Abstract
Cervids are known to be reservoirs of zoonotic bacteria transmitted by ticks. This study aimed to identify the Anaplasma species carried by captive red deer and swamp deer in a wild fauna reserve in France. Blood from 59 red deer and 7 swamp deer was collected and analyzed over a period of two years. A semi-nested PCR targeting the 23S rRNA was performed to detect and characterize Anaplasma spp. and determine the presence of zoonotic species. Anaplasma phagocytophilum was identified in 14/59 red deer (23.7%) but it was not identified in any of the swamp deer (7 animals). Three sequences could not be assigned to any particular species based on the 23S rRNA sequences. Complementary nested PCR targeting 16S rRNA, gltA and groEL genes and sequencing analysis then identified these sequences as a recently reported zoonotic species, Anaplasma capra; this species was found in 2 red deer (Cervus elaphus) and 1 swamp deer (Rucervus duvaucelii). This is the first report of the tick-borne zoonotic bacterium A. capra in France, a species otherwise described only in China, Japan, Malaysia and South Korea in goats, sheep, deer, cattle and Japanese serows (Capricornis crispus). While this bacterium may have been introduced into the reserve by infected imported animals, its local epidemiological cycle via tick transmission seems possible as locally born deer were found infected. Diagnostic methods, especially molecular ones, should take into account the potential infection of animals and humans with this species.
Introduction
Bacteria of the genus Anaplasma are obligate intracellular parasites that replicate within the vacuoles of diverse eukaryotic cells (monocytes, granulocytes, erythrocytes, endothelial cells). These bacteria are mainly transmitted by Ixodid ticks and multiply in both invertebrate and vertebrate hosts [1]. The genus Anaplasma includes six recognized species (A. phagocytophilum, A. bovis, A. centrale, A. marginale, A. ovis and A. platys) responsible for anaplasmosis worldwide in a large range of wild and domesticated vertebrates [1]. One of these species, A. phagocytophilum, described in 1994 in the USA as the agent of human granulocytic anaplasmosis (HGA), is now increasingly detected worldwide [1]. In 2015, a new zoonotic species, provisionally named A. capra, was described in humans in China [2]. In a population of 477 hospital patients with a tick-bite history, 28 (6%) were found infected with A. capra with non-specific febrile manifestations. Five people were hospitalized due to severe symptoms. The general clinical features in the patients included fever, headache, and malaise, as well as eschar, lymphadenopathy and gastrointestinal symptoms [2].
Both A. phagocytophilum and A. capra infect diverse domestic (sheep, cattle and goats) and wild ruminant (deer) species, which are considered as reservoirs. In a survey of tick-borne diseases conducted in the Réserve de la Haute Touche, a French wildlife reserve, we investigated the presence of Anaplasma species infecting captive red deer (Cervus elaphus) and swamp deer (Rucervus duvaucelii). Several endangered species such as the swamp deer (CITES appendix I) are maintained ex-situ on the reserve. It is surrounded by a large, forested, wetland area, a biotope favorable to Ixodid ticks, vectors of A. phagocytophilum.
Methods
Animal sampling
In 2015, a molecular survey of Anaplasma spp. infecting deer was started in the Réserve de la Haute Touche, a nature reserve in Indre, France (National Museum of Natural History). Blood samples from 59 red deer and 7 swamp deer were collected between 2015 and 2017. They were used for molecular detection and characterization of Anaplasma spp.. Blood was sampled at the jugular vein on the occasion of animal care (treatments, vaccinations, transfers within the reserve) (authorization 36-145-002). This study has not been reviewed by an ethics committee, as animal samples were taken by the veterinarians from the Park as diagnostic samples. The authorization 36-145-002 is the authorization for the veterinarians to treat and care for the animals in the Park. Animals in this park were suffering from emaciation and sometimes recurrent fever without clear reasons, with unexplained death and weaknesses. As ticks were quite frequent on the vegetation and animals, veterinarians suspected tick-borne diseases. So each time they had to isolate a deer (treatments) they took a blood sample and sent this sample to our lab to check for tick-borne diseases.
Molecular detection and characterization of Anaplasma spp.
