Nanoliter acoustic dispensing technology is an efficient way to synthesize large, diverse, and complex libraries of boronic acids.
Abstract
The compatibility of free boronic acid building blocks in multicomponent reactions to readily create large libraries of diverse and complex small molecules was investigated. Traditionally, boronic acid synthesis is sequential, synthetically demanding, and time-consuming, which leads to high target synthesis times and low coverage of the boronic acid chemical space. We have performed the synthesis of large libraries of boronic acid derivatives based on multiple chemistries and building blocks using acoustic dispensing technology. The synthesis was performed on a nanomole scale with high synthesis success rates. The discovery of a protease inhibitor underscores the usefulness of the approach. Our acoustic dispensing–enabled chemistry paves the way to highly accelerated synthesis and miniaturized reaction scouting, allowing access to unprecedented boronic acid libraries.
INTRODUCTION
Boron is a unique element of great versatility and individuality, although it seems that nature and evolution have generally bypassed it (with the exception of a few natural products, e.g., boromycin) (1). Boron plays an exquisite role in synthetic chemistry, with boronic acids and their esters of paramount importance to all facets of chemical science. Since the introduction of the Pd-catalyzed C─C Suzuki-Miyaura couplings (2) that brought boronate esters into vogue, the boronic acid moiety has become a very important functional group (3). Other highly useful transformations based on boronic acids include the Petasis reaction (4), C─N and C─O coupling (Chan-Lam coupling) (5, 6), Liebeskind-Srogl coupling (7), regioselective deuteration, or sulfonamide formation (8). Boronic acids as mild electrophiles are also investigated as reversible covalent inhibitors (9, 10), and thousands of different building blocks are now commercially available. As a result, boronic acids are increasingly being seen in approved drugs, e.g., vaborbactam or bortezomib (Fig. 1, A and B) (11, 12).
Fig. 1. Importance of boronic acids, commonly used synthetic methods for the ─B(OH)2 introduction, and our proposed building block–centered approach.
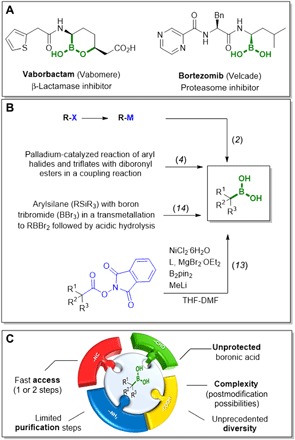
(A) Marketed drugs containing free ─B(OH)2 moieties. (B) Common methods for late-stage introduction of the ─B(OH)2 moiety. THF-DMF, tetrahydrofuran-dimethylformamide. (C) Building block approach to prepare complex ─B(OH)2 moiety containing molecules in large numbers.
However, these boron building blocks comprise almost exclusively low–molecular weight compounds, as the late-stage functionalization of high–molecular weight boronic acids is synthetically demanding due to their tedious introduction, modest functional group compatibility, regioselectivity issues, and difficulty to parallelize (13, 14). Because of the exquisite differential properties of boronic acids, an easy access to high–molecular weight elaborated compounds is highly desirable. Isocyanide-based multicomponent reactions (IMCRs) are well established for functional group compatibility that accounts for the immense scaffold diversity that can be generated on the basis of some handful primary IMCRs (15, 16). Furthermore, IMCRs are useful to access a drug-like chemical space and many marketed or experimental drugs (17, 18). Thus, we hypothesized that unprotected boronic acids are compatible with the reaction conditions of IMCR and can be introduced into complex high–molecular weight compounds of use (19). The use of unprotected boronic acids directly could enable a faster access with limited protecting steps to a large number of boron-based derivatives. In addition, the screening of these compounds (e.g., as covalent inhibitors) could be performed directly without further deprotection (Fig. 1C). To test this hypothesis, we used an acoustic droplet ejection (ADE)–enabled synthesis platform. In ADE, acoustic waves are applied to eject nanoliter droplets from a source plate with building block stock solutions to a destination plate in which the reaction occurs. While ADE is an established dispensing technology in many other scientific areas (e.g., crystallography), it is uncommon in organic synthesis (20). The ADE platform is based on microliter volume chemistry, uses minimal resources, is highly automatable, and is useful to screen many building block combinations in a shorter time frame than other current technologies (21).
