Abstract
Objectives:
There are limited screening tools to predict adverse post-operative outcomes for the geriatric surgical fracture population. Frailty is increasingly recognized as a risk assessment to capture complexity. The goal of this study was to utilize a short screening tool, the FRAIL scale, to categorize the level of frailty of older adults admitted with a fracture to determine the association of each frailty category with post-operative and 30-day outcomes.
Design:
Retrospective cohort study
Setting:
Level 1 Trauma Center
Participants:
175 consecutive patients over age 70 admitted to co-managed orthopaedic trauma and geriatrics service
Measurements:
The FRAIL scale (short five-questions assessment of fatigue, resistance, aerobic capacity, illnesses and loss of weight) classified the patients into three categories: robust (score=0), prefrail (score=1–2), and frail (score=3–5). Postoperative outcome variables collected were: postoperative complications, unplanned ICU admission, length of stay (LOS), discharge disposition, and orthopedic follow-up after surgery. 30-day outcomes measured were 30-day readmission and 30-day mortality. Analysis of variance (one-way ANOVA) and Kruskal-Wallis tests were used to compare continuous variables across the three FRAIL categories. Fisher exact tests were used to compare categorical variables. Multiple regression analysis, adjusted by age, gender and Charlson index, was conducted to study the association between frailty category and outcomes.
Results:
FRAIL scale categorized the patients into three groups: robust (n=29), prefrail (n=73), and frail (n=73). There were statistically significant differences between groups in terms of age, comorbidity, dementia, functional dependency, polypharmacy and rate of institutionalization, being higher in the frailest patients. Hip fracture was the most frequent fracture, and it was more frequent as the frailty of the patient increased (48%, 61% and 75% in robust, prefrail and frail groups, respectively). The American Society of Anesthesiologists (ASA) preoperative risk significantly correlated with the frailty of the patient (ASA score 3–4: 41%, 82% and 86%, in robust, prefrail and frail groups, p<0.001). After adjustment by age, gender and comorbidity, there was a statistically significant association between frailty and both, length of stay and the development of any complication after surgery (length of stay: 4.2, 5.0, and 7.1 days, p=0.002; any complication: 3.4, 26 and 39.7%, p=0.03; in robust, prefrail and frail groups). There were also significant differences in discharge disposition (31% of robust and 4.1% of frail patients were discharged home (p 0.001)) and follow-up completion (97% of robust versus 69% of the frail ones). Differences in time to surgery, unplanned ICU admission, and 30-day readmission and mortality, although showing a trend, didn’t reach statistical significance.
Conclusion:
Frailty, measured by the FRAIL scale, was associated with increase length of stay, complications after surgery, and discharge to rehabilitation facility in geriatric fracture patients. The FRAIL scale is a promising short screen to stratify and help operationalize the perioperative care of older surgical patients.
Keywords: frailty, fracture, fall, delirium, older adult
Introduction
With the population aging, there is a larger impact on the healthcare system of older patients admitted to the hospital with fractures. Understanding who is at risk for adverse outcomes in the aging surgical fracture population is essential to allow for informed conversations and targeted programs to assist this vulnerable population.
To date, there are limited screening tools for predicting adverse post-operative outcomes for the geriatric surgical fracture population. Preoperative risk assessments are traditionally carried out using the American Society of Anesthesiology (ASA) level and a cardiac evaluation such as a Revised Cardiac Risk Index1,2, and Gupta Index.3 However, these screening tools are limited for the older adult population because they fail to capture the complexity of the elderly population, in other words, they do not assess for frailty. The presence of frailty in elderly patients is becoming increasingly recognized as a contributing element in the outcome of treatment.4–8 Frailty, defined as a state of increased vulnerability to stress, has been one way for clinicians to characterize the physiological reserve of geriatric patients. Assessment of frailty of geriatric patients prior to surgery can aid in prognosis and optimize care plans. The two most commonly used concepts of frailty include the Fried criteria and the Rockwood frailty index.9,10 One disadvantage to screening for frailty using these diagnostic tools is they are both time consuming and labor intensive. Due to these limitations, simplified screening tools are needed to operationalize frailty management in this vulnerable population.
Given that there is currently a lack of consensus regarding the appropriate assessment of frailty in older adults admitted for surgical repair of fracture11, our goal was to utilize a screening frail questionnaire the FRAIL scale to categorize the level of frailty of elderly patients admitted with a fractures and determine the association of frailty category with post-operative and 30-day outcomes.
