardiopathies. Curr Drug Targets. 2015;16(4):342–60. Epub 2014/11/28. . [DOI] [PubMed] [Google Scholar]
- 2.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10(9):531–47. Epub 2013/08/01. 10.1038/nrcardio.2013.105 . [DOI] [PubMed] [Google Scholar]
- 3.Caforio AL, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S. Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev. 2013;18(6):715–32. Epub 2012/11/02. 10.1007/s10741-012-9364-5 . [DOI] [PubMed] [Google Scholar]
- 4.Nussinovitch U, Shoenfeld Y. Anti-troponin autoantibodies and the cardiovascular system. Heart. 2010;96(19):1518–24. Epub 2010/09/15. 10.1136/hrt.2010.195255 . [DOI] [PubMed] [Google Scholar]
- 5.Nussinovitch U, Shoenfeld Y. The diagnostic and clinical significance of anti-muscarinic receptor autoantibodies. Clin Rev Allergy Immunol. 2012;42(3):298–308. Epub 2011/01/06. 10.1007/s12016-010-8235-x . [DOI] [PubMed] [Google Scholar]
- 6.Bornholz B, Wallukat G, Roggenbuck D, Schimke I. Chapter 3—Autoantibodies Directed Against G-Protein-Coupled Receptors in Cardiovascular Diseases: Basics and Diagnostics In: Nussinovitch U, editor. The Heart in Rheumatic, Autoimmune and Inflammatory Diseases: Academic Press; 2017. p. 49–63. [Google Scholar]
- 7.Müller J, Wallukat G, Schimke I. Chapter 27—Autoantibody-Directed Therapy in Cardiovascular Diseases In: Nussinovitch U, editor. The Heart in Rheumatic, Autoimmune and Inflammatory Diseases: Academic Press; 2017. p. 659–79. [Google Scholar]
- 8.Becker NP, Goettel P, Mueller J, Wallukat G, Schimke I. Functional autoantibody diseases: Basics and treatment related to cardiomyopathies. Frontiers In Bioscience Landmark. 2019;(24):14-. [DOI] [PubMed] [Google Scholar]
- 9.Becker NP, Muller J, Gottel P, Wallukat G, Schimke I. Cardiomyopathy—An approach to the autoimmune background. Autoimmun Rev. 2017;16(3):269–86. Epub 2017/02/07. 10.1016/j.autrev.2017.01.012 . [DOI] [PubMed] [Google Scholar]
- 10.Fu ML. Anti-M2 muscarinic receptor autoantibodies and idiopathic dilated cardiomyopathy. Int J Cardiol. 1996;54(2):127–35. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 11.Wallukat G, Wollenberger A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed Biochim Acta. 1987;46(8–9):S634–9. Epub 1987/01/01. . [PubMed] [Google Scholar]
- 12.Dandel M, Wallukat G, Englert A, Lehmkuhl HB, Knosalla C, Hetzer R. Long-term benefits of immunoadsorption in beta(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur J Heart Fail. 2012;14(12):1374–88. Epub 2012/08/16. 10.1093/eurjhf/hfs123 . [DOI] [PubMed] [Google Scholar]
- 13.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347(22):1806 Epub 2002/11/29. 10.1056/NEJM200211283472220 . [DOI] [PubMed] [Google Scholar]
- 14.Jahns R, Jahns V, Lohse M, Palm D, inventors; Julius-Maximilians-Universitaet Wuerzburg, assignee. MEANS FOR THE INHIBITION OF ANTI- ss1-ADRENERGIC RECEPTOR ANTIBODIES patent EP1866335 (A2) Abstract of corresponding document: WO2006103101 (A2). 2007 2007/12/19/.
- 15.Schimke I, Haberland A, Wallukat G, inventors; Charite—Universitaetsmedizin Berlin; Max-Delbrueck-Centrum Fuer Molekulare Medizin; Schimke, Ingolf; Haberland, Annekathrin; Wallukat, Gerd, assignee. Use of Aptamers in Therapy and/or Diagnosis of Autoimmune Diseases patent WO2012119938 (A2). 2012 2012/09/13/.
