Abstract
Rabies is a neglected disease mostly affecting the developing world. Accurate and reliable diagnostic and surveillance data forms the foundation for the formulation and monitoring of control strategies. Although various sensitive and specific tests are available for detection of rabies virus, implementation of these tests in low-resource settings are challenging and remains limited. In this study, we describe the developed of a reverse transcription recombinase polymerase amplification assay for the detection of rabies virus. The analytical sensitivity of this assay was determined to be 562 RNA copies and was performed in 20 minutes. The diagnostic sensitivity of the RT-RPA was 100% for detection of rabies virus in field samples. In conclusion, the RT-RPA assay allowed for very quick and sensitive detection of rabies virus and could be adapted for use in low-source settings.
Introduction
Rabies is a fatal viral infection that causes progressive encephalitis in all mammals and is caused by 16 officially recognized viral species [1,2]. It is estimated that approximately 59 000 human deaths occur each year due to rabies and that over 99% of these are caused by rabies virus (RABV) transmitted by domestic dogs [3]. RABV is established worldwide in various hosts and can be divided into two phylogenic groups i.e. bat-related RABV and dog-related RABV. The latter group can be further divided into several major clades i.e. the cosmopolitan, Africa 2, Africa 3, Arctic-related, Asian and Indian subcontinent clades [4]. As rabies is 100% preventable the United Against Rabies collaboration; consisting of the World Health Organization (WHO), the World Organisation for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO) and the Global Alliance for Rabies Control (GARC); have compiled a global strategic plan in order to eliminate dog-mediated human rabies by 2030 [5] that emphasizes the need for accurate data. To assess the true burden of disease, routine and accurate diagnosis of rabies is required, this however, has been shown to be a major limitation in developing countries that are plagued by limited diagnostic capability that leads to poor surveillance, underreporting and misdiagnosis [6–10]. The current gold standard for rabies diagnosis is the fluorescent antibody test (FAT) recommended by the OIE and WHO [11,12]. This test relies on the visualization of RABV antigens in fresh or frozen brain material by fluorescent microscopy. Implementation of the FAT in resource-limited countries, where rabies diagnosis is most needed, is hindered by the high cost of acquiring and maintaining fluorescent microscopes, lack of trained technicians and difficulty in collection and preservation of fresh specimens [7–9]. More recently a streptavidin-biotin peroxidase based test, the direct rapid immunohistochemical test (dRIT) [13], has been recommended as an alternative to the FAT in resource-limited laboratories [11] with a few developing countries beginning to implement the dRIT as a primary test for rabies diagnosis [14].
Molecular techniques for rabies diagnosis have become more wide-spread and accepted [15] and has been shown to be more reliable for decomposed tissues than the FAT [16,17]. However, similar to the FAT, several factors have prevented its implementation in resource-limited settings including the high cost of equipment and reagents, electricity requirements, skilled technicians and the need to maintain the cold chain. As such, the implementation of diagnostic tests in the developing world will be reliant on resource availability and simplicity [7,15] with members of the United Against Rabies collaboration promoting a shift towards point-of-care (POC) diagnostic tools [5].
The use of lateral flow devices or rapid immunodiagnostic tests has been suggested as an alternative to laboratory testing. However, the evaluation of six different commercially available lateral flow devices indicated poor performance with regards to sensitivity and specificity [18]. Due to these limitations, further improvement and standardization of these tests were recommended before they could be considered as a potential POC test [18]. Recombinase polymerase amplification (RPA) is a molecular method that is initiated when recombinase binds to primers to from a nucleoprotein filament that forms a D-loop structure where homologous sequences are present in duplex DNA. The D-loop structure then initiates a strand exchange reaction. The recombinase can then disassemble from the nucleoprotein filament and is available for the next pair of primers. Recombinase disassembly then allows DNA polymerase to initiate synthesis from the free 3’ end of the primer. As polymerization continues, strands can then separate and form two duplexes after which the whole process is repeated [19,20]. RPA reactions are carried out at constant temperature (usually 37–42°C), the lyophilised reaction pellets have a shelf-life of several months and can be stored at room temperature with the option of different detection methods including lateral flow devices and portable incubation and detection instruments [20]. RPA has therefore been recommended as a potential POC test due to its affordability, simplicity, sensitivity, speed and minimal equipment requirements [21]. Its effectiveness as a POC test has been demonstrated for human immunodeficiency virus type 1 [22], and as a portable or field test for foot-and-mouth disease virus [23] and avian influenza A (H7N9) virus [24]. The potential of RT-RPA for use in enhanced surveillance for rabies has been shown in a previous proof-of-principle study [25] by evaluating a small panel (n = 33) of samples with a reported diagnostic sensitivity of 97% and limit of detection (LOD) of 1000 RNA copies.
This study aimed to develop an RT-RPA assay for the detection of lyssaviruses in animal samples from Africa. During assay design our main priority was focused on detection of dog-related RABV since it is responsible for the majority of human rabies cases. This assay was shown to be quick and simple with detection of rabies virus within 20 minutes, sensitivity of 562 RNA copies and specificity of 100%. Furthermore, the RT-RPA assay shows promise for further development as a pan-lyssavirus assay for potential use in low-resource settings.
Material and methods
Ethics statement and samples
This research was conducted with the approval of the University of Pretoria Animal Ethics Committee (Project number: H005-16). A panel of brain material (n = 109) collected from naturally infected animals [8,9,26–29] that tested positive with the FAT [30] and the direct, rapid immunohistochemical test (DRIT) [8] and four negative samples were included for diagnostic evaluation of the RT-RPA. RNA was extracted from all samples (from approximately 50 mg tissue) using Trizol reagent (Invitrogen) according to the manufacturer’s instructions and eluted in 50 μl nuclease free water (Ambion). To determine cross-reactivity of the assay, RNA from 18 virus cell culture supernatants were also included.
qRT-PCR
The One Step PrimeScript RT-PCR kit (Takara Bio Inc) was used for all qRT-PCR reactions on a QuantStudio 5 real-time PCR system (Thermo Fisher Scientific) as described previously [31,32] with modifications. Briefly, 1 μl extracted RNA was amplified in a final volume of 10 μl containing 1x One step RT-PCR Buffer III, 1U TaKaRa Ex Taq HS, 0.2 μl PrimeScipt RT enzyme mix II, 0.8 μM of each primer (541lys: 5’-CACMGSNAAYTAYAARACNAA-3’ and 550B: 5’-GTRCTCCARTTAGCRCACAT-3’) and 0.4 μM probe 620lyssaC (5’: 6-carboxyfluorescein (FAM)–CAYCAYACHYTVATGACHACHCAYAA–non-fluorescent quencher (QSY) 3’). First-strand synthesis was achieved by incubation at 42°C for 30 minutes and subsequent denaturation at 95°C for 10 seconds. Reactions were cycled 45 times at 95°C for 5 seconds, 42°C for 5 seconds and 72°C for 5 seconds. Viral RNA copy numbers were estimated using external standard curves as described previously [31,32] using the QuantStudio Design and Analysis Software version 1.4 (Thermo Fisher Scientific).