Methods
This retrospective cohort study included all consecutive patients over age 70 admitted to a geriatric fracture co-management service at a Level 1 Trauma center, between August 2015 and May 2016. Patients were evaluated by one of three board certified geriatricians and seen daily by orthopedic surgeons. The FRAIL scale was completed as part of the routine comprehensive geriatric assessment performed on admission as part of a new initiative.
During the study period, our geriatricians assessed 369 patients. Patients were excluded if they did not have an orthopedic fracture diagnosis (i.e., no fracture or only rib, nasal, or facial fractures) or if their injury did not require surgery. 175 geriatric fracture patients met the inclusion criteria (Figure 1). They were categorized using FRAIL scale into 3 groups: robust (N=29), pre-frail (N=73) and frail (N=73). The study was approved by the hospital Institutional Review Board.
Figure 1.
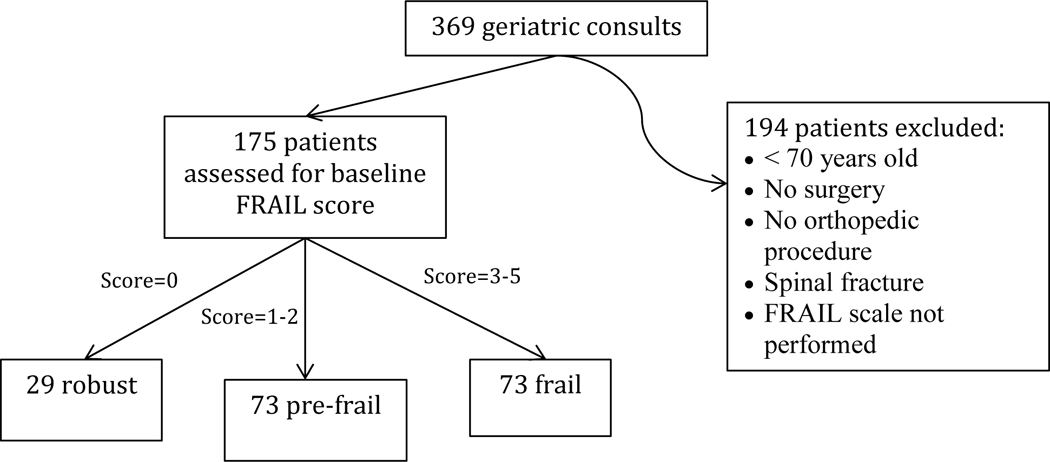
Patient inclusion algorithm
Assessment of Frailty
The FRAIL scale is a short five-question assessment that can screen for frailty (Figure 2).12–14 The scale contains 4 questions directed at components of the Cardiovascular Health Study Frailty Index and one (number of illnesses) at the Rockwood Scale.15 Individuals care considered robust (score=0), prefrail (score=1–2), and frail (score=3–5). The questionnaire has been shown to be an optimal screening test for clinicians to identify frail persons at risk of decline in health and mortality.16–18
Figure 2.
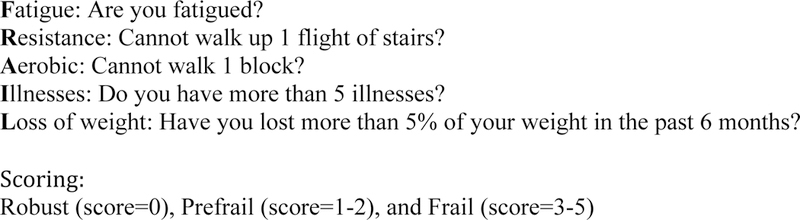
FRAIL Scale
Preoperative Variables
Preoperative variables that were obtained from the medical records included the following: demographics, marital status, living situation, Charlson index19, activities of daily living (BADL)20 and instrumental activities of daily living (IADL)21, Mini-Cog score22, number of medications on admission, self-reported history of falls, basic code status, albumin level, ASA score, and fracture type. All preoperative variables were obtained from the initial geriatric consult note with the exception of ASA score and comorbidities, which were collected from the anesthesiology and orthopedic surgery notes.