Genomic DNA was extracted from blood following previously described protocols [3]. We detected Anaplasmataceae by semi-nested PCR based on the 23S rRNA gene [4] and determined the species by sequencing PCR positive amplicons. A new detected Anaplasma species was further characterized using nested PCR and sequencing of the 16S rRNA, gltA and groEL genes (Table 1). PCR (reagents as well as cycling conditions) and amplicons purification were performed as previously described [3]. Each step (DNA extraction, preparation of PCR mix, PCR, sample dilution for the second PCR, gel electrophoresis) was performed in separate rooms or even buildings. Material was decontaminated from DNA by using hypochlorite. A negative control was included in each extraction and amplification, to control potential contaminations at each of these two procedures. Positive controls were not included to avoid PCR contamination and false positive results. Extraction and PCR were considered as efficient as several positive samples were obtained in each extraction and PCR run (see results section). Bidirectional sequencing was performed to ensure reliable sequences that were further analyzed using the BLASTn (http://www.ncbi.nlm.nih.gov/BLAST/) and CLUSTAL-Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) programs.
Table 1. Nucleotide sequence of primers used in the study.
| Target gene | Primer name | Sequence (5’-3’) | Tm | Amplicon length | Reference |
|---|---|---|---|---|---|
| 23S rRNA | Ana23S-212F | ATAAGCTGCGGGGAATTGTC | 58°C | 696 bp | [4] |
| Ana23S-908R | TGGAGGACCGAACCTGTTAC | [4] | |||
| Ana23S-212F | ATAAGCTGCGGGGAATTGTC | 59°C | 541 bp | [4] | |
| Ana23S-753R | GTGACAGCGTACCTTTTGCA | [4] | |||
| 16S rRNA | Ana16Sup1 | CGGGTGAGTAATGCATAGGA | 58°C | 1089 bp | This study* |
| Ana16Sdo3 | TAGCACGTGTGTAGCCCAC | This study* | |||
| Ana16sIntup1 | AACTCCGTGCCAGCAGCCGCG | 59°C | 581 bp | This study* | |
| Ana16Sdo1 | CCCAACATCTCACGACAC | This study* | |||
| gltA | Outer-F | GCGATTTTAGAGTGYGGAGATTG | 50°C | 1077 bp | [2] |
| Outer-R | TACAATACCGGAGTAAAAGTCAA | [2] | |||
| Inner-F | GGGTTCCTGTCCACTGCTGCGTG | 52°C | 793 bp | [2]** | |
| Inner-R | TTGGATCGTAATTCTTGTAGACC | [2]** | |||
| groEL | Ac-groEL-F1 | GCGAGGCGTTAGACAAGTCCATT | 50°C | 1264 bp | [2] |
| Ac-groEL-R3 | TCCAGAGATGCGAGCGTGTATAG | [2] | |||
| Ac-groEL-F2 | TGCACTGCTGGTCCAAAGGGGCT | 52°C | 1087 bp | This study*** | |
| Ac-groEL-R2 | CAACTTCGCTAGAGCCGCCAACC | This study*** | |||
Phylogenetic analysis
With the aim of identification at the species level, we compared the 16S rRNA gene sequences that we obtained with a set of 25 published sequences from the 8 species of the Anaplasma genus (A. capra, A. phagocytophilum, A. ovis, A. bovis, A. platys, A. marginale, A. centrale, A. odocoilei). The sequence of Ehrlichia chaffeensis was used as an outgroup. We used the Maximum Likelihood method and the bayesian inference method on a length of ca. 458 bp, with the settings described in detail in Fig 1, S1 and S2 Figs. The same analyses were performed using gltA and groEL sequences with a subset of reference sequences representing different Anaplasma species (S1 and S2 Figs).
Fig 1. Evolutionary analysis by Maximum Likelihood method.
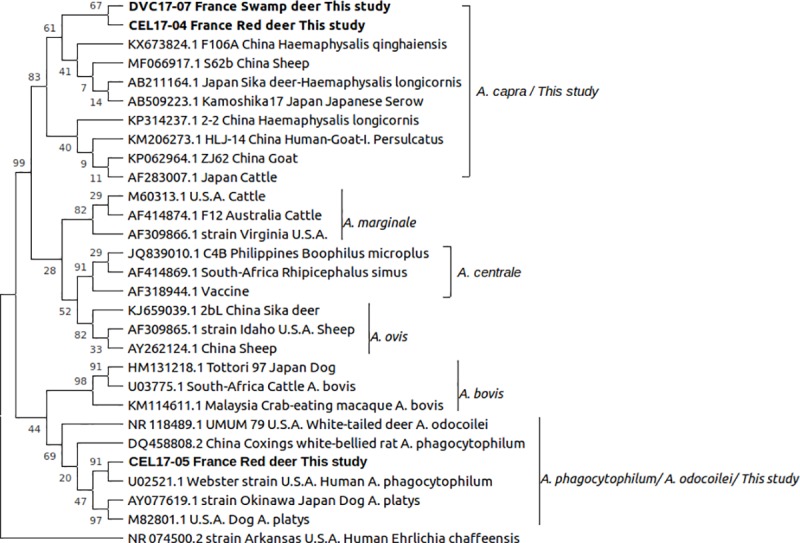
Phylogenetic relationships were inferred by using the Maximum Likelihood method and Tamura-Nei model [6]. The tree with the highest log likelihood (-1030.85) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 1.0683)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 43.67% sites). This analysis involved 29 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 458 positions in the final dataset Evolutionary analyses were conducted in MEGAX [7], with 500 bootstrap interations [8].