Generation of standard RNA
The complete nucleoprotein gene of challenge virus standard (CVS-11) was amplified using primers Lys001 (5’-ACGCTTAACGAMAAA-3’) and 304 (5’-TTGACAAAATCTTCTCAT-3’) as described previously [33]. Amplification products were purified using the Zymoclean Gel DNA Recovery Kit (Zymo Research) followed by cloning using the pGEM-T Easy vector system (Promega) according to the manufacturer’s instructions. Recombinant clones were characterized by sequencing using the BigDye Terminator V3.1 cycle sequencing kit (Thermo Fisher Scientific) and an ABI3500xL genetic analyser (Applied Biosystems) to determine orientation. A single recombinant clone containing the insert in the correct orientation with regard to the SP6 promoter was selected, and the insert was in vitro transcribed using the MegaScript kit (Ambion) according to the manufacturer’s instructions. In vitro transcribed RNA was purified using the RNA Clean and Concentrator-25 kit (Zymo Research) and quantified using the Qubit 3.0 fluorometer (Invitrogen). Contamination with plasmid DNA was ruled out with no-RT controls.
RT-RPA
Primer and probe design
The ClustalW subroutine of BioEdit Sequence Alignment Editor version 7 [34] was used to create a multiple alignment of representative sequences for RABV available on GenBank (https://www.ncbi.nlm.nih.gov/) (S1 Table). Regions of homology were identified, and primers and probes were designed targeting the nucleoprotein gene.
RT-RPA reaction conditions
All RT-RPA reactions were performed using the TwistAmp exo RT kit (TwistDx) and optimized regarding magnesium acetate concentration (MgOAc), time of shaking, probe concentration, primer selection and concentration. The optimized master mix (46.16 μl) contained 600 nM of each primer (RPA_RV_N497F and RPA_RV_N681R, Table 1), 200 nM exo-probe (Table 1), 40U RiboLock RNase Inhibitor (Thermo Scientific) and 29.5 μl rehydration buffer that was added to the lyophilized reaction pellet followed by the addition of 1 μl RNA. The reaction was initiated by the addition of 2.86 μl magnesium acetate (280 mM). The reactions were incubated for a total of 20 minutes at 42°C in an ESEQuant Tube Scanner (Qiagen) with brief vortexing after 6 minutes. Fluorescent signal (FAM channel) was measured at 20 second intervals and analyzed using the Tube Scanner Studio software (Qiagen) with regards to threshold validation (fluorescence increases three standard deviations above the background during the first minute of the reaction) and slope validation (set at 15 mV/min) that was verified with calculation of the second derivative.
Table 1. Details of probe and primers used in the RT-RPA assay.
| Primer or probe | Sequence (5’-3’) | Position on genomeg |
|---|---|---|
| RPA_RV_N461Fa | CAGGACAAAACACCGGCAACTATAAGACAAAC | 461 |
| RPA_RV_N497Fb | CAGATAGGATAGAGCAGATTTTCGAGACAGC | 497 |
| RPA_RV_N528F | CCCCTTTTGTTAAAATCGTGGAACACCATAC | 528 |
| RPA_RV_N557F | ACAAACATYGCRGATAGRATAGAGCAGATTTTY | 488 |
| RPA_RV_N645R | CTGATTGCTGAATATCTCTGCTCAATCCGG | 645 |
| RPA_RV_N681R | GAGCAGTCTTCATAAGCAGTGACAACTGTG | 681 |
| RPA_RV_N692R | GYTCAATCCGGGAGAAAWACATGTCRTTTCC | 622 |
| RPA_RV_N562exoprobe | ATGACAACTCAYAARATGTGYGCYAATTGGAGYAC(FAMdTcc/THFdd/BHQ-1dTee)ACCRAAYTT(C3Spf) | 562 |
a R: reverse primer
b F: forward primer
c FAMdTC: thymidine nucleotide carrying 6-carboxyfluorescein
d THFd: tetrahydrofuran residue
e BHQ-1dTe: thymidine nucleotide carrying black hole quencher 1
f C3Sp: C3 spacer to block elongation
g Nucleotide positions are numbered according to rabies virus, CVS-11 (GenBank accession number GQ918139)
RT-RPA assay characteristics
The analytical sensitivity of the RT-RPA assay was determined by testing serially diluted in vitro transcribed rabies virus (CVS-11) RNA (200, 300, 500, 103−108 copies). Probit regression analysis was performed on four replicates of the various dilutions using MedCalc Statistical software version 18.10.2 (MedCalc Software bvba). Diagnostic sensitivity and specificity were evaluated by testing rabies positive and negative brain material from Africa (Table 2) with RT-RPA and compared with qRT-PCR. Additionally, RNA representing different rabies-related viruses and bat-related RABV lineages (Table 3) were also tested with both methods to determine cross-reactivity.
Table 2. Detection of different rabies positive and negative field samples using RT-RPA and qRT-PCR.
| Laboratory number | Country | Host | RT-RPA | qRT-PCR | ||||
|---|---|---|---|---|---|---|---|---|
| Result | Time | Result | Copy nrh | Cp | Timeg | |||
| 99/14a | Lesotho | Canine | Pos | 5 | Pos | 1,90E7 | 14,8 | 46,8 |