RESULTS AND DISCUSSIONS
The mechanism-based functional groups required in IMCRs are carboxylic acids, amines, oxo components, and isocyanides. We synthesized and purchased a number of the first three building block categories. In addition, we also synthesized an unknown isocyanide boronic acid in one example (Fig. 2). We planned to perform four IMCRs and investigate the reaction success rate depending on the reactions, the components, and the substitution pattern (e.g., o-, m-, and p-) in combination with multiple complementary building blocks chosen in a random fashion.
Fig. 2. Boronic acid building blocks used in this study, first synthesis of boronic acid isocyanide, and evaluated reactions.
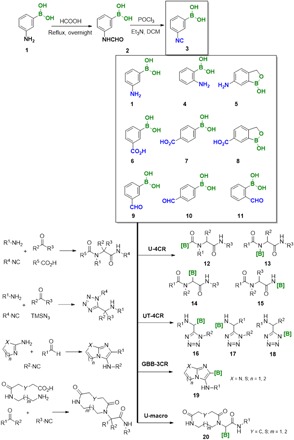
[B], phenyl boronic acid moiety.
Optimizing the compatibility of boronic acids for MCR is an interesting synthetic challenge, as the C─B bond is well known to react with common MCR starting materials and intermediates under mild conditions, e.g., primary, secondary amines, and carbonyl compounds (Petasis reaction and others) (22). Moreover, the electrophilic boron could unproductively complex to nucleophilic key functional groups of MCRs and thereby interrupt the reaction progress (23). Here, we used different building blocks (amines, aldehydes, carboxylic acids, and isocyanides) with free boronic acids in different positions to investigate their compatibility with a number of IMCRs (Fig. 2). The stability of the boronic acid moiety in the presence of the isocyanide in one molecule in the absence of such molecules is unknown. To this end, we also synthesized the first free boronic acid–containing isocyanide. We have extensively investigated the compatibility of multiple free boronic acid–containing building blocks in multiple IMCRs, including the classical Ugi four-component reaction (U-4CR) (24), the Ugi tetrazole (UT-4CR) (25), the Gröbcke-Blackburn-Bienaymé (GBB-3CR) (26–28), and the Ugi-based macrocycles (scaffolds 12 to 20; Fig. 3) (29). In addition, we studied the suitability of the corresponding boronic acid libraries in a secondary Suzuki cross-coupling reaction. We performed the project under extreme resource- and time-saving conditions using the ADE-enabled chemistry platform in a 384-well format.
Fig. 3. HT synthesis of boronic acids using the building block approach.
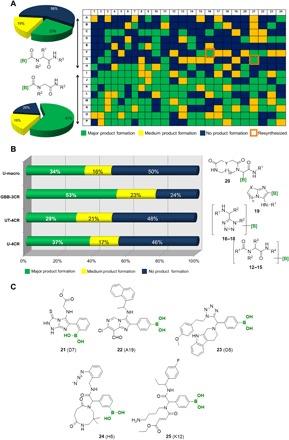
(A) Exemplary analytical 384-well plate of the U-4CR scaffold 12 (green, major product formation; yellow, product present; blue, product not present). (B) Statistical analysis of the quality of reactions of the different scaffolds. (C) Structures of some unusual reaction products from different IMCRs.
