Abstract
High-valent ferryl species (e.g., (Por)FeIV=O, Cmpd-II) are observed or proposed key oxidizing intermediates in the catalytic cycles of heme-containing enzymes (P-450s, peroxidases, catalases, and cytochrome c oxidase) involved in biological respiration and oxidative metabolism. Herein, various axially ligated iron(IV)-oxo complexes were prepared to examine the influence of the identity of the base. These were generated by addition of various axial ligands (1,5-dicyclohexylimidazole (DCHIm), a tethered-imidazole system, and sodium derivatives of 3,5-dimethoxyphenolate and imidazolate). Characterization was carried out via UV−vis, electron paramagnetic resonance (EPR), 57Fe Mössbauer, Fe X-ray absorption (XAS), and 54/57Fe resonance Raman (rR) spectroscopies to confirm their formation and compare the axial ligand perturbation on the electronic and geometric structures of these heme iron(IV)-oxo species. Mössbauer studies confirmed that the axially ligated derivatives were iron(IV) and six-coordinate complexes. XAS and 54/57Fe rR data correlated with slight elongation of the iron-oxo bond with increasing donation from the axial ligands. The first reported synthetic H-bonded iron(IV)-oxo heme systems were made in the presence of the protic Lewis acid, 2,6-lutidinium triflate (LutH+), with (or without) DCHIm. Mössbauer, rR, and XAS spectroscopic data indicated the formation of molecular Lewis acid ferryl adducts (rather than full protonation). The reduction potentials of these novel Lewis acid adducts were bracketed through addition of outer-sphere reductants. The oxidizing capabilities of the ferryl species with or without Lewis acid vary drastically; addition of LutH+ to F8Cmpd-II (F8 = tetrakis (2,6-difluorophenyl) porphyrinate) increased its reduction potential by more than 890 mV, experimentally confirming that H-bonding interactions can increase the reactivity of ferryl species.
Graphical Abstract

INTRODUCTION
Heme metalloenzymes activate dioxygen or hydrogen peroxide to generally form two intermediates: the highly oxidizing oxoiron(IV) porphyrin cation radical formally known as Compound-I (Cmpd-I) and the one-electron reduced ferryl porphyrin Compound-II (Cmpd-II). These high-valent intermediates can be found in peroxidases, catalases, oxidases, P450s, and globins.1–3 Many of these metalloenzymes have various proximal axial bases that allow the formation of a protonated ferryl derivative or a valence tautomer of Compound-I ((Por•+)FeIV=O) or -II as a key reactive intermediate (Figure 1). High-valent oxoiron(IV) complexes are key oxidative reactive intermediates in heme and non-heme iron enzymes, catalyzing various essential biochemical processes (e.g., catabolism, angiogenesis, respiration, and apoptosis).1,3–11 The oxidative and oxygenation reactions catalyzed by heme metalloenzymes and the nature of intermediate ferryl species have been the focus of many studies, but due to the transient nature of these intermediates, synthetic iron porphyrin models have played an essential role in understanding the biological intermediates in dioxygen activation and oxygen-atom-transfer reactions in nature.3,12
Figure 1.
Heme FeIV=O enzyme intermediates with various axial ligands. The “push” from strongly electron-donating axial bases with anionic character found in cytochrome P450 and catalase results in the greater “pull” on an H-atom so that their Cmpd-II species are formally protonated, giving an iron(IV)-hydroxide.13 Due to hydrogen-bonding interactions with a nearby proximal residue, the axial histidine ligand in most peroxidases has partial anionic character, which may result in the formation of an FeIV−OH (APX) or FeIV=O (HRP) species.
Synthetic systems of these ferryl intermediates allow for greater insight into the effects of various factors (e.g., nearby amino acid residues, proximal axial bases, or pKa values) that mitigate their formation, stability, efficiency, and reactivity. Synthetic high-valent iron(IV)-oxo heme systems have been reported to be able to mimic the oxidative behavior of analogous enzymatic reactive intermediates performing reactions such as alkene epoxidation, aliphatic and aromatic hydroxylation, N- or O-dealkylation, S-oxidation, and halogenation of alkanes.2–4,12,14–24 Synthetic model studies have been found to give important mechanistic insight into the behavior of cytochrome P450 oxygenases (e.g., their roles in drug metabolism); however, the specifics of key steps involving highly reactive intermediates remain to be fully elucidated.3,25,26 More efficient and biomimetic model systems of these enzymes are needed, especially of cytochrome P450, which exhibits promising catalytic oxidative properties for various biosynthetic pathways. This would allow for the application of these enzymes beyond their normal functions in selective catalytic oxidation of strong aliphatic C−H bonds (e.g., hydroxylation of alkanes) for renewable energy/fuel research;25,27 for example, heme metalloenzymes have been employed as electrocatalysts to develop an alternative energy source to replace the scarce fossil fuels.28
Balch and co-workers were the first to report on a synthetic oxoiron(IV) porphyrin species,23,29,30 and Groves and coworkers were the first to prepare synthetic oxoiron(IV) heme cation radical complexes.24,31–33 A number of synthetic model studies focus on investigation of the nature/reactivity of iron(IV)-oxo porphyrin π-cation radicals;34–39 however, reports on iron(IV)-oxo heme complexes are more limited. Efforts have been predominantly directed toward studying the protonation state of Cmpd-II as its basicity has been shown to drive the reactivity of Cmpd-I (having implications for two-electron oxidations).1 Studies by Green and co-workers have shown, employing rapid-mixing pH-jump experiments coupled with spectroscopy, that the identity of the axial ligand in heme enzymes plays a critical role in the ferryl protonation of Cmpd-II.40–43 Notably, heme-thiolate proteins such as chloroperoxidase (CPO) and P450s have been shown to have a very basic Cmpd-II ferryl oxygen due to the strong electron donation by the axial cysteine ligand.40 This “push” effect from the proximal ligand has been reported to be key to ferryl protonation, leading to Fe−O elongation and shortening of the Fe−S bond.42–44 Work on catalase and ascorbate peroxidase (APX) has demonstrated that tyrosinate and histidine with anionic character, respectively, are electron-donating enough to maintain a highly basic ferryl capable of generating an FeIV−OH species.41,45 Most peroxidases contain a well-conserved aspartate near the Nδ atom of the proximal histidine (Figure 1), resulting in either FeIV=O formation for horseradish peroxidase (HRP) Cmpd-II or an FeIV−OH species for APX Cmpd-II.33,46–48 In globins, where the proximal histidine is only engaged in a weak hydrogen bond interaction and has no anionic character, Cmpd-II is an FeIV=O species, even at pH’s as low as 3.49 Collectively, this indicates not only the identity of the axial ligand but also that its secondary coordination sphere tunes the FeIV=O/OH unit.
In spite of these important enzymatic studies, there still remains a need for further enzymatic and synthetic model studies to examine the influence of axial ligation on facilitating protonation, verify the protonation state of Cmpd-II in various enzymes, and determine the basicity of metal-oxo moieties.3 Many enzymatic and synthetic investigations remain controversial,1,3 and significant efforts are still necessary to interrogate the properties of heme ferryl intermediates. Synthetic high-valent model systems are useful in understanding how ferryl intermediates dictate enzyme reactivity and direct catalysis toward hydroxylation of unactivated C−H bonds in P450s or electron transfer in peroxidases.7,12 Efforts have also explored the use of Lewis acids, particularly in nonheme or porphyrinoid systems, to examine the role of Lewis acidic ions in modulating chemistry, for instance, the H+ in cytochrome P450 or the Ca2+ ion of the oxygen-evolving Mn4O5 cluster of photosystem II. Reports have experimentally demonstrated the importance of Lewis acidic ions (or secondary coordination sphere interactions) in enhancing reactivity.50–58 There is no conclusive report on synthetic heme FeIV−OH species, which would be paramount in understanding P450 chemistries, and knowing the conditions which would allow for the generation of such species (and their characterization) would be significant toward our understanding of such a key reactive intermediate.
Herein, the full spectroscopic characterization of seven synthetic high-valent heme iron(IV)-oxo (Compound II) species is reported, wherein the physical properties of the iron(IV)-oxo species were examined by addition of axial ligands, a Lewis acid, or both. A catalase Cmpd-II synthetic model has been prepared, possessing a phenolate ligand, although, as will be discussed below, its physical properties turn out to be somewhat unexpected (vide infra). Ferryl derivatives were prepared with the strong electron-donating exogenous bases 1,5-dicyclohexylimidazole (DCHIm), sodium 3,5-dimethoxyphenolate (hereafter referred to as ArO−), and sodium imidazolate (Im−) to act as the axial ligand with tetrakis(2,6-difluorophenyl)porphyrinate (F8) heme (Figure 2). A comparison was also made between a tethered (PIm) and untethered (DCHIm) imidazole axial base, (DCHIm)-F8Cmpd-II vs PImCmpd-II, to explore how the tether addition affects the imidazole coordination to iron and its effect on the bond length of the iron(IV)-oxo moiety (Figure 2). Interestingly, we report the first (that we know of) Mössbauer and extended X-ray absorption fine structure (EXAFS) spectroscopic data of a synthetic imidazole tethered heme Cmpd-II system. Further, the effects of the relative basicity of the ligands and their character (anionic or neutral) on the structural and electronic properties are assessed and compared to known synthetic models and enzymatic systems. Results discussed in this work provide a significant expansion on the heretofore limited axially ligated heme Cmpd-II synthetic model system literature and EXAFS data to date comprised of a single report by Penner-Hahn et al.33
Figure 2.
Synthetic Cmpd-II complexes generated and studied as part of this report, including (1) the parent ferryl species, (2) derivatives formed by adding various exogenous strongly electron-donating axial ligands and (3) the tethered derivative PImCmpd-II.
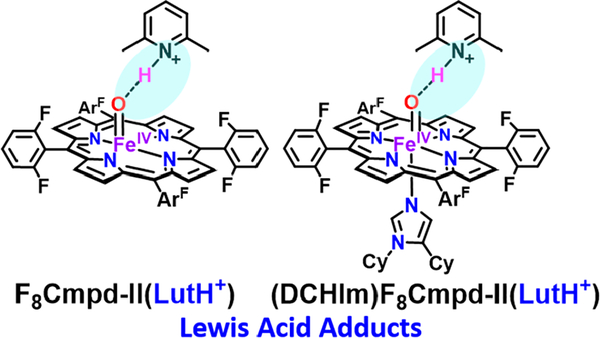
This study also includes two synthetic heme iron(IV)-oxo derivatives formed from addition of the protic Lewis acid (LA) 2,6-lutidinium triflate (LutH+)), yielding two models to study the interaction of a proton/acid molecule with an iron(IV)-oxo species (Figure 3). This can give important insights into the established protonated Compound II intermediate complex found in key enzymes such as cytochrome P450. The structure and electronic properties of these seven various ferryl derivatives were characterized by UV−vis, 2H NMR, continuous-wave (CW) X-band EPR, 54/57Fe-labeled resonance Raman (rR), 57Fe Mössbauer, Fe K-edge X-ray absorption spectroscopy (XAS), and complementary computational analysis experiments to obtain a spectroscopic handle on various axially ligated ferryl models and Lewis acid Cmpd-II adducts; such data are lacking in the literature. XAS and 57Fe Mössbauer provide atom-specific information about the geometric and electronic structures around Fe. The pre-edge region of the Fe K-edge XAS spectrum has intensity directly correlated to the dipole character of the 1s→3d transition caused by 4p-3d mixing in non-centrosymmetric systems.59 In our application, besides distinguishing five-versus sixcoordination, the pre-edge intensity increases as the Fe=O bond length decreases due to increased 4p mixing. 57Fe Mössbauer isomer shifts are connected to the oxidation of the 57Fe atom with lower values correlating to higher oxidation. Low-spin Fe(IV) complexes are distinguishable from other oxidation and spin states, providing a good handle on sample purity. Quadrupole splitting parameters in Mössbauer spec troscopy have less straightforward correlations, and these depend on the electric field gradient tensor. An approximate trend related to our application here is that strong bonding along the molecular z-axis adds a negative contribution to the quadrupole splitting, which in this case would lower the magnitude of the splitting.60
Figure 3.
Generation of high-valent ferryl Lewis acid adducts by addition of 2,6-lutidinium triflate (LutH+) to F8Cmpd-II and (DCHIm)F8Cmpd-II.
Application of these spectroscopic methods leads to the following, as described in this report: The purity of the Lewis acid and axially ligated ferryl derivatives is confirmed (via Mössbauer spectroscopy), which also supports that they are iron(IV) species (from the isomer shift) and five- or sixcoordinate complexes based on the quadrupole splitting. XAS and 54/57Fe rR data correlate with slight elongation of the iron-oxo bond with increasing electron donation from the axial ligands. Mössbauer, rR, and XAS spectroscopic data support the formation of molecular Lewis acid ferryl adducts (rather than full proton transfer occurring), wherein rR and XAS data indicate that the Lewis acid adducts possess a slightly elongated iron(IV)-oxo bond for F Cmpd-II(LutH+).
Through the use of outer-sphere chemical reductants, bracketed reduction potentials of (DCHIm)F8Cmpd-II and F8Cmpd-II, with and without LutH+, have been obtained. It was found that addition of the Lewis acid greatly increases the oxidizing capabilities of these synthetic iron(IV)-oxo complexes; for example, the reduction potential of F8Cmpd-II-(LutH+) is >890 mV more positive than that of F8Cmpd-II. The results obtained provide leads toward a systematic approach to the development of additional biomimetic synthetic models to answer many of the controversial questions concerning high-valent species in heme enzymes.
RESULTS AND DISCUSSION
Tethered/Exogenous Imidazole Axial Base: F8Cmpd-II, (DCHIm)F8Cmpd-II, and PImCmpd-II.
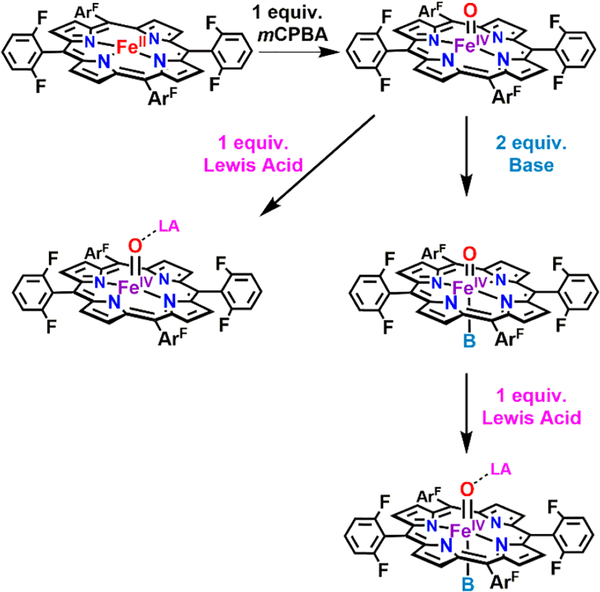
The UV−vis spectra of F8Cmpd-II, (DCHIm)F8Cmpd-II, and PImCmpd-II were previously reported;61–66 however, no further spectroscopic evidence was provided, and the conditions described there were different than those used in the present work. The reduced iron complex F8FeII or PImFeII was cooled to −90 °C in a 1:9 MeTHF:toluene (MeTHF = 2-methyltetrahydrofuran) solvent mixture and 1 equiv of meta-chloroperbenzoic acid (mCPBA) oxidant was added to form F8Cmpd-II or PImCmpd-II, respectively. Subsequently, 2 equiv of the strongly electron-donating exogenous axial base DCHIm was added to F8Cmpd-II, yielding (DCHIm)F8Cmpd-II (Scheme 1).