| 60/14a | Lesotho | Bovine | Pos | 5 | Pos | 2,27E6 | 18,2 | 50,5 |
| 45a/14a | Lesotho | Bovine | Pos | 4 | Pos | 2,92E7 | 14,2 | 46,0 |
| 45b/14a | Lesotho | Bovine | Pos | 3,7 | Pos | 6,69E7 | 12,9 | 44,6 |
| 08/14a | Lesotho | Bovine | Pos | 3,7 | Pos | 9,13E7 | 12,4 | 44,1 |
| 195/14a | Lesotho | Bovine | Pos | 3,7 | Pos | 6,63E7 | 12,9 | 44,6 |
| 190/12a | Lesotho | Caprine | Pos | 4 | Pos | 3,62E7 | 13,8 | 45,7 |
| 21/15a | Lesotho | Bovine | Pos | 15,3 | Pos | 4,00E4 | 24,6 | 57,5 |
| 24/15a | Lesotho | Canine | Pos | 5,3 | Pos | 4,86E5 | 20,6 | 53,2 |
| 17/15a | Lesotho | Canine | Pos | 5,7 | Pos | 5,06E5 | 20,6 | 53,1 |
| 22/16a | Lesotho | Bovine | Pos | 5,3 | Pos | 8,83E5 | 19,7 | 52,1 |
| 298/93b | Mozambique | Canine | Pos | 5 | Pos | 4,00E5 | 20,9 | 53,5 |
| 186/99b | Mozambique | Canine | Pos | 5,3 | Pos | 3,90E5 | 21,9 | 54,6 |
| 572/99b | Mozambique | Canine | Pos | 4,7 | Pos | 8,50E5 | 19,8 | 52,2 |
| 633/00b | Mozambique | Canine | Pos | 5,3 | Pos | 9,04E5 | 19,7 | 52,1 |
| 315/04b | Mozambique | Canine | Pos | 5,3 | Pos | 3,19E5 | 21,3 | 53,9 |
| 232/05b | Mozambique | Canine | Pos | 8,7 | Pos | 1,24E4 | 26,4 | 59,5 |
| 558/05b | Mozambique | Canine | Pos | 5,3 | Pos | 6,13E5 | 20,3 | 52,8 |
| 659/05b | Mozambique | Canine | Pos | 4,3 | Pos | 9,38E5 | 19,6 | 52,0 |
| 687/05b | Mozambique | Canine | Pos | 4 | Pos | 5,59E5 | 20,4 | 52,9 |
| 131/12b | Mozambique | Feline | Pos | 5,3 | Pos | 1,06E6 | 19,4 | 51,8 |
| 482/12b | Mozambique | Canine | Pos | 4,7 | Pos | 1,36E6 | 19,0 | 51,4 |
| 1018/12b | Mozambique | Bovine | Pos | 4,3 | Pos | 1,26E6 | 19,1 | 51,5 |
| 233/13b | Mozambique | Canine | Pos | 5 | Pos | 1,35E6 | 19,0 | 51,4 |
| 191K09c | Namibia | Kudu | Pos | 3,7 | Pos | 4,03E7 | 13,7 | 45,5 |
| 212K09c | Namibia | Kudu | Pos | 4 | Pos | 2,45E7 | 14,4 | 46,4 |
| 234K09c | Namibia | Kudu | Pos | 5,3 | Pos | 1,42E7 | 15,3 | 47,3 |
| 240K09c | Namibia | Kudu | Pos | 1 | Pos | 8,08E5 | 19,8 | 52,3 |
| 151J09c | Namibia | Jackal | Pos | 5 | Pos | 3,47E7 | 13,9 | 45,8 |
| 179J09c | Namibia | Jackal | Pos | 4 | Pos | 8,58E7 | 12,5 | 44,2 |
| 192J09c | Namibia | Jackal | Pos | 3 | Pos | 1,78E8 | 11,3 | 42,9 |
| 193J09c | Namibia | Jackal | Pos | 4 | Pos | 3,64E6 | 17,5 | 49,7 |
| 197J09c | Namibia | Jackal | Pos | 8 | Pos | 4,34E5 | 20,8 | 53,4 |
| 204J09c | Namibia | Jackal | Pos | 5,7 | Pos | 1,39E6 | 19,0 | 51,3 |
| 236J09c | Namibia | Jackal | Pos | 4 | Pos | 1,42E7 | 15,3 | 47,3 |
| NIG49d | Nigeria | Canine | Pos | 18,3 | Pos | 7,90E8 | 8,9 | 40,3 |
| NIG50d | Nigeria | Canine | Pos | 4,3 | Pos | 4,92E8 | 9,7 | 41,2 |
| NIG65d | Nigeria | Canine | Pos | 2 | Pos | 2,66E8 | 10,7 | 42,2 |
| NIG95d | Nigeria | Canine | Pos | 1 | Pos | 1,11E8 | 12,0 | 43,7 |
| NIG140d | Nigeria | Canine | Pos | 3,3 | Pos | 1,77E8 | 11,3 | 42,9 |
| NIG142d | Nigeria | Canine | Pos | 3,3 | Pos | 6,52E8 | 9,3 | 40,7 |
| NIG176d | Nigeria | Canine | Pos | 8 | Pos | 1,62E8 | 11,5 | 43,1 |
| NIG185d | Nigeria | Canine | Pos | 3,7 | Pos | 5,97E8 | 9,4 | 40,8 |
| NIG249d | Nigeria | Canine | Pos | 9 | Pos | 2,53E8 | 10,7 | 42,3 |
| NIG251d | Nigeria | Canine | Pos | 8,3 | Pos | 5,83E8 | 9,4 | 40,9 |
| 71/14e | Zimbabwe | Canine | Pos | 4,7 | Pos | 1,21E7 | 15,6 | 47,6 |
| 168/14e | Zimbabwe | Feline | Pos | 15,3 | Pos | 2,67E3 | 28,9 | 62,2 |
| 307/15e | Zimbabwe | Canine | Pos | 13 | Pos | 6,28E6 | 16,6 | 48,7 |
| 406/15e | Zimbabwe | Canine | Pos | 4,7 | Pos | 4,27E7 | 13,6 | 45,4 |
| 424/15e | Zimbabwe | Canine | Pos | 4 | Pos | 2,23E7 | 14,6 | 46,5 |
| 427/15e | Zimbabwe | Canine | Pos | 14,7 | Pos | 9,51E7 | 12,3 | 44,0 |
| 464/15e | Zimbabwe | Canine | Pos | 4 | Pos | 3,70E7 | 13,8 | 45,6 |
| 515/15e | Zimbabwe | Canine | Pos | 5 | Pos | 1,18E7 | 15,6 | 47,6 |
| 245/16e | Zimbabwe | Bovine | Pos | 7,7 | Pos | 4,59E5 | 20,7 | 53,3 |
| 12/526f | South Africa | Caprine | Pos | 3,7 | Pos | 6,21E7 | 13,0 | 44,7 |
| 12/621f | South Africa | Jackal | Pos | 4,7 | Pos | 1,63E6 | 18,7 | 51,1 |
| 12/616f | South Africa | Jackal | Pos | 4,7 | Pos | 2,38E6 | 18,1 | 50,4 |
| 12/730f | South Africa | Canine | Pos | 3,3 | Pos | 8,42E7 | 12,5 | 44,2 |
| 12/849f | South Africa | Bovine | Pos | 17,3 | Pos | 2,08E3 | 29,3 | 62,6 |
| 15/17f | South Africa | Canine | Pos | 4,3 | Pos | 1,59E7 | 15,1 | 47,1 |
| 15/142f | South Africa | Mongoose | Pos | 4,3 | Pos | 1,26E7 | 15,5 | 47,5 |
| 15/170f | South Africa | Mongoose | Pos | 1 | Pos | 1,12E8 | 12,0 | 43,7 |
| 15/173f | South Africa | Mongoose | Pos | 8 | Pos | 2,24E8 | 10,9 | 42,5 |
| 15/181f | South Africa | Duiker | Pos | 5 | Pos | 4,87E6 | 17,0 | 49,2 |