We accomplished the synthesis of m-isocyanophenyl boronic acid by a classical formylation/dehydration procedure (Fig. 2). Because of the presumed instability, we immediately used the isocyanide after preparation. Using the boronic acid building blocks of Fig. 2, we investigated different IMCRs on a nanomole scale using ADE technology. The analytics of the four 384-well format plates were performed using mass spectrometry (MS) as described previously (30), which allowed us to classify the reactions into three groups: major (green color), mediocre (yellow color), or no product formation (blue color). The outcome of the high-throughput (HT) analytics for the different reactions is shown in Fig. 3, and a detailed analysis of the different building blocks is given in the Supplementary Materials. The rapid collection of information facilitated the ability to predict outcomes from other possible combinations of reagents. For example, it was found that the ortho substituted building blocks 4 and 11 reacted less efficiently than the corresponding meta or para substituted in all MCRs (Fig. 2). This can be rationalized by the neighbor group effect of boronic acid that might hamper formation or reduce reactivity of the key Schiff base. It was also found that boronic acid monoesters 5 and 8 were less reactive than their boronic acid counterparts, probably due to the introduction of ring strain around the boron center, leading to slightly different electronic properties (31). In addition, it was demonstrated that the U-4CR of the three carboxyphenyl boronic acids 6 to 8 (heat map shown in Fig. 3A) was greatly enhanced when p-formaldehyde was used (>60% of the reactions worked; see the Supplementary Materials). Last, it is noteworthy that formylphenyl boronic acids behave well in the GBB-3CR, since more than 50% of the reactions that were performed were successful (see the Supplementary Materials). In general, the use of building blocks without the free ─B(OH)2 moieties was less successful than those with boronic acids. This could point to a potential catalytic activity of boronic acids in the GBB-3CR as a Brønsted acid as there are many cases of GBB catalysis by Brønsted acids (26–28). Detailed analysis of the rich data of the complementary building blocks can help to uncover subtle reactivity details.
In our approach, novel substrates could be generated. In the GBB-3CR, compound 21 reacted repeatedly well despite the existence of a hitherto unreported triazolidine-5-thione moiety in this context. Another interesting finding is the good reactivity of building block 22 in the GBB-3CR, in which the formyl group did not react, as the additional formyl group could theoretically undergo alternative reaction pathways such as condensation and addition reactions. Another pleasant finding is the good reactivity of tetrahydro-β-carboline 23 in the UT-4CR, which is a pharmacophore in multiple natural products and drugs (e.g., harman and tadalafil). Complex medium-sized and macrocycles gave, unexpectedly, very good results (e.g., medium-sized cycle 24). Last, we observed functional group tolerance and selectivity. In the case of U-4CR 25, we used a diamine, which reacted only once, leaving a primary amine behind. The HT synthesis approach displayed here is a treasure trove to uncover interesting unknown reactivities that deserve further investigation and detailed analysis in a narrower compound series.
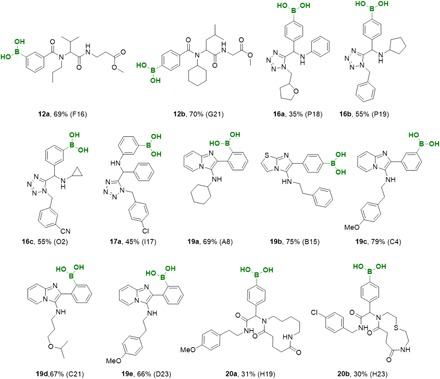
The scalability from the nanomole to the millimole scale is often problematic. Therefore, we resynthesized multiple examples of each compound series (compounds 12 to 20) on a millimole scale (including full characterization) to verify our ADE results (Fig. 4) and to identify any potential bottlenecks into transferring synthesis from ADE technology to classical approaches.
Fig. 4. Resynthesized complex boronic acid derivatives based on different scaffolds on a millimole scale and corresponding yields.
Boronic acids are exceedingly useful functional groups and the starting materials for many reactions. To underscore the usefulness of our building block approach, we investigated multiple boronic acid building blocks in a subsequent Suzuki C─C coupling. We performed reaction scouting in 384-well polypropylene plates using ADE; after the transfer of starting materials, we incubated the plates at 50°C overnight. Again, we resynthesized an example on a millimole scale in a one-pot fashion (compound 28; Fig. 5).
Fig. 5. HT Suzuki reaction of boronic acids using the building block approach.
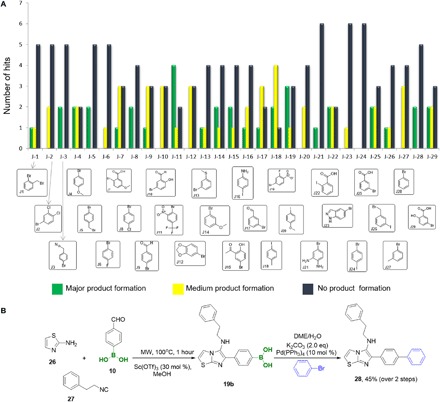
(A) Statistical analysis of the aryl halides that were used in the HT screening (green, major peak in MS; yellow, product present; blue, product not present). (B) A one-pot resynthesized compound 28 on a millimole scale and isolated yield. DME, dimethoxyethane.
