As a minimally invasive treatment option, intra-articular injection of platelet-rich plasma (PRP) has been widely used in clinical therapy. Our meta-analysis showed that PRP injections constitute an effective and safe alternative treatment for long-term pain relief and functional improvement in patients with knee osteoarthritis (KOA). The optimal dosage, timing intervals and frequency of injections and the ideal treatment for different stages of KOA remain areas of concern for future investigation future.
Keywords: Platelet-Rich Plasma, Osteoarthritis, Knee, Hyaluronic Acid, Randomized Controlled Trials, Randomized Control Trials, Meta-analysis
Abstract
Purpose
The purpose of this meta-analysis was to compare platelet-rich plasma (PRP) and hyaluronic acid (HA) in patients with knee osteoarthritis (KOA).
Methods
Randomized controlled trials (RCTs) comparing the use of PRP and HA in KOA patients were retrieved from each database from the establishment date to April 2018. Outcome measurements were the Western Ontario and McMaster Universities Arthritis Index (WOMAC), visual analog scale (VAS), International Knee Documentation Committee, and Lequesne Index scores and adverse events. The pooled data were evaluated with Review Manager 5.3.5.
Results
Fifteen RCTs (N = 1,314) were included in our meta-analysis. The present meta-analysis indicated that PRP injections reduced pain more effectively than HA injections in patients with KOA at six and 12 months of follow-up, as evaluated by the WOMAC pain score; the VAS pain score showed a significant difference at 12 months. Moreover, better functional improvement was observed in the PRP group, as demonstrated by the WOMAC function score at three, six, and 12 months. Additionally, PRP injections did not display different adverse event rates compared with HA injections.
Conclusion
In terms of long-term pain relief and functional improvement, PRP injections might be more effective than HA injections as a treatment for KOA. The optimal dosage, the timing interval and frequency of injections, and the ideal treatment for different stages of KOA remain areas of concern for future investigations.
Introduction
Knee osteoarthritis (KOA) is a progressive joint disease that often involves intra- and periarticular structures [1] and is considered pathology characterized by articular cartilage lesions, synovitis, subchondral sclerosis, and osteophytes [2]. In addition, KOA is one of the most common causes of joint pain and loss of motor function among middle-aged and elderly people in the United States [3,4]. Despite advances in medical technology, no drug or surgical intervention is currently available for delaying the development of KOA [5]. Oral anti-inflammatory drugs, physiotherapy, topical anti-inflammatory gels, and intra-articular injections are currently routine treatments for patients with symptomatic KOA [5,6]. Nonsurgical treatments, such as exercise and weight loss, are recommended because surgical treatment may lead to increased symptoms and poor functional outcomes [7]. However, compliance with nonsurgical treatment is lower in KOA, and medications, such as simple analgesics and nonsteroidal anti-inflammatory drugs, are often associated with adverse events [8,9]. Many studies have reported that hyaluronic acid (HA) has visco-induction properties and can increase intra-articular lubrication; therefore, intra-articular HA injection is widely used to treat KOA [10]. However, although intra-articular drug therapy is often associated with reduced pain and increased joint function in patients, it is not effective in patients with KOA [11].
In the past decade, the use of autologous growth factors to treat knee osteoarthritis, such as intra-articular injection of platelet-rich plasma (PRP), has received increasing attention [12]. Recent studies have demonstrated that growth factors and other cytokines released by platelets during injury can modulate the inflammatory process and help maintain or regenerate tissue structure [13,14]. The subchondral bone contributes to the cartilage repair process and KOA. PRP is an autologous blood product containing a high concentration of platelets, and it has become an emerging treatment for ligament, tendon, cartilage, and bone injuries in orthopedics and sports medicine [15–18]. The intra-articular injection of PRP as a minimally invasive treatment has been widely used in the treatment of clinically related diseases. Sanchez et al. [19] reported that a new technique for the delivery of PRP is intraosseous infiltration combined with intra-articular injections to treat severe KOA; moreover, no adverse reactions occurred in their study. Many clinical studies have reported good clinical efficacy of PRP injections [20–22], and some previously published systematic reviews and meta-analyses have also suggested that PRP is a safe and effective orthopedic treatment compared with other intra-articular injections [23–27]. However, these reviews did not reach a consensus in terms of the effects of PRP on pain relief and functional recovery and concluded that more high-quality randomized controlled trials (RCTs) are still needed. Previous reviews either included non-RCTs or only analyzed a small number of RCTs (<10), and additional randomized trials have since been published. Therefore, further systematic reviews and meta-analyses are needed to fully investigate the effects of PRP on knee pain relief and functional improvement. Our aim is to identify all prospective RCTs published to date to provide up-to-date insights into the efficacy of PRP in the treatment of KOA.
Methods
According to the PRISMA criteria, we created a prospective protocol including search strategies, selection criteria, outcome measurements, and methods of statistical analysis before commencing the study. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. This review is registered in PROSPERO: CRD42018108825.
Search Strategy
A comprehensive search of the PubMed (1966–April 2018), EMBASE (1980–April 2018), and Cochrane library (1966–April 2018) databases was conducted. The search terms included “platelet-rich plasma,” “PRP,” “knee,” “osteoarthritis,” “arthritis,” and “arthritic.” No language exclusions were applied. Manual searches were performed on the references of the identified studies.
Selection Criteria
The inclusion criteria were as follows: 1) RCTs comparing PRP with HA as a treatment for patients with KOA and 2) data that include at least one key outcome indicator, including the Western Ontario and McMaster Universities Arthritis Index (WOMAC), International Knee Documentation Committee (IDKC), visual analog scale (VAS), Lequesne Index, or adverse events.
The exclusion criteria were as follows: 1) retrospective studies, cohort studies, or nonrandomized studies; 2) case reports, letters, editorials, and animal experimental studies; and 3) inability to extract relevant data from the included studies.