Scheme 1.
Addition of 1 Equiv of mCPBA to F8FeII Species in 1:9 MeTHF:Toluene at −90 °C Yielded F8Cmpd-II; Further Treatment with an Exogenous Base or Lewis Acid Resulted in the Formation of an Axial Base Ferryl Derivative and/or a Lewis Acid Iron(IV)-Oxo Species
Initial Observations: Cmpd-II Complex Generation.
Preliminary synthetic experimentation revealed that preparing F8Cmpd-II in coordinating solvents such as tetrahydrofuran, MeTHF, or n-butyronitrile (vs non-coordinating solvents as dichloromethane or toluene) resulted in a stable and pure complex. This was supported by the lack of decomposition and by the lack of ferric impurities observed by UV−vis and EPR spectroscopies. However, F8Cmpd-II was less reactive toward exogenous bases (e.g., phenolate, imidazolate, various imidazoles), substrates (i.e., phenols, C−H substrates), or Lewis acids in coordinating solvents; thus, exploration of the use of different solvent systems was carried out to optimize reactivity and stability to form stable synthetic ferryl model complexes with interesting oxidative capabilities (Figures 2 and 3). Interestingly, it was found that the mixture 1:9 MeTHF:toluene resulted in a solvent system that allowed for the generation of F8Cmpd-II, in which it was stable, pure, and reactive toward exogenous bases/Lewis acids as observed by UV−vis, EPR, rR, EXAFS, and Mössbauer spectroscopies (vide infra). It was originally postulated that F8Cmpd-II’s high stability and purity in coordinating solvents was due to the solvent acting as a weakly bound axial ligand to help stabilize the FeIV=O moiety. However, EXAFS spectroscopy indicates that F8Cmpd-II is five-coordinate, which was further corroborated by the pre-edge intensity and Mössbauer spectroscopy (Table 1, vide infra). Thus, the increased thermal stability imparted by the addition of a small percentage of MeTHF in the solvent may be a result of more favorable solvation of the iron(IV)-oxo moiety.
Table 1.
Spectroscopic Characterization of Axially Ligated F8 and PIm Heme System (Porphyrinate)FeIV-Oxo Cmpd-II Complexesa
| UV-vis | Mössbauerb | Fe XAS | rR | ||
|---|---|---|---|---|---|
| Soret, Q-bands (nm) | δ (mm/s) | ΔEQ (mm/s) | pre-edge areaf | ν(57Fe=O) (Δ57−54Fe) (cm−1) | |
| F8Cmpd-II | 415, | 0.11 | 2.06 | 54.3 (2.6) | 833(5) |
| 544 | ν4: 1370 | ||||
| ν2: 1574 | |||||
| (DCHIm)F8Cmpd-II | 421, | 0.11 | 1.07 | 27.6 | 811(5) |
| 552 | (1.1) | ν4: 1370 | |||
| ν2: 1573 | |||||
| PImCmpd-IIc | 422, | 0.11 | 1.23 | N/A | -d |
| 554 | |||||
| (Im−)F8Cmpd-IIe | 420, | 0.12 | 1.550 | 33.6 | 828(6) |
| 556 | (0.7) | ν4: 1368 | |||
| ν2: 1571 | |||||
| (ArO−)F8Cmpd-II | 420, | 0.12 | 1.57 | 42.1 | 829(6) |
| 548 | (0–9) | ν4: 1369 | |||
| ν2: 1572 | |||||
All complexes are EPR silent as determined by using X-band EPR spectroscopy at 10 K.
The values for the major species are reported in this table. See SI for minor Mössbauer species fits and details.
The Mössbauer spectrum of PImCmpd-II is best fit to three ferryl species due to the flexibility of the tethered imidazole base; see the text for more details.
rR spectroscopic data for PImCmpd-II were not collected.
The Mössbauer spectrum of (Im−)F8Cmpd-II shows a 13% impurity of F8 Cmpd-II.
Pre-edge area (standard deviations for the fits).
Addition of 1 equiv of mCPBA to F8FeII resulted in a UV− vis spectral shift from 422 to 415 nm in its Soret band and from 542 to 544 nm in the Q-band, forming the parent F8FeIV(=O) species (Scheme 1, Figures 2 and 4). The subsequent addition of DCHIm was characterized by a red shift in the λmax of the Soret (415 to 421 nm) and of the Q-band (544 to 552 nm) generating (DCHIm)F8Cmpd-II. A similar red shift was observed upon addition of 1 equiv of mCPBA to PImFeII (419, 525 to 422, 554 nm), forming PImCmpd-II (Table 1 and Figures 2, 4, and S4) The complexes (DCHIm)F8Cmpd-II and PImCmpd-II were stable in solution at −90 °C (>2 h); note that 2 equiv of DCHIm was needed to ensure full incorporation of the axial ligand (no F8Cmpd-II remained). These spectral values are consistent with previous reports (Table S1), wherein six-coordinate or imidazole-ligated synthetic heme iron(IV)-oxo species exhibit red-shifted UV− vis absorption λmax values (vs analogous five-coordinate species).
Figure 4.
UV−vis monitoring of the formation of (DCHIm)F8Cmpd-II at 0.01 mM: the Soret band (A) and the Q-band (B) at −90 °C in 1:9 MeTHF:toluene.
Mössbauer Spectroscopic Characterization.
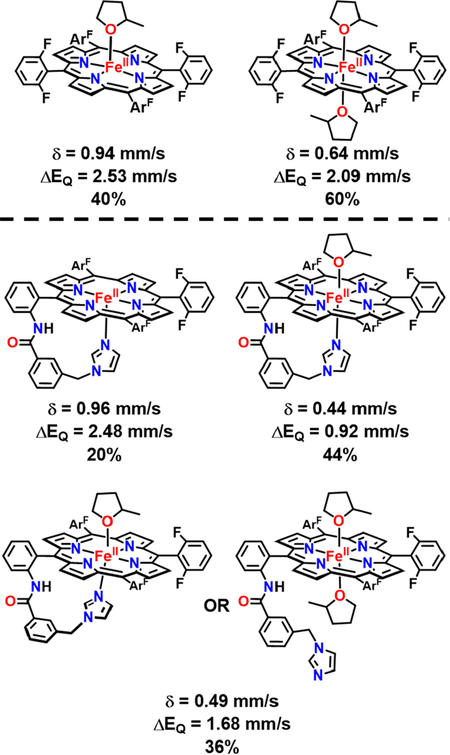
To confirm the species’ purity, determine the oxidation state (or electronic density at the metal center) via the isomer shift [δ (mm/s)], and the electric field gradient at the iron via the quadrupole splitting [ΔEQ (mm/s)], 57Fe Mössbauer spectroscopy was employed, in which all spectra were recorded at 80 K in the absence of a magnetic field.67 The Mössbauer spectrum of the ferrous precursor F 57FeII in 1:9 MeTHF:toluene was a mixture of two quadrupole doublets with parameters [major species (60%) ΔEQ = 2.09 mm/s and δ =0.64 mm/s and minor species (40%) ΔEQ = 2.53 mm/s and δ = 0.94 mm/s] that are typical for high-spin ferrous heme complexes (Table S2 and Figure S11).68–70 These parameters of the minor species were similar to the F857FeII spectrum previously reported by Karlin and co-workers71 in acetone at −80 °C where they observed one species with parameters ΔEQ = 2.66 mm/s and δ = 0.93 mm/s and suggested that one solvent molecule is bound. Thus, F857FeII in 1:9 MeTHF:toluene was determined to be a mixture of the five-coordinate (MeTHF)F857FeII (due to the similarity of its parameters to (acetone)F857FeII) and the six-coordinate (MeTHF)2F857FeII species (due to its decreased quadrupole splitting and its similarity to other high-spin ferrous species68–70) (Chart 1).
Chart 1. Proposed Ferrous Species Based on Mössbauer Parameters and Similar Assignments Found in the Literaturea.
aTwo ferrous species were identified for F8 57FeII, with one or two MeTHF solvent molecules bound. Three ferrous species were detected for Pim57FeII, with approximately 80% of the sample favoring a six-coordinate geometry. The six-coordinate complexes may be ascribed to (1) a tethered base and one solvent molecule bound (with the tethered base possibly taking on two conformations; i.e., coming out of the plane in one conformer) or (2) two solvent molecules bound to the iron center.
PIm57FeII was prepared for the first time in this work. (Note: See synthetic details in the Experimental Section.) The Mössbauer spectrum of PIm57FeII indicated three different species (Chart 1), where there are two different six-coordinate low-spin ferrous species (ΔEQ = 1.68 mm/s and δ = 0.49 mm/s and ΔEQ = 0.92 mm/s and δ = 0.44 mm/s) and one five-coordinate high-spin ferrous species (ΔEQ = 2.48 mm/s and δ = 0.96 mm/s) (Table S2 and Figure S13). The high-spin parameters of PIm57FeII are in accordance with the values of a typical high-spin five-coordinate ferrous compound,68–70 with either the tethered imidazole or solvent binding, and with the parameters for the high-spin five-coordinate complex (MeTHF)F857FeII. It was previously established that the tethered imidazole of the PIm system can easily coordinate to iron or persist free in solution. This was demonstrated by the EPR of PImFeIIISbF6 or PImFeIIIOH wherein two sets of signals were observed, that of a high-spin iron(III) species and that of a low-spin iron(III) complex.63 Previous 1H NMR data on PImFeII in various solvents showed that it can exist in either a high-spin five-coordinate (in noncoordinating solvents, with the imidazole tether bound axially) or low-spin six-coordinate (in coordinating solvent, with the tether and one solvent molecule bound axially) geometry.72 While the exact nature of the second low-spin species observed in our Mössbauer (36%) cannot be determined, we suggest that it could be (MeTHF)2PImFeII, with the tether unbound or a species with one solvent molecule and the tether bound in a different orientation (Chart 1).
Samples for Mössbauer spectroscopy of F 578FeIV O, (DCHIm)F857 FeIV=O, and PIm57FeIV=O were all prepared in a manner similar to those utilized for UV−vis experiments in 1:9 MeTHF:toluene at −90 °C. The results confirm that all three complexes possess the heme-iron in the FeIV oxidation, based on the fitted isomer shifts found to be around 0.1 mm/s (Tables 1 and S2), a hallmark of other known synthetic and enzymatic ferryl systems understood as lowered 3d electron shielding causing increased electron density at the nucleus, which results in a decreased isomer shift relative to lower oxidations (Tables 1, S1, and S2 and Figures 5 and S13).11,42,44 F857FeIV=O (ΔEQ = 2.06 mm/s and δ = 0.11 mm/s) and (DCHIm)F857FeIV=O (ΔEQ = 1.073 mm/s and δ = 0.11 mm/s) were both fit with one quadrupole doublet and, under the aforementioned conditions, were of high purity (Figure 5 and Tables 1 and S2). Addition of DCHIm to F8Cmpd-II resulted in a decrease in the quadrupole splitting from 2.06 to 1.07 mm/s, which is consistent with the formation of a six-coordinate species as the covalency of the axial base affects the dz2 orbital due to an increased electron density upon binding (Tables 1 and S2 and Figure 5).
Figure 5.
80 K, zero applied magnetic field 57Fe Mössbauer spectra (blue) with corresponding fits (orange) at 2 mM in 1:9 MeTHF:toluene: (top) F8Cmpd-II and (bottom) (DCHIm)-F8Cmpd-II. The fits for these Mössbauer spectra indicated a single quadrupole doublet, implying high purity of the samples. Mössbauer parameters are given in Table 1.
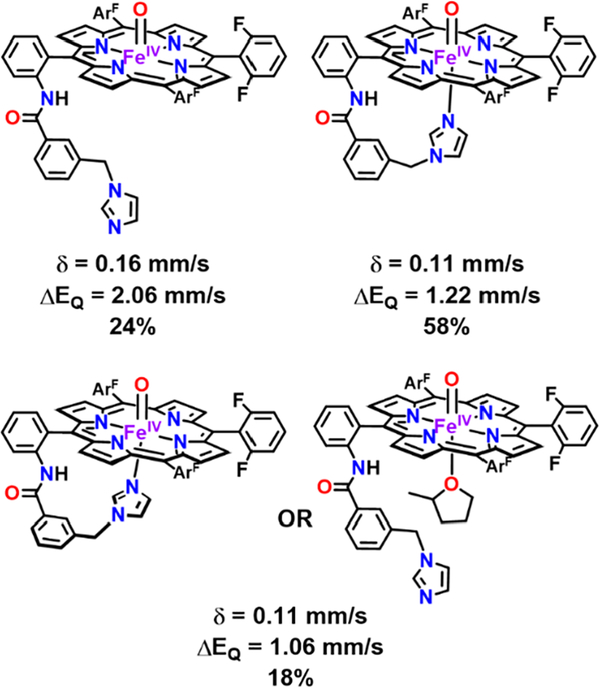
In previous reports, synthetic iron(IV)-oxo species with various porphyrins and with/without 1-methylimidazole (1-MeIm) serving as an axial base ligand have been characterized. Their Mössbauer spectroscopic parameters are summarized in Table S1. Every synthetic ferryl system with 1-MeIm has an isomer shift around 0.1 mm/s and a quadrupole splitting around 1 mm/s in accordance with the values reported here for (DCHIm)F8Cmpd-II (Tables 1, S1, and S2 and Figure 5). Consistent with the three PImFeII species observed in the Mössbauer spectrum, there were three ferryl PImCmpd-II species (ΔEQ = 1.06 mm/s and δ = 0.11 mm/s; ΔEQ = 1.22 mm/s and δ = 0.11 mm/s; and ΔEQ = 2.06 mm/s and δ = 0.16 mm/s) ascribed to the fluxional nature of the tether (Chart 2, Figure S13, and Tables 1 and S2). Comparison of F8Cmpd-II and other five-coordinate synthetic heme ferryl systems (Tables 1 and S1) helped us to assign the five-coordinate PImCmpd-II species with the tether free in solution to the complex with the higher quadrupole splitting around 2 mm/s. One might consider the possibility that the tethered imidazole interacts with the oxo atom ligand (since it is not coordinated to iron in the five-coordinate ferryl species), but this is most likely not occurring; the isomer shift and quadrupole splitting parameters for F8Cmpd-II and the five-coordinate PImCmpd-II are very similar, and greater changes might be expected if this interaction occurred. The sixcoordinate imidazole-tethered PImCmpd-II species had values in agreement with (DCHIm)F8Cmpd-II (Chart 2 and Table 1), so the imidazole being tethered or not does not seem to have a major effect on the electron density or electric field gradient at the iron center as observed by Mössbauer spectroscopy. The second six-coordinate species may arise from a different conformation of the tethered axial base when bound to the iron ion (e.g., the tether may be coming out of the plane) or from solvent binding (see Chart 2). DFT calculations support the conclusion that for PImCmpd-II the major species in solution favors the tethered imidazole coordinated to the iron metal center (vide infra).
Chart 2. Proposed PIm Ferryl Derivatives Based on Mössbauer Parameters (Table S2 andFigure S13) and Previous Reports of PIm-Based Species63,72,73 a.
aApproximately 76% of the sample favored being six-coordinate, wherein we assign 58% of the sample to the tethered imidazole axial base coordinated to iron. The second six-coordinate species may be a different conformer that the tethered base could adopt when bound to the iron ion (bottom left; e.g., the tether could be coming out of the plane) or a MeTHF solvent molecule bound to the iron ion (bottom right).
Resonance Raman Spectroscopic Characterization of F8Cmpd-II and (DCHIm)F8Cmpd-II.