- 16.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113(10):1419–29. Epub 2004/05/18. 10.1172/JCI20149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui S, Fu ML, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Card Fail. 1999;5(3):246–54. Epub 1999/09/25. . [DOI] [PubMed] [Google Scholar]
- 18.Camacho P, Fan H, Liu Z, He JQ. Small mammalian animal models of heart disease. Am J Cardiovasc Dis. 2016;6(3):70–80. Epub 2016/09/30. . [PMC free article] [PubMed] [Google Scholar]
- 19.Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Commun. 2007;31 Suppl 1:35–41. Epub 2007/10/10. 10.1007/s11259-007-0005-8 . [DOI] [PubMed] [Google Scholar]
- 20.Milani-Nejad N, Brunello L, Gyorke S, Janssen PM. Decrease in sarcoplasmic reticulum calcium content, not myofilament function, contributes to muscle twitch force decline in isolated cardiac trabeculae. J Muscle Res Cell Motil. 2014;35(3–4):225–34. Epub 2014/07/25. 10.1007/s10974-014-9386-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wess G, Schulze A, Butz V, Simak J, Killich M, Keller LJ, et al. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med. 2010;24(3):533–8. Epub 2010/03/06. 10.1111/j.1939-1676.2010.0479.x . [DOI] [PubMed] [Google Scholar]
- 22.Hamlin RL. Animal models of ventricular arrhythmias. Pharmacol Ther. 2007;113(2):276–95. Epub 2006/10/31. 10.1016/j.pharmthera.2006.08.006 . [DOI] [PubMed] [Google Scholar]
- 23.Hensley MT, Tang J, Woodruff K, Defrancesco T, Tou S, Williams CM, et al. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J Cell Mol Med. 2017;21(8):1503–12. Epub 2017/03/16. 10.1111/jcmm.13077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Grady MR, O’Sullivan ML. Dilated cardiomyopathy: an update. Vet Clin North Am Small Anim Pract. 2004;34(5):1187–207. Epub 2004/08/25. 10.1016/j.cvsm.2004.05.009 . [DOI] [PubMed] [Google Scholar]
- 25.Petric AD, Stabej P, Zemva A. Dilated cardiomyopathy in Doberman Pinschers: Survival, Causes of Death and a Pedigree Review in a Related Line. J Vet Cardiol. 2002;4(1):17–24. Epub 2002/05/01. 10.1016/S1760-2734(06)70019-4 . [DOI] [PubMed] [Google Scholar]
- 26.Smucker ML, Kaul S, Woodfield JA, Keith JC, Manning SA, Gascho JA. Naturally occurring cardiomyopathy in the Doberman pinscher: a possible large animal model of human cardiomyopathy? J Am Coll Cardiol. 1990;16(1):200–6. Epub 1990/07/01. . [DOI] [PubMed] [Google Scholar]
- 27.Mausberg TB, Wess G, Simak J, Keller L, Drogemuller M, Drogemuller C, et al. A locus on chromosome 5 is associated with dilated cardiomyopathy in Doberman Pinschers. PLoS One. 2011;6(5):e20042 Epub 2011/06/01. 10.1371/journal.pone.0020042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owczarek-Lipska M, Mausberg TB, Stephenson H, Dukes-McEwan J, Wess G, Leeb T. A 16-bp deletion in the canine PDK4 gene is not associated with dilated cardiomyopathy in a European cohort of Doberman Pinschers. Anim Genet. 2013;44(2):239 Epub 2012/07/28. 10.1111/j.1365-2052.2012.02396.x . [DOI] [PubMed] [Google Scholar]
- 29.Lopes R, Solter PF, Sisson DD, Oyama MA, Prosek R. Characterization of canine mitochondrial protein expression in natural and induced forms of idiopathic dilated cardiomyopathy. Am J Vet Res. 2006;67(6):963–70. Epub 2006/06/03. 10.2460/ajvr.67.6.963 . [DOI] [PubMed] [Google Scholar]
- 30.Calvert CA, Meurs KM. Cardiomyopathy in Doberman Pinschers In: Bonagura JD, Twedt DC, editors Current veterinary therapy: St. Louis: Saunders Elsevier; 2009. p. 800–3. [Google Scholar]
- 31.Wess G, Domenech O, Dukes-McEwan J, Haggstrom J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J Vet Cardiol. 2017;19(5):405–15. Epub 2017/10/03. 10.1016/j.jvc.2017.08.006 . [DOI] [PubMed] [Google Scholar]
- 32.Geraghty NK. Vergleich verschiedener Holterkriterien zur Diagnose des arrhythmischen Stadiums der dilatativen Kardiomyopathie beim Dobermann [Text.PhDThesis]: Ludwig-Maximilians-Universität München; 2011.