Data Extraction
Two authors (YHH and HTH) independently extracted relevant data from the included studies, including authors’ names, publication date, patient age and gender, patient body mass index (BMI), sample size, radiographic classification, follow-up period, details of PRP treatment protocols and controls, and related clinical results. In the event that data were missing, we obtained detailed information by contacting the appropriate author.
Quality Assessment
Two authors (YHH and JKP) independently assessed the methodological quality of the included studies using the Cochrane Collaboration Tool for assessing the risk of bias (ROB). Each included study was scored as having a high, low, or unclear ROB according to the following evaluation criteria: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessments, 5) incomplete outcome data, 6) selective reporting, and 7) other sources of bias. Any discrepancies between the reviewers’ findings were arbitrated by the appropriate senior experts.
Statistical Analysis
Review Manager 5.3.5 software (Cochrane Collaboration, Oxford, UK) was used for all analyses. Continuous data for the meta-analysis were calculated and are expressed as the mean differences (MDs) with 95% confidence intervals (CIs), and dichotomous data for the meta-analysis are presented as risk ratios (RRs) with 95% CIs. Heterogeneity between studies was estimated with the I2 test. If the heterogeneity was at an I2 value of 75% or higher, a random-effects model was used; otherwise, a fixed-effects model was used. P values <0.05 were considered to indicate statistical significance in all the results.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Search Results
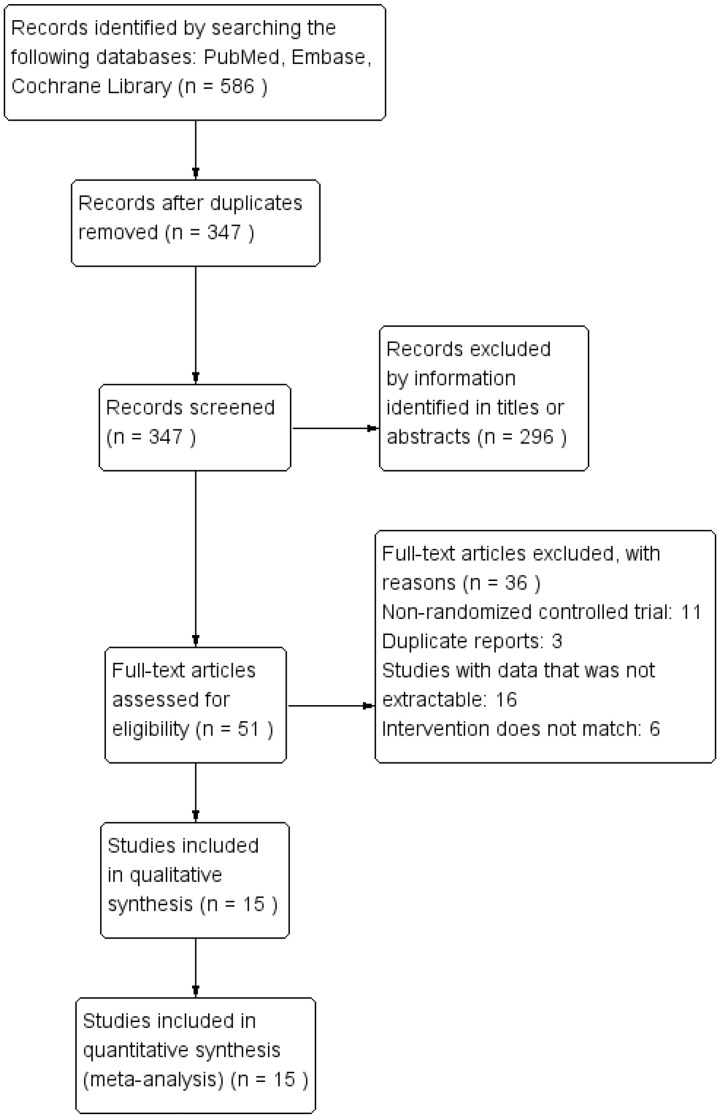
Figure 1 shows the flow diagram of study selection. A total of 586 records were identified after the initial literature search. Of these, 296 records were excluded after a thorough screen of the titles and abstracts. Ultimately, the remaining 51 studies were assessed by performing a full-text review, and 15 RCTs [2,21,22,28–39] were included in the meta-analysis.
Figure 1.
Flow diagram of study selection.
Study Characteristics
Table 1 shows the characteristics of the included studies. The sample sizes of the studies ranged from 10 to 94, with a total of 1314 individuals; 643 individuals were included in the HA group and 671 individuals in the PRP group. One of the studies [28] had a total follow-up period of three months, seven studies [29,30,34,35,37–39] had follow-up periods of six months, six studies [21,22,31–33,36] had follow-up periods of 12 months, and only one study [2] had a follow-up period of 18 months. With the exception of one study [37], all studies used the Kellgren and Lawrence grading scales to classify the severity of KOA. The patients in most studies had KOA with a severity of class II or III. The demographic characteristics between the two groups in each of the included studies were similar.
Table 1.