Postoperative and 30-day Outcome Variables
Postoperative outcome variables included postoperative complications (pulmonary, cardiac, delirium, deep vein thrombosis or pulmonary embolism, renal insufficiency [twice more than baseline creatinine] and unplanned intensive care unit (ICU) admission [new admission to ICU after surgery], LOS, discharge disposition location, and orthopedic follow-up at any point after surgery. Thirty-day outcomes included readmission, and mortality. All postoperative variables were recorded from the geriatric and discharge notes found in patients’ electronic medical records (EMR). Follow-up appointment and 30-day readmission data were ascertained by reviewing patients’ longitudinal data in the EMR, as was the 30-day mortality.
Statistical analysis
Analysis of variance (one-way ANOVA) and Kruskal Wallis tests were used to compare continuous variables across the three FRAIL categories: robust, prefrail and frail. Chi-square and Fisher exact tests were used to compare categorical variables. Multiple regression analysis, adjusted by age, gender and Charlson index, was conducted to study the association between frailty category and outcomes. All the analyses were performed using IBM SPSS Statistics for Macintosh, Version 20 (Armonk, NY: IBM Corp.)
Results
The baseline characteristics of the study population are shown in Table 1. The mean (SD) age of the cohort was 82.3 (7.4) years, and the majority of the patients were female (74.9%). Several variables demonstrated a statistically significant difference according to frailty categories. Age increased in parallel to the frailty of the patients (mean age in robust patients: 77.8 years, versus 84 years in the frail group). While 83% of robust patients were living at home before the admission, only 49% of frail patients could do the same. Robust patients had less number of comorbidities compared to prefrail and frail patients (Charlson index mean: 1, 2.2, and 2.4, respectively), and they were on fewer medications at the time of admission (6.6, 10.5, and 10.5, respectively).
Table 1.
Preoperative Characteristics by Frailty Category
| Characteristics | Total N=175 |
Robust N= 29 |
Prefrail N=73 |
Frail N=73 |
P- Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 82.3 (7.4) | 77.8 (5.7) | 82.3 (7.2) | 84 (7.5) | 0.01 |
| Female, n (%) | 131(74.9) | 23 (79.3) | 54 (74) | 54 (74) | 0.83 |
| Married, n (%) | 72 (42.4) | 17 (58.6) | 27 (38) | 28 (40) | 0.57 |
| Living at home prior to admission, n (%) | 116(66.3) | 24 (83) | 56 (77) | 36 (49) | 0.001 |
| Charlson index, mean (SD) | 2.1 (1.9) | 1.0 (1.0) | 2.2 (1.9) | 2.4 (2.0) | 0.001 |
| Dementia, n (%) | 27(15.4) | 0 | 11 (15.1) | 16 (21.9) | 0.02 |
| Comprehensive Geriatric Assessment (CGA) | |||||
| Independent for all ADL, n (%) | 101(57.7) | 29 (100) | 50 (68.5) | 22 (30.1) | <0.001 |
| Independent for all IADL, n (%) | 54 (30.9) | 23 (79.3) | 26 (35.6) | 5 (6.8) | <0.001 |
| Mini-cog abnormal, n (%) | 80 (51.3) | 3 (10.7) | 28 (43.1) | 49 (77.8) | <0.001 |
| No. medications, mean (SD) | 9.8 (5.6) | 6.6 (5.6) | 10.5 (5.7) | 10.5 (5.0) | 0.03 |
| History of falls in past year, n (%) | 96 (55.8) | 15 (51.5) | 36 (49.3) | 45 (64.3) | 0.17 |
| Incontinence, n (%) | 66 (38) | 3 (10.3) | 25 (34.2) | 38 (53) | 0.002 |
| DNR code, n (%) | 43 (24.6) | 3 (10.3) | 13 (17.8) | 27 (37) | 0.02 |
| Albumin level, mean (SD) | 3.6 (0.5) | 3.8 (0.4) | 3.7 (0.5) | 3.5 (0.5) | 0.10 |
| ASA score 3–4, n (%) | 135(77.1) | 12 (41.4) | 60 (82.2) | 63 (86.3) | <0.001 |
| Fracture type, n (%) | |||||
| Proximal femoral intertrochanteric | 51 (29.8) | 6 (22.2) | 21 (29.6) | 24(32.9) | |