Similar to the work by Bajdor and Nakamoto, who compared rR spectra of unlabeled (56Fe) and 54Fe tetraphenylporphyrin iron(IV)-oxo complexes to identify Fe=O stretches,74 this study utilized57/54Fe derivatives in synthetic heme iron(IV)-oxo systems. There are examples in the literature of labeling the oxidant or adding labeled water to exchange iron(IV)-oxo groups (Table S1), but these approaches proved unsuccessful here. Further, 57/54Fe rR spectroscopy was employed to aid the Mössbauer results and to examine the effects of the axial ligands on the iron(IV)-oxo stretching vibration (Table 1 and Figure 6). The heme complex, F8FeIII(Cl), was prepared by metalating the F8 ligand with either 57FeCl or 54FeCl2, followed by reduction to form the previously published F857FeII and the newly synthesized (for this report) heme complex F854FeII.71
Figure 6.
Resonance Raman spectra in the mid-frequency region for the F854/57 FIVFe =O complexes (57Fe spectra in black and 54Fe spectra in red) without and with phenolate, imidazolate, and DCHIm axial bases. (All spectra are normalized with respect to the 1083 cm−1band.) The ν(Fe=O) for each derivative is labeled and was observed to be in the expected 800 cm−1 region. See the SI for additional details (e.g., rR spectra showing the oxidation and spin state features).
The rR data of F8Cmpd-II and (DCHIm)F8Cmpd-II confirmed their iron(IV) oxidation state by the oxidation state marker band v4 around 1370 cm−1 (Table 1 and Figure S10). The mid-frequency region also provides clear evidence for the isotope sensitive ν(Fe=O) mode in the 800–850 cm−1 region (Figure 6 and Table 1). The iron(IV)-oxo stretching frequency of F8Cmpd-II shifted from 833 to 811 cm−1 (Δ =−22 cm−1) upon addition of DCHIm, consistent with electron donation from the exogenous axial base causing slight elongation of the Fe=O bond (Figures 6, S7, and S8 and Table 5). This is in good agreement with previous reports (Table S1), wherein the addition of an axial base, either 1-methylimidazole or a strongly donating ligand, lowered the observed ν(Fe=O). For instance the addition of 1-MeIm to (TPP)FeIV(=O) or to (OEP)FeIV(=O) [TPP = tetraphenylporphyrinate; OEP = octaethylporhyrinate], showed that the ν(Fe=O) frequency decreased from 852 to 820 cm−1 (Δ = −32 cm−1) (Table S1).74–77 Application of Badger’s rule with the parameters determined by Green,78 re = 55.702/ve2/3 + 1.003, indicates that based on the rR data the Fe=O bond is slightly elongated, from 1.632 to 1.644 Å, upon addition of DCHIm to F8Cmpd-II. As the dielectric constants for MeTHF and toluene are 7.0 and 2.4, respectively, and protein interiors are modeled with a dielectric of 4–10, we do not expect our application of Badger’s rule to deviate significantly from Green’s application to biological systems. Fe=O bond distance determinations coming from XAS are discussed below.
Anionic Axial Ligands: (ArO−)F8Cmpd-II and (Im−)-F8Cmpd-II.
Since various axial ligands, many of them having an anionic nature, are present in heme-containing enzymes, attempts to synthesize F8Cmpd-II derivatives with axially ligated substituted phenolate (ArO−) and unsubstituted imidazolate (Im−) donors were carried out. It is noteworthy that phenolates without electron-donating moieties are unreactive toward F8Cmpd-II in 1:9 MeTHF:toluene at −90 °C, failing to form a new species as determined by UV−vis spectroscopy (no spectral change). However, when 2 equiv of sodium 3,5-dimethoxyphenolate, in a mixture of 15-crown-5 ether and butyronitrile, was added to F8Cmpd-II in 1:9 MeTHF:toluene at −90 °C, there was a red shift in the Soret and Q-band from (415, 544 nm to 420, 548 nm), the latter values being similar to t h o s e o b s e r v e d f o r (DCHIm)F8Cmpd-II (Figures 5 and S2 and Table 1). When 3,5-dimethoxyphenol was added to F8Cmpd-II, there was an immediate spectral change to a known ferric species (the resulting UV−vis was similar to the authentic UV−vis signature of F FeIII OH, see Figure S35), further confirmed by observance of a high-spin ferric g = 6 signal via EPR spectroscopy. This control experiment provided evidence that 3,5-dimethoxyphenolate (not phenol) binds the iron center. The existence of the Cmpd-II system, (ArO−)F8Cmpd-II (Figure 2), and its characterization (here), may well facilitate future efforts toward designing a protonated catalase Cmpd-II model, to further elucidate compound structure, electronic structure/bonding, and reactivity, as pertains to the findings uncovered by Green and co-workers, as mentioned in the Introduction.
Addition of 2 equiv of sodium imidazolate, in a mixture of 15-crown-5 ether and butyronitrile, to F8Cmpd-II in 1:9 MeTHF:toluene at −90 °C formed the (Im−)F8Cmpd-II derivative (Figure 2), which showed the characteristic red shift i n t h e S o r e t a n d Q - b a n d (a s o b s e r v e d f o r (DCHIm)F8Cmpd-II and (ArO−)F8Cmpd-II, Figures 4 and S2) from 415, 544 nm to 420, 556 nm (Figure S3 and Table 1). Although addition of imidazole (as a neutral unsubstituted compound) to F8Cmpd-II resulted in a similar UV−vis signature as compared to the (Im−)F8Cmpd-II derivative, different Mössbauer parameters were observed, supporting the supposition that anionic imidazolate was bound in what we formulate as (Im−)F8Cmpd-II (Figure S12 and Table S2).
The Mössbauer spectrum of (ArO−)F8Cmpd-II showed one quadrupole doublet, supporting the compound’s purity (ΔEQ = 1.57 mm/s and δ = 0.12 mm/s); however, (Im−)F8Cmpd-II (ΔEQ = 1.55 mm/s and δ = 0.12 mm/s) had a minor (13%) ferryl impurity of unreacted F8Cmpd-II (Figure 7 and Tables 1 and S2). Both derivatives were confirmed to be of the iron(IV) oxidation state by the isomer shift around 0.1 mm/s and six-coordinate from the decreased quadrupole splitting (~2.0 to1.5 mm/s) upon addition of imidazolate or phenolate to F8Cmpd-II (Table 1). The isomer shift and quadrupole splitting increased with imidazolate (vs the neutral imidazole, DCHIm), which is not consistent with the expected increase in Fe−O elongation and shortening of Fe−NIm bond imparted by a better electron-donating axial ligand. The values for the phenolate Cmpd-II derivative (vs (DCHIm)F8Cmpd-II) were also not consistent with the expected parameters imparted by a better electron-donating axial ligand. These observations may be accounted for by considering the steric effects of the phenolate methoxy groups affecting the angle at which the axial ligand interacts with the iron center, which thus may hinder electron donation from the phenolate. It may also be due to the solvent mixture (ArO−)F8Cmpd-II was prepared in, in that it is predominantly toluene (a nonpolar solvent), which could well favor ion pairing in solution. Thus, the phenolate may be interacting with both the iron and the crowned sodium ion, which may also be affecting the angle at which the phenolate coordinates to the iron (this could also affect the donation observed from (Im−)F8Cmpd-II). See also, below, the discussion of DFT calculations, since computations for both (ArO−)F8Cmpd-II and (Im−)F8Cmpd-II resulted in values closer to the experimental spectroscopic data when a sodium ion was included.
Figure 7.
80 K, zero applied magnetic field 57Fe Mössbauer spectra (blue) with corresponding fits (orange) at 2 mM in 1:9 MeTHF:toluene: (top) (Im−)F8Cmpd-II major (black) and minor (purple) species (unreacted F8Cmpd-II) and (bottom) (ArO−)-F8Cmpd-II (one quadrupole doublet, indicating high purity). Changes in the absorption are due to differences in sample cell path length. Major species parameters given in Table 1, and the parameters for the minor species are given in Table S2.
The iron(IV) oxidation state and lack of strong electron donation from the phenolate or imidazolate axial ligands was also confirmed by rR spectroscopy employing 57/54Fe labeling, wherein DCHIm effected a greater change on the Fe=O bond, which does not follow the expected trend (Figure 6 and Table 1). Phenolate coordination to F8Cmpd-II slightly weakened the ν(Fe=O) from 833 to 829 cm−1, and (Im−)F8Cmpd-II showed a similar 5 cm−1 downshift from 833 to 828 cm−1. Based on Badger’s rule78 and the rR spectroscopic data, (ArO−)F8Cmpd-II has an Fe=O bond length of 1.634 Å, and (Im−)F8Cmpd-II has an Fe=O bond length of 1.635 Å, both of which are typical for iron(IV)-oxo systems, as seen in Table S1. As the results reported herein demonstrate, we have established conditions to generate synthetic Compound-II heme models with anionic axial ligands, and future studies with a more basic phenolate or different porphyrin system may be able to better mimic catalase.
EXAFS/XANES Spectroscopy of the Axially Ligated Ferryl Derivatives.
EXAFS spectroscopic literature is extremely limited for synthetic heme iron(IV)-oxo Cmpd-II systems relative to Mössbauer or rR literature; there is only one report to date, by Penner-Hahn and co-workers in 1986, for (1-MeIm)(TTP)FeIV(=O) [TTP = meso-tetratolylporphyrin], where the Fe=O bond distance was determined to be 1.64–1.66 Å.33 Due to early difficulties modeling the significant multiple scattering in hemes, there are few examples of synthetic heme Fe=O EXAFS spectra; however, numerous EXAFS spectroscopy reports on heme enzyme intermediates have been reported by Green and co-workers.1,3,40 The present report will significantly add to the limited EXAFS reports for synthetic heme iron(IV)-oxo compounds.
The Fe EXAFS spectroscopy (k = 2–15.9 Å−1) fits for the synthetic F8Cmpd-II models confirms the formation of Fe=O units with an Fe=O distance of 1.66 Å for (DCHIm)-F8Cmpd-II and all other models at 1.65 Å, with the diffierences not being significant (Figure S16 and Table S3). With the exception of the (ArO−)F8Cmpd-II, fits were unable to resolve the axial ligand bond length from the pyrrole nitrogens in the first coordination sphere. For the ArO− axial ligand system, a marginally improved fit can be achieved by separating the axial ligand scattering path from the pyrrole nitrogen path slightly above the experimental resolution (Raxial−Rpyrrole = 0.12 Å;resolution = 0.11 Å). Only the F8Cmpd-II complex had a best fit with an Fe−Np/N coordination number of 4 (Table S3), substantiated by the large pre-edge intensity (Table 1 and see discussions below). The tethered PIm porphyrinate reveals a similar fit as the (DCHIm)F8Cmpd-II, but with a lower σ2 factor for the pyrrole/axial nitrogen paths.
While the EXAFS data are similar when comparing F8Cmpd-II and its derivatives (due to the predominance of the signals from the pyrrole structure), there are key diffierences in the intensities of the pre-edge region (X-ray absorption near-edge spectroscopy, XANES) for each model (Figures 8 and S15A). The pre-edge quadrupole intensity is governed by the 3d-hole metal character of the 1s→3d transition, and dipole intensity arises from 4p orbital mixing with the 3d manifold for non-centrosymmetric molecules:59 in this case due to the strong axial distortion caused by the short Fe(IV)=O bond.
Figure 8.
XANES spectra of the tethered imidazole complex (magenta), the five-coordinate F8Cmpd-II complex (black) and axially ligated derivatives: (DCHIm)F8Cmpd-II (blue), (Imidazolate)F8Cmpd-II (red), and (3,5-dimethoxy-phenolate)-F8Cmpd-II (green). Decreased pre-edge intensity was indicative of axial ligand strength, which correlated well with the electronic influence of the various axial ligands on the ν(Fe=O) obtained from rR.
Peak fitting of the pre-edge intensities reveals that the five-coordinate F8Cmpd-II has the largest pre-edge area (Table 1), due to a large dipole contribution caused by 4p mixing into the 3d orbitals. Of the six-coordinate models, the ArO− derivative has the largest pre-edge area, followed by that for the imidazolate, then the neutral imidazole (i.e., DCHIm) axially ligated species. The integrated pre-edge intensity area positively correlates with the 57Fe=O stretching mode frequency observed by rR, which maps naturally onto a shortening of the Fe=O bond length, increased 4p-3d orbital mixing, and concomitant increase in the dipole intensity of the pre-edge (Table 1). It is noteworthy that the difference in pre-edge area between ArO− and Im− axial ligand systems is not mirrored in the negligible difference in the Fe=O frequency determined by rR spectroscopy. This may coincide with the increased Fe−Oaxial bond length determined by EXAFS spectroscopy (Table S3) relative to the unresolvable Fe−Naxial distance in the Im− system. Attempts to fit the PIm pre-edge resulted in a significantly different background relative to the F8 complexes, likely related to the sample being a mixture of species (Table S2); thus we considered the pre-edge unreliable for comparative purposes. Although most obvious for the DCHIm complex (near 7117.8 eV), the spectra featured an inflection of the rising edge region, but could not be acceptably resolved by standard Fe K-edge XAS. However, these features will be explored by high-energy resolution fluorescence detected (HERFD) K-edge spectroscopy.
Synthetic Heme Lewis Acid Adduct Ferryl Derivatives.
UV−vis spectroscopic monitoring of the addition of 1 equiv of the Brønsted (and Lewis) acid 2,6-lutidinium triflate (LutH+), dissolved in butyronitrile, to F8Cmpd-II or (DCHIm)F8Cmpd-II resulted in the formation of the Cmpd-II Lewis acid adducts, F8Cmpd-II(LutH+) and (DCHIm)-F8Cmpd-II(LutH+), respectively (Figures 3, S5, and S6 and Table 2). The former adduct exhibited slightly shifted Soret and Q-band absorptions (i.e., changes from 415, 544 nm to 413, 546 nm), while addition of LutH+ to (DCHIm)-F8Cmpd-II caused a blue shift in the Soret and Q-band peaks (i.e., from 421, 552 to 418, 539 nm) (Figures S5 and S6 and Table 2). Interestingly, the interaction between LutH+ and F8Cmpd-II or (DCHIm)F8Cmpd-II resulted in a decreased molar absorptivity in the Soret and Q-band (vs the increased molar absorptivity seen when an axial base was added, vide supra).
Table 2.
Spectroscopic Characterization of Lewis Acid F8Compound-II Derivativesa
| UV-vis | Mössbauerb | Fe XAS | rR | ||
|---|---|---|---|---|---|
| Soret, Q-band (nm) | δ (mm/s) | ΔEQ (mm/s) | pre-edge aread | ν(57Fe=O) (Δ57−54Fe) (cm−1) | |
| F8Cmpd-Il(LutH+) | 413, 546 | 0.08 | 2.23 | 48.8(1.5) | 819(4) |
| ν4: 1371 | |||||
| ν2: 1573 | |||||
| (DCHIm)F8Cmpd-Il(LutH+) | 418, | 0.07 | 1.30 | 25.0 | -c |
| 539 | (1.0) | ||||
All these complexes are EPR silent via perpendicular mode EPR spectroscopy at 10 K.
The values for the major species are reported in this table. See SI for minor Mössbauer species fits and details.
Attempts to obtain the rR spectrum were unsuccessful for (DCHIm)F8Cmpd-II(LutH+).
Pre-edge area (standard deviations for the fits).