Characteristics of the included studies
| Author | Date | Sample Size |
Gender (F/M) |
Age, y |
BMI, kg/m2 |
Radiographic Classification (K-L) |
Follow-up Periods, mo | Clinical Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRP | HA | PRP | HA | PRP | HA | PRP | HA | PRP | HA | ||||
| Paterson et al. [28] | 2016 | 11 | 10 | 8/3 | 7/3 | 49.9±13.7 | 52.7±10.3 | 27.9±11.9 | 30.8±5.6 | II, III | II, III | 3 | VAS, Adverse events |
| Li et al. [29] | 2011 | 15 | 15 | 6/9 | 7/8 | 36∼76 | 39∼76 | 19.4∼29.5 | 17.6∼28.9 | I, II, III, IV | I, II, III, IV | 6 | WOMAC, IDKC, Lequesne Index, Adverse events |
| Cerza et al. [30] | 2012 | 60 | 60 | 25/35 | 28/32 | 66.5±11.3 | 66.2±10.6 | NA | NA | I, II, III | I, II, III | 6 | WOMAC, Adverse events |
| Cole et al. [31] | 2017 | 49 | 50 | 28/21 | 20/30 | 55.9±10.4 | 56.8±10.5 | 27.4±3.9 | 29.0±6.4 | I, II, III | I, II, III | 12 | VAS, WOMAC, IDKC |
| Duymus et al. [32] | 2016 | 33 | 34 | 32/1 | 33/1 | 60.4±5.1 | 60.3±9.1 | 27.6±4.6 | 28.4±3.6 | II, III | II, III | 12 | VAS, WOMAC |
| Filardo et al. [33] | 2015 | 94 | 89 | 34/60 | 37/52 | 53.3±13.2 | 57.6±11.8 | 26.6±4.0 | 26.9±4.4 | I, II, III | I, II, III | 12 | IDKC |
| Gormeli et al. [34] | 2015 | 39 | 39 | 23/16 | 22/17 | 57.3±13.1 | 53.5±14 | 28.7±4.8 | 29.7±3.7 | I, II, III, IV | I, II, III, IV | 6 | IDKC |
| Montanez-Heredia et al. [35] | 2016 | 27 | 26 | 15/12 | 17/9 | 66.3±8.3 | 61.5±8.6 | 29.0±5.5 | 30.4±4.9 | I, II, III | I, II, III | 6 | Adverse events |
| Raeissadat et al. [36] | 2015 | 77 | 62 | 69/8 | 47/15 | 56.9±9.1 | 61.1±7.5 | 28.2±4.6 | 27.0±4.2 | I, II, III, IV | I, II, III, IV | 12 | WOMAC |
| Sanchez et al. [37] | 2012 | 79 | 74 | 46/33 | 45/29 | 60.5±7.9 | 58.9±8.2 | 27.9±2.9 | 28.2±2.7 | NA | NA | 6 | WOMAC, Lequesne Index, Adverse events |
| Vaquerizo et al. [21] | 2013 | 48 | 42 | 32/16 | 26/16 | 62.4±6.6 | 64.8±7.7 | 30.7±3.6 | 31.0±4.6 | II, III, IV | II, III, IV | 12 | WOMAC, Lequesne Index, Adverse events |
| Raeissadat et al. [38] | 2017 | 36 | 33 | 29/7 | 27/6 | 57.0±7.2 | 59.5±7.5 | 28.6±2.82 | 27.5±2.9 | II, III | II, III | 6 | VAS, WOMAC, Adverse events |
| Su et al. [2] | 2018 | 25 | 30 | 14/11 | 18/12 | 54.2±6.6 | 53.1±6.4 | 28.2±1.4 | 28.7±1.1 | II, III | II, III | 18 | VAS, WOMAC, Adverse events |
| Filardo et al. [22] | 2012 | 54 | 55 | 17/37 | 24/31 | 55 | 58 | 27 | 26 | I, II, III | I, II, III | 12 | IDKC |
| Louis et al. [39] | 2018 | 24 | 24 | 10/14 | 13/11 | 53.2±11.7 | 48.5±11.5 | 25.6±2.9 | 27.0±2.9 | III, IV | III, IV | 6 | VAS, WOMAC, Adverse events |
BMI = body mass index; HA = hyaluronic acid; IDKC = International Knee Documentation Committee; K-L = Kellgren and Lawrence grading scale; NA = data not available; PRP = platelet-rich plasma; VAS = visual analog scale, WOMAC = Western Ontario and McMaster Universities Arthritis Index.
The preparation and administration of PRP varied among these studies. Table 2 shows the details of PRP preparation and administration protocols specific to each included study, such as the PRP category, the use of exogenous activators, and the injection regimen, including the doses, times, and intervals.
Table 2.
Details of the PRP treatment protocols and the controls
| Author | Date | PRP |
HA |
||||
|---|---|---|---|---|---|---|---|
| Category | Activation | Injection Dose, Times, and Intervals | Fresh/Frozen | Type | Injection Dose, Times, and Intervals | ||
| Paterson et al. [28] | 2016 | PA-PRP | Ultraviolet light | 3 mL, 3 times, weekly | Fresh | Hylan G-F 20 | 3 mL, 3 times, weekly |
| Li et al. [29] | 2011 | PRP | Calcium chloride | 3.5 mL, 3 times, 3 weeks | Fresh | Sofast | 2 mL, 3 times, 3 weeks |
| Cerza et al. [30] | 2012 | ACP | NA | 5.5 mL, 4 times, weekly | Fresh | Hyalgan | 20 mg, 4 times, weekly |
| Cole et al. [31] | 2017 | PRP | NA | 4 mL, 3 times, weekly | Fresh | Hylan G-F 20 | 16 mg, 3 times, weekly |
| Duymus et al. [32] | 2016 | PRP | NO | 5 mL, 2 times, monthly | Fresh | Ostenil Plus | 40 mg, 1 time, monthly |
| Filardo et al. [33] | 2015 | PRP | 10% calcium chloride | 5 mL, 3 times, weekly | Fresh | Hyalubrix | 30 mg, 3 times, weekly |
| Gormeli et al. [34] | 2015 | PRP | 1 mL of calcium chloride | 5 mL, 3 times, weekly | 1 fresh/2 frozen | Orthovisc | 2 mL, 3 times, weekly |
| Montanez-Heredia et al. [35] | 2016 | PRP | NA | NA, 3 times, 15 days | Frozen | Adant | NA, 3 times, 15 days |
| Raeissadat et al. [36] | 2015 | LR-PRP | NO | 4–6 mL, 2 times, 4 weeks | Fresh | Hyalgan | 20 mg, 3 times, weekly |
| Sanchez et al. [37] | 2012 | PRGF | 400 uL of calcium chloride | 8 mL, 3 times, weekly | Fresh | Euflexxa | NA, 3 times, weekly |
| Vaquerizo et al. [21] | 2013 | PRGF | 400 uL of calcium chloride | 8 mL, 3 times, weekly | Fresh | Durolane | NA, 1 time |
| Raeissadat et al. [38] | 2017 | PRGF | 1.5 mL of Rooyagen | 5 mL, 2 times, 3 weeks | Fresh | Hyalgan | 20 mg, 3 times, weekly |
| Su et al. [2] | 2018 | LR-PRP | Calcium chloride | 6 mL, 2 times, 14 days | Fresh | Freda | 2 mL, 5 times, weekly |
| Filardo et al. [22] | 2012 | PRP | NA | 5 mL, 3 times, weekly | Frozen | Hyalubrix | NA, 3 times, weekly |
| Louis et al. [39] | 2018 | PRP | Calcium chloride | 3 mL, 1 time | Fresh | Durolane | 3 mL, 1 time |
ACP = autologous conditioned plasma; HA = hyaluronic acid; LP-PRP = leukocyte-poor platelet-rich PRP; LR-PRP = leukocyte-rich platelet-rich PRP; NA = data not available; NO = No Activation; PA-PRP = photo-activation and platelet-rich plasma; PRGF = plasma rich in growth factors; PRP = platelet-rich plasma.