| Proximal femoral subtrochanteric | 12 (7) | 3 (11.1) | 4 (5.6) | 5 (6.8) | |
| Proximal femoral neck | 49 (28.7) | 4 (14.8) | 19 (26.8) | 26 (35.6) | |
| Proximal femoral periprosthetic | 11 (6.4) | 1 (3.7) | 5 (7) | 5 (6.8) | |
| Distal femur | 10 (5.8) | 1 (3.7) | 4 (5.6) | 5 (6.8) | |
| Distal femoral periprosthetic | 7 (4.1) | 2 (7.4) | 3 (4.2) | 2 (2.7) | |
| Ankle | 10 (5.8) | 1 (3.7) | 6 (8.5) | 3 (4.1) | |
| Tibia/Fibula | 7 (4.1) | 2 (7.4) | 4 (5.6) | 1 (1.4) | |
| Upper extremity | 14 (8.2) | 7 (25.9) | 5 (7.0) | 2 (2.7) | |
IADL: Instrumental Activities of daily living
DNR: Do-not-resuscitate code
Regarding the Comprehensive Geriatric Assessment, robust patients were more independent for BADL and IADL than pre-frail or frail patients (independent for BADL: 100%, 68.5%, and 30.1%, respectively; IADL: 79.3%, 35.6%, and 6.8%, respectively). There were no patients with the diagnosis of baseline dementia in the robust group compared to 15.1% in the prefrail group, and 21.9% in the frail group. However, 10.7% of robust patients had an abnormal Mini-Cog test during admission, compared to 43.1% and 77.8% in the pre-frail and frail groups, respectively. The Do-not-resuscitate code (DNR code) was in place for 10.3% of the robust patients, compared to 17.8% and 37% in prefrail and frail patients. Only 10.3% of robust patients were incontinent, while that percent increased to 53% in the frail group.
Preoperative risk as defined by ASA score also correlated with the frailty of the patients, as 41% of robust patients had high pre-surgical risk (ASA score 3 or 4) compared to 86% of the patients in the frail group. The majority of the fracture patients presented with proximal femoral fracture (65.5%), and its prevalence increased as the frailty of the patient increased (48.1%, 62% and 75.3% in robust, prefrail and frail patients). The total prevalence of upper extremity fractures was 8.2%. They constituted 25.9% of all fractures in robust patients while only a 2.7% of all in frail patients.
Table 2 show frail patients had a slightly longer time to surgery than prefrail and robust ones; however, the difference was not statistically significant (mean time to surgery in the frail group: 1.96 versus 1.34 and 1.38 in the prefrail and robust groups, respectively). While the average number of complications experienced by patients was less than one, there does appear to be an increasing number of complications with frailty. The overall incidence of post-operative delirium was 20%. More specifically, post-operative delirium is significantly greatest in the frail group, in which it was present in almost 29% of the patients. Ninety percent of the entire cohort patients were discharged to a rehabilitation facility, while 31% of robust patients were discharged home; frail and pre-frail patients had a greater percentage of rehabilitation facility discharges. In our sample, all robust patients (100%) attended some form of follow-up compared to pre-frail patients (91.7%) and frail patients (71.8%).
Table 2.
Time to surgery, complications and discharge disposition by Frailty Category
| Characteristic | Total N=175 |
Robust N=29 |
Prefrail N=73 |
Frail N=73 |
P- Value |
|---|---|---|---|---|---|
| Time to surgery, mean(SD) (days) | 1.6 (2.0) | 1.4 (0.9) | 1.3 (1.0) | 2.0 (2.9) | 0.42 |
| No. Complications, mean (SD) | 0.4 (0.8) | 0 (0.2) | 0.4 (1.0) | 0.6 (0.9) | 0.01 |
| Complications, n (%) | |||||
| Respiratory | 7 (4) | 0 | 2 (2.8) | 5 (6.8) | 0.22 |
| Cardiac | 10 (5.7) | 0 | 4 (5.5) | 6 (8.2) | 0.27 |
| Delirium | 35 (20) | 1 (3.4) | 13 (17.8) | 21 (28.8) | 0.01 |
| DVT/PE | 2 (1.1) | 0 | 2 (2.7) | 0 | 0.25 |