| 15/182f | South Africa | Lynx | Pos | 5 | Pos | 2,67E7 | 14,3 | 46,2 |
| 15/295f | South Africa | Bovine | Pos | 3,7 | Pos | 5,11E7 | 13,3 | 45,1 |
| 15/479f | South Africa | Bovine | Pos | 5 | Pos | 1,17E6 | 19,2 | 51,6 |
| 15/495f | South Africa | Canine | Pos | 3,7 | Pos | 3,97E7 | 13,7 | 45,5 |
| 15/305f | South Africa | Bovine | Pos | 3,7 | Pos | 1,55E7 | 15,2 | 47,1 |
| 15/538f | South Africa | Bovine | Pos | 4,3 | Pos | 3,61E7 | 13,8 | 45,7 |
| 15/543f | South Africa | Canine | Pos | 5,7 | Pos | 5,06E5 | 20,6 | 53,1 |
| 15/553f | South Africa | Otter | Pos | 5 | Pos | 1,33E7 | 15,4 | 47,4 |
| 15/563f | South Africa | Canine | Pos | 3,7 | Pos | 1,50E6 | 18,9 | 51,2 |
| 15/595f | South Africa | Jackal | Pos | 3,7 | Pos | 5,02E6 | 13,3 | 45,1 |
| 15/634f | South Africa | Mongoose | Pos | 4 | Pos | 3,36E7 | 13,9 | 45,8 |
| 15/636f | South Africa | Bovine | Pos | 4 | Pos | 4,20E6 | 17,2 | 49,4 |
| 15/643f | South Africa | Canine | Pos | 4,7 | Pos | 4,19E6 | 17,2 | 49,4 |
| 15/644f | South Africa | Bovine | Pos | 4 | Pos | 8,50E8 | 12,5 | 44,2 |
| 16/010f | South Africa | Jackal | Pos | 4,7 | Pos | 3,76E6 | 17,4 | 49,6 |
| 16/051f | South Africa | Canine | Pos | 8 | Pos | 3,09E5 | 21,4 | 53,9 |
| 16/069f | South Africa | Jackal | Pos | 5,7 | Pos | 2,03E6 | 18,4 | 50,7 |
| 16/094f | South Africa | Mongoose | Pos | 4,3 | Pos | 3,29E6 | 17,6 | 49,8 |
| 16/102f | South Africa | Jackal | Pos | 4,7 | Pos | 8,27E6 | 16,2 | 48,2 |
| 17/298f | South Africa | Mongoose | Pos | 3,3 | Pos | 1,84E8 | 11,3 | 42,9 |
| 17/330f | South Africa | Mongoose | Pos | 4,7 | Pos | 4,44E6 | 17,1 | 49,3 |
| 17/336f | South Africa | Mongoose | Pos | 3,3 | Pos | 6,99E8 | 9,1 | 40,5 |
| 15/304d | South Africa | Jackal | Pos | 4 | Pos | 8,48E5 | 19,8 | 52,2 |
| 15/467d | South Africa | Canine | Pos | 14,3 | Pos | 6,69E6 | 16,5 | 48,6 |
| 15/474d | South Africa | Canine | Pos | 14,3 | Pos | 7,23E6 | 16,4 | 48,5 |
| 15/519d | South Africa | Bovine | Pos | 14 | Pos | 9,19E6 | 16,6 | 48,7 |
| 16/239d | South Africa | Bovine | Pos | 4,7 | Pos | 4,50E7 | 13,5 | 45,3 |
| 16/247d | South Africa | Canine | Pos | 3,7 | Pos | 8,37E7 | 12,5 | 44,2 |
| 16/256d | South Africa | Caprine | Pos | 3,3 | Pos | 1,30E8 | 11,8 | 43,5 |
| 16/260d | South Africa | Ovine | Pos | 4 | Pos | 9,62E7 | 12,3 | 44,0 |
| 16/286d | South Africa | Bovine | Pos | 4 | Pos | 4,26E7 | 13,6 | 45,4 |
| 16/318d | South Africa | Canine | Pos | 5,3 | Pos | 2,49E7 | 14,4 | 46,3 |
| 16/343d | South Africa | Bovine | Pos | 4,3 | Pos | 4,46E7 | 13,5 | 45,3 |
| 14/406d | South Africa | Caprine | Pos | 5 | Pos | 1,37E8 | 11,7 | 43,4 |
| 14/424d | South Africa | Canine | Pos | 4 | Pos | 2,80E8 | 10,6 | 42,1 |
| 15/130d | South Africa | Bovine | Pos | 4,3 | Pos | 1,84E7 | 14,9 | 46,9 |
| 15/205d | South Africa | Canine | Pos | 5 | Pos | 1,51E7 | 15,2 | 47,2 |
| 13/339f | South Africa | Canine | Pos | 1 | Pos | 1,88E8 | 11,2 | 42,8 |
| 13/310f | South Africa | Canine | Pos | 1 | Pos | 3,87E5 | 21,0 | 53,6 |
| 13/107f | South Africa | Canine | Pos | 3 | Pos | 5,25E8 | 9,6 | 41,0 |
| 13/522f | South Africa | Canine | Pos | 1 | Pos | 9,03E7 | 12,4 | 44,1 |
| 13/256f | South Africa | Canine | Pos | 1,3 | Pos | 4,18E8 | 10,0 | 41,4 |
| 13/355f | South Africa | Canine | Pos | 3,7 | Pos | 2,76E8 | 10,6 | 42,2 |
| 13/104f | South Africa | Canine | Pos | 1 | Pos | 3,14E8 | 10,4 | 41,9 |
| 13/79f | South Africa | Canine | Pos | 1 | Pos | 2,33E8 | 10,9 | 42,4 |
| RK002 | South Africa | Jackal | Neg | Neg | ||||
| RK010 | South Africa | Fox | Neg | Neg | ||||
| RK018 | South Africa | Civet | Neg | Neg | ||||
| RK023 | South Africa | Canine | Neg | Neg | ||||
a Obtained from the Central Veterinary Laboratory, Maseru, Lesotho; samples positive with the FAT [30] and DRIT [8]
b Obtained from the Central Veterinary Laboratory, Maputo, Mozambique; samples positive with the FAT [30] and DRIT [8]
c Obtained from the Central Veterinary Laboratory, Windhoek, Namibia; samples positive with the FAT [30] and DRIT [8]
d Obtained from the Agricultural Research Council-Onderstepoort Veterinary Research, Gauteng Province, South Africa; samples positive with the FAT [30] and DRIT [8]
e Obtained from the Central Veterinary Laboratory, Harare, Zimbabwe; samples positive with the FAT [30] and DRIT [8]
f Obtained from Allerton Provincial Veterinary Laboratory, KwaZulu-Natal Province, South Africa; samples positive with the FAT [30] and DRIT [8]
g For better comparability between assays, qRT-PCR crossing point values were converted into estimated detection time
h RNA copies/μl eluate
Table 3. Detection of RNA from representative lyssavirus species using RT-RPA and qRT-PCR.