Assessment of the ROB
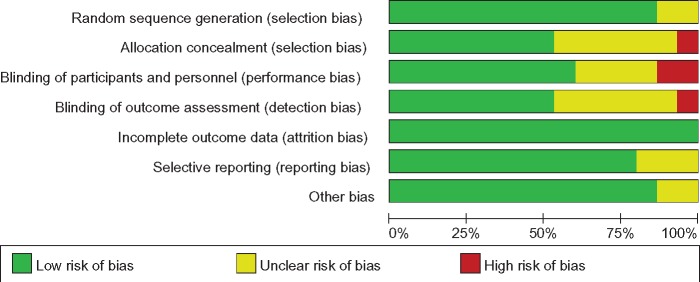
The summary of the ROB assessment of all included studies is illustrated in Figure 2. Adequate randomized sequences were generated in 13 studies [2,19,22,28,31–39]. However, two studies [29,30] referred to random assignments but did not report the details of these random assignments. Appropriate allocation concealment was reported in eight studies [21,22,28,31,33,34,37,39], the blinding of participants and personnel was clear in nine studies [21,22,28,31,33,34,37–39], and the blinding of outcome assessors was reported in eight studies [21,22,33–35,37–39]. Incomplete outcome data were not observed in all included studies. In addition, no selective reporting or other obvious sources of bias were identified in these studies.
Figure 2.
Risk of bias assessment.
Outcomes of the Meta-analysis
WOMAC Pain Score
WOMAC pain scores were reported by three [2,31,32], four [2,31,32,39], six [2,21,31,32,37,38], and five studies [2,21,31,32,36] at one, three, six, and 12 months, respectively. The pooled analysis did not reveal significant differences between the PRP and HA groups at one (MD = −0.19, 95% CI = −0.65 to 0.27, I2 = 67%, P = 0.42) and three months (MD = −0.31, 95% CI = −1.16 to 0.54, I2 = 88%, P = 0.47). However, subjects in the PRP group experienced significantly more pain relief than those in the HA group at six (MD = −1.24, 95% CI = –1.94 to −0.53, I2 = 83%, P = 0.0006) and 12 months (MD = −1.75, 95% CI = −2.50 to −1.01, I2 = 89%, P < 0.000001). Table 3 presents all of these details.
Table 3.
Details of PRP treatment protocols and control
| Follow-up, mo | WOMAC | No. of Studies | No. of Cases PRP/HA | MD | (95% CI) | Heterogeneity, P; I2 | P Value of Effect Size, Z (P) |
|---|---|---|---|---|---|---|---|
| 1 | Pain | 3 | 108/114 | – | (–0.65 to 0.27) | 0.05; 67% | 0.80 (0.42) |
| Stiffness | 2 | 58/64 | −0.13 | (–0.41 to 0.15) | 0.21; 35% | 0.89 (0.37) | |
| Function | 2 | 58/64 | −2.35 | (–5.28 to 0.57) | 0.12; 59% | 1.57 (0.12) | |
| Total | 3 | 119/124 | −3.81 | (–7.98 to 0.36) | 0.03; 71% | 1.79 (0.07) | |
| 3 | Pain | 4 | 129/138 | −0.31 | (–1.16 to 0.54) | <0.0001; 88% | 0.72 (0.47) |
| Stiffness | 3 | 80/88 | −0.35 | (–0.63 to −0.08) | 0.44; 0% | 2.50 (0.01) | |
| Function | 4 | 80/88 | −1.92 | (–2.57 to −1.27) | 0.80; 0% | 5.82 (<0.000001) | |
| Total | 5 | 155/163 | −5.02 | (–10.79 to 0.76) | <0.00001; 89% | 1.70 (0.09) | |
| 6 | Pain | 6 | 270/263 | −1.24 | (–1.94 to −0.53) | <0.0001; 83% | 3.42 (0.0006) |
| Stiffness | 5 | 221/213 | −0.46 | (–0.92 to 0.01) | 0.04; 60% | 1.93 (0.05) | |
| Function | 5 | 221/213 | −3.71 | (–7.21 to −0.22) | <0.0001; 84% | 2.08 (0.04) | |
| Total | 7 | 296/288 | −10.78 | (–17.51 to −4.04) | <0.00001; 94% | 3.13 (0.002) | |
| 12 | Pain | 5 | 232/218 | −1.75 | (–2.50 to −1.01) | <0.00001; 89% | 4.61 (<0.000001) |
| Stiffness | 4 | 183/168 | −0.99 | (–1.57 to −0.42) | 0.001; 81% | 3.40 (0.0007) | |
| Function | 4 | 183/168 | −8.90 | (–14.82 to −2.99) | <0.00001; 94% | 2.95 (0.003) | |
| Total | 4 | 183/168 | −12.11 | (–20.21 to −4.01) | <0.00001; 94% | 2.93 (0.003) |
WOMAC Stiffness Score
WOMAC stiffness scores were reported by two [2,32], three [2,32,39], five [2,21,32,37,38], and four studies [2,21,32,36] at one, three, six, and 12 months, respectively. The pooled analysis did not reveal significant differences between the PRP and HA groups at one (MD = −0.13, 95% CI = −0.41 to 0.15, I2 = 35%, P = 0.37) and six months (MD = −0.46, 95% CI = −0.92 to 0.01, I2 = 60%, P = 0.05). However, the subjects in the PRP group experienced significantly greater improvement in knee stiffness than those in the HA group at three (MD = –0.35, 95% CI = −0.63 to −0.08, I2 = 0%, P = 0.01) and 12 months (MD = −0.99, 95% CI = −1.57 to −0.42, I2 = 81%, P = 0.0007). Table 3 presents all of these details.