- 33.Wallukat G, Munoz Saravia SG, Haberland A, Bartel S, Araujo R, Valda G, et al. Distinct patterns of autoantibodies against G-protein-coupled receptors in Chagas’ cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J Am Coll Cardiol. 2010;55(5):463–8. Epub 2010/02/02. 10.1016/j.jacc.2009.06.064 . [DOI] [PubMed] [Google Scholar]
- 34.Wallukat G, Pruss H, Muller J, Schimke I. Functional autoantibodies in patients with different forms of dementia. PLoS One. 2018;13(3):e0192778 Epub 2018/03/15. 10.1371/journal.pone.0192778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27(1):397–406. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 36.Schimke I, Haberland A, Kage A, Wallukat G, Dahmen C, inventors; Charite—Universitaetsmedizin Berlin; Max-Delbrueck-Centrum Fuer Molekulare Medizin; Aptares Ag; Schimke, Ingolf; Haberland, Annekathrin; Kage, Andreas; Wallukat, Gerd; Dahmen, Claudia, assignee. Aptamers That Inhibit Interaction Between Antibody and 2nd Extracellular Loop of Human Beta-1-Adrenergic Receptor patent WO2012000889 (A1). 2012 2012/01/05/.
- 37.Haberland A, Wallukat G, Berg S, Schulz AM, Freyse EJ, Vetter R, et al. Neutralization of pathogenic beta1-receptor autoantibodies by aptamers in vivo: the first successful proof of principle in spontaneously hypertensive rats. Mol Cell Biochem. 2014;393(1–2):177–80. Epub 2014/04/20. 10.1007/s11010-014-2057-8 . [DOI] [PubMed] [Google Scholar]
- 38.Haberland A, Wallukat G, Dahmen C, Kage A, Schimke I. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ Res. 2011;109(9):986–92. Epub 2011/08/27. 10.1161/CIRCRESAHA.111.253849 . [DOI] [PubMed] [Google Scholar]
- 39.Mueller J, Haberland A, Becker N-P, Wenzel K, Wallukat G, Goettel P, et al. THE DNA-BASED THERAPEUTIC AGENT BC 007 COMPLETELY NEUTRALIZES AGONISTIC AUTOANTIBODIES DIRECTED AGAINST β1-ADRENOCEPTORS: RESULTS OF A PHASE 1 TRIAL. Journal of the American College of Cardiology. 2018;71(11 Supplement):A645 10.1016/S0735-1097(18)31186-0 [DOI] [Google Scholar]
- 40.Wallukat G, Muller J, Haberland A, Berg S, Schulz A, Freyse EJ, et al. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis. 2016;244:44–7. Epub 2015/11/20. 10.1016/j.atherosclerosis.2015.11.001 . [DOI] [PubMed] [Google Scholar]
- 41.Calvert CA. Dilated congestive cardiomyopathy in doberman pinschers. Comp Cont Educ Pract. 1986;8(6):417–30. [Google Scholar]
- 42.Meurs KM, Fox PR, Norgard M, Spier AW, Lamb A, Koplitz SL, et al. A prospective genetic evaluation of familial dilated cardiomyopathy in the Doberman pinscher. J Vet Intern Med. 2007;21(5):1016–20. Epub 2007/10/18. . [DOI] [PubMed] [Google Scholar]
- 43.Tidholm A, Jonsson L. A retrospective study of canine dilated cardiomyopathy (189 cases). J Am Anim Hosp Assoc. 1997;33(6):544–50. Epub 1997/11/14. 10.5326/15473317-33-6-544 . [DOI] [PubMed] [Google Scholar]