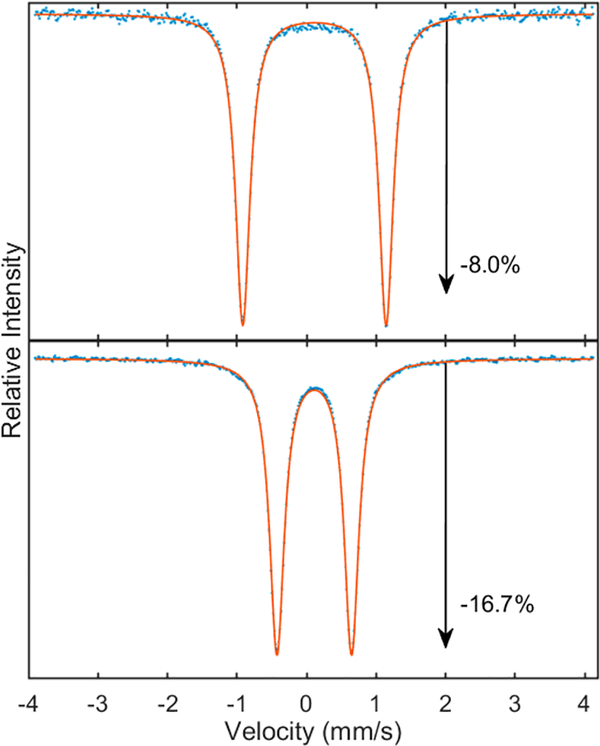
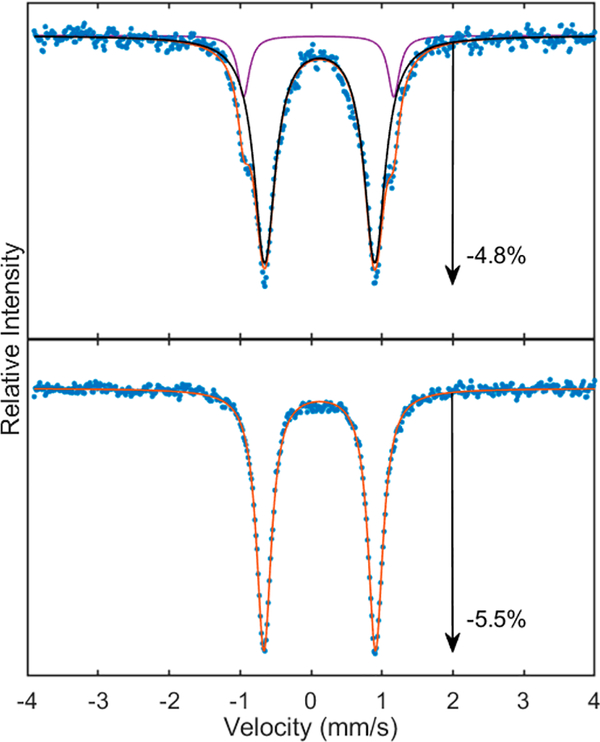
57Fe Mössbauer spectroscopy of (DCHIm)F8Cmpd-II-(LutH+) indicated the complex consisted of one species of high purity as seen by the observation of one quadrupole doublet (ΔEQ = 1.30 mm/s and δ = 0.07 mm/s) (Figure 9). However, F8Cmpd-II(LutH+) (ΔEQ = 2.23 mm/s and δ = 0.08 mm/s) had an impurity of unreacted F8Cmpd-II starting material (Figure 9). The identity of this impurity was later confirmed by 57/54Fe-labeled rR spectroscopy (vide infra, Figures S7 and S9, and Table 2). Both of the Lewis acid ferryl derivatives were confirmed to be of the iron(IV) oxidation state as reflected by the isomer shift around ~0.1 mm/s, and for F8Cmpd-II(LutH+) this was further confirmed by rR spectroscopy from the oxidation state marker band being ~1370 cm−1 (Figure S10 and Table 2). Thus, addition of LutH+ to both parent species resulted in a slight decrease in the isomer shift and a slight increase in the quadrupole splitting observed by Mössbauer spectroscopy. The increase in quadrupole splitting in both cases is consistent with a weakened Fe=O bond. Further, the decreased isomer shift values are as expected due to the Lewis acid withdrawing electron density away from the iron nucleus through its interaction with the oxo ligand.
Figure 9.
80 K zero applied magnetic field 57Fe Mössbauer spectra (blue) with corresponding fits (orange) at 2 mM in 1:9 MeTHF:toluene: (top) F8Cmpd-II(LutH+) and (bottom) (DCHIm)- F8Cmpd-II(LutH+). Major species in black and minor species in purple. Changes in absorption are due to differences in sample cell path length. Parameters ascribed to the Lewis acid species are given in Table 2, and parameters for the other species seen are given in Table S2. Both species were fitted to two quadrupole doublets, of which one was ascribed to the Lewis acid ferryl complex and the other to F8Cmpd-II. F8Cmpd-II(LutH+) was close to a 50/50 mixture with F8Cmpd-II; however, (DCHIm)F8Cmpd-II(LutH+) was of high purity with only 10% being F8Cmpd-II.
Resonance Raman spectroscopic data were only successfully collected for F8Cmpd-II(LutH+) (Figure S9 and Table 2). Upon addition of LutH+ to F8Cmpd-II, the ν(Fe=O) decreased from 833 to 819 (Δ = −14 cm−1) and required addition of 5 equiv of LutH+ to fully convert F8Cmpd-II to the F8Cmpd-II(LutH+) complex (Figure S9). This ν(57Fe=O) downshift to 819 cm−1 indicates that the Lewis acid caused a slight elongation of the Fe=O bond of 0.007 Å (from 1.632 to 1.639 Å) according to Badger’s rule.
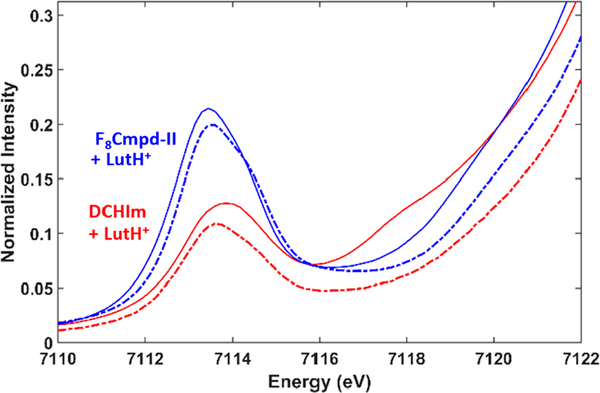
EXAFS spectroscopy is only accurate to ~0.02 Å and could not detect this slight difference between F8Cmpd-II and F8Cmpd-II(LutH+); however, the pre-edge intensity decreases for the LutH+ adduct, indicating small elongation of the Fe−O bond (Figure 10). EXAFS spectroscopy of F8Cmpd-II(LutH+) and (DCHIm)F8Cmpd-II(LutH+) indicated that both species were Lewis acid adducts and not fully protonated FeIV−OH species, which is also inferred by the only slight spectroscopic changes observed (Figure S17 and Table S4). This is due to the presence of the very short Fe=O bond (1.64 Å), while protonated Cmpd-II species have Fe−O bond lengths greater than or equal to 1.8 Å.1 The interaction of the Lewis acid with the oxo ligand causes the pre-edge intensity to decrease from 54.3 to 48.8 units from F8Cmpd-II to F8Cmpd-II(LutH+) and from 27.6 to 25.0 units from (DCHIm)F8Cmpd-II to (DCHIm)F8Cmpd-II(LutH+), indicative of a small reduction in dipole character, caused by a reduction in ligand field axial distortion (elongating Fe−O). Although there is not full protonation, these may be promising systems for better understanding the interaction of hydrogen bonding with ferryl complexes in enzymes, which is known to contribute to the increased stability and reactivity of analogous high-valent intermediates.
Figure 10.
X-ray absorption edge spectra of F8Cmpd-II (blue) and (DCHIm)F8Cmpd-II (red) and as dashed lines F8 Cmpd-II(LutH+) (blue) and (DCHIm)F8 Cmpd-II(LutH+) (red). Addition of LutH+ decreases pre-edge intensity, which is indicative of slight elongation of the iron(IV)oxo bond length.
Electronic Structure Calculations.
To obtain further insights into the geometric perturbations caused by the presence of the various axial ligands, geometry optimizations with DFT were performed for each of the F8-derived complexes (Table 3 and Figure S40). For each complex, we calculated the Mössbauer parameters of the geometry-optimized structures and determined the p-orbital contributions to the metal-based frontier molecular orbitals (FMOs) through Mulliken population analysis. In all cases, the quadrupole splitting was calculated to be positive agreeing with our assumption that strong axial bonding will lower the observed magnitude of ΔEQ. Further, utilizing the Badger’s rule parameters of Green,78 we estimate the Fe=O bond stretching vibrational frequency from the geometry optimized bond length.
Table 3.
DFT-Derived Spectroscopic Parametersa
| Mössbauer | ν(Fe=O) | metal FMOs | |||
|---|---|---|---|---|---|
| Cmpd-IIderivative | Fe=O (Å) | σ (mm/s) | ΔEQ (mm/s) | Badger’s rule (cm−1) | p-orbital % |
| F8Cmpd-II | 1.630 | 0.09 | 2.23 | 838.1 | 18.157 |
| F8Cmpd-Il(LutH+) | 1.657 | 0.09 | 2.58 | 786.8 | 11.816 |
| (DCHIm)F8Cmpd-II | 1.652 | 0.15 | 0.89 | 796.1 | 11.618 |
| (D CHIm) F8 Cmpd-II (LutH+) | 1.679 | 0.12 | 1.36 | 748.8 | 5.904 |
| (Im−)F8Cmpd-II | 1.677 | 0.18 | 0.02 | 750.6 | 12.208 |
| (Na+Im−)F8Cmpd-II | 1.659 | 0.17 | 0.48 | 782.4 | 12.424 |
| (H+Im−)F8Cmpd-II | 1.649 | 0.15 | 1.00 | 800.1 | 10.766 |
| (ArO−)F8Cmpd-II | 1.669 | 0.20 | 0.45 | 765.7 | 10.523 |
| (Na+ArO−)F8 Cmpd-II | 1.655 | 0.18 | 0.84 | 789.8 | 11.444 |
| PImCmpd-II | 1.649 | 0.15 | 0.99 | 799.9 | 4.793 |
See Figure S40 for DFT structures.
The calculations reveal the five-coordinate compound to have the shortest Fe−O bond, which corresponds to the experimentally observed highest rR stretching frequency and pre-edge intensity relative to the other F8 models. Likewise, predicted isomer shift and quadrupole splitting values (0.09 and 2.23 mm/s, respectively) correspond well to the observed experimental values for the major Mössbauer species (Tables 1 and 3). The short Fe−O bond also coincides with the greatest p-orbital metal-based hole character, which is reflected in the high experimental pre-edge intensity. Addition of the DCHIm axial ligand results in an increase of the calculated Fe−O bond length and decrease in quadrupole splitting, reproducing the observed experimental trends and coinciding with the addition of the ligand along the z-axis. The calculations show that addition of the axial ligand also decreases the p-orbital character of the metal-based FMOs by 42% relative to the five-coordinate parent complex, which maps well to the analogous 49% decrease in experimental pre-edge intensity.
Calculations including either of the ligands, ArO− or Im−, result in longer Fe−O bonds (Table 3), coinciding with decreased Fe−O Badger’s rule stretching frequencies relative to the DCHIm complex. This contrasts with the experimental observations of higher stretching frequencies for the anionic ligands than the six-coordinate DCHIm model. Likewise, the anionic computational models demonstrate poor agreement with the experimental Mössbauer parameters. Inclusion of a Na+ counterion near the anionic axial ligands improves the correlation to the experimental Fe−O frequency, with a concomitant shift in predicted Mössbauer parameters toward the experimental values. These calculations suggest that the experimentally investigated anionic ligand models are potentially forming ion-pair complexes with the cation.
The geometry optimized structure of the tethered imidazole complex, PIm, reveals a slight shortening of the Fe−O bond, coupled with an increase in the predicted quadrupole splitting relative to the DCHIm-bound complex, mirroring the experimental data for the major component of the PIm Mössbauer sample. The Fe−Nax bond is longer for PIm and the average Fe−Neq bonds are shorter, with the net effect being a higher ΔEQ despite having a slightly shorter Fe=O bond than the DCHIm-bound model.
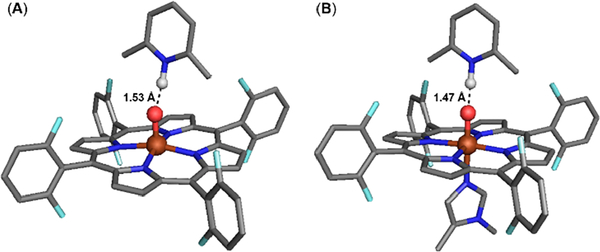
Inclusion of the LutH+ H-bonding to the oxo of the ferryl, for the five-coordinate and DCHIm-ligated models, elongates the Fe−O bond by ~0.02 Å (Chart 3). Addition of LutH+ also increases the predicted quadrupole splitting of both adducts relative to their respective non-LutH+ counterparts and are in good agreement with the experimental Mössbauer parameters, consistent with an elongation of the Fe=O bond. The Lewis acid adducts, relative to their non-Lewis acid counterparts, have lower p-orbital character of the metal-based FMOs by 35 and 49%, respectively (Table 3). This calculated reduction in p-orbital character is not reflected by the change in pre-edge intensity for the six-coordinate Lewis acid adduct. The five-coordinate Lewis acid adduct XAS sample was a 50% mixture of the non-LA species, therefore the DFT-derived p-orbital character cannot be compared.
Chart 3. DFT Structures of F8Cmpd‑II(LutH+) (A) and (DCHIm)F8Cmpd‑II(LutH+) (B)a.
aHydrogen atoms (except for the proton of LutH+) and the cyclohexyl groups of DCHIm were omitted for clarity.
Outer-Sphere Electron-Transfer Reduction of the Lewis Acid Ferryl Derivatives.
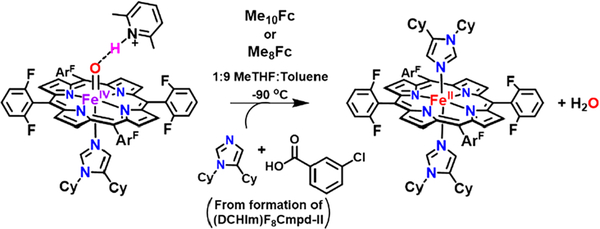
Various outer-sphere reductants (ferrocene derivatives, see SI for depicted structures) were added to F8Cmpd-II, (DCHIm)F8Cmpd-II, F8Cmpd-II(LutH+), and (DCHIm)F8Cmpd-II(LutH+) to approximate the reduction potential of the iron(IV)-oxo functionality (Schemes 2 and S1–S12 and Figures 11 and S18–S29), and to compare and contrast the effects of the presence of a strongly donating axial ligand (i.e., DCHIm) and/or the Lewis acid interacting with the oxo ligand. Reactivity studies, monitored by UV−vis spectroscopy, showed that the Lewis acid derivative (DCHIm)F8Cmpd-II(LutH+) could react with 10 equiv of decamethylferrocene (Me10Fc, E1/2 = −0.53 V vs Fc+/0 in MeTHF)79 or 10 equiv of octamethylferrocene (Me8Fc, E1/2 = −0.43 V vs Fc+/0 in MeTHF),79 but not 10 equiv of dimethylferrocene (Me2Fc, E1/2= −0.115 vs Fc+/0 in MeTHF)79 (Schemes 2, S2, and S3 and Figures 11, S19, and S20). The reactions with Me10Fc and Me8Fc showed the immediate spectral change from 418, 539 nm to 420, 530 nm, with two unique low-energy bands in the 600–800 nm region assignable to their respective ferrocenium products (Schemes S2 and S3 and Figures 11, S19, and S20).