WOMAC Function Score
WOMAC function scores were reported by two [2,32], three [2,32,39], five [2,21,32,37,38], and four studies [2,21,32,36] at one, three, six, and 12 months, respectively. The pooled analysis showed that the subjects in the PRP and HA groups exhibited similar functional recovery after one month (MD = −2.35, 95% CI = −5.28 to 0.57, I2 = 59%, P = 0.12) of treatment. However, the subjects in the PRP group performed better than those in the HA group at three (MD = −1.92, 95% CI = −2.57 to −1.27, I2 = 0%, P < 0.000001), six (MD = −3.71, 95% CI = −7.21 to −0.22, I2 = 84%, P = 0.04), and 12 months (MD = −8.90, 95% CI = −14.82 to −2.99, I2 = 94%, P = 0.003). Table 3 presents all of these details.
WOMAC Total Score
WOMAC total scores were reported by three [2,30,32], five [2,29,30,32,39], seven [2,21,29,30,32,37,38], and four studies [2,21,32,36] at one, three, six, and 12 months, respectively. The pooled analysis did not identify significant differences between the PRP and HA groups at one (MD = −3.81, 95% CI = −7.98 to 0.36, I2 = 71%, P = 0.07) and three months (MD = −5.02, 95% CI = −10.79 to 0.76, I2 = 89%, P = 0.09). However, the subjects in the PRP group experienced significantly greater improvement in total scores than those in the HA group at six (MD = −10.78, 95% CI = −17.51 to −4.04, I2 = 94%, P = 0.002) and 12 months (MD = −12.11, 95% CI = −20.21 to −4.01, I2 = 94%, P = 0.003). All these details are presented in Table 3.
VAS
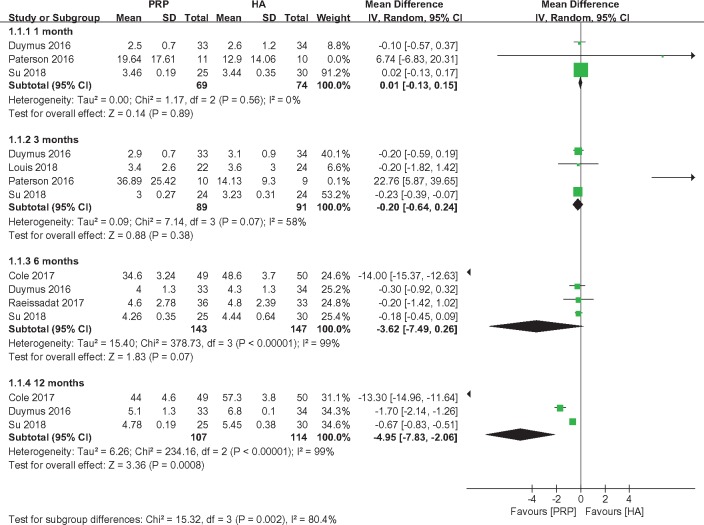
VAS scores for pain were reported by three [28,32], four [2,28,32,39], four [2,31,32,38], and three studies [2,31,32] at one, three, six, and 12 months, respectively. Therefore, a subgroup meta-analysis was performed to compare the VAS scores for pain based on the length of follow-up. No significant differences were observed between the PRP and HA groups at one (MD = 0.01, 95% CI = −0.13 to 0.15, I2 = 0%, P = 0.89), three (MD = −0.20, 95% CI = −0.64 to 0.24, I2 = 58%, P = 0.38), and six months (MD = −3.62, 95% CI = −7.49 to 0.26, I2 = 99%, P = 0.07). However, the treatment in the PRP groups exhibited better efficacy than that in the HA groups at 12 months (MD = −4.95, 95% CI = −7.83 to −2.06, I2 = 99%, P = 0.0008) (Figure 3).
Figure 3.
Forest plot and meta-analysis of VAS score.
IDKC
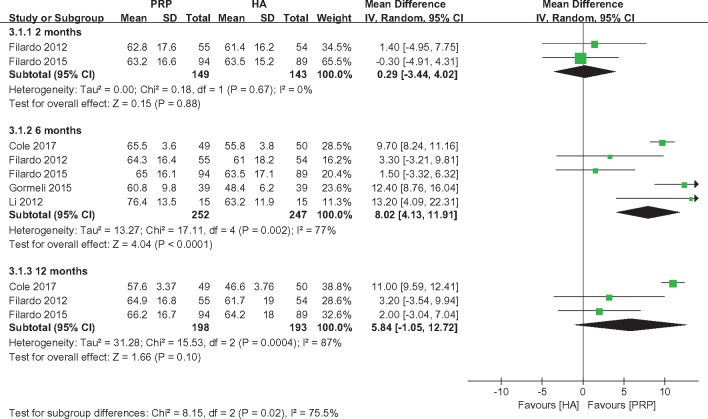
IDKC scores were reported by two [22,33], five [22,29,31,33,34], and three studies [22,31,33] at two, six, and 12 months, respectively. Therefore, a subgroup meta-analysis was performed to compare the IDKC scores based on the length of follow-up. No significant differences were observed between the PRP and HA groups at two (MD = 0.29, 95% CI = −3.44 to 4.02, I2 = 0%, P = 0.88) and 12 months (MD = 5.84, 95% CI = −1.05 to 12.72, I2 = 87%, P = 0.10). However, the subjects in the PRP group had significantly better IDKC scores than those in the HA group at six months (MD = 8.02, 95% CI = 4.13 to 11.91, I2 = 77%, P < 0.0001) (Figure 4).