- 44.O’Grady MR, Minors SL, O’Sullivan ML, Horne R. Effect of pimobendan on case fatality rate in Doberman Pinschers with congestive heart failure caused by dilated cardiomyopathy. J Vet Intern Med. 2008;22(4):897–904. Epub 2008/06/10. 10.1111/j.1939-1676.2008.0116.x . [DOI] [PubMed] [Google Scholar]
- 45.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123(1):19–26. Epub 2013/01/03. 10.1172/JCI62862 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L, et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J. 2018;39(20):1784–93. Epub 2018/01/30. 10.1093/eurheartj/ehx819 . [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–95. Epub 2015/07/28. 10.1111/joim.12395 . [DOI] [PubMed] [Google Scholar]
- 48.Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, et al. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity. 2008;41(1):35–45. Epub 2008/01/08. 10.1080/08916930701619235 . [DOI] [PubMed] [Google Scholar]
- 49.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37(2):418–24. Epub 2001/02/24. . [DOI] [PubMed] [Google Scholar]
- 50.Pei J, Li N, Chen J, Li X, Zhang Y, Wang Z, et al. The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure. Eur J Heart Fail. 2012;14(8):887–94. Epub 2012/06/21. 10.1093/eurjhf/hfs082 . [DOI] [PubMed] [Google Scholar]
- 51.Stork S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152(4):697–704. Epub 2006/09/26. 10.1016/j.ahj.2006.05.004 . [DOI] [PubMed] [Google Scholar]
- 52.Magnusson Y, Marullo S, Hoyer S, Waagstein F, Andersson B, Vahlne A, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86(5):1658–63. Epub 1990/11/01. 10.1172/JCI114888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikolaev VO, Boivin V, Stork S, Angermann CE, Ertl G, Lohse MJ, et al. A novel fluorescence method for the rapid detection of functional beta1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol. 2007;50(5):423–31. Epub 2007/07/31. 10.1016/j.jacc.2007.03.051 . [DOI] [PubMed] [Google Scholar]
- 54.Fu LX, Magnusson Y, Bergh CH, Liljeqvist JA, Waagstein F, Hjalmarson A, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91(5):1964–8. Epub 1993/05/01. 10.1172/JCI116416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallukat G, Nissen E, Morwinski R, Muller J. Autoantibodies against the beta- and muscarinic receptors in cardiomyopathy. Herz. 2000;25(3):261–6. Epub 2000/07/25. . [DOI] [PubMed] [Google Scholar]
- 56.Haberland A, Holtzhauer M, Schlichtiger A, Bartel S, Schimke I, Muller J, et al. Aptamer BC 007—A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur J Pharmacol. 2016;789:37–45. Epub 2016/07/05. 10.1016/j.ejphar.2016.06.061 . [DOI] [PubMed] [Google Scholar]
- 57.Wenzel K, Schulze-Rothe S, Muller J, Wallukat G, Haberland A. Difference between beta1-adrenoceptor autoantibodies of human and animal origin-Limitations detecting beta1-adrenoceptor autoantibodies using peptide based ELISA technology. PLoS One. 2018;13(2):e0192615 Epub 2018/02/10. 10.1371/journal.pone.0192615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.