Scheme 2.
Addition of Me10Fc or Me8Fc to (DCHIm)F8Cmpd-II(LutH+) Results in a Two-Electron Reduction To Give (DCHIm)2F8FeII as the Final Product, As Supported by UV−Vis and Mössbauer Spectroscopic Measurements
Figure 11.
UV−vis spectroscopy following the addition of 10 equiv of Me10Fc to 0.1 mM (DCHIm)F8Cmpd-II(LutH+) in 1:9 MeTHF:toluene at −90 °C, wherein there was an immediate change from the LA ferryl adduct in orange to the resulting reaction mixture in navy (top). The products in the reaction mixture were independently generated by adding 1 equiv of DCHIm to 0.1 mM F8FeII in 1:9 MeTHF:toluene at −90 °C (red) and by adding a second equiv of DCHIm to allow the favorable formation of the major product (DCHIm)2F8FeII (blue) (bottom), a low-spin six-coordinate d6 complex. The top reaction mixture in navy and the bottom (DCHIm)2F8FeII complex in light blue had comparable UV−vis features, Soret at 420 nm and Q-band at 530 nm (Figures S19 and Figure S32). Quantification of the low-energy decamethylferrocenium peaks observed in the 700–800 nm region indicated two electrons were transferred at the end of the reaction (Figures S19 and S30 and Table S5).
However, (DCHIm)F8Cmpd-II did not react with any of these reductants (Scheme S1 and Figure S18). The authentic B(C6F5)4 ¯(BAr) salts of the oxidized forms of the reductants, decamethylferrocenium BArF (Me10Fc+) and octamethylferro cenium BArF (Me Fc+), were prepared in order to quantify the number of electrons transferred to (DCHIm)F8Cmpd-II-(LutH+) in these reactions. Quantification of the low-energy ferrocenium peaks observed after completion of the reduction reactions indicated that 2 equiv of Me10Fc or Me8Fc was consumed in the reaction with (DCHIm)F8Cmpd-II(LutH+) (Figures S19 and S20), based on standard curves produced from the authentic ferrocenium salts (Figures S30 and S31 and Tables S5 and S7). To further confirm this result, sequential addition of 0.5 equiv of Me10Fc to (DCHIm)F8Cmpd-II-(LutH+) showed that no further reaction occurred after 2 equiv was added. Various ferrous derivatives were prepared independently to identify the resulting heme reaction product. It was thought that, due to the 2 equiv of DCHIm employed to form (DCHIm)F8Cmpd-II, the final ferrous product might be coordinated by DCHIm. (DCHIm)2F8FeII was prepared independently by addition of 2 equiv of DCHIm to F8FeIIin 1:9 MeTHF:toluene at −90 °C. The UV−vis absorption features of (DCHIm)2F8Fe II (Figures 11 (bottom) and S32) correlate directly with the spectrum observed for the final reaction mixture of (DCHIm)F8Cmpd-II(LutH+) with Me10Fc or Me8Fc (Figures 11 (top), S19 and S20), all exhibiting a Soret and a Q-band at 420 and 530 nm, respectively. Further, EPR spectroscopy (X-band, run at 10 K) of the reaction mixtures after 5 equiv of Me10Fc or Me8Fc was added to (DCHIm)F8Cmpd-II(LutH+) was silent, analogous to the EPR silent spectra of (DCHIm)2F8 Fe IIin 1:9 MeTHF:toluene at −90 °C.
The Mössbauer spectrum of the reaction mixture of (DCHIm)F8Cmpd-II(LutH+) with 10 equiv of Me10Fc showed two quadrupole doublets with a major species of 90% [(ΔEQ = 1.01 mm/s and δ = 0.45 mm/s)] and minor species contributing 10% [(ΔEQ = 2.45 mm/s and δ = 0.50 mm/s)] (Figure 12, Table S2). Similar products were identified in the Mössbauer spectrum of the reaction mixture of (DCHIm)F8Cmpd-II(LutH+) with 10 equiv of Me8Fc (Figure S14 and Table S2). In combination with the similar UV−vis spectral features, the comparable Mössbauer parameters of the authentic standard of (DCHIm)2F8FeII (Table S2) determined the product mixture is predominantly composed (90%) of the (DCHIm) 2F8FeII species (Figure 12). It is postulated that the presence of 2 equiv of reductant and two protons, one from LutH+ and one from 3-chlorobenzoic acid (the byproduct of mCPBA used to originally generate the ferryl complexes), allows for the reduction of the iron(IV) species to iron(II) (rather than iron(III) due to the thermodynamically favorable concomitant formation of the low-spin d6 (DCHIm2F8FeII product, and, of course, the release of water (Scheme 2).
Figure 12.
80 K, zero applied magnetic field 57Fe Mössbauer spectra (blue) with total fits (red): addition of 10 equiv of Me10Fc to 2 mM (DCHIm)F8 Cmpd-II(LutH+) in 1:9 MeTHF:toluene at −90 °C (top). (DCHIm)2F8FeII was prepared by adding 2 equiv of DCHIm to 2 mM F8FeII in 1:9 MeTHF:toluene at −90 °C (bottom). The values in the resulting reaction mixture were comparable to those for (DCHIm)2F8FeII and thus (DCHIm)F8 Cmpd-II(LutH+) was reduced by two electrons, forming the corresponding low-spin ferrous species and water. Mössbauer parameters are given in Table S2.
With all the data taken together, the reduction potential of (DCHIm)F8Cmpd-II(LutH+) can be approximated. Unfortunately, cyclic voltammetry was not able to be obtained for the ferrocene derivatives in the 1:9 MeTHF:toluene reaction mixture due to the insolubility of common electrolytes in this solvent mixture. Thus, the reduction potential values of the ferrocene derivatives in MeTHF79 were used to approximate the reduction potential of the iron(IV)-oxo species (Table S9). The reduction potential of the iron(IV)-oxo moiety of (DCHIm)F8Cmpd-II(LutH+) can then be bracketed, −0.43 < E1/2 < −0.115 V vs Fc+/0, based on the reactivity studies described above (Table S9). Since (DCHIm)-F8Cmpd-II did not react with any of the reductants utilized (Scheme S1 and Figure S18), its reduction potential must be less than −0.53 V vs Fc+/0, at least 100 mV more negative than that of (DCHIm)F8Cmpd-II(LutH+). This substantiates that (DCHIm)F8Cmpd-II is a worse oxidant than (DCHIm)-F8Cmpd-II(LutH+), as expected, and provides indirect evidence for interaction of the Lewis acid with the oxo ligand. It also shows that even though small structural changes were observed (vide supra), large reactivity differences may be seen. Thus, (DCHIm)F8Cmpd-II(LutH+) can be used to examine the interaction of a proton with the oxo ligand of a ferryl species to give insights into the protonated Cmpd-II intermediate in cytochrome P450 monooxygenase and/or Lewis acid adducts of other enzyme or synthetic high-valent heme iron(IV)-oxo species. Also, due to hydrogen bonding being a prevalent factor in metalloenzymes to stabilize the formation of high-valent species, and/or modulate their reactivity, these or related compounds can be used as good models to support how hydrogen-bonded interactions help dictate an enzyme’s reactivity.
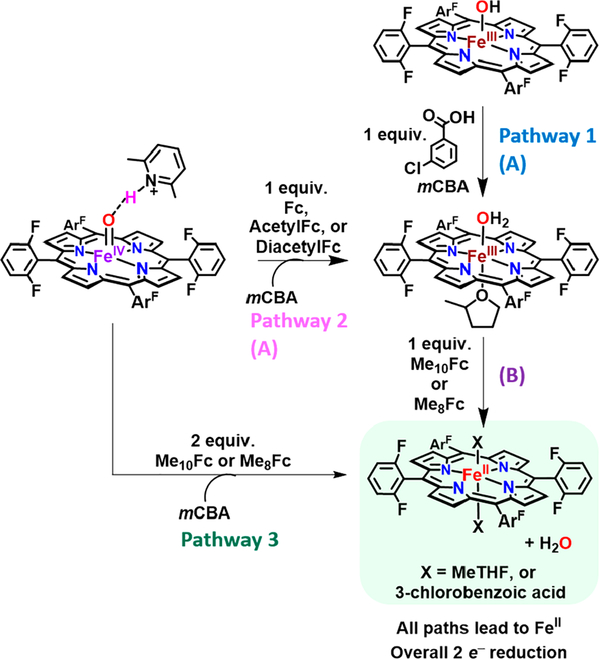
Analogous reactivity studies were carried out for F8Cmpd-II and F8Cmpd-II(LutH+). In contrast to (DCHIm)F8Cmpd-II, these studies showed that F8Cmpd-II can be reduced by Me10Fc, but not Me8Fc (Schemes S4 and S5 and Figures S21 and S22). Addition of 10 equiv of Me10Fc to F8Cmpd-II, monitored by UV−vis spectroscopy (Scheme S4 and Figure S21), resulted in the formation of (MeTHF)F FeIII(X), with X = solvent or 3-chlorobenzoate. Quantification of the Me10Fc+ peaks was not possible due to the overlapping intense low-energy ferric band; however, the ferric state was confirmed by low-temperature 2H NMR spectroscopy employing the deuterated pyrrole d8-F8 analogue (Figure S37). Though the reaction stopped at iron(III) and exhibited a similar 2H NMR shift as (MeTHF) 2F8FeII (OH), the final product was found to not be the iron(III)-hydroxide complex due to differences in UV−vis absorption features (Scheme S4 and Figures S21, S33, and S34).
Interestingly, especially based on the small structural changes observed for F8Cmpd-II(LutH+) compared to F8Cmpd-II, the addition of LutH+ greatly increased this species’s reduction potential. Previous reports,80–84 on nonheme iron(IV)- or manganese(IV)-oxo complexes have shown that addition of Lewis acids such as Sc3+ or HClO4 increased the reduction potential of these species by 0.5–0.9 mV (however, the greater differences in magnitude, as observed herein, were usually only detected when excess amounts of the Lewis acid were added). Thus, the unusually large increase in reduction potential observed here, with the addition of 1 equiv of LutH+ (as a molecular adduct and not just a proton transferred, vide supra), is notable. In fact, F8Cmpd-II(LutH+) was found to react with Me10Fc, Me8Fc, Me2Fc, ferrocene (Fc), acetylferrocene (AcetylFc, E1/2 = +0.235 V vs Fc+/0 in MeTHF79), and diacetylferrocene (DiacetylFc, E1/2 = +0.460 V vs Fc+/0 in MeTHF (Table S9, Schemes 3, S6–S9, and S11, and Figures S23–S26 and S28). However, F8Cmpd-II(LutH+) did not react with tris(4-bromophenyl)amine (E1/2 = +0.695 V vs Fc+/0 in MeTHF (Table S9, Scheme S11, and Figure S28). These reactions with F8Cmpd-II(LutH+) resulted in the formation of more complicated reaction mixtures. The stronger reductants Me10Fc and Me8Fc were able to predominately reduce the iron(IV)-oxo Lewis acid adduct by two electrons, forming (X)2F8FeII, with X = MeTHF or 3-chlorobenzoic acid (Scheme 3, Pathway 3, Schemes S6 and S7, and Figures S23 and S24), whose assignment is based on similar absorption features (λmax of 428 and 529 nm) as (DCHIm))2F8FeII(λmax of 420 and 530 nm). The low-energy ferrocenium peaks were quantified and supported the conclusion that ~2 equiv of reductant was consumed (Tables S6 and S8); a minor ferric impurity could be observed in the Soret peak region at 411 nm and slightly in the Q-band (Figures S23 and S24). Also, EPR spectroscopy (X-band, run at 10 K) of the reaction mixtures after 5 equiv of Me10Fc or Me8Fc was added to F8Cmpd-II(LutH+) was silent, analogous to the EPR-silent spectrum of (DCHIm))2F8FeII in 1:9 MeTHF:toluene at −90 °C. Thus, a six-coordinate ferrous complex was the product when employing these stronger reductants.
Scheme 3. F8Cmpd-II(LutH+) Can Be Reduced by Various Ferrocene Derivativesa.
aWhen stronger reductants (Me10Fc or Me8Fc) are used, the iron(IV) is reduced by two electrons to (X) 2F8FeII Fe (Pathway 3). However, when weaker reductants (Fc, AcetylFc, or DiacetylFc) are used, one-electron reduction occurs, giving (MeTHF)F FeIII (OH2) (Pathway 2A), as determined by comparison with the independently generated (MeTHF)F8 FeIII (OH2) prepared by addition of 3-chlorobenzoic acid to F8 FeIII (OH) (Pathway 1A). Further, the assignment of (MeTHF)-F8 FeIII (OH2) is supported due to its ability to be reduced by Me10Fc or Me8Fc to give (X)2F8Fe II, which similarly occurs when those reductants are added to the Fc, AcetylFc, or DiacetylFc reaction mixture (B). All reactions were carried out in 1:9 MeTHF:toluene at −90 °C. See Supporting Information for further details.
However, addition of weaker reductants such as Me2Fc, Fc, AcetylFc, and DiacetylFc did not result in two-electron reduction of the iron(IV)-oxo to iron(II); predominately a ferric species was observed via UV−vis spectroscopy (Scheme 3 Pathway 2A, Figures S25, S26, and S28 and Schemes S8, S9, and S11). To determine the final product from the reactions with the weaker reductants, 3-chlorobenzoic acid was added to F8FeIII OH, supporting that (MeTHF)F8FeIII (OH2) is the resulting species based on the similar Soret of 411 nm and the highly characteristic multiple peak Q-band signature between the authentically generated species and the reaction mixtures (Schemes S8, S9, S11, S13, and S14 and Figures S25, S26, S28, S33, and S34). However, due to the relatively intense, broad low-energy band of the ferric species spanning ~650–1100 nm, the ferrocenium products for the weaker reductants were not able to be quantified due to the overlap of the peaks. The ferric nature of the reaction product was supported by 2H NMR spectroscopy, which showed a paramagnetic shift around 124 ppm (Figure S38). Also, addition of 5 equiv of AcetylFc and 10 equiv DiacetylFc to the EPR-silent F Cmpd-II(LutH+) complex resulted in a high-spin ferric signal (g = 6 and 2) (Figure S28). To further confirm the iron(III) oxidation state, 10 equiv of Me10Fc was added to the Fc and AcetylFc reaction mixtures (Schemes S10 and S12 and Figures S27, S29 and S38), resulting in an immediate spectral change to 529 nm, similar to the UV−vis signature and 2H NMR shift of the reaction mixtures when Me10Fc or Me8Fc was used (Scheme 3 Pathway 2B, Figures S23, S24 and S39). Further evidence for the reduction of the ferric species to ferrous was the quantification of the decamethylferrocenium peaks, which showed a one-electron reduction (as observed by the low-energy bands, Schemes S10 and S12 and Figures S27 and S29). The independently generated (MeTHF)-F8FeIII(OH2) was also able to be reduced by Me10Fc (Scheme 3, Pathway 1B), forming a similar 529 nm UV−vis signature, and the low-energy Me10Fc+ peaks were quantified to indicate a 1-electron reduction occurred (Scheme S14 and Figure S34). The comparable UV−vis (Figures S25, S26, S28, S33, and S34) and 2H NMR signatures (Figure S38 and S39) of the independently generated (MeTHF)F8FeIII(OH2) and the Me2Fc, Fc, AcetylFc reaction mixtures supports that the ferric aqua complex was formed; additional support is that both can be further reduced by one electron (Figures S27, S29, S34, S38, and S39).