Results
Detection of Anaplasma spp. in deer blood
Of the 66 heparin blood samples from red deer and swamp deer, 23S rRNA amplicons of the right size were obtained for 28 samples. A BLASTn search of 23S rRNA sequences identified A. phagocytophilum in 14 red deer (4/21 in 2015, 7/23 in 2016 and 3/15 in 2017) (infection rate of 23.7%) but not in swamp deer. Sequences (lengths between 430–476 bp) were more than 99.5% identical (maximum two mismatches) to A. phagocytophilum strain HZ (GenBank accession number NR_076399). Sequences from 11 amplicons (367 to 476 bp) were identical, with 99.8% identities with Ralstonia pickettii (GenBank accession number CP001644). Three identical sequences obtained from two red deer and one swamp deer (3/66—infection rate 4.5%) gave the highest identities with “Candidatus Anaplasma mediterraneum” sequence (KY498330), described as a potentially new Anaplasma species infecting sheep in Corsica [5] (Table 2). Other genetic markers often used to identify Anaplasma species were then tested to further characterize this Anaplasma species which had never before been described in deer.
Table 2. Percentages of homology by pairwise alignment with published Anaplasma spp. sequences, on the length of the partial sequences obtained in this study.
| Gene | Sequence names, lengths and GenBank accession numbers | Reference organisms and sequences | Identity rate |
|---|---|---|---|
| 23S rRNA | CEL15–367 bp | Cand. A. mediterraneum KY498330 | 99.6% |
| CEL17–506 bp—MH084724 | A. ovis KM021411 | 95.00% | |
| DVC17–515 bp—MH084723 | A. centrale NR-076686 | 94.60% | |
| A. marginale KY498332 | 93.80% | ||
| A. platys KM021412 | 91.60% | ||
| A. phagocytophilum KM021418 | 90.80% | ||
| 16S rRNA | CEL17–531 bp—MH084721 | A. capra KM206273—MF066917 | 99.6% - 99.8% |
| DVC17–518 bp—MH084722 | A. marginale AF414874 | 98.70% | |
| A. centrale AF318944 | 98.50% | ||
| A. ovis AJ633049 | 98.30% | ||
| A. phagocytophilum NR-044762 | 96.40% | ||
| A. platys AY077619 | 96.20% | ||
| groEL | CEL15–559 bp | A. capra KM206275—AB454078 | 91.4% - 97.7% |
| CEL17–1008 bp—MH084718 | A. marginale AF414864 | 83.20% | |
| DVC17–1087 bp—MH084717 | A. centrale EF520691 | 83.20% | |
| A. ovis AF441131 | 83.20% | ||
| A. platys AY044161 | 77.50% | ||
| A. phagocytophilum JF494833 | 76.20% | ||
| gltA | CEL15–729 bp | A. capra KM206274—MG940872 | 87.9% - 98% |
| CEL17–707 bp—MH084720 | A. marginale AF304139 | 74.60% | |
| DVC17–725 bp—MH084719 | A. ovis PKOE01000003 | 74.20% | |
| A. centrale CP001759 | 73.30% | ||
| A. phagocytophilum AY464132 | 65.40% | ||
| A. platys EU516387 | 61.40% |
CEL: Cervus elaphus. DVC: Rucervus duvaucelii.