| RT-RPA | qRT-PCR | ||||||
|---|---|---|---|---|---|---|---|
| Lyssavirus species | Country | Result | Time | Result | Copy nr | Cp | Timea |
| ARAV | Kyrgyzstan | Pos | 8 | Pos | 2.7E8 | 10.7 | 42.2 |
| DUVV | South Africa | Neg | Pos | 9,0E7 | 12.4 | 44.1 | |
| EBLV-1 | Denmark | Neg | Pos | 4.2E6 | 17.2 | 49.4 | |
| EBLV-2 | United Kingdom | Pos | 18.7 | Pos | 4.2E7 | 13.6 | 45.4 |
| IKOV | Tanzania | Neg | Pos | 2.7E6 | 17.9 | 50.1 | |
| IRKV | Russia | Pos | 16.7 | Pos | 2.4E8 | 10.9 | 42.4 |
| KHUV | Tajikistan | Pos | 18.7 | Pos | 6.1E8 | 9.4 | 40.8 |
| LBV (lineage A) | Unknown | Pos | 15.3 | Pos | 2.5E8 | 10.8 | 42.3 |
| LBV (lineage C) | South Africa | Pos | 19 | Pos | 1.2E7 | 15.6 | 47.6 |
| LBV (lineage D) | Kenya | Pos | 16.7 | Pos | 4.3E5 | 20.8 | 53.4 |
| MOKV | South Africa | Pos | 5.7 | Pos | 1.7E6 | 18.7 | 51 |
| RABV (free-tailed bat strain) | Americas | Pos | 3,7 | Pos | 9,2E6 | 15.2 | 47.2 |
| RABV (silver-haired bat strain) | Americas | Pos | 15.7 | Pos | 1.1E7 | 15.6 | 47.6 |
| RABV (Vampire strain) | Americas | Pos | 15 | Pos | 5.8E7 | 12.2 | 43.9 |
| RABV (Eastern big brown bat strain) | Americas | Pos | 15.3 | Pos | 1.2E8 | 11.9 | 43.5 |
| RABV (Western big brown bat strain) | Americas | Pos | 17 | Pos | 5.5E7 | 13.2 | 45 |
| SHIBV | Kenya | Neg | Pos | 2.1E5 | 21.4 | 54 | |
| WCBV | Russia | Pos | 15.3 | Pos | 2.5E8 | 10.8 | 48.7 |
a For better comparability between assays, qRT-PCR crossing point values were converted into estimated detection time
Results
RT-RPA Primer evaluation and assay optimization
For the development of a rabies RT-RPA assay, seven primers (four forward and three reverse) and a degenerate RPA exo probe (Table 1) were designed and evaluated using conditions recommended by the manufacturer.
To identify the optimal primer pair, a total of seven primer pairs were evaluated using in vitro transcribed CVS RNA (108 copies/μl) with final RT-RPA amplicon lengths ranging from 117–220 bp. All primer sets were able to amplify rabies virus (CVS-11) RNA, albeit with different efficiencies and were detected within 6 minutes. Primer set RPA_RV_N497F and RPA_RV_N681R performed the best with detection after 2.7 minutes and was subsequently used for further evaluation of the assay.
Analytical sensitivity of the RT-RPA assay
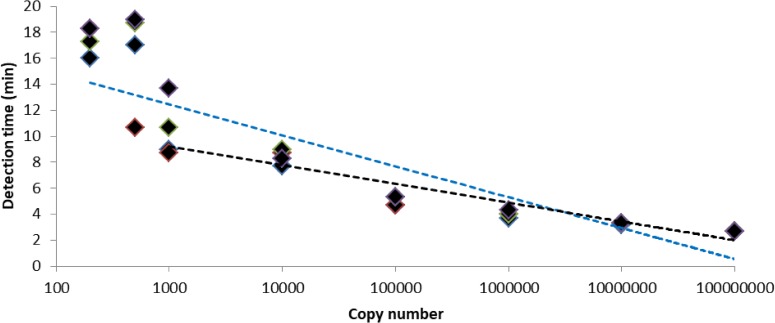
Serially diluted in vitro transcribed rabies virus (CVS-11) RNA was used to determine the analytical sensitivity of the RT-RPA assay using optimized conditions. The calculated LOD using probit regression analysis at 95% probability was 562 RNA copies (95% confidence interval: 331–933 RNA copies). Good correlation was seen between copy number and time to positive (R2 = 0.93) when 103−108 copies were considered, however, this correlation decreased when lower copy numbers (<103 copies) were included (Fig 1).
Fig 1. RT-RPA performance.
Detection times of four replicates of serially diluted in vitro transcribed rabies virus (CVS-11) RNA. Correlation between detection time and copy number is indicated by semi-logarithmic regression lines of all dilutions evaluated i.e. 200−108 copies (blue dotted line, R2 = 0.82) and where the two lowest dilutions were excluded i.e. 103−108 copies (black dotted line, R2 = 0.93).
Diagnostic sensitivity of the RT-RPA assay
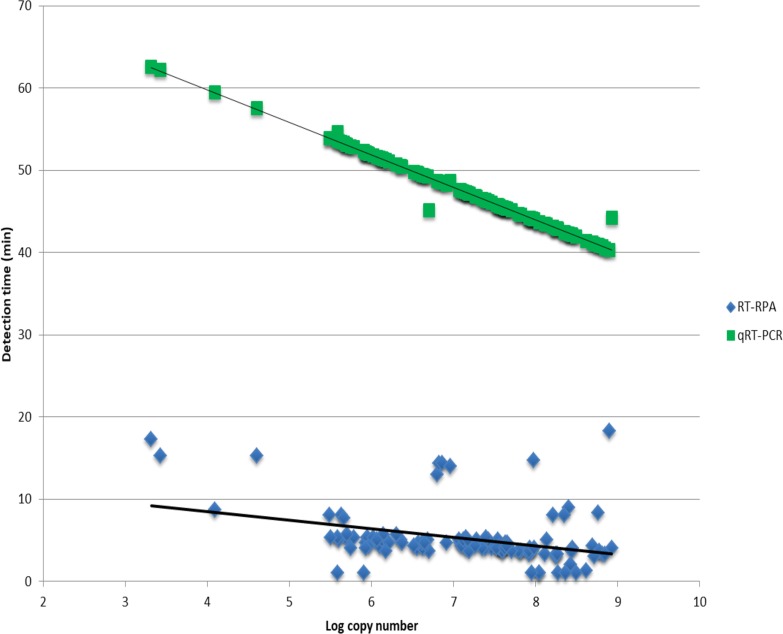
The RT-RPA assay was evaluated by testing a cohort of rabies positive and negative samples and compared to qRT-PCR (Table 2). All samples were detected with qRT-PCR and the RT-RPA resulting in a diagnostic sensitivity of 100%. The average detection time of the RT-RPA was 5.3 minutes compared to 47.6 minutes for qRT-PCR. Good correlation was observed for qRT-PCR copy number and detection time (R2 = 0.99), however, linear regression analysis demonstrated poor correlation between estimated copy number and RT-RPA detection time (R2 = 0.13, Fig 2). This poor correlation indicates that RT-RPA detection time should not be applied to quantitative analysis.
Fig 2. Correlation between estimated copy number and detection time of field samples tested with RT-RPA and qRT-PCR.
Linear regression analysis of RT-RPA and qRT-PCR detection times and estimated copy numbers for 109 rabies positive brain material.
To determine if the RT-RPA assay could be used as a pan-lyssavirus detection assay, representatives of 11 lyssavirus species and representatives of bat-related RABV lineages were tested (Table 3). The RT-RPA showed cross detection of 7 lyssavirus species, i.e., ARAV, EBLV-2, IRKV, KHUV, LBV, MOKV, WCBV and could detect 5 bat-related RABV lineages from the Americas. However, detection with the RT-RPA occurred much later than expected for the majority of samples (compared to the estimated copy number determined with qRT-PCR).