These reactivity studies allow us to bracket the reduction potential of F8Cmpd-II, −0.53 < E1/2 < −0.43 V vs Fc+/0, demonstrating that F8Cmpd-II is a stronger oxidant than (DCHIm)F8Cmpd-II (Tables 4 and S9). However, the reduction potential of F8Cmpd-II(LutH+) is bracketed to be between +0.460 and +0.695 V vs Fc+/0, since DiacetylFc was capable of reducing this species, but no reaction occurred with tris(4-bromophenyl)amine (Scheme S11 and Figure S28C). Thus, addition of LutH+ to F8Cmpd-II increases the reduction potential of the complex by greater than 0.890 V, indicating that F8Cmpd-II(LutH+) is a strong oxidant (Tables 4 and S9). These reduction reactions are not expected to be reversible, e.g., FeIII(OH2) → FeIV(OH2) is thermodynamically unfavorable due to the likely instability of a protonated iron(IV)-oxo complex, while (DCHIm)2F8FeII → (DCHIm)F8Cmpd-II-(LutH+) would be unfavorable because the former is a highly stable low-spin d6 complex. These synthetic heme iron(IV)-oxo Lewis acid derivatives may pave the way for the preparation of a fully protonated FeIV−OH model
Table 4.
Approximation of the Reduction Potential of Various Ferryl Derivatives by Reacting Them with Ferrocene Derivatives
 | |||
|---|---|---|---|
| reductant | E° vs Fc+/0 (mV)a | oxidant, paired with strongest reacting reductant | bracketed E1/2 of FeIV=O (mV) |
| N(ArBr)3 | 695 | ||
| DiacetylFc | 460 | F8Cmpd-II(LutH+) | +460 < E1/2 < +695 |
| AcetylFc | 235 | ||
| Fc | 0 | ||
| Me2Fc | −115 | ||
| Me8Fc | −430 | (DCHIm)F8Cmpd-II(LutH+) | −430 < E1/2 < −115 |
| Me10Fc | −530 | F8Cmpd-II | −530 < E1/2 < −430 |
| (DCHIm)F8Cmpd-II | <−530 | ||
CONCLUSIONS
High-valent ferryl intermediates with various axial ligands catalyze numerous oxidative processes in metalloenzymes. Investigations utilizing synthetic systems may allow for a greater understanding of structures and bonding (i.e., electronic structure), plus mechanistic insights into enzymatic pathways. This aids in the understanding of the nature of these highly oxidizing intermediates, which can be harnessed for numerous practical applications (drug development, catalysis, synthesis, fuel cells, etc.). However, there have been limited reports on synthetic heme Cmpd-II models with varying axial ligation, with no synthetic model systems studied for catalase. Herein, the full characterization of synthetic Cmpd-II models with and without various axial bases was achieved, and the electronic properties were examined using a wide array of spectroscopic techniques, including UV−vis, EPR, 2H NMR, Mössbauer, 57/54Fe-labeled rR, and EXAFS.
Addition of axial bases such as imidazole, imidazolate, or phenolate causes a slight elongation of the Fe=O bond. This is the first report of a synthetic heme Cmpd-II model containing phenolate axial ligation as a model for the enzyme catalase. This synthetic model complex could also potentially be used in future electrocatalytic studies to understand how catalase (and analogous enzymes) disproportionates reactive oxygen species (e.g., H2O2), minimizing harmful ROS reactions.28 The first Mössbauer and EXAFS data of a synthetic heme axially ligated phenolate or tethered imidazole iron(IV)-oxo species are also reported. The work reported herein offers significantly expanded EXAFS characterization of synthetic heme iron(IV)-oxo complexes beyond that currently reported in the literature. Further, the iron(IV)-oxo vibrational frequency was identified by rR employing 54Fe and 57Fe derivatives (vs needing to synthesize 18-O-labeled oxidant (e.g., mCPBA) or using 18-O-labeled water for an exchange reaction, which leads to adverse reactions).
This work reports on the first hydrogen-bonded synthetic heme Lewis acid iron(IV)-oxo adducts with and without an axial base. Addition of the protic Lewis acid, 2,6-lutidinium triflate, to F8Cmpd-II or (DCHIm)F8Cmpd-II resulted in a slight elongation of the Fe=O bond, which was supported by rR and/or XAS analysis. These novel ferryl Lewis acid adducts, F8Cmpd-II(LutH+) and (DCHIm)F8Cmpd-II(LutH+), showed increased (more positive) reduction potentials compared to their counterparts without LutH+; in fact, the reduction potential of F8Cmpd-II increased by almost a volt upon addition of LutH+. Thus, addition of LutH+ causes a direct interaction with the oxo ligand and makes these synthetic Cmpd-II compounds much more oxidizing, further supporting the importance of the protonation or hydrogen-bonding state of Cmpd-II in enzymatic systems.
EXPERIMENTAL SECTION
General.
All reagents and solvents purchased and used were of commercially available quality except as noted. Inhibitor-free 2-methyltetrahydrofuran (MeTHF) and tetrahydrofuran (THF) were distilled over Na/benzophenone under Ar and deoxygenated with Ar before use. Toluene was distilled over calcium hydride and deoxygenated with Ar before use. Butyronitrile was distilled over sodium carbonate and potassium permanganate and deoxygenated with Ar before use. Solvent deoxygenation was achieved by bubbling Ar through the desired solvent for ≥45 min via an addition funnel connected to a receiving Schlenk flask. All solvents were stored in amber bottles under 4 Å sieves. The oxidant, 3-chloroperbenzoic acid (mCPBA) was purified according to published procedures.85 Air-free manipulations were performed in a Vac atmosphere OMNI-LAB drybox or under argon atmosphere using standard Schlenk techniques. Low-temperature UV−vis spectroscopy experiments were carried out by using a Cary-50 Bio spectrophotometer equipped with an Unisoku USP-203A cryostat with a modified Schlenk cuvette with a 1 cm path quartz cell. The spectrometer was equipped with Cary WinUV Scanning Kinetics software. All NMR spectra were recorded in 9 in., 5 mm o.d. NMR tubes on a Bruker 300 MHz NMR instrument equipped with a tunable deuterium probe to enhance deuterium detection. The 2H chemical shifts were calibrated to natural abundance deuterium solvent peaks. EPR spectra were collected with an ER 073 magnet equipped with a Bruker ER041 X-band microwave bridge and a Bruker EMX 081 power supply: microwave frequency = 9.42 GHz, microwave power = 0.201 mW, attenuation = 30 db, modulation amplitude = 10 G, modulation frequency = 100 kHz, temperature = 10 K. Mössbauer spectra were recorded on a spectrometer from SEE Co. (Edina, MN) operating in the constant acceleration mode in a transmission geometry. The sample was kept in an SVT-400 cryostat from Janis (Wilmington, MA), using liquid N2 as a cryogen for 80 K measurements. Isomer shifts were determined relative to the centroid of the spectrum of a metallic foil of α-Fe collected at room temperature. Data analysis was performed using version F of the program WMOSS (www.wmoss.org), and quadrupole doublets were fit to Lorentzian line shape. XAS data were collected at beamlines 7–3 and 9–3 at the Stanford Synchrotron Radiation Lightsource (SSRL) at the SLAC National Accelerator Laboratory under ring operating conditions of 500 mA over an energy range of 6785–8100 eV, with the samples maintained at 10 K. To mitigate X-ray induced sample damage, we parsed each sample into 6–8 spots and collected 1–2 scans per spot. However, consistent in our experience with many synthetic model complexes frozen in dry organic solvents, we observed no significant scan-to-scan changes in the pre-edge nor edge positions at the Fe K-edge energies, and we were able to utilize the first and second scans for nearly all the models. The exception was the six-coordinate lutidinium complex, which had a ~4% scan-to-scan reduction in maximum pre-edge intensity and a slight (<0.1 eV) change in the edge–the EXAFS analysis presented here utilized both scans per spot, but no significant differences were observed by fitting the first scans only. The pre-edge area analysis for the six-coordinate lutidinium complex utilized only the first measured scans. Data were background subtracted, splined, and normalized by the PYSPLINE software.86 Theoretical EXAFS scattering amplitudes were calculated by FEFF (version 7.0).87 These amplitudes were then used in the fitting performed through the EXAFSPAK suite for the parameters: Fe−X path distance R, path mean square deviation σ2, and threshold energy E0. Two multiple scattering paths (Fe−N−Cα/β) were included with σ2 fixed to that of the pertinent Fe−Cα/β paths consistent with previous heme EXAFS studies.88 For the first coordination sphere, alternative fits were attempted to isolate the axial ligand path from the equatorial pyrrole nitrogens path—but resulted in unreasonable σ2 values and/or distances not resolvable by the experimental parameters, except for the ArO− axial ligand as discussed in the text. Pre-edge areas were determined by fitting one or two pre-edge peaks, two background peaks to model the rising-edge, and one peak for the edge jump utilizing least-squares fitting in the Igor Pro version 6.37 with Pearson VII functions, and integrals were taken using the Igor program “areaxy” trapezoidal integration function. The averages and standard deviations were taken from 11 best fits for each spectrum (in the range 7105–7122 eV), where the width of the edge jump peak was fixed from the first fit and adjusted by alternating steps of ±0.1 eV, and then all other parameters were refit again to a maximum of 5 fits in each direction.
Density Functional Theory geometry optimizations were performed in vacuum with the program ORCA version 4.0,89 using the BP8690 functional and Ahlrich’s triple-ζ basis set Def2-TZVPP91 for all atoms at the grid6 integration grid level and the CP(PPP) basis set for Fe at the grid7 integration grid level. 57Fe isomer shift calibration coefficients used were those calculated previously.92 Inclusion of dispersion effects (D3)93 for the calculations caused no significant deviation from observables of the non-corrected calculations (Table S10) presented in the main text, except in the case of the Lewis-acid adducts where addition of dispersion correction caused a larger tilt in the Lewis-acid relative to the Fe−O axis. The difference does not significantly change the Fe−O bond length, but it changes the calculated quadrupole splitting for the six-coordinate complex by −0.32 mm/s. For (ArO−)F8Cmpd-II and (Im−)F8Cmpd-II, calculations were performed with and without the presence of a sodium ion. Calculations with a sodium ion present yielded results closer to the experimentally observed parameters. See Supporting Information for further details.
Synthesis.
The complexes F8FeII, F857FeII, PImFeII and d8-F8FeII were synthesized as previously described.63,71,72,94 54FeIICl2 and the compounds (F8TPP)54FeIII−Cl and (F8TPP)54FeII were synthesized according to the literature methods described by Karlin and coworkers to make the 57Fe derivative,71 with the only difference being that 54FeIICl2 was utilized in the metalation of F8.
(F8TPP)54FeIII−Cl.
UV−vis (CH2Cl2): 411, 503, 639, 772. 1H NMR (CDCl3): δ 80.1 (s, br, 8 H), 13.8 (s, 4 H), 12.5 (s, 4 H), 7.6 (s, 4 H). Calculated for C44H20ClF8FeN4: C, 62.32; H, 2.38; N, 6.61. Found: C, 62.44; H, 2.51; N 6.58.
(F8TPP)54FeII.
UV−vis (1:9 MeTHF:toluene): 422, 542. 1H NMR (THF-d8): δ 56.1 (s, 8 H), 8.4 (s, 4H), 7.2 (s, 8H). Calculated for C44H20F8FeN4: C, 65.04; H, 2.48; N, 6.90. Found: C, 64.56; H, 2.83; N 6.87.
The compound PImFeIII_Cl was synthesized by using the same procedure Karlin and co-workers used to prepare the previously reported PImFeIIIOH.72 However, the only difference was that the solution was stirred in HCl and then neutralized to pH 7 with sodium bicarbonate, instead of NaOH at the end of metalation procedure. Likewise, PIm57FeIII−Cl was made using the same procedure, except starting with 57FeIICl2 instead of 56 FeIICl2. The newly synthesized PIm57FeII was formed using the same procedure72 Karlin and coworkers used to prepare the previously reported PIm56FeII.
(PIm)57FeIIICl·CH2Cl2.
Calculated for C56H33N7Cl3F6FeO: C, 61.36; H, 3.30; N, 8.95. Found: C, 61.45; H, 3.28; N 9.18.
(PIm)57FeII·2H2O.
Calculated for C55H35N7F6FeO3: C, 65.29; H, 3.49; N, 9.69. Found: C, 64.65; H, 3.54; N 9.45.
Sodium 3,5-Dimethoxyphenolate.
In the glovebox, a vial of 3,5-dimethoxyphenol (0.510 g, 3.31 mmol) was dissolved in 2 mL of THF. Next, 1 equiv of sodium hydride (0.079 g, 3.31 mmol) was added, and the reaction mixture was stirred for 20 min. Over the course of the reaction, bubbles were observed, indicating release of hydrogen, and the vial became slightly warm. Subsequently, the reaction mixture was concentrated to give a white solid. 1H NMR (DMSO): δ 5.3 (d, 2.12 Hz, 2 H), 5.1 (t, 2.16 Hz, 1 H), 3.5 (s, 6 H).
Decamethylferrocenium BArF.
To a suspension of 350 mg (1.07 mmol) of decamethylferrocene in 125 mL of acetonitrile were added 173.1 mg (1.02 mmol) of solid AgNO3 and 731.8 mg (1.02 mmol) of KBC24F20, causing an immediate color change. The solution was allowed to react for 30 min, after which time the acetonitrile solution was extracted with (2 × 75 mL) of hexane. The acetonitrile solution was saved and solvent removed. The solid was suspended in ~120 mL of tetrahydrofuran and filtered through a pad of Celite. The tetrahydrofuran was removed, yielding a green solid which was crystallized by chilling a hot methanol:ethanol:acetonitrile:water solution. After filtration and drying, the yield was 825 mg (76% yield) of Me10FcBArF. Calculated for C44H30BF20Fe: C, 52.57; H,3.01. Found: 52.37; H, 3.49.
Octamethylferrocenium BArF.
Synthesized by the same procedure as Me10FcBArF above, using octamethylferrocene in place of decamethylferrocene. Calculated for C42H26BF20Fe: C, 51.62; H, 2.68. Found: 51.67; H, 2.67.
Spectroscopic Sample Preparations.
UV−Vis.
F8Cmpd-II, (DCHIm)F8Cmpd-II, and PImCmpd-II were synthesized as previously described.62–65,72 High-valent iron(IV)-oxo complexes were generated by preparing a 3.0 mL 1:9 MeTHF:toluene solution (0.01 mM or 0.1 mM) of F8FeII or PImFeII in a 10 mm path length quartz Schlenk cuvette, which was then sealed with a rubber septum in the glovebox. The cuvette was then cooled in the cryostat chamber to −90 °C, and the solution was then allowed to thermally equilibrate (~10 min) before various reagents were added. The reduced iron(II) precursors were subjected to 1 equiv of mCPBA dissolved in toluene at −90 °C, forming the F8Cmpd-II or PImCmpd-II species after 5 min (denoted by a characteristic increase in the extinction coe-cient of the Q-band). Subsequently, 2 equiv of 1,5-dicyclohexylimidazole (DCHIm) dissolved in toluene, 2 equiv of sodium 3,5-dimethoxyphenolate (ArO−), or 2 equiv of sodium imidazolate (Im−) was added to F8Cmpd-II, resulting in a red shift in the Soret and Q-band, yielding (DCHIm)F8Cmpd-II, (ArO−)F8Cmpd-II, or (Im−)-F8Cmpd-II, respectively. Due to solubility issues, sodium 3,5-dimethoxyphenolate and sodium imidazolate were added in a 4:1 15-crown-5 ether:butyronitrile solution instead of in toluene.