Further molecular characterization of the new Anaplasma spp. from deer in France
Two 16S rRNA identical sequences were obtained from red and swamp deer blood samples. They shared similarity values of over 99.6% with numerous 16S rRNA Anaplasma capra sequences deposited in GenBank. These sequences were obtained from sheep, goat, human blood and ticks from China (MF066917, FJ389574, KM206273, KF728355 respectively), as well as from cattle, sika deer (Cervus nippon), Japanese serows and ticks from Japan (AF283007, AB211164, AB509223, AB454075 respectively). Sequence similarities with other known Anaplasma 16S rRNA sequences were lower than 99% (Table 2).
As Anaplasma capra groEL and gltA sequences were also deposited in GenBank, we amplified and sequenced these genes from our deer blood samples to better characterize this new Anaplasma. The three groEL Anaplasma sequences from the deer were identical and identity rates ranged from 91.4 to 97.7% with the A. capra groEL sequences from China and Japan available in GenBank (Table 2). The similarities with groEL sequences from other related Anaplasma species (A. centrale, A. marginale, A. platys, A. phagocytophilum and A. ovis) fell under 84%. The three gltA Anaplasma sequences from the deer differed by one nucleotide. The identity rates of the longest sequence (729 bp) ranged from 87.9 to 98% with published A. capra gltA sequences from Japan and China. They were lower than 75% (61.4 to 74.6%) with gltA sequences from other Anaplasma species (Table 2). All these data confirmed the identity of the Anaplasma from the French deer as belonging to the A. capra species.
Partial sequences of the 16S rRNA, 23S rRNA, groEL and gltA from A. capra identified from the swamp deer and one red deer were deposited in GenBank (accession numbers MH084717-MH084724 with details in Table 2).
Phylogenetic position of the Anaplasma species detected in this study, based on 16S rRNA, gltA and groEL sequences
In the phylogenetic trees built by the Maximum Likelihood method and by Bayesian inference, two of the sequences (obtained on red deer and swamp deer) clearly clustered among sequences of A. capra, based on 16S rRNA, gltA or groEL genes. Another (obtained from red deer) was close to the sequence of the A. phagocytophilum human reference strain “Webster” (Fig 1 and S1 and S2 Figs). With both methods and the three analyzed gene sequences, there was a strong statistical support for the corresponding subtrees, even when the discrimination between other species in the trees remained unresolved (Fig 1 and S1 and S2 Figs)[6–13].
Persistence of A. capra
The persistence of deer infection by A. capra was analyzed by sampling blood from one of the two infected red deer four months after the initial detection of this unexpected bacterial species. We detected A. capra, with 23S rRNA, 16S rRNA, groEL and gltA sequences 100% identical to the first identified A. capra, with the same distinct nucleotide in the gltA sequence as characterized four months earlier.
Discussion
For about 40% of the positive conventional Anaplasmataceae spp. specific semi-nested PCRs, sequences indicated the amplification of Ralstonia pickettii 23S rRNA, highlighting a lack of specificity of the semi-nested PCR used. As some of our negative controls were also positive, we decided to sequence them, and some sequences corresponding to Ralstonia picketii were found. As this bacterium is a frequent contaminant of all kind of solutions, including ultrapure water [14], our results most probably correspond to R. pickettii contamination of the solutions we used for extraction or PCR, combined with a low specificity of the 23S rRNA nPCR used.
Nested PCR is prone to DNA contamination as amplified PCR products are re-amplified to increase the sensitivity of detection. To avoid these risks, we used different rooms to perform each step, from sample treatment, DNA extraction, PCR to gel electrophoresis. We also made the choice of not using positive controls in our PCR experiments. It was anyway difficult to decide which Anaplasma species we should use as positive controls, as we analyzed blood from very different animals (cervids, but also in the same Reserve bovids, canids, camelids.) that could carry different and “exotic” Anaplasma species (as described in this study with A. capra). As a high proportion of the PCR was positive, and as the sequences were all of good quality, we concluded that the quality of extraction and amplification was sufficient. Moreover, false negative results due to a bad DNA quality are not really a concern in our study, that aims to describe the presence of a new species in two deer species in France, and not to provide a precise infection rate.