| Acute renal failure | 7 (4) | 0 | 4 (5.5) | 3 (4.1) | 0.44 |
| Discharge disposition, n (%) | <0.001 | ||||
| Home | 15 (8.6) | 9 (31) | 3 (4.2) | 3 (4.1) | |
| Rehaba | 157 (90.2) | 20 (69) | 68 (94.4) | 69 (94.5) | |
| Otherb | 2 (1.2) | 0 | 1 (1.4) | 1 (1.4) | |
| Orthopedic follow-up, n (%) | 0.008 | ||||
| Yes | 142 (82.1) | 28 (96.6) | 65 (89) | 49 (69) | |
| No | 9 (5.2) | 0 | 2 (2.7) | 7 (9.9) | |
| Off-site | 5 (2.9) | 1 (3.4) | 2 (2.7) | 2 (2.8) | |
| No showc | 17 (9.8) | 0 | 4 (5.5) | 13 (18.3) | |
Rehabilitation hospital or Skilled Nursing Facility
Hospice or Cognitive rehab facility
Patients who did not follow up with an orthopedic surgeon while their medical records remained active with other appointments
The association between frailty and postoperative and 30-days outcomes is shown in Table 3. The unadjusted analysis evidenced a statistically significant difference between robust, prefrail and frail groups in terms of length of stay (mean LOS: 4.2, 5 and 7.1 days, respectively), and the development of any complication during admission (3.4, 26 and 39.7%, respectively). While 90.4% of frail patients were likely to stay 6 or more days only 51.7% of robust patients required that extended length of stay. There were also significant differences when comparing the percent of patients discharged to home (31, 4.2 and 4.1%, in robust, prefrail and frail patients), and the percentage of patients completing a follow-up appointment (100, 91.8, and 71.8%, respectively). Finally, non-significant differences between groups were found in unplanned ICU admission, 30-day readmissions and mortality with a trend toward worse outcomes in the pre-frail and frail groups.
Table 3.
Association between Frailty and Postoperative and 30-day Outcomes
| Outcomes Variable | Total N=175 |
Robust N= 29 |
Prefrail N=73 |
Frail N=73 |
P-Value | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Length of stay, mean (SD) (days) | 5.5 (3.4) | 4.2 (1.7) | 5.0 (3.0) | 7.1 (5.7) | <0.001 | 0.002 |
| Length of stay ≥ 6 days, n (%) | 127 (72.6) | 15 (51.7) | 46 (63) | 66 (90.4) | <0.001 | <0.001 |
| Any complication, n (%) | 49 (28) | 1 (3.4) | 19 (26) | 29 (39.7) | 0.001 | 0.03 |
| Unplanned ICU admission, n (%) | 13 (7.4) | 0 | 6 (8.2) | 7 (9.6) | 0.24 | 0.98 |
| Discharged home, n (%) | 15 (8.6) | 9 (31) | 3 (4.2) | 3 (4.1) | <0.001l | 0.01 |
| Orthopedic follow-up, n (%) | 147 (84) | 29 (100) | 67 (91.8) | 51 (71.8) | <0.001 | 0.02 |
| 30-day readmission, n (%) | 20 (11.4) | 2 (6.9) | 9 (12.5) | 9 (12.5) | 0.69 | 0.96 |
| 30-day mortality, n (%) | 5 (2.9) | 0 | 1 (1.4) | 4 (5.5) | 0.20 | 0.43 |
Adjusted by age, gender and Charlson index
Discussion
This study demonstrated that frailty is common among fracture patients as well as investigated the relationship between a physiological frailty screening tool, FRAIL scale, and postoperative and 30-day outcomes. It is unique in that we found that this brief screening tool identifies vulnerable patients with a relationship between level of frailty and outcomes independent of age, gender and comorbidity in fracture patients. The results of this study are consistent with the small body of literature on frailty and hip fracture outcomes in older adults. Specifically, alternative frailty indices have shown a significant association between frailty and outcomes after hip fracture including increased LOS23, postoperative complications24,25, and mortality at 1 and 2 years post-fracture.26 The advantage of the FRAIL scale compared to other scales is that it is a brief, easy to use tool18 that assesses physiological reserve in geriatric surgical fracture patients to stratify risk.
Overall, there is a general lack of consensus on how to best categorize frailty; nonetheless, it is increasingly being used for clinical risk assessments.6,27 The outcomes associated with frail and prefrail patients on the FRAIL scale have implications for where resources should be employed within the growing number of geriatric orthopedic co-management services.28–31 We discovered significant findings that non-robust patients (prefrail, frail) have greater needs than robust ones. Delirium was occurred in a greater percentage of non-robust patients. Delirium has been shown to be associated with worse postoperative outcomes in the postsurgical setting.32,33 Almost a third of frail patients (28.2%) did not make it to their outpatient surgical follow up. This has implications for outpatient follow up resources designated for these patients and may be better served with house visit or community outreach services for these targeted patients. Early surgery is optimal for fracture patients34, and from our internal quality metrics in our co-management group, on average, even frail patients can be assessed and stabilized for surgery within 2 days, but require more time postoperatively (on average 2 more days LOS) before discharge. This has implications when designing surgical bundles where robust and non-robust patients will require different number of postoperative days. Additionally, a third of robust patients were able to go home from hospital, not something normally associated with acute fractures in older adults. This is an important finding for those designing transitions of care. Frail patients had a trend, although non-significant, toward greater of 30-day readmission and 30-day mortality than robust patients, which agrees with the findings of other similar studies.35,36 All of these findings increase the value of implementing a simple FRAIL scale screening.
As expected, proximal femoral fractures, intertrochanteric and femoral neck fractures, were the most prevalent in frail patients. Frail patients likely have less associated capacity to avoid falling onto their pelvis. Additionally, we found that in the robust group upper extremity fractures more frequent (25.9% vs 2.7%). This suggests that the robust patients likely had greater bone density, were more active and that they may have greater strength and reflex to catch themselves upon falling; therefore, resulting in an upper extremity fracture rather than a lower extremity fracture.37,38
The use of Mini-Cog, measure of cognitive frailty, has previously been demonstrated to identify vulnerable geriatric ortho-patients pre-operatively.39 There is a significant relationship between cognitive impairment, represented by an abnormal Mini-Cog, and frailty. Mini-Cog is a complimentary assessment to the FRAIL scale in that is assesses cognitive reserve instead of physiological reserve. FRAIL scale does not replace the Mini-Cog, rather it should be viewed as an explicit cognitive assessment directly identifying delirium risk and the capacity for the patient providing consent around hip fractures. This argument is supported by our current data that demonstrates that 10.7% of robust patients had abnormal Mini-Cog. Among this group of robust patients, 3.4% developed delirium, the dominant complication in robust patients. This unlikely combination of robust physiological reserve combined with vulnerable cognitive state justifies the benefit of an explicit assessment for delirium risk even in robust patients. Finally, it is also important to point out that the overall delirium incidence was only 20%, compared with 41% in usual care, and is consistent with previous studies demonstrating the benefits of geriatric orthopedic co-management.29
There are several noteworthy strengths to this study. First, the same three geriatricians performed the comprehensive geriatric assessment at time of admission, including the FRAIL scale, thereby maintaining consistency throughout the duration of the study. Second the study was performed in a geriatric orthopedic co-management service where care pathways are standardized and interdisciplinary care is optimized for the acute care of the elderly. Third, medical records of consecutive patients were analyzed, which reduced any type of bias. Lastly, this study used a novel approach to screening geriatric fracture patients with a simple FRAIL scale, in order to predict their risk of post-operative complications. Our study was limited in that it was a retrospective study utilizing one institution’s electronic medical record. We were also unable to obtain information on if a patient was admitted to a different institution within the 30-day discharge period. Additionally, our sample size might have limited the study power to detect significant associations between frailty and other postoperative outcomes.
Future studies should compare the frail screen to a full frailty assessment such as a calculated frailty index from the comprehensive geriatric assessment in a larger sample to further validate this screen in the fracture population.
Conclusion
Frailty screening, measured by the FRAIL scale, can help predict postoperative outcomes in fracture patients. The FRAIL scale is a promising short screen to stratify patients by frailty and help operationalize the perioperative care of older surgical fracture patients.
Acknowledgments
Funding sources: This work was supported by the MSTAR Program (American Federation for Aging Research/NIH Grant #1T35AG38027-02 9) for EB as medical student awardee. LG has support from HRSA U1QHP28728, Geriatric Workforce Enhancement Program.
References:
- 1.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. The New England journal of medicine 1977;297:845–50. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. [DOI] [PubMed] [Google Scholar]
- 3.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011;124:381–7. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiology and drug safety 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons 2010;210:901–8. [DOI] [PubMed] [Google Scholar]
- 6.Cooper Z, Rogers SO Jr., Ngo L, et al. Comparison of Frailty Measures as Predictors of Outcomes After Orthopedic Surgery. Journal of the American Geriatrics Society 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC geriatrics 2016;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colburn JL, Mohanty S, Burton JR. Surgical Guidelines for Perioperative Management of Older Adults: What Geriatricians Need to Know. Journal of the American Geriatrics Society 2017. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology Series A, Biological sciences and medical sciences 2007;62:722–7. [DOI] [PubMed] [Google Scholar]
- 11.Soong JT, Poots AJ, Bell D. Finding consensus on frailty assessment in acute care through Delphi method. BMJ open 2016;6:e012904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kan Abellan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. The journal of nutrition, health & aging 2008;12:29–37. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association 2013;14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. Journal of the American Geriatrics Society 2012;60:1478–86. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology Series A, Biological sciences and medical sciences 2001;56:M158–66. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. The journal of nutrition, health & aging 2012;16:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaehr E, Visvanathan R, Malmstrom TK, Morley JE. Frailty in nursing homes: the FRAIL-NH Scale. Journal of the American Medical Directors Association 2015;16:87–9. [DOI] [PubMed] [Google Scholar]
- 18.Aprahamian I, Lin SM, Suemoto CK, et al. Feasibility and Factor Structure of the FRAIL Scale in Older Adults. Journal of the American Medical Directors Association 2017;18:367.e11-.e18. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Maryland state medical journal 1965;14:61–5. [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- 22.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. International journal of geriatric psychiatry 2000;15:1021–7. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan M, Beck S, Havelock W, Eeles E, Hubbard RE, Johansen A. Predicting outcome after hip fracture: using a frailty index to integrate comprehensive geriatric assessment results. Age and ageing 2014;43:122–6. [DOI] [PubMed] [Google Scholar]
- 24.Dayama A, Olorunfemi O, Greenbaum S, Stone ME Jr., McNelis J. Impact of frailty on outcomes in geriatric femoral neck fracture management: An analysis of national surgical quality improvement program dataset. International journal of surgery (London, England) 2016;28:185–90. [DOI] [PubMed] [Google Scholar]
- 25.Folbert EC, Hegeman JH, Gierveld R, et al. Complications during hospitalization and risk factors in elderly patients with hip fracture following integrated orthogeriatric treatment. Archives of orthopaedic and trauma surgery 2017;137:507–15. [DOI] [PubMed] [Google Scholar]
- 26.Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clinical orthopaedics and related research 2014;472:1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Manas L, Feart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. The journals of gerontology Series A, Biological sciences and medical sciences 2013;68:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. Journal of orthopaedic trauma 2014;28:e49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. Journal of the American Geriatrics Society 2008;56:1349–56. [DOI] [PubMed] [Google Scholar]
- 30.Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--a literature review comparing different models. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2010;21:S637–46. [DOI] [PubMed] [Google Scholar]
- 31.Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet 2015;385:1623–33. [DOI] [PubMed] [Google Scholar]
- 32.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. Journal of the American Geriatrics Society 2000;48:618–24. [DOI] [PubMed] [Google Scholar]
- 33.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellelli G, Mazzola P, Corsi M, et al. The combined effect of ADL impairment and delay in time from fracture to surgery on 12-month mortality: an observational study in orthogeriatric patients. Journal of the American Medical Directors Association 2012;13:664.e9-.e14. [DOI] [PubMed] [Google Scholar]
- 35.Shin JI, Keswani A, Lovy AJ, Moucha CS. Simplified Frailty Index as a Predictor of Adverse Outcomes in Total Hip and Knee Arthroplasty. The Journal of arthroplasty 2016;31:2389–94. [DOI] [PubMed] [Google Scholar]
- 36.Revenig LM, Canter DJ, Kim S, et al. Report of a Simplified Frailty Score Predictive of Short-Term Postoperative Morbidity and Mortality. Journal of the American College of Surgeons 2015;220:904–11.e1. [DOI] [PubMed] [Google Scholar]
- 37.Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol 1992;135:477–89. [DOI] [PubMed] [Google Scholar]
- 38.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. Journal of the American Geriatrics Society 1993;41:1226–34. [DOI] [PubMed] [Google Scholar]
- 39.Heng M, Eagen CE, Javedan H, Kodela J, Weaver MJ, Harris MB. Abnormal Mini-Cog Is Associated with Higher Risk of Complications and Delirium in Geriatric Patients with Fracture. The Journal of bone and joint surgery American volume 2016;98:742–50. [DOI] [PubMed] [Google Scholar]