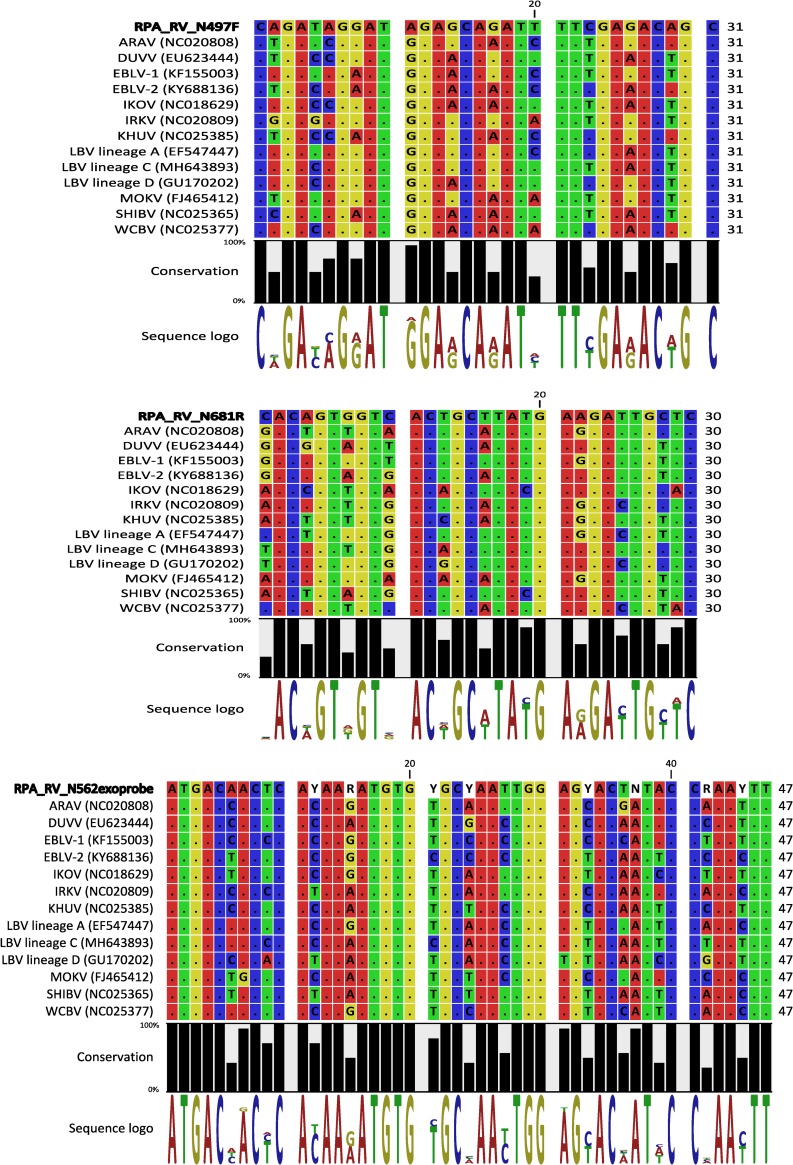
Evaluation of the probe and primer binding regions indicated 4–8 mismatches with the primers and 2–7 mismatches with the probe (Fig 3). In general, mismatches up to 14% were tolerated by the assay and detection failure was only noted when this value was exceeded (S2 Table).
Fig 3. Binding regions of the RT-RPA primers and probe to rabies-related viruses.
Dots represent identity to the sequence in the first row i.e. RT-RPA forward primer (RPA_RV_N497F), reverse complement of the reverse primer (RPA_RV_N681R) and the probe (RPA_RV_N562exoprobe). The conservation percentage and the sequence logo created using CLC Genomic Workbench software version 6 (Qiagen) is displayed beneath the alignment. The conservation graph shows the conservation percentage of all sequence positions, the height of the bars shows how conserved that particular position is in the alignment. The sequence logo displays the information content of all positions in the alignment as nucleotides stacked on top of each other. The height of the individual letters represents the sequence information content in that particular position of the alignment.
Discussion
The lack of inexpensive, rapid and simple diagnostic tests has been cited as a major constraint for assessment of the true burden of rabies [7]. Molecular-based methods have received increasing attention in recent years with several assays for broad-spectrum or targeted detection of lyssaviruses (reviewed in [35]) and have been indicated to be more sensitive than the gold standard, FAT [36]. Molecular methods, such as RT-PCR and real-time RT-PCR, although sensitive and specific, rely on constant power supply, expensive equipment, skilled personnel and sophisticated laboratories. To overcome some of these obstacles isothermal molecular techniques have been developed such as loop-mediated isothermal amplification (LAMP) and nucleic acid sequence-based amplification (NASBA). Several RT-LAMP assays have been used for rabies virus detection [37–40] and has been shown to be a quick (>1 hour) and sensitive (approximately 1000 RNA copies) method, however, this assay is limited by the considerable sequence variation between and within lyssavirus species that can make designing broadly reactive primer sets difficult [15,40]. In contrast, primer design for NASBA is relatively simple, but this assay requires longer running times (2-3h) and is more expensive than other molecular methods [41].
RPA has been identified as a promising tool for the quick, cost-effective identification of pathogens with several assays being developed that can detect a wide variety of RNA and DNA pathogens (reviewed in [42]) including rabies virus [25]. In this study, we developed and evaluated an RPA assay for the detection of rabies, with a specific focus on dog-related RABV in Africa. The length of RPA primers exceeds that of standard PCR primers and could, therefore, be problematic for variable viruses. Additionally, the performance of various primer sets in RPA assays cannot be determined a priori and should be experimentally evaluated, the influence of the number and distribution of mismatches with target sequences is also not well understood [43]. Thus, several primer sets based on the conserved nucleoprotein gene were designed, according to the kit manufacturer’s website (http://www.twistdx.co.uk), including a degenerate primer set to compensate for variability. The combination of non-degenerate primers RPA_RV_N497F and RPA_RV_N681R was shown to be the most effective for amplification and yielded the highest sensitivity of 562 RNA copies as determined with probit regression analysis. This assay shows improved sensitivity compared to previously published isothermal methods, i.e., RT-LAMP [40] and RT-RPA [25] assays (1000 RNA copies). The diagnostic sensitivity of this assay was 100% for a sample cohort collected across Africa. Samples on average were detected in <6 minutes with estimated RNA copy numbers ranging from 2080 to >108. The sensitivity of the assay (LOD of 562 RNA copies) would therefore be adequate for use as a rabies diagnostic tool using brain material. Linear regression analysis demonstrated poor correlation between estimated copy number and RT-RPA detection time. RT-RPA has been reported to produce non-linear curves that are unsuitable for quantification [23,44,45]. Several explanations have been proposed for this observation including the use of a chemical start (addition of magnesium acetate) rather than a thermal start (as with PCR assays) for the reaction; and since no thermal cycling is employed in RPA, synchronization is absent and annealing will occur continuously resulting in quantitative variation when using real-time fluorescent probes [20]. To determine the applicability of the current assay as a pan-lyssavirus surveillance tool, several lyssavirus species were tested. All bat-related RABV lineages (from the Americas) were detected, but only 64% of the rabies-related lyssaviruses were detected with the RT-RPA. Detection times of the RT-RPA occurred much later than expected for almost all rabies-related viruses (except MOKV). This indicates that the assay in its current form has low replication efficiency and would not be sensitive enough for use as a surveillance tool for rabies-related lyssaviruses or bat-related RABV. A previous study reported that up to 8% mismatches across primer pairs were tolerable [22] and comparable results (11% mismatches) were obtained when evaluating 87 primers [43]. Although no direct correlation between the amount and location of mismatches and RPA amplification could be determined, it was shown that mismatches at the 3’ end of both primers usually resulted in reduced efficiency [43]. Evaluating the mismatches between the RT-RPA primers and probe indicated mismatches of 13–26% and 2–15% respectively to rabies-related viruses. However, similar to Daher et al. [43], no correlation was observed between the number and location of mismatches and detection failure/success. Nevertheless, these results indicate the moderate tolerability of RPA to polymorphisms. This feature could lend itself towards the development of pan-lyssavirus or region-specific lyssavirus assays by modification of the primers described or inclusion of multiple primer sets in the reaction.
In conclusion, we developed an RT-RPA assay that was shown to be sensitive and specific for the detection of dog-related RABV in Africa. The assay demonstrated 100% diagnostic sensitivity compared to an established qRT-PCR. Although the sample cohort tested do not cover the full genetic diversity of RABV, the simplified approach and reduced turnaround time (approximately 9 times faster than qRT-PCR) of this assay suggests that it should be considered as a supplementary tool, where basic laboratory infrastructure is available, for enhanced surveillance efforts that could contribute towards rabies control efforts in a timeous manner. The TwistAmp exo RT kit (TwistDx) is no longer available; however, the assay described can be reproduced by adding a reverse transcriptase enzyme (such as Murine Leukemia virus reverse transcriptase) to the TwistAmp exo kit. This assay also shows promise as a valuable tool and possibly portable test for rabies diagnosis in resource-limited settings pending further development and evaluation.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We wish to thank Dr. C.T. Sabeta (Agricultural Research Council-Onderstepoort Veterinary Research) and Dr. U. Eze (University of Nigeria, Nsukka) for the provision of the viral RNA from Nigeria and the Centres for Disease Control and Prevention, USA and the Animal Plant Health Agency, UK for providing lyssavirus isolates. This work is based on research supported by the South African Research Chair initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98339) and the Poliomyelitis Research Foundation (Grant No 16/27).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is based on research supported by the South African Research Chair initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98339, WM) and the Poliomyelitis Research Foundation (Grant No 16/27, JW).
References
- 1.Amarasinghe GK, Aréchiga Ceballos NG, Banyard AC, Basler CF, Bavari S, Bennett AJ, et al. Taxonomy of the order Mononegavirales: update 2018. Arch Virol. 2018;163: 2283–2294. 10.1007/s00705-018-3814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markotter W, Coertse J. Bat lyssaviruses. Rev Sci Tech Off Int Epiz. 2018;37: 385–400. doi:0.20506/rst.37.2.2809 [DOI] [PubMed] [Google Scholar]
- 3.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the Global Burden of Endemic Canine Rabies. Carvalho MS, editor. PLoS Negl Trop Dis. 2015;9: e0003709 10.1371/journal.pntd.0003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troupin C, Dacheux L, Tanguy M, Sabeta C, Blanc H, Bouchier C, et al. Large-Scale Phylogenomic Analysis Reveals the Complex Evolutionary History of Rabies Virus in Multiple Carnivore Hosts. Parrish C, editor. PLOS Pathog. 2016;12: e1006041 10.1371/journal.ppat.1006041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization, Food and Agricultural Organization of the United Nations, World Organisation for Animal Health, Global Alliance for Rabies Control. Zero by 30: the global strategic plan to end human deaths from dog-mediated rabies by 2030 [Internet]. 2018 [cited 10 Jan 2019]. Available: https://www.who.int/rabies/resources/9789241513838/en/
- 6.WHO. WHO expert consultation on Rabies. WHO Expert Consultation on Rabies: Second Report. 2013. doi:92 4 120931 3
- 7.Duong V, Tarantola A, Ong S, Mey C, Choeung R, Ly S, et al. Laboratory diagnostics in dog-mediated rabies: an overview of performance and a proposed strategy for various settings. Int J Infect Dis. 2016;46: 107–114. 10.1016/j.ijid.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 8.Coetzer A, Sabeta CT, Markotter W, Rupprecht CE, Nel LH. Comparison of Biotinylated Monoclonal and Polyclonal Antibodies in an Evaluation of a Direct Rapid Immunohistochemical Test for the Routine Diagnosis of Rabies in Southern Africa. Zinsstag J, editor. PLoS Negl Trop Dis. 2014;8: e3189 10.1371/journal.pntd.0003189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coetzer A, Coertse J, Makalo MJ, Molomo M, Markotter W, Nel L. Epidemiology of Rabies in Lesotho: The Importance of Routine Surveillance and Virus Characterization. Trop Med Infect Dis. 2017;2: 30 10.3390/tropicalmed2030030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallewa M, Fooks AR, Banda D, Chikungwa P, Mankhambo L, Molyneux E, et al. Rabies Encephalitis in Malaria-Endemic Area, Malawi, Africa. Emerg Infect Dis. 2007;13: 136–139. 10.3201/eid1301.060810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Organisation for Animal Health. Rabies (infection with rabies virus and other lyssaviruses) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organisation for Animal Health; 2018. Available: http://www.oie.int/standard-setting/terrestrial-manual/access-online/ [Google Scholar]
- 12.World Health Organization. Laboratory techniques in rabies. 5th ed Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Geneva: World Health Organization; 2018. [Google Scholar]
- 13.Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006;12: 310–313. 10.3201/eid1202.050812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupprecht CE, Xiang Z, Servat A, Franka R, Kirby J, Ertl HCJ. Additional progress in the development and application of a direct, rapid immunohistochemical test for rabies diagnosis. Vet Sci. 2018;5 10.3390/vetsci5020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fooks AR, Johnson N, Freuling CM, Wakeley PR, Banyard AC, McElhinney LM, et al. Emerging Technologies for the Detection of Rabies Virus: Challenges and Hopes in the 21st Century. Rupprecht CE, editor. PLoS Negl Trop Dis. 2009;3: e530 10.1371/journal.pntd.0000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markotter W, Coertse J, Le Roux K, Peens J, Weyer J, Blumberg L, et al. Utility of forensic detection of rabies virus in decomposed exhumed dog carcasses. J S Afr Vet Assoc. 2015;86: 1–5. 10.4102/jsava.v86i1.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David D, Yakobson B, Rotenberg D, Dveres N, Davidson I, Stram Y. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet Microbiol. 2002;87: 111–118. 10.1016/S0378-1135(02)00041-X [DOI] [PubMed] [Google Scholar]
- 18.Eggerbauer E, de Benedictis P, Hoffmann B, Mettenleiter TC, Schlottau K, Ngoepe EC, et al. Evaluation of Six Commercially Available Rapid Immunochromatographic Tests for the Diagnosis of Rabies in Brain Material. Williams M, editor. PLoS Negl Trop Dis. 2016;10: e0004776 10.1371/journal.pntd.0004776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA Detection Using Recombination Proteins. Haber J, editor. PLoS Biol. 2006;4: e204 10.1371/journal.pbio.0040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Macdonald J, von Stetten F. Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. Royal Society of Chemistry; 2019;144: 31–67. 10.1039/C8AN01621F [DOI] [PubMed] [Google Scholar]