In this survey, we detected two Anaplasma species infecting deer. A. phagocytophilum was detected with a rather moderate prevalence (23.7%) only in red deer. These animals are captive in fenced enclosures. The prevalence of A. phagocytophilum infection in wild red deer varies widely across Europe, from 1.5% in Austria, 10.9% in Portugal, 40–75% in Italy, 80.8% in Spain, and 97.9 to 100% in central Europe (respectively in Slovakia and Hungary) [15–21]. Captive deer are probably less prone to tick bites than wild deer due to grazing area management. This result nonetheless indicates the contact of red deer with ticks and the transmission of A. phagocytophilum in the reserve. Swamp deer were not found infected with A. phagocytophilum, a result which could be attributed to the small number of animals analyzed (7) combined with a low infection rate. There are no data about tick-transmitted pathogens for this endangered species, so the susceptibility of swamp deer to A. phagocytophilum is unknown. A recently described Anaplasma species, A. capra, was detected and identified in both deer species, in a much lower proportion of animals (4.5%). A. capra has already been detected in various wild and domestic ruminant hosts: sheep, goats, cattle, sika deer, Japanese serows, takin (Budorcas taxicolor), forest musk deer (Moschus berezovskii) and Reeves's muntjac (Muntiacus reevesi) but its localization was up to now geographically restricted to Asia (China, Japan, Malaysia and South Korea) [22–28]. Human infection by this newly-described species has been reported in northeast China, leading to the hospitalization of some individuals [2]. The detection and identification of A. capra based on several molecular markers in our study represents the first evidence of this potentially new zoonotic species in Europe (France) in two new hosts, red deer and swamp deer. The assignation to the A. capra species was based on 16S rRNA homologies, since differences lower than 0.5% were obtained with previously characterized A. capra 16S rRNA sequences (homologies of 99.6 to 99.8% on the length of the sequenced fragment, i.e. about 458 bp)[29]. All six phylogenetic trees based on three different genes also support the clustering of french deer sequences within the A. capra species.
The 23S rRNA sequence from A. capra described in our study aligned by BLASTn with an unknown Anaplasma species provisionally named “Candidatus Anaplasma mediterraneum” from sheep in Corsica (France)[5]. Whether “Candidatus Anaplasma mediterraneum” corresponds in fact to A. capra could not be determined, as the only other marker used in the Corsica study was rpoB, whereas we, like most authors, used a combination of 16S rRNA, groEL, gltA and msp4 sequences to identify and subtype Anaplasma capra [2, 23–28, 30–32].
We have detected A. capra in three different deer since 2015. The first infected and detected red deer was a male originating from France (Theix), while the two others (one red deer and one swamp deer) detected in 2017 and 2018 were both born inside the reserve. It is therefore probable that these two deer acquired A. capra through local transmission, even if A. capra may have been originally introduced into the reserve from an external source. The epidemiological cycle of A. capra seems therefore to be completed locally. The low prevalence of infected deer in the reserve might be due to the introduction having taken place recently. Ticks are the main vectors for Anaplasma species even though other transmission routes have been described for some species (blood-sucking flies and transplacental transmissions) [1]. Although A. capra has been detected in several tick species, Ixodes persulcatus [2], Rhipicephalus microplus [30], Haemaphysalis longicornis [23,31] and Haemaphysalis qinghaiensis [32], vector competence has not yet been proven. As most of these tick species are not present in France, another tick species may be responsible for A. capra transmission in France. The Réserve de la Haute Touche is located in a forested preserve area suitable for ticks, and ticks are commonly found feeding on the animals as well as questing on the vegetation (not shown). Vector identification and vector competence remain to be elucidated.
In this study, we demonstrated the presence in France of the new species A. capra on two new hosts. New studies are required to examine its zoonotic ability, as non-zoonotic genetic variants may exist as described in the case of A. phagocytophilum [1,3]. Diagnostic methods, especially molecular ones, should take into account the potential of infection of animals and humans with this species, as molecular tools are often designed to specifically detect A. phagocytophilum. The vector tick species should be identified to improve our knowledge of the epidemiological cycle of this bacterium in France and to assess the risk of transmission to humans. Deer should therefore be considered as a potential reservoir for A. capra.
Supporting information
The evolutionary history was inferred by using the Maximum Likelihood method and Tamura-Nei model [6]. The tree with the highest log likelihoods are shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites. The rate variation model allowed for some sites to be evolutionarily invariable. This analysis involved respectively 22 and 18 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). Evolutionary analyses were conducted in MEGA X [7, 8]. The reference AF304141 referred as Anaplasma centrale Aomori cattle–Japan in the gltA tree corresponds to a misclassification of this sequence and is not truly A. centrale.