One equivalent of 2,6-lutidinium triflate, prepared as previously described by Tilley and co-workers,95 was added in butyronitrile to F8Cmpd-II or (DCHIm)F8Cmpd-II, generating F8Cmpd-II(LutH+) or (DCHIm)F8Cmpd-II(LutH+), respectively. Various ferrocene derivatives (Me10Fc, Me8Fc, Me2Fc, Fc, AcetylFc, DiacetylFc) or tris(4-bromophenyl)amine were added to F8Cmpd-II, (DCHIm)-F8Cmpd-II, F8Cmpd-II(LutH+), and (DCHIm)F8Cmpd-II(LutH+), wherein the reactions were monitored by UV−vis, 2H NMR, or Mössbauer spectroscopies. The absorbance at 784 nm was monitored for various concentrations of decamethylferrocenium BArF (0.025–0.29 mM) and at 779 nm for octamethylferrocenium BArF (0.025–0.20 mM), forming standard curves (Figures S30 and S31) to quantify the number of electrons transferred at the end of the reaction. See Supporting Information for further details.
X-Band EPR.
Compound-II derivatives were generated by adding 1 equiv of mCPBA and, depending on the derivative, 1 equiv of axial ligand to a 1:9 MeTHF:toluene solution containing 1 mM of F8FeII or PImFeII (total volume = 0.6 mL) at −90 °C (acetone/liquid nitrogen cold bath). Upon addition of reagent(s), the solution was bubbled with Ar for 20 s to ensure thorough mixing. In each case, upon addition of mCPBA, the solution was allowed to react for 5 min (F8Cmpd-II and PImCmpd-II were frozen after 5 min), following the addition of 2 equiv of axial ligand to form (DCHIm)F8Cmpd-II, (ArO−)F8Cmpd-II, and (Im−)F8Cmpd-II. Next, 1 equiv of 2,6-lutidinium triflate was added in butyronitrile to F8Cmpd-II or (DCHIm)F8Cmpd-II, generating F8Cmpd-II(LutH+) and (DCHIm)-F8Cmpd-II(LutH+). After the complexes were generated, tubes were frozen in N2(liq), and all spectra were recorded at 10 K. All complexes were EPR silent (X-band), consistent with an integer spin electronic spin value and the typical EPR signature of an S = 1 Compound-II species.
54/57Fe-Labeled rR.
The oxoiron(IV) complexes were generated in the same fashion as the EPR spectroscopy samples; however, samples were made at 2 mM and employed the 57Fe and 54Fe derivatives with a total volume of 0.3 mL at −90 °C (acetone/liquid nitrogen bath) in a 7-in., 5 mm, rubber septum-capped, NMR tube. Resonance Raman spectra were obtained with a 407 nm laser excitation (4 mW) on samples maintained at 110 K and with constant spinning as described previously.94
2H NMR.
The oxoiron(IV) complexes were generated in the same fashion as the EPR spectroscopy samples; however, they were prepared using the pyrrole-deuterated analogue, d8-F8FeII, at 5.0 mM concentrations with a total volume of 0.6 mL at −90 °C (acetone/liquid N2) in a 9-in., 5 mm, rubber septum-capped, NMR tube. Upon addition of reagent(s), the solution was bubbled with Ar for 20 s to ensure thorough mixing. 2H NMR spectra were acquired on a Bruker AVANCE 300 MHz NMR spectrometer at 46.072 MHz. Experiments were carried out at −90 °C using a recycle delay of 0.01s, and a total of 5120 scans were collected. The peaks were referenced to the one of toluene solvent peaks at 7.1 ppm (the −CH group of toluene, which is the most upfield).
57Fe Mössbauer.
Samples were prepared similarly to how they were generated for UV−vis spectroscopy. However, the reduced F 578FeII or PIm57FeII complexes were employed. The iron starting material was prepared as a 2.0 mL 1:9 MeTHF:toluene solution at 2 mM under Ar in a 10 mL Schlenk flask in the glovebox, which was then subsequently cooled to −90 °C for 10 min. Reagents were then added accordingly to form desired complexes as described in the UV−vis section. See Supporting Information for further details. Samples were prepared by cooling a pipet in liquid nitrogen and then quickly filling a pre-chilled Delrin Mössbauer cup. All Mössbauer spectra were taken at 80 K in the absence of a magnetic field using liquid nitrogen as a cryogen.
XAS.
Samples were prepared in a similar fashion to the Mössbauer samples as above. All samples except the DCHIm ligated sample were prepared in 10% MeTHF and 90% toluene with a concentration at 2.0 mM employing the F 578FeII analogue. Mössbauer spectroscopy was run on all the XAS samples to confirm the purity of that particular batch, and the reported Mössbauer purities reflect the purity of the corresponding XAS spectra. The DCHIm samples were the only exception; the samples were prepared in 100% toluene and were 90% pure by Mössbauer with the impurity being the ferrous precursor. Samples were prepared by cooling a pipet in liquid nitrogen and then quickly filling pre-chilled Delrin cell, and immediately frozen in liquid nitrogen. Every compound was generated twice and measured for XAS.
Supplementary Material
ACKNOWLEDGMENTS
The research support of the U.S. National Institutes of Health (GM60353 to K.D.K.) is gratefully acknowledged. This research was supported by the National Institute of General Medical Sciences under award numbers R01GM040392 (E.I.S.), R01GM074785 (P.M.-L.), and F32GM122194 (L.B.G.). The SSRL Structural Molecular Biology Program is supported by the U.S. Department of Energy Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences, Grant P41GM103393 (to K.O.H. and B.H.). Some computational work was performed under the XSIM project on the CORI computing system at NERSC, a U.S. Department of Energy Office of Science User Facility operated under Contract No. DE-AC02–05CH11231. We also gratefully acknowledge Dr. David P. Goldberg for the use of his Mössbauer instrument and Jesse B. Gordon for assistance in running the samples. M.A.E. would also like to especially thank Dr. David A. Quist for helpful discussions and for aiding in sample preparation. We acknowledge the help of Drs. Matthew Latimer and Erik Nelson for assistance at SSRL beamlines 9–3 and 7–3. L.B.G. also acknowledges Dr. Thomas Kroll for assistance in pre-edge fitting.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b00795.
Synthetic and analytical details (methodologies and UV−vis, rR, Mössbauer, EXAFS, EPR, and NMR spectra), including Figures S1–S40, Tables S1–S10, and Schemes S1–S15 (PDF)
Notes
The authors declare no competing financial interest
REFERENCES
- (1).Behan RK; Green MT On the Status of Ferryl Protonation. J. Inorg. Biochem 2006, 100, 448–459. [DOI] [PubMed] [Google Scholar]
- (2).Adam SM; Wijeratne GB; Rogler PJ; Diaz DE; Quist DA; Liu JJ; Karlin KD Synthetic Fe/Cu Complexes: Toward Understanding Heme-Copper Oxidase Structure and Function. Chem. Rev 2018, 118, 10840–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Huang X; Groves JT Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev 2018, 118, 2491–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Huang X; Groves JT Beyond Ferryl-Mediated Hydroxylation: 40 Years of the Rebound Mechanism and C−H Activation. JBIC, J. Biol. Inorg. Chem 2017, 22, 185–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Rittle J; Younker JM; Green MT Cytochrome P450: The Active Oxidant and Its Spectrum. Inorg. Chem 2010, 49, 3610–3617. [DOI] [PubMed] [Google Scholar]
- (6).Krest CM; Onderko EL; Yosca TH; Calixto JC; Karp RF; Livada J; Rittle J; Green MT Reactive Intermediates in Cytochrome P450 Catalysis. J. Biol. Chem 2013, 288, 17074–17081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gumiero A; Metcalfe CL; Pearson AR; Raven EL; Moody PCE Nature of the Ferryl Heme in Compounds I and II. J. Biol. Chem 2011, 286, 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Denisov IG; Makris TM; Sligar SG; Schlichting I Structure and Chemistry of Cytochrome P450. Chem. Rev 2005, 105, 2253–2278. [DOI] [PubMed] [Google Scholar]
- (9).Sono M; Roach MP; Coulter ED; Dawson JH Heme-Containing Oxygenases. Chem. Rev 1996, 96, 2841–2888. [DOI] [PubMed] [Google Scholar]
- (10).Ortiz de Montellano PR Hydrocarbon Hydroxylation by Cytochrome P 450 Enzymes. Chem. Rev 2010, 110, 932–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hohenberger J; Ray K; Meyer K The Biology and Chemistry of High-Valent Iron-Oxo and Iron-Nitrido Complexes. Nat. Commun 2012, 3, 720. [DOI] [PubMed] [Google Scholar]
- (12).Groves JT High-Valent Iron in Chemical and Biological Oxidations. J. Inorg. Biochem 2006, 100, 434–447. [DOI] [PubMed] [Google Scholar]
- (13).Groves JT Enzymatic C-H Bond Activation: Using Push to Get Pull. Nat. Chem 2014, 6, 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nam W; Ryu YO; Song WJ Oxidizing Intermediates in Cytochrome P450 Model Reactions. JBIC, J. Biol. Inorg. Chem 2004, 9, 654–660. [DOI] [PubMed] [Google Scholar]
- (15).Nam W High-Valent Iron(IV)−Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions. Acc. Chem. Res 2007, 40, 522–531. [DOI] [PubMed] [Google Scholar]
- (16).Dawson JH; Sono M Cytochrome P-450 and Chloroperoxidase: Thiolate-Ligated Heme Enzymes. Spectroscopic Determination of Their Active Site Structures and Mechanistic Implications of Thiolate Ligation. Chem. Rev 1987, 87, 1255–1276. [Google Scholar]
- (17).Groves JT; Viski P Asymmetric Hydroxylation, Epoxidation, and Sulfoxidation Catalyzed by Vaulted Binaphthyl Metalloporphyrins. J. Org. Chem 1990, 55, 3628–3634. [Google Scholar]
- (18).Groves JT; Nemo TE; Myers RS Hydroxylation and Epoxidation Catalyzed by Iron-Porphine Complexes. Oxygen Transfer from Iodosylbenzene. J. Am. Chem. Soc 1979, 101, 1032–1033. [Google Scholar]
- (19).Groves JT; McClusky GA; White RE; Coon MJ Aliphatic Hydroxylation by Highly Purified Liver Microsomal Cytochrome P-450. Evidence for a Carbon Radical Intermediate. Biochem. Biophys. Res. Commun 1978, 81, 154–160. [DOI] [PubMed] [Google Scholar]
- (20).Traylor TG; Kim C; Richards JL; Xu F; Perrin CL Reactions of Iron(III) Porphyrins with Oxidants. Structure−Reactivity Studies. J. Am. Chem. Soc 1995, 117, 3468–3474. [Google Scholar]
- (21).Dicken CM; Woon TC; Bruice TC Kinetics and Mechanisms of Oxygen Transfer in the Reaction of P-Cyano-N,NDimethylaniline N-Oxide with Metalloporphyrin Salts. 3. Catalysis by [Meso-Tetrakis(2,6-Dichlorophenyl)Porphinato]Iron(III) Chloride. J. Am. Chem. Soc 1986, 108, 1636–1643. [Google Scholar]
- (22).Nam W; Park S; Lim IK; Lim MH; Hong J; Kim J First Direct Evidence for Stereospecific Olefin Epoxidation and Alkane Hydroxylation by an Oxoiron(IV) Porphyrin Complex. J. Am. Chem. Soc 2003, 125, 14674–14675. [DOI] [PubMed] [Google Scholar]
- (23).Chin DH; Balch AL; La Mar GN Formation of Porphyrin Ferryl (FeO2+) Complexes through the Addition of Nitrogen Bases to Peroxo-Bridged Iron(III) Porphyrins. J. Am. Chem. Soc 1980, 102, 1446–1448. [Google Scholar]
- (24).Groves JT; Gross Z; Stern MK Preparation and Reactivity of Oxoiron(IV) Porphyrins. Inorg. Chem 1994, 33, 5065–5072. [Google Scholar]
- (25).Guo M; Dong H; Li J; Cheng B; Huang YQ; Feng YQ; Lei A Spectroscopic Observation of Iodosylarene Metalloporphyrin Adducts and Manganese(V)-Oxo Porphyrin Species in a Cytochrome P450 Analogue. Nat. Commun 2012, 3, 1190–1199. [DOI] [PubMed] [Google Scholar]
- (26).Moody PCE; Raven EL The Nature and Reactivity of Ferryl Heme in Compounds I and II. Acc. Chem. Res 2018, 51, 427–435. [DOI] [PubMed] [Google Scholar]
- (27).Ray K; Heims F; Schwalbe M; Nam W High-Valent Metal-Oxo Intermediates in Energy Demanding Processes: From Dioxygen Reduction to Water Splitting. Curr. Opin. Chem. Biol 2015, 25, 159–171. [DOI] [PubMed] [Google Scholar]
- (28).Sengupta K; Chatterjee S; Dey A In Situ Mechanistic Investigation of O2 Reduction by Iron Porphyrin Electrocatalysts Using Surface-Enhanced Resonance Raman Spectroscopy Coupled to Rotating Disk Electrode (SERRS-RDE) Setup. ACS Catal. 2016, 6, 6838–6852. [Google Scholar]
- (29).Balch AL; Chan YW; Cheng RJ; La Mar GN; Latos-Grazynski L; Renner MW Oxygenation Patterns for Iron(II) Porphyrins. Peroxo and Ferryl (FeIVO) Intermediates Detected by Proton Nuclear Magnetic Resonance Spectroscopy during the Oxygenation of (Tetramesitylporphyrin)Iron(II). J. Am. Chem. Soc 1984, 106, 7779–7785. [Google Scholar]
- (30).Balch AL; Latos-Grazynski L; Renner MW Oxidation of Red Ferryl [(FeIVO)2+] Porphyrin Complexes to Green Ferryl [(FeIVO)2+] Porphyrin Radical Complexes. J. Am. Chem. Soc 1985, 107, 2983–2985 [Google Scholar]
- (31).Groves JT; Haushalter RC; Nakamura M; Nemo TE; Evans BJ High-Valent Iron-Porphyrin Complexes Related to Peroxidase and Cytochrome P-450. J. Am. Chem. Soc 1981, 103, 2884–2886. [Google Scholar]
- (32).Groves JT; Watanabe Y Reactive Iron Porphyrin Derivatives Related to the Catalytic Cycles of Cytochrome P-450 and Peroxidase. Studies of the Mechanism of Oxygen Activation. J. Am. Chem. Soc 1988, 110, 8443–8452. [Google Scholar]
- (33).Penner-Hahn JE; Dawson JH; Renner M; Groves JT; Eble KS; Hodgson KO; McMurry TJ; Balch AL Structural Characterization of Horseradish Peroxidase Using EXAFS Spectroscopy. Evidence for Fe=O Ligation in Compounds I and II. J. Am. Chem. Soc 1986, 108, 7819–7825. [DOI] [PubMed] [Google Scholar]
- (34).Fujii H Model Complexes of Heme Peroxidases In Heme Peroxidases; Raven E, Dunford B, Eds.; The Royal Society of Chemistry: Cambridge, 2016; pp 181–217. [Google Scholar]
- (35).Fujii H Electronic Structure and Reactivity of High-Valent Oxo Iron Porphyrins. Coord. Chem. Rev 2002, 226, 51–60. [Google Scholar]