Figure 4.
Forest plot and meta-analysis of IDKC.
Lequesne Index
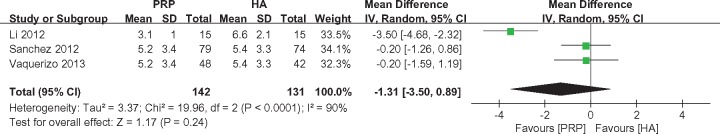
Lequesne Index scores were reported by three studies [21,29,37], with 142 patients treated with PRP and 131 with HA. The pooled analysis did not reveal a significant difference between the PRP and HA groups at six months (MD = −1.31, 95% CI = −3.50 to 0.89, I2 = 90%, P = 0.24) (Figure 5).
Figure 5.
Forest plot and meta-analysis of Lequesne index scores.
Adverse Events
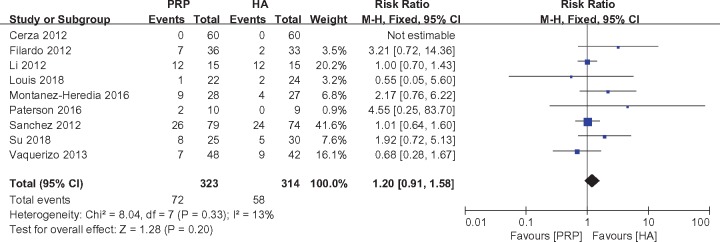
Adverse events were reported by nine studies [2,21,28–30,35,37–39], with 323 patients treated with PRP and 314 with HA. The pooled analysis did not detect a significant difference between the PRP and HA groups (RR = 1.20, 95% CI = 0.91 to 1.58, I2 = 13%, P = 0.20) (Figure 6).
Figure 6.
Forest plot and meta-analysis of adverse events.
Discussion
As a common degenerative joint disease, KOA is the second leading cause of loss of joint function [4], which causes enormous economic and social burdens worldwide [40]. The understanding of KOA in terms of etiology and pathogenesis remains unclear [41]; the main pathological changes involve components around the joints and degeneration of articular cartilage [42].
HA is mainly composed of important factors such as D-glucosamine and N-acetyl-D-glucosamine, and these factors play an important role in nourishing cartilage and protecting joints [43]. HA is degraded and diluted by exudates, resulting in a decrease in the molecular weight and endogenous concentration of HA in the knee joint cavity of patients with KOA. These changes reduce the viscoelasticity of the knee cartilage and reduce the knee’s ability to resist mechanical stress and damage [11,44]. Many clinical studies have also shown that HA can effectively relieve knee pain and improve knee function [45–47]. However, some researchers have shown that HA does not improve regeneration of damaged cartilage, particularly in elderly patients with severe KOA [44,48]. PRP is highly concentrated autologous plasma containing a variety of growth factors that promote the healing of ligaments, tendons, and bones, including platelet-derived growth factor, transforming growth factor–b, vascular endothelial growth factor, basic fibroblast growth factor, and bioactive proteins [49–51]. As a vector for large growth factors [52], PRP promotes tissue repair [53] and is increasingly used to treat KOA. PRP plays an important role in mesenchymal stem cell proliferation and cartilage formation through a combination of various growth factors [54]. It has been reported that PRP may have a positive effect on inducing migration, proliferation, and differentiation of precursor cells in cartilage. Therefore, PRP can effectively repair the damaged cartilage of the knee joint [55] while reducing the effects of knee pain and inflammatory responses [56]. These effects of PRP make it a new drug treatment for KOA.
Most of the previous systematic reviews have shown that PRP is an effective alternative treatment for long-term relief of knee pain and improved joint function in KOA patients. However, the previous conclusions were reached on the basis of a small number of RCTs [23–26,27,57], and thus, the time effect of PRP injection therapy on KOA has not been fully investigated. Chang et al. [58] found that patients receiving PRP injections showed better, longer-lasting improvements than patients receiving HA treatment; however, most of the studies that were included in the analysis were case series, and only five were RCTs. Laudy et al. [27] pooled 10 trials, including six RCTs, and found that PRP injections were more effective than placebo or HA injections for alleviating pain symptoms and improving joint motor function in KOA patients. Another recent meta-analysis [11] included 13 studies (10 RCTs) and synthesized the WOMAC scores to compare the efficacy of PRP injections with that of HA injections. Based on a large number of RCTs, this study evaluated the effects of PRP treatment on knee joint pain and physiological function at different times after injection.
In our study, the data from 15 RCTs showed that long-term pain relief and functional improvement in the PRP group were superior to those in the HA group. Divergent results were observed in the WOMAC, VAS, IKDC, and Lequesne Index scores, as well as in the occurrence of adverse events. The principal findings of this study were that within one to three months postinjection, subjects in the PRP group and HA group had similar experiences with respect to pain relief (WOMAC pain score and VAS pain score) and functional improvement (WOMAC total score, WOMAC function score, and IKDC score). In addition, no significant differences in the Lequesne Index were observed between the PRP and HA groups after six months. However, subjects in the PRP group experienced significantly better pain relief (WOMAC pain score and VAS pain score) and functional improvement (WOMAC function score, WOMAC total score, and IKDC score) between six and 12 months postinjection than those in the HA group. In addition, the HA and PRP groups did not display different adverse event rates.