(PDF)
The analyses were performed for each of the three genes (A: rRNA 16S, B: groEL and C: gltA) on the Phylogeny.fr platform [10] and comprised the following steps. Sequences were aligned with MUSCLE (v3.8.31) configured for highest accuracy (MUSCLE with default settings) [11]. After alignment, positions with gaps were removed from the alignment. The phylogenetic tree was reconstructed using the bayesian inference method implemented in the MrBayes program (v3.2.6) [12]. The number of substitution types was fixed to 6. The standard (4by4) model of nucleotide substitution was used, while rates variation across sites was fixed to "invgamma". Four Markov Chain Monte Carlo (MCMC) chains were run for 10000 generations, sampling every 10 generations, with the first 250 sampled trees discarded as "burn-in". Finally, a 50% majority rule consensus tree was constructed. The trees were drawn and annotated with the Mega X software [7].
(PDF)
Acknowledgments
Special thanks go to Alice Brunet, Héloïse Duchêne, Emmanuel Maréchal and Christophe Jubert for handling the deer and for their help in this survey as well as to the entire staff of the La Haute Touche nature reserve for their care of the animals. We also thank Roland Simon for helping to make this study possible.
Data Availability
Gene sequences are available in GenBank under accession numbers MH084717-MH084724.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Battilani M, De Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect Genet Evol. 2017;49:195–211. 10.1016/j.meegid.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 2.Li H, Zheng YC, Ma L, Jia N, Jiang BG, Jiang RR, et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis. 2015;15:663–70. 10.1016/S1473-3099(15)70051-4 [DOI] [PubMed] [Google Scholar]
- 3.Jouglin M, Chagneau S, Faille F, Verheyden H, Bastian S, Malandrin L. Detecting and characterizing mixed infections with genetic variants of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) by developing an ankA cluster-specific nested PCR. Parasit Vectors. 2017;10:377 10.1186/s13071-017-2316-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahmani M, Davoust B, Benterki MS, Fenollar F, Raoult D, Mediannikov O. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp Immunol Microbiol Infect Dis. 2015;39:39–45. 10.1016/j.cimid.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Dahmani M, Davoust B, Tahir D, Raoult D, Fenollar F, Mediannikov O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasit Vectors. 2017;10:302 10.1186/s13071-017-2233-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura K and Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993;10:512–26. 10.1093/oxfordjournals.molbev.a040023 [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Stecher G, Li M, Knyaz C, and Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol and Evol. 2018;35:1547–9. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–91. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 9.Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol. 2010;10:8 10.1186/1471-2148-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–9. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- 13.Chevenet F, Brun C, Banuls AL, Jacq B, Chisten R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439 10.1186/1471-2105-7-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan MP, Pembroke JT, Adley CC. Ralstonia pickettii: a persistent gram-negative nosocomial infectious organism. J Hosp Infect. 2006,62(3):278–84. 10.1016/j.jhin.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 15.Cézanne R, Mrowietz N, Eigner B, Duscher GG, Glawischnig W, Fuehrer HP. Molecular analysis of Anaplasma phagocytophilum and Babesia divergens in red deer (Cervus elaphus) in Western Austria. Mol Cell Probes. 2017;31:55–58. 10.1016/j.mcp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Pereira A, Parreira R, Nunes M, Casadinho A, Vieira ML, Campino L, Maia C. Molecular detection of tick-borne bacteria and protozoa in cervids and wild boars from Portugal. Parasit Vectors. 2016;9:251 10.1186/s13071-016-1535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebani VV, Rocchigiani G, Bertelloni F, Nardoni S, Leoni A, Nicoloso S, Mancianti F. Molecular survey on the presence of zoonotic arthropod-borne pathogens in wild red deer (Cervus elaphus). Comp Immunol Microbiol Infect Dis. 2016;7:77–80. 10.1016/j.cimid.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Di Domenico M, Pascucci I, Curini V, Cocco A, Dall'Acqua F, Pompilii C, Cammà C. Detection of Anaplasma phagocytophilum genotypes that are potentially virulent for human in wild ruminants and Ixodes ricinus in Central Italy. Ticks Tick Borne Dis. 2016;7:782–787. 10.1016/j.ttbdis.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 19.García-Pérez AL, Oporto B, Espí A, del Cerro A, Barral M, Povedano I, Barandika JF, Hurtado A. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick Borne Dis. 2016;7(2):264–9. 