To further underscore the usefulness of our fast, convergent, and highly diverse access of boronic acid libraries, we screened for inhibition of the biological target MptpB, a virulence factor from Mycobacterium tuberculosis (32). MptpB belongs to the notoriously undruggable target class of phosphatases that, despite their overarching relevance in medicine, suffer from having no approved drug (33). This is generally attributed to the highly positively charged active site of phosphatases requiring negatively charged inhibitors that cannot overcome membrane penetration issues (34). Looking for a potential covalent interaction between the active-site nucleophiles Cys160, Thr223, and Ser57 of MptpB and an electrophilic boronic acid, we screened the library in a colorimetric enzyme assay (see the Supplementary Materials). In this assay, we found several hits, the most potent one 18a (Fig. 6). The exact binding mode of 18a is unclear due to the large number of reactive Ser, Cys, and Thr on the surface and in the active site of MptpB (the Supplementary Materials). Modeling studies of 18a in MptpB with Cys160, Ser57, and Thr223 were performed and suggest a covalent adduct to a tetrahedral boron (Fig. 6C and see the Supplementary Materials).
Fig. 6. Covalent inhibition of tuberculosis target MptpB.
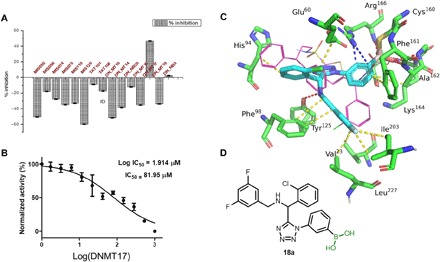
(A) Screening of the boronic acid library by a colorimetric enzyme assay. (B) Median inhibitory concentration (IC50) of compound 18a. (C and D) Modeling of compound 18a into MptpB [Protein Data Bank (PDB) ID: 2OZ5], where it forms a covalent adduct with active-site cysteine. Van der Waals interactions, hydrogen bonding, and cation-π interactions are indicated by yellow, red, and blue dotted lines, respectively.
Classical access to boronic acids by late-stage functionalization of complex molecules suffers from a lack in functional group compatibility and regioselectivity and often requires harsh conditions that are incompatible with molecule stability (35–38). Here, we introduced the concept of boronic acid building blocks combined with the diversity of MCRs as a valid approach for the synthesis of large and unprecedented libraries of boronic acids. Our studies go much beyond a singleton report on the use of a few free boronic acids in the Ugi reaction as we also investigated the GBB-3CR, UT-4CR, and several different IMCR variations more in an unprecedented breadth of building block combinations (35, 36). In other reports, isocyanide-bearing boronic acids are only known in their protected ester form that would need another, often harsh, deprotecting step to yield boronic acids suitable for screening (39, 40). Here, we found that IMCR generally runs under such mild conditions that free boronic acids are widely tolerated. We systematically investigated 10 different boronic acid building blocks with complementary functional groups (primary amine, aldehyde, carboxylic acid, and isocyanide) and combined them with 353 different reactants in four IMCRs. More than 1300 different combinations were investigated in a nanomole miniaturized and automated fashion using ADE technology. HT analytics using MS revealed that the different reactions worked better than satisfactorily in 714 cases (458 giving the main product and 256 cases a satisfactory yield). Many subtle reactivities were uncovered, which in a classical millimole scale reaction, evaluation approach could never been elucidated in a reasonable time frame. Upscaling of a substantial number of diverse products revealed the synthetic usefulness of the approach. Last, we probed our library to uncover previously unknown boronic acid–based covalent inhibitors for a notoriously undruggable phosphatase target, identifying a micromolar inhibitor. We believe our described building block approach will widen the accessibility of the boronic acid chemical space markedly for applications in synthesis, chemical biology, and drug discovery. This is also true in light of the recently found catalytic enantioselective Ugi reaction (41).