- (36).Takahashi A; Kurahashi T; Fujii H Redox Potentials of Oxoiron(IV) Porphyrin π-Cation Radical Complexes: Participation of Electron Transfer Process in Oxygenation Reactions. Inorg. Chem 2011, 50, 6922–6928 [DOI] [PubMed] [Google Scholar]
- (37).Takahashi A; Kurahashi T; Fujii H Effect of Imidazole and Phenolate Axial Ligands on the Electronic Structure and Reactivity of Oxoiron(IV) Porphyrin π-Cation Radical Complexes: Drastic Increase in Oxo-Transfer and Hydrogen Abstraction Reactivities. Inorg. Chem 2009, 48, 2614–2625. [DOI] [PubMed] [Google Scholar]
- (38).Bell SR; Groves JT A Highly Reactive P450 Model Compound I. J. Am. Chem. Soc 2009, 131, 9640–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Boaz NC; Bell SR; Groves JT Ferryl Protonation in Oxoiron(IV) Porphyrins and Its Role in Oxygen Transfer. J. Am. Chem. Soc 2015, 137, 2875–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Green MT; Dawson JH; Gray HB Oxoiron(IV) in Chloroperoxidase Compound II Is Basic: Implications for P450 Chemistry. Science 2004, 304, 1653–1656. [DOI] [PubMed] [Google Scholar]
- (41).Yosca TH; Langston MC; Krest CM; Onderko EL; Grove TL; Livada J; Green MT Spectroscopic Investigations of Catalase Compound II: Characterization of an Iron(IV) Hydroxide Intermediate in a Non-Thiolate-Ligated Heme Enzyme. J. Am. Chem. Soc 2016, 138, 16016–16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Behan RK; Hoffart LM; Stone KL; Krebs C; Green MT Evidence for Basic Ferryls in Cytochromes P450. J. Am. Chem. Soc 2006, 128, 11471–11474. [DOI] [PubMed] [Google Scholar]
- (43).Stone KL; Behan RK; Green MT Resonance Raman Spectroscopy of Chloroperoxidase Compound II Provides Direct Evidence for the Existence of an Iron(IV)-Hydroxide. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 12307–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Stone KL; Hoffart LM; Behan RK; Krebs C; Green MT Evidence for Two Ferryl Species in Chloroperoxidase Compound II. J. Am. Chem. Soc 2006, 128, 6147–6153. [DOI] [PubMed] [Google Scholar]
- (45).Kwon H; Basran J; Casadei CM; Fielding AJ; Schrader TE; Ostermann A; Devos JM; Aller P; Blakeley MP; Moody PCE; Raven EL Direct Visualization of a Fe(IV)−OH Intermediate in a Heme Enzyme. Nat. Commun 2016, 7, 13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Schulz CE; Sage JT; Debrunner PG; Hager LP; Rutter R Mössbauer and Electron Paramagnetic Resonance Studies of Horseradish Peroxidase and Its Catalytic Intermediates. Biochemistry 1984, 23, 4743–4754. [DOI] [PubMed] [Google Scholar]
- (47).Hasinoff BB; Dunford HB Kinetics of the Oxidation of Ferrocyanide by Horseradish Peroxidase Compounds I and II. Biochemistry 1970, 9, 4930–4939. [DOI] [PubMed] [Google Scholar]
- (48).Sitter AJ; Reczek CM; Terner J Observation of the FeIVO Stretching Vibration of Ferryl Myoglobin by Resonance Raman Spectroscopy. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol 1985, 828, 229–235. [DOI] [PubMed] [Google Scholar]
- (49).Zeng W; Barabanschikov A; Zhang Y; Zhao J; Sturhahn W; Alp EE; Sage JT Synchrotron-Derived Vibrational Data Confirm Unprotonated Oxo Ligand in Myoglobin Compound II. J. Am. Chem. Soc 2008, 130, 1816–1817. [DOI] [PubMed] [Google Scholar]
- (50).Baglia RA; Krest CM; Yang T; Leeladee P; Goldberg DP High-Valent Manganese−Oxo Valence Tautomers and the Influence of Lewis/Brönsted Acids on C−H Bond Cleavage. Inorg. Chem 2016, 55, 10800–10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yano J; Yachandra V Mn 4 Ca Cluster in Photosynthesis: Where and How Water Is Oxidized to Dioxygen. Chem. Rev 2014, 114, 4175–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Umena Y; Kawakami K; Shen J-R; Kamiya N Crystal Structure of Oxygen-Evolving Photosystem II at a Resolution of 1.9 Å. Nature 2011, 473, 55–60. [DOI] [PubMed] [Google Scholar]
- (53).Rivalta I; Brudvig GW; Batista VS Oxomanganese Complexes for Natural and Artificial Photosynthesis. Curr. Opin. Chem. Biol 2012, 16, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kanady JS; Tsui EY; Day MW; Agapie T A Synthetic Model of the Mn3Ca Subsite of the Oxygen-Evolving Complex in Photosystem II. Science 2011, 333, 733–736 [DOI] [PubMed] [Google Scholar]
- (55).Tsui EY; Tran R; Yano J; Agapie T Redox-Inactive Metals Modulate the Reduction Potential in Heterometallic Manganese-Oxido Clusters. Nat. Chem 2013, 5, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Tsui EY; Agapie T Reduction Potentials of Heterometallic Manganese-Oxido Cubane Complexes Modulated by Redox-Inactive Metals. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 10084–10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Fukuzumi S Electron-Transfer Properties of High-Valent Metal-Oxo Complexes. Coord. Chem. Rev 2013, 257, 1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Park J; Morimoto Y; Lee YM; Nam W; Fukuzumi S Unified View of Oxidative C-H Bond Cleavage and Sulfoxidation by a Nonheme Iron(IV)-Oxo Complex via Lewis Acid-Promoted Electron Transfer. Inorg. Chem 2014, 53, 3618–3628. [DOI] [PubMed] [Google Scholar]
- (59).Westre TE; Kennepohl P; DeWitt JG; Hedman B; Hodgson KO; Solomon EI A Multiplet Analysis of Fe K-Edge 1s → 3d Pre-Edge Features of Iron Complexes. J. Am. Chem. Soc 1997, 119, 6297–6314. [Google Scholar]
- (60).Gütlich P; Bill E; Trautwein AX Hyperfine Interactions. Mössbauer Spectroscopy and Transition Metal Chemistry; Springer: Berlin/Heidelberg, 2011; pp 73–135. [Google Scholar]
- (61).Pan Z; Newcomb M Kinetics and Mechanism of Oxidation Reactions of Porphyrin-Iron(IV)-Oxo Intermediates. Inorg. Chem 2007, 46, 6767–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Adam SM; Garcia-Bosch I; Schaefer AW; Sharma SK; Siegler MA; Solomon EI; Karlin KD Critical Aspects of Heme−Peroxo−Cu Complex Structure and Nature of Proton Source Dictate Metal−O Peroxo Breakage versus Reductive O−O Cleavage Chemistry. J. Am. Chem. Soc 2017, 139, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Garcia-Bosch I; Sharma SK; Karlin KD A Selective Stepwise Heme Oxygenase Model System: An Iron(IV)-Oxo Porphyrin π-Cation Radical Leads to a Verdoheme-Type Compound via an Isoporphyrin Intermediate. J. Am. Chem. Soc 2013, 135, 16248–16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Halime Z; Kieber-Emmons MT; Qayyum MF; Mondal B; Gandhi T; Puiu SC; Chufán EE; Sarjeant AAN;Hodgson KO; Hedman B; Solomon EI; Karlin KD Heme− Copper−Dioxygen Complexes: Toward Understanding Ligand-Environmental Effects on the Coordination Geometry, Electronic Structure, and Reactivity. Inorg. Chem 2010, 49, 3629–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Sharma SK; Schaefer AW; Lim H; Matsumura H; Moenne-Loccoz P; Hedman B; Hodgson KO; Solomon EI; Karlin KD A Six-Coordinate Peroxynitrite Low-Spin Iron(III) Porphyrinate Complex - The Product of the Reaction of Nitrogen Monoxide (·NO(g)) with a Ferric-Superoxide Species. J. Am. Chem. Soc 2017, 139, 17421–17430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Jeong YJ; Kang Y; Han AR; Lee YM; Kotani H; Fukuzumi S; Nam W Hydrogen Atom Abstraction and Hydride Transfer Reactions by Iron(IV)-Oxo Porphyrins. Angew. Chem., Int. Ed 2008, 47, 7321–7324. [DOI] [PubMed] [Google Scholar]
- (67).Sharma VK; Zboril R Ferryl and Ferrate Species: Mössbauer Spectroscopy Investigation. Croat. Chem. Acta 2015, 88, 363–368 [Google Scholar]
- (68).Hu C; Schulz CE; Scheidt WR All High-Spin (S = 2) Iron(II) Hemes Are NOT Alike. Dalton Trans. 2015, 44, 18301–18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Münck E; Champion PM Heme Proteins and Model Compounds: Mossbauer Absorption and Emission Spectroscopy. Ann. N. Y. Acad. Sci 1975, 244, 142–162. [DOI] [PubMed] [Google Scholar]
- (70).Champion PM; Chiang R; Muenck E; Debrunner P; Hager LP Moessbauer Investigations of High-Spin Ferrous Heme Proteins. II. Chloroperoxidase, Horseradish Peroxidase, and Hemoglobin. Biochemistry 1975, 14, 4159–4166. [DOI] [PubMed] [Google Scholar]
- (71).Ghiladi RA; Chufán EE; del Río D; Solomon EI; Krebs C; Huynh BH; Huang H; Moënne-Loccoz P; Kaderli S;Honecker M; Zuberbühler AD; Marzilli L; Cotter RJ; Karlin KD Further Insights into the Spectroscopic Properties, Electronic Structure, and Kinetics of Formation of the Heme−Peroxo−Copper Complex [(F8 TPP)Fe III − (O 2-2-)−Cu II (TMPA)] +. Inorg. Chem 2007, 46, 3889–3902. [DOI] [PubMed] [Google Scholar]
- (72).Li Y; Sharma SK; Karlin KD New Heme−dioxygen and Carbon Monoxide Adducts Using Pyridyl or Imidazolyl Tailed Porphyrins. Polyhedron 2013, 58, 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Sharma SK; Kim H; Rogler PJ; Siegler MA; Karlin KD Isocyanide or Nitrosyl Complexation to Hemes with Varying Tethered Axial Base Ligand Donors: Synthesis and Characterization. JBIC, J. Biol. Inorg. Chem 2016, 21, 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Bajdor K; Nakamoto K Formation of Ferryltetraphenylporphyrin by Laser Irradiation. J. Am. Chem. Soc 1984, 106, 3045–3046. [Google Scholar]
- (75).Nakamoto K Resonance Raman Spectra and Biological Significance of High-Valent Iron(IV,V) Porphyrins. Coord. Chem. Rev 2002, 226, 153–165. [Google Scholar]
- (76).Proniewicz LM; Bajdor K; Nakamoto K Resonance Raman Spectra of Ferrylporphyrins and Related Compounds in Dioxygen Matrixes. J. Phys. Chem 1986, 90, 1760–1766. [Google Scholar]
- (77).Kean RT; Oertling WA; Babcock GT Characterization of Six-Coordinate Ferryl Protoheme by Resonance Raman and Optical Absorption Spectroscopy. J. Am. Chem. Soc 1987, 109, 2185–2187. [Google Scholar]
- (78).Green MT Application of Badger’s Rule to Heme and Non-Heme Iron-Oxygen Bonds: An Examination of Ferryl Protonation States. J. Am. Chem. Soc 2006, 128, 1902–1906. [DOI] [PubMed] [Google Scholar]
- (79).Peterson RL; Ginsbach JW; Cowley RE; Qayyum MF; Himes RA; Siegler MA; Moore CD; Hedman B; Hodgson KO; Fukuzumi S; Solomon EI; Karlin KD Stepwise Protonation and Electron-Transfer Reduction of a Primary Copper-Dioxygen Adduct. J. Am. Chem. Soc 2013, 135, 16454–16467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Yoon H; Lee Y-M; Wu X; Cho K-B; Sarangi R; Nam W; Fukuzumi S Enhanced Electron-Transfer Reactivity of Nonheme Manganese(IV)−Oxo Complexes by Binding Scandium Ions. J. Am. Chem. Soc 2013, 135, 9186–9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Park J; Morimoto Y; Lee Y-M; Nam W; Fukuzumi S Proton-Promoted Oxygen Atom Transfer vs Proton-Coupled Electron Transfer of a Non-Heme Iron(IV)-Oxo Complex. J. Am. Chem. Soc 2012, 134, 3903–3911. [DOI] [PubMed] [Google Scholar]
- (82).Wang D; Zhang M; Bühlmann P; Que L Redox Potential and C−H Bond Cleaving Properties of a Nonheme FeIV=O Complex in Aqueous Solution. J. Am. Chem. Soc 2010, 132, 7638–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Chen J; Yoon H; Lee Y-M; Seo MS; Sarangi R; Fukuzumi S; Nam W Tuning the Reactivity of Mononuclear Nonheme ManganeseIV-Oxo Complexes by Triflic Acid. Chem. Sci 2015, 6, 3624–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Fukuzumi S; Ohkubo K; Lee Y-M; Nam W Lewis Acid Coupled Electron Transfer of Metal-Oxygen Intermediates. Chem. -Eur. J 2015, 21, 17548–17559. [DOI] [PubMed] [Google Scholar]
- (85).Chai C; Armarego WLF Purification of Laboratory Chemicals, 6th ed.; Elsevier Inc.: Amsterdam, 2009. [Google Scholar]
- (86).Tenderholt A; Hedman B; Hodgson KO PySpline: A Modern, Cross-Platform Program for the Processing of Raw Averaged XAS Edge and EXAFS Data. AIP Conf. Proc. 2006, 882, 105–107. [Google Scholar]
- (87).Ankudinov AL; Rehr JJ Relativistic Calculations of Spin-Dependent x-Ray-Absorption Spectra. Phys. Rev. B: Condens. Matter Mater. Phys 1997, 56, R1712–R1716. [Google Scholar]
- (88).Wilson SA; Green E; Mathews II; Benfatto M; Hodgson KO; Hedman B; Sarangi R X-Ray Absorption Spectroscopic Investigation of the Electronic Structure Differences in Solution and Crystalline Oxyhemoglobin. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 16333–16338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Neese F Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci 2018, 8, No. e1327. [Google Scholar]
- (90).Becke AD Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A: At., Mol., Opt. Phys 1988, 38, 3098–3100. [DOI] [PubMed] [Google Scholar]
- (91).Weigend F; Ahlrichs R Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys 2005, 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- (92).Römelt M; Ye S; Neese F Calibration of Modern Density Functional Theory Methods for the Prediction of 57Fe Mössbauer Isomer Shifts: Meta-GGA and Double-Hybrid Functionals. Inorg. Chem 2009, 48, 784–785. [DOI] [PubMed] [Google Scholar]
- (93).Grimme S; Antony J; Ehrlich S; Krieg H A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys 2010, 132, 154104. [DOI] [PubMed] [Google Scholar]
- (94).Ghiladi RA; Hatwell KR; Karlin KD; Huang H; Moënne-Loccoz P; Krebs C; Huynh BH; Marzilli LA; Cotter RJ; Kaderli S; Zuberbühler AD Dioxygen Reactivity of Mononuclear Heme and Copper Components Yielding A High-Spin Heme−Peroxo−Cu Complex. J. Am. Chem. Soc 2001, 123, 6183–6184. [DOI] [PubMed] [Google Scholar]
- (95).Curley JJ; Bergman RG; Tilley TD Preparation and Physical Properties of Early-Late Heterobimetallic Compounds Featuring Ir-M Bonds (M = Ti, Zr, Hf). Dalton Trans. 2012, 41, 192–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.