10.1016/j.ttbdis.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 20.Kazimírová M, Hamšíková Z, Špitalská E, Minichová L, Mahríková L, Caban R, et al. Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia. Parasit Vectors. 2018;11:495 10.1186/s13071-018-3068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornok S, Sugár L, Fernández de Mera IG, de la Fuente J, Horváth G, Kovács T, et al. Tick- and fly-borne bacteria in ungulates: the prevalence of Anaplasma phagocytophilum, haemoplasmas and rickettsiae in water buffalo and deer species in Central Europe, Hungary. BMC Vet Res. 2018;14:98 10.1186/s12917-018-1403-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inokuma H, Terada Y, Kamio T, Raoult D, Brouqui P. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other Ehrlichiae. Clin Diagn Lab Immunol. 2001;8:241–4. 10.1128/CDLI.8.2.241-244.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, et al. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol. 2006;72:1102–9. 10.1128/AEM.72.2.1102-1109.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Nishizawa I, Fujihara M, Nishimura T, Matsubara K, Harasawa R. Phylogenetic analysis of the 16S rRNA gene of Anaplasma species detected from Japanese serows (Capricornis crispus). J Vet Med Sci. 2009;71:1677–9. 10.1292/jvms.001677 [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Liu Z, Niu Q, Liu J, Han R, Guan G, Hassan MA, Liu G, Luo J, Yin H. A novel zoonotic Anaplasma species is prevalent in small ruminants: potential public health implications. Parasit Vectors. 2017;10:264 10.1186/s13071-017-2182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh FX, Panchadcharam C, Sitam FT, Tay ST. Molecular investigation of Anaplasma spp. in domestic and wildlife animals in Peninsular Malaysia. Vet. Parasitol. Regional Studies Rep. 2018;13:141–147. 10.1016/j.vprsr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 27.Seo MG, Ouh IO, Lee H, Geraldino PJL, Rhee MH, Kwon OD, Kwak D. Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen. Vet Microbiol. 2018;226:15–22. 10.1016/j.vetmic.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Liu Z, Niu Q, Mukhtar MU, Guan G, Liu G, Luo J, Yin H. A novel genotype of "Anaplasma capra" in wildlife and its phylogenetic relationship with the human genotypes. Emerg Microbes Infect. 2018;7(1):210 10.1038/s41426-018-0212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarridge JE III. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17:840–862. 10.1128/CMR.17.4.840-862.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu M, Tian JH, Yu B, Guo WP, Holmes EC, Zhang YZ. Extensive diversity of rickettsiales bacteria in ticks from Wuhan, China. Ticks Tick Borne Dis. 2017;8:574–580. 10.1016/j.ttbdis.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 31.Sun XF, Zhao L, Wen HL, Luo LM, Yu XJ. Anaplasma species in China. Lancet Infect Dis. 2015;15:1263–4. 10.1016/S1473-3099(15)00377-1 [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Liu Z, Niu Q, Liu J, Han R, Liu G, et al. Molecular survey and characterization of a novel Anaplasma species closely related to Anaplasma capra in ticks, northwestern China. Parasit Vectors. 2016;9:603 10.1186/s13071-016-1886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The evolutionary history was inferred by using the Maximum Likelihood method and Tamura-Nei model [6]. The tree with the highest log likelihoods are shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites. The rate variation model allowed for some sites to be evolutionarily invariable. This analysis involved respectively 22 and 18 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). Evolutionary analyses were conducted in MEGA X [7, 8]. The reference AF304141 referred as Anaplasma centrale Aomori cattle–Japan in the gltA tree corresponds to a misclassification of this sequence and is not truly A. centrale.
(PDF)
The analyses were performed for each of the three genes (A: rRNA 16S, B: groEL and C: gltA) on the Phylogeny.fr platform [10] and comprised the following steps. Sequences were aligned with MUSCLE (v3.8.31) configured for highest accuracy (MUSCLE with default settings) [11]. After alignment, positions with gaps were removed from the alignment. The phylogenetic tree was reconstructed using the bayesian inference method implemented in the MrBayes program (v3.2.6) [12]. The number of substitution types was fixed to 6. The standard (4by4) model of nucleotide substitution was used, while rates variation across sites was fixed to "invgamma". Four Markov Chain Monte Carlo (MCMC) chains were run for 10000 generations, sampling every 10 generations, with the first 250 sampled trees discarded as "burn-in". Finally, a 50% majority rule consensus tree was constructed. The trees were drawn and annotated with the Mega X software [7].
(PDF)
Data Availability Statement
Gene sequences are available in GenBank under accession numbers MH084717-MH084724.