MATERIALS AND METHODS
All the reagents and solvents were purchased from Sigma-Aldrich, AK Scientific, Fluorochem, abcr GmbH, and Acros and were used without further purification. All isocyanides were prepared in-house (see the Supplementary Materials). All microwave irradiation reactions were carried out in a Biotage Initiator microwave synthesizer. Thin-layer chromatography was performed on Millipore precoated silica gel plates (thickness, 0.20 mm; particle size, 25 μm). Nuclear magnetic resonance spectra were recorded on Bruker Avance 500 spectrometers [1H NMR (nuclear magnetic resonance; 500 MHz), 13C NMR (126 MHz)]. Chemical shifts for 1H NMR were reported as δ values, and coupling constants were in hertz. The following abbreviations were used for spin multiplicity: s, singlet; br s, broad singlet; d, doublet; t, triplet; q, quartet; quin, quintet; dd, double of doublets; ddd, double doublet of doublets; and m, multiplet. Chemical shifts for 13C NMR were reported in parts per million relative to the solvent peak. Flash chromatography was performed on a Reveleris X2 flash chromatography system, using Grace Reveleris Silica flash cartridges (12 g). Mass spectra were measured on a Waters Investigator Supercritical Fluid Chromatograph with a 3100 MS detector (ESI) using a solvent system of methanol and CO2 on a Viridis silica gel column (4.6 × 250 mm, 5-μm particle size) or Viridis 2-ethyl pyridine column (4.6 × 250 mm, 5-μm particle size). High-resolution mass spectra were recorded using an LTQ Orbitrap XL (Thermo Fisher Scientific) at a resolution of 60,000 at m/z 400. The Echo 555 liquid handler (Labcyte) was used to transfer nanoliter droplets of starting materials from the 384-well source plate to the 384-well destination plate.
Supplementary Material
Acknowledgments
Funding: This research has been supported (to A.D.) by the NIH (2R01GM097082-05), the European Lead Factory (IMI) under grant agreement no. 115489, and the Qatar National Research Foundation (NPRP6-065-3-012). Moreover, funding was received through ITN “Accelerated Early staGe drug dIScovery” (AEGIS; grant agreement no. 675555) and COFUND ALERT (grant agreement no. 665250), Hartstichting (ESCAPE-HF, 2018B012), and KWF Kankerbestrijding grant (grant agreement no. 10504). L.G. and R.X. are grateful for the CSC Fellowships from the Chinese government. Labcyte Inc. has provided access to the Echo 555 instrument as an in-kind contribution for this work. Author contributions: A.D. conceived of and directed the project. S.S., M.A., L.G., and R.X. performed the HT synthesis of boronic acids using the building block approach with ADE technology. C.G.N., T.Z.-T., M.N., and T.M. resynthesized the complex boronic acid derivatives. C.G.N., S.S., and M.A. performed the data analysis. A.R.R. and M.I.I. performed the screening of the boronic acid library. A.R.R. did the computational studies. J.O., R.E., V.H., and M.K. helped the direction of the project. A.D., C.G.N., S.S., and M.R.G. cowrote the manuscript. Competing interests: J.O. and R.E. are employees of Labcyte Inc., the manufacturer of the Echo liquid handler. R.E. is an inventor on several U.S. patents related to this work (no. 6,416,164, 9 July 2002; no. 6,612,686, 2 September 2003; no. 6,666,541, 23 December 2003; no. 6,802,593, 12 October 2004; no. 6,938,987, 6 September 2005; no. 6,938,995, 6 September 2005; no. 7,090,333, 15 August 2006; no. 7,354,141, 08 April 2008; no. 7,454,958, 25 November 2008; no. 7,900,505, 08 March 2011; and no. 7,901,039, 08 March 2011). The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/7/eaaw4607/DC1
Supplementary Materials and Methods
Fig. S1. Isocyanide syntheses.
Fig. S2. Reactions in destination plate I.
Fig. S3. Reactions in destination plate II.
Fig. S4. Reactions in destination plate III.
Fig. S5. Reactions in destination plate IV.
Fig. S6. Labcyte Echo plate reformat software.
Fig. S7. Heat plots with product structures, green for major product formation, yellow for medium product formation, and blue for no product formation.
Fig. S8. Stabilization effect of 18a as proof of interaction with MptpB as assessed by DSF.
Fig. S9. Binding curve of 18a to the fluorescently labeled MptpB sample as assessed by MST.
Fig. S10. Three-dimensional structure of the target phosphatase.
Fig. S11. Proposed docking model for 18a covalently bound to Cys160 (PDB ID: 2OZ5).