- 21.Lobato IM O ’Sullivan CK. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal Chem. 2018;98: 19–35. 10.1016/j.trac.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, et al. Rapid Detection of HIV-1 Proviral DNA for Early Infant Diagnosis Using Recombinase Polymerase Amplification. MBio. 2013;4: e00135–13. 10.1128/mBio.00135-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abd El Wahed A, El-Deeb A, El-Tholoth M, Abd El Kader H, Ahmed A, Hassan S, et al. A Portable Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of Foot-and-Mouth Disease Virus. Meng X-J, editor. PLoS One. 2013;8: e71642 10.1371/journal.pone.0071642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd El Wahed A, Weidmann M, Hufert FT. Diagnostics-in-a-Suitcase: Development of a portable and rapid assay for the detection of the emerging avian influenza A (H7N9) virus. J Clin Virol. Elsevier B.V.; 2015;69: 16–21. 10.1016/j.jcv.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlottau K, Freuling CM, Müller T, Beer M, Hoffmann B. Development of molecular confirmation tools for swift and easy rabies diagnostics. Virol J. Virology Journal; 2017;14: 184 10.1186/s12985-017-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coetzer A, Gwenhure L, Makaya P, Markotter W, Nel L. Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010–2017) in Harare, Zimbabwe. Samy AM, editor. PLoS One. 2019;14: e0210018 10.1371/journal.pone.0210018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coetzer A, Anahory I, Dias PT, Sabeta CT, Scott TP, Markotter W, et al. Enhanced diagnosis of rabies and molecular evidence for the transboundary spread of the disease in Mozambique. J S Afr Vet Assoc. 2017;88: 1–9. 10.4102/jsava.v88i0.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coertse J, Markotter W, le Roux K, Stewart D, Sabeta CT, Nel LH. New isolations of the rabies-related Mokola virus from South Africa. BMC Vet Res. 2016;13: 37 10.1186/s12917-017-0948-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott TP, Fischer M, Khaiseb S, Freuling C, Höper D, Hoffmann B, et al. Complete Genome and Molecular Epidemiological Data Infer the Maintenance of Rabies among Kudu (Tragelaphus strepsiceros) in Namibia. Dutilh BE, editor. PLoS One. 2013;8: e58739 10.1371/journal.pone.0058739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dean DJ, Abelseth MK, Atanasiu P. The fluoresent antibody test In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed World Health Organization; 1996. pp. 88–95. 10.1136/jcp.49.11.955-b [DOI] [Google Scholar]
- 31.Coertse J, Markotter W, Nel LH. Demonstration of African lyssavirus RNA with real-time polymerase chain reaction In: Rupprecht C, Nagarajan T, editors. Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention. 1st ed Elsevier; 2015. pp. 63–73. 10.1016/C2013-0-12670-0 [DOI] [Google Scholar]
- 32.Coertse J, Weyer J, Nel LH, Markotter W. Improved PCR Methods for Detection of African Rabies and Rabies-Related Lyssaviruses. J Clin Microbiol. 2010;48: 3949–3955. 10.1128/JCM.01256-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markotter W, Kuzmin I, Rupprecht C, Randles J, Sabeta C, Wandeler A, et al. Isolation of Lagos Bat Virus from Water Mongoose. Emerg Infect Dis. 2006;12: 1913–1918. 10.3201/eid1212.060514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999. pp. 95–98. 10.1108/S1876-0562(2012)0000006007 10780396 [DOI] [Google Scholar]
- 35.Fischer M, Hoffmann B, Freuling CM, Muller T, Beer M. Perspectives on molecular detection methods of lyssaviruses. Berl Munch Tierarztl Wochenschr. 2012;6: 264–271. 10.2376/0005-9366-125-266 [DOI] [PubMed] [Google Scholar]
- 36.Robardet E, Picard-Meyer E, Andrieu S, Servat A, Cliquet F. International interlaboratory trials on rabies diagnosis: An overview of results and variation in reference diagnosis techniques (fluorescent antibody test, rabies tissue culture infection test, mouse inoculation test) and molecular biology techniques. J Virol Methods. Elsevier B.V.; 2011;177: 15–25. 10.1016/j.jviromet.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Muleya W, Namangala B, Mweene A, Zulu L, Fandamu P, Banda D, et al. Molecular epidemiology and a loop-mediated isothermal amplification method for diagnosis of infection with rabies virus in Zambia. Virus Res. 2012;163: 160–168. 10.1016/j.virusres.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 38.Saitou Y, Kobayashi Y, Hirano S, Mochizuki N, Itou T, Ito FH, et al. A method for simultaneous detection and identification of Brazilian dog- and vampire bat-related rabies virus by reverse transcription loop-mediated isothermal amplification assay. J Virol Methods. Elsevier B.V.; 2010;168: 13–17. 10.1016/j.jviromet.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 39.Hayman DTS, Johnson N, Horton DL, Hedge J, Wakeley PR, Banyard AC, et al. Evolutionary History of Rabies in Ghana. Zinsstag J, editor. PLoS Negl Trop Dis. 2011;5: e1001 10.1371/journal.pntd.0001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boldbaatar B, Inoue S, Sugiura N, Noguchi A, Orbina JRC, Demetria C, et al. Rapid detection of rabies virus by reverse transcription-loop-mediated isothermal amplification. Jpn J Infect Dis. 2009;62: 187–191. 10.1111/j.1348-0421.2010.00286.x [DOI] [PubMed] [Google Scholar]
- 41.Wacharapluesadee S, Phumesin P, Supavonwong P, Khawplod P, Intarut N, Hemachudha T. Comparative detection of rabies RNA by NASBA, real-time PCR and conventional PCR. J Virol Methods. Elsevier B.V.; 2011;175: 278–282. 10.1016/j.jviromet.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 42.Daher RK, Stewart G, Boissinot M, Bergeron MG. Recombinase Polymerase Amplification for Diagnostic Applications. Clin Chem. 2016;62: 947–958. 10.1373/clinchem.2015.245829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG. Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol Cell Probes. 2015;29: 116–121. 10.1016/j.mcp.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 44.Patel P, Abd El Wahed A, Faye O, Prüger P, Kaiser M, Thaloengsok S, et al. A Field-Deployable Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of the Chikungunya Virus. Williams M, editor. PLoS Negl Trop Dis. 2016;10: e0004953 10.1371/journal.pntd.0004953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma L, Zeng F, Cong F, Huang B, Zhu Y, Wu M, et al. Development and evaluation of a broadly reactive reverse transcription recombinase polymerase amplification assay for rapid detection of murine norovirus. BMC Vet Res. BMC Veterinary Research; 2018;14: 399 10.1186/s12917-018-1736-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.