Heterogeneity was different between all measured parameters, indicating that the effects were inconsistent throughout the studies. Based on a careful review and evaluation of all included studies, we found some common problems. First, the pathology of KOA in the PRP and HA groups was different among all these included studies, and the distribution of KOA severity among the studies varied between grades I and IV (K-L grading scale). As shown in the studies by Chang et al. [58] and Filardo et al. [59], PRP has a better effect on patients with early or moderate forms of KOA but has a limited effect on patients with advanced forms of KOA. Therefore, patients with different stages of KOA may not exhibit the same responses to PRP or HA treatment [11].
Additionally, the number of injections and the length of the time between the two injections also varied among all these included studies. Among the studies that included multiple injections, an interval of two weeks was used in the studies by Montanez-Heredia et al. [35] and Su et al. [2], three weeks was adopted by Li et al. [29] and Raeissadat et al. [38], and one month was adopted by Duymus et al. [32] and Raeissadat et al. [36]. In the study by Gormeli et al. [34], no differences were observed between patients who received an HA injection and those who received a single-dose PRP injection, whereas the patients receiving multiple-dose PRP experienced greater improvement than the patients receiving either of the other two treatments. However, Patel et al. [53] concluded that a single dose of PRP injection and a double dose of PRP injection had the same therapeutic effect. The study by Görmeli et al. [34] further confirmed this conclusion in patients with advanced KOA, as there was no difference between treatment methods. These findings might provide guidance for future treatment options, because a consensus regarding treatment methods is currently unavailable.
As this study was a systematic review, it inevitably had certain limitations. Limitations exist in the recommendation of routine PRP injections as a treatment for KOA because the composition of PRP remains uncertain. Different types of PRP preparations have been used; thus, these preparations differ in the platelet counts, growth factor concentrations, and leukocyte counts depending on the patient characteristics and the preparation kit. Furthermore, the interval between injections and the optimal dosage are still areas of concern in the future because they might exert an effect on the postinjection process, and there are currently no published studies supporting any specific injection protocol. In the present study, insufficient data were available for us to conduct a subgroup analysis to confirm whether multiple injections are necessary for patients with different stages of KOA.
Conclusions
Based on the current evidence, the use of PRP to treat KOA has a positive effect on pain levels and functional outcomes. In addition, PRP injections do not display different adverse event rates compared with HA injections. There is a lack of clarity regarding the number and frequency of PRP injections required to achieve maximum results and in the ideal treatment regimens for different severities of KOA.
Authors’ Contributions
Conceived and designed the experiments: Yanhong Han and Jun Liu; performed the experiments: Jianke Pan, Weiyi Yang, and Lingfeng Zeng; analyzed the data: Hetao Huang, Jiongtong Lin, and Guihong Liang; wrote the paper: Yanhong Han and Jianke Pan.
Acknowledgments
We are very grateful to the American Journal Experts for their language assistance in the process of writing this manuscript.
Funding sources: This study was supported by the National Ministry of Industry and the State Health Planning Commission “Orthopedic Surgery Robot Application Center” construction projects (No. 2017MHDOSR1008), the TCM Standardization Projects of the State Administration of Traditional Chinese Medicine of China (No. SATCM-2015-BZ115, No. SATCM-2015-BZ173), the Science and Technology Planning Project of Guangdong Province (No. 2012B061700037, No. 2017ZC0185), the Project of Guangdong Provincial Department of Finance (No. [2014]157, No. [2018]8), the Medical Scientific Research Foundation of Guangdong Province (No. A2017215), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YKYN2015MS15).
Conflicts of interest: We declare that we have no conflicts of interest.
References
- 1. Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19(5):478–82. [DOI] [PubMed] [Google Scholar]
- 2. Su K, Bai Y, Wang J, et al. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol 2018;37(5):1341–50. [DOI] [PubMed] [Google Scholar]
- 3. Suri P, Morgenroth DC, Hunter DJ.. Epidemiology of osteoarthritis and associated comorbidities. Pm R 2012;4(5 Suppl):S10–9. [DOI] [PubMed] [Google Scholar]
- 4. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis & Rheumatism 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartilage 2008;16(2):137–62. [DOI] [PubMed] [Google Scholar]
- 6. Pham T, Maillefert JF, Hudry C, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis. A two-year prospective randomized controlled study. Osteoarthritis Cartilage 2004;12(1):46–55. [DOI] [PubMed] [Google Scholar]
- 7. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD.. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin Orthop Relat Res 2010;468(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjordal JM, Ljunggren AE, Klovning A, Slordal L.. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: Meta-analysis of randomised placebo controlled trials. BMJ 2004;329(7478):1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvey WF, Hunter DJ.. The role of analgesics and intra-articular injections in disease management. Med Clin N Am 2009;93(1):201–11. [DOI] [PubMed] [Google Scholar]
- 10. Jüni P, Reichenbach S, Trelle S, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: A randomized controlled trial. Arthritis Rheum 2007;56(11):3610–9. [DOI] [PubMed] [Google Scholar]
- 11. Zhang HF, Wang CG, Li H, Huang YT, Li ZJ.. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Drug Des Devel Ther 2018;12:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cugat R, Cusco X, Seijas R, et al. Biologic enhancement of cartilage repair: The role of platelet-rich plasma and other commercially available growth factors. Arthroscopy 2015;31(4):777–83. [DOI] [PubMed] [Google Scholar]
- 13. Andia I, Maffulli N.. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 2013;9(12):721–30. [DOI] [PubMed] [Google Scholar]
- 14. Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: Intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 2010;18(4):472–9. [DOI] [PubMed] [Google Scholar]
- 15. Sampson S, Gerhardt M, Mandelbaum B.. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr Rev Musculoskelet Med 2008;1(3–4):165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA.. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med 2009;37(11):2259–72. [DOI] [PubMed] [Google Scholar]
- 17. Sundman EA, Cole BJ, Fortier LA.. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med 2011;39(10):2135–40. [DOI] [PubMed] [Google Scholar]
- 18. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH.. The use of platelet-rich plasma in arthroscopy and sports medicine: Optimizing the healing environment. Arthroscopy 2010;26(2):269–78. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez M, Fiz N, Guadilla J, et al. Intraosseous infiltration of platelet-rich plasma for severe knee osteoarthritis. Arthrosc Tech 2014;3(6):e713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rayegani SM, Raeissadat SA, Taheri MS, et al. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev (Pavia) 2014;6(3):112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy 2013;29(10):1635–43. [DOI] [PubMed] [Google Scholar]
- 22. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012;13:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dold AP, Zywiel MG, Taylor DW, Dwyer T, Theodoropoulos J.. Platelet-rich plasma in the management of articular cartilage pathology: A systematic review. Clin J Sport Med 2014;24(1):31–43. [DOI] [PubMed] [Google Scholar]
- 24. LaPrade CM, James EW, LaPrade RF, Engebretsen L.. How should we evaluate outcomes for use of biologics in the knee? J Knee Surg 2015;28(1):35–44. [DOI] [PubMed] [Google Scholar]
- 25. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24(5):1665–77. [DOI] [PubMed] [Google Scholar]
- 26. Ornetti P, Nourissat G, Berenbaum F, et al. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine 2016;83(1):31–6. [DOI] [PubMed] [Google Scholar]
- 27. Laudy ABM, Bakker EWP, Rekers M, Moen MH.. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Brit J Sport Med 2015;49(10):657–72. [DOI] [PubMed] [Google Scholar]
- 28. Paterson KL, Nicholls M, Bennell KL, Bates D.. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 2016;17:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee-articular injection of platelet-rich plasma on knee articular cartilage degeneration [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011;25:1192–6. [PubMed] [Google Scholar]
- 30. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med 2012;40(12):2822–7. [DOI] [PubMed] [Google Scholar]
- 31. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: A prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med 2017;45(2):339–46. [DOI] [PubMed] [Google Scholar]
- 32. Duymus TM, Mutlu S, Dernek B, et al. Choice of intra-articular injection in treatment of knee osteoarthritis: Platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc 2017;25(2):485–92. [DOI] [PubMed] [Google Scholar]
- 33. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation. A randomized controlled trial. Am J Sports Med 2015;43(7):1575–82. [DOI] [PubMed] [Google Scholar]
- 34. Gormeli G, Gormeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:958–65. [DOI] [PubMed] [Google Scholar]
- 35. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: A randomized clinical trial in the context of the Spanish National Health Care System. Int J Mol Sci 2016;17(7):1064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: Platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord 2015;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012;28:1070–8. [DOI] [PubMed] [Google Scholar]
- 38. Raeissadat SA, Rayegani SM, Ahangar AG, et al. Efficacy of intra-articular injection of a newly developed plasma rich in growth factor (PRGF) versus hyaluronic acid on pain and function of patients with knee osteoarthritis: A single-blinded randomized clinical trial. Clin Med Insights Arthritis Musculoskelet Disord 2017;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Louis ML, Magalon J, Jouve E, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: A randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy 2018;34(5):1530–40. [DOI] [PubMed] [Google Scholar]
- 40. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R.. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003;290(18):2443–54. [DOI] [PubMed] [Google Scholar]
- 41. Gobbi A, Lad D, Karnatzikos G.. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2015;23(8):2170–7. [DOI] [PubMed] [Google Scholar]
- 42. Aigner T, Kim HA.. Apoptosis and cellular vitality: Issues in osteoarthritic cartilage degeneration. Arthritis Rheum 2002;46(8):1986–96. [DOI] [PubMed] [Google Scholar]
- 43. Reitinger S, Lepperdinger G.. Hyaluronan, a ready choice to fuel regeneration: A mini-review. Gerontology 2013;59(1):71–6. [DOI] [PubMed] [Google Scholar]
- 44. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE.. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthritis Cartilage 2011;19(6):611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hunter DJ, Lo GH.. The management of osteoarthritis: An overview and call to appropriate conservative treatment. Rheum Dis Clin North Am 2008;34(3):689–712. [DOI] [PubMed] [Google Scholar]
- 46. Campbell J, Bellamy N, Gee T.. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthritis Cartilage 2007;15(12):1424–36. [DOI] [PubMed] [Google Scholar]
- 47. Miller LE, Block JE.. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord 2013;6:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dagenais S. Intra-articular hyaluronic acid (viscosupplementation) for knee osteoarthritis. Issues Emerg Health Technol 2006;(94):1–4. [PubMed] [Google Scholar]
- 49. Kabiri A, Esfandiari E, Esmaeili A, et al. Platelet-rich plasma application in chondrogenesis. Adv Biomed Res 2014;3:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levi D, Horn S, Tyszko S, et al. Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: Preliminary results from a prospective trial. Pain Med 2016;17:1010–22. [DOI] [PubMed] [Google Scholar]
- 51. Lutz GE. Increased nuclear T2 signal intensity and improved function and pain in a patient one year after an intradiscal platelet-rich plasma injection. Pain Med 2017;18(6):1197–9. [DOI] [PubMed] [Google Scholar]
- 52. Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA.. Platelet-rich plasma: Where are we now and where are we going? Sports Health 2010;2(3):203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A.. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am J Sports Med 2013;41(2):356–64. [DOI] [PubMed] [Google Scholar]
- 54. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD.. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy 2016;32(3):495–505. [DOI] [PubMed] [Google Scholar]
- 55. Manferdini C, Maumus M, Gabusi E, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum 2013;65(5):1271–81. [DOI] [PubMed] [Google Scholar]
- 56. de Vries-van MM, Narcisi R, Kops N, et al. Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone. Tissue Eng Part A 2014;20(1–2):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lai LP, Stitik TP, Foye PM, et al. Use of platelet-rich plasma in intra-articular knee injections for osteoarthritis: A systematic review. Pm R 2015;7(6):637–48. [DOI] [PubMed] [Google Scholar]
- 58. Chang KV, Hung CY, Aliwarga F, et al. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: A systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95(3):562–75. [DOI] [PubMed] [Google Scholar]
- 59. Filardo G, Kon E, Roffi A, et al. Platelet-rich plasma: Why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23(9):2459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]