Fig. S12. Proposed docking model for 18a covalently bound to Ser57 (PDB ID: 2OZ5).
Fig. S13. Proposed docking model for 18a covalently bound to Thr223 (PDB ID: 2OZ5).
Fig. S14. ADE technology.
Table S1. Summary table of the docking scores for Covdock and Scorpion.
Scheme S1. Quality control results for destination plate I.
Scheme S2. Performance of formylphenyl boronic acids in destination plate I.
Scheme S3. Performance of isocyanides in GBB-3CR reaction in destination plate I.
Scheme S4. Performance of isocyanides in Ugi-based macrocycles in destination plate I.
Scheme S5. Performance of isocyanides in U-4CR in destination plate I.
Scheme S6. Performance of isocyanides in UT-4CR in destination plate I.
Scheme S7. Performance of carboxylic acids in U-4CR in destination plate I.
Scheme S8. Performance of amines in U-4CR in destination plate I.
Scheme S9. Performance of amines in UT-4CR in destination plate I.
Scheme S10. Performance of amidines in GBB-3CR reaction in destination plate I.
Scheme S11. Performance of α,ω-amino carboxylic acids in Ugi-based macrocycles in destination plate I.
Scheme S12. Quality control results for destination plate II.
Scheme S13. Performance of aminophenyl boronic acids in destination plate II.
Scheme S14. Performance of isocyanides in U-4CR in destination plate II.
Scheme S15. Performance of isocyanides in UT-4CR in destination plate II.
Scheme S16. Performance of oxo components in U-4CR in destination plate II.
Scheme S17. Performance of oxo components in UT-4CR in destination plate II.
Scheme S18. Performance of carboxylic acids in U-4CR in destination plate II.
Scheme S19. Quality control results for destination plate III.
Scheme S20. Performance of carboxyphenyl boronic acids in destination plate III.
Scheme S21. Performance of isocyanides in U-4CR in destination plate III.
Scheme S22. Performance of isocyanides in U-4CR with CH2O in destination plate III.
Scheme S23. Performance of oxo component in U-4CR in destination plate III.
Scheme S24. Performance of amines in U-4CR in destination plate III.
Scheme S25. Performance of amines in U-4CR with CH2O in destination plate III.
Scheme S26. Quality control results for destination plate IV.
Scheme S27. Performance of MCR boronic acid building blocks in destination plate IV.
Scheme S28. Performance of aryl halides in destination plate IV.
REFERENCES AND NOTES
- 1.Diaz D. B., Yudin A. K., The versatility of boron in biological target engagement. Nat. Chem. 9, 731–742 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Miyaura N., Yamada K., Suzuki A., A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 20, 3437–3440 (1979). [Google Scholar]
- 3.Miyaura N., Suzuki A., Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995). [Google Scholar]
- 4.Petasis N. A., Akritopoulou I., The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 34, 583–586 (1993). [Google Scholar]
- 5.Chan D. M. T., Monaco K. L., Wang R.-P., Winters M. P., New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 39, 2933–2936 (1998). [Google Scholar]
- 6.Lam P. Y. S., Clark C. G., Saubern S., Adams J., Winters M. P., Chan D. M. T., Combs A., New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 39, 2941–2944 (1998). [Google Scholar]
- 7.Liebeskind L. S., Srogl J., Thiol ester−boronic acid coupling. A mechanistically unprecedented and general ketone synthesis. J. Am. Chem. Soc. 122, 11260–11261 (2000). [Google Scholar]
- 8.Chen Y., Murray P. R. D., Davies A. T., Willis M. C., Direct copper-catalyzed three-component synthesis of sulfonamides. J. Am. Chem. Soc. 140, 8781–8787 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Lanier M., Cole D. C., Istratiy Y., Klein M. G., Schwartz P. A., Tjhen R., Jennings A., Hixon M. S., Repurposing Suzuki coupling reagents as a directed fragment library targeting serine hydrolases and related enzymes. J. Med. Chem. 60, 5209–5215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leśnikowski Z. J., Recent developments with boron as a platform for novel drug design. Expert Opin. Drug Discovery 11, 569–578 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Groll M., Berkers C. R., Ploegh H. L., Ovaa H., Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure 14, 451–456 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Smoum R., Rubinstein A., Dembitsky V. M., Srebnik M., Boron containing compounds as protease inhibitors. Chem. Rev. 112, 4156–4220 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Li C., Wang J., Barton L. M., Yu S., Tian M., Peters D. S., Kumar M., Yu A. W., Johnson K. A., Chatterjee A. K., Yan M., Baran P. S., Decarboxylative borylation. Science 356, eaam7355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp M. J., Cheng W., Snieckus V., Synthetic connections to the aromatic directed metalation reaction. Functionalized aryl boronic acids by ipso borodesilylation. General syntheses of unsymmetrical biphenyls and m-terphenyls. Tetrahedron Lett. 28, 5093–5096 (1987). [Google Scholar]
- 15.Dömling A., Ugi I., Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39, 3168–3210 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Slobbe P., Ruijter E., Orru R. V. A., Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm. 3, 1189 (2012). [Google Scholar]
- 17.Dömling A., Wang W., Wang K., Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Znabet A., Polak M. M., Janssen E., de Kanter F. J. J., Turner N. J., Orru R. V. A., Ruijter E., A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem. Commun. 46, 7918–7920 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Solleder S. C., Meier M. A. R., Sequence control in polymer chemistry through the Passerini three-component reaction. Angew. Chem. Int. Ed. 53, 711–714 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Yin X., Scalia A., Leroy L., Cuttitta C. M., Polizzo G. M., Ericson D. L., Roessler C. G., Campos O., Ma M. Y., Agarwal R., Jackimowicz R., Allaire M., Orville A. M., Sweet R. M., Soares A. S., Hitting the target: Fragment screening with acoustic in situ co-crystallization of proteins plus fragment libraries on pin-mounted data-collection micromeshes. Acta Crystallogr., Sect. D: Struct. Biol. 70, 1177–1189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellson R., Picoliter: Enabling precise transfer of nanoliter and picoliter volumes. Drug Discov. Today 7, S32–S34 (2002). [Google Scholar]
- 22.Candeias N. R., Montalbano F., Cal P. M. S. D., Gois P. M. P., Boronic acids and esters in the Petasis-borono Mannich multicomponent reaction. Chem. Rev. 110, 6169–6193 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Tan J., Yudin A. K., Borylated reagents for multicomponent reactions. Drug Discov. Today Technol. 29, 51–60 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Ugi I., Steinbrückner C., Über ein neues Kondensations-Prinzip. Angew. Chem. Int. Ed. 72, 267–268 (1960). [Google Scholar]
- 25.Neochoritis C. G., Zhao T., Dömling A., Tetrazoles via multicomponent reactions. Chem. Rev. 119, 1970–2042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groebke K., Weber L., Mehlin F., Synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines by a novel three-component condensation. Synlett 1, 661–663 (1998). [Google Scholar]
- 27.Blackburn C., Guan B., Fleming P., Shiosaki K., Tsai S., Parallel synthesis of 3-aminoimidazo[1,2-a]pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 39, 3635–3638 (1998). [Google Scholar]
- 28.Bienaymé H., Bouzid K., A new heterocyclic multicomponent reaction for the combinatorial synthesis of fused 3-aminoimidazoles. Angew. Chem. Int. Ed. 37, 2234–2237 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Madhavachary R., Abdelraheem E. M. M., Rossetti A., Twarda-Clapa A., Musielak B., Kurpiewska K., Kalinowska-Tłuścik J., Holak T. A., Dömling A., Two-step synthesis of complex artificial macrocyclic compounds. Angew. Chem. Int. Ed. 56, 10725–10729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S., Dikler S., Blincoe W. D., Ferguson R. D., Sheridan R. P., Peng Z., Conway D. V., Zawatzky K., Wang H., Cernak T., Davies I. W., DiRocco D. A., Sheng H., Welch C. J., Dreher S. D., Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS. Science 361, eaar6236 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Gamrat J. M., Mancini G., Burke S. J., Colandrea R. C., Sadowski N. R., Figula B. C., Tomsho J. W., Protection of the benzoxaborole moiety: Synthesis and functionalization of zwitterionic benzoxaborole complexes. J. Org. Chem. 83, 6193–6201 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Beresford N., Patel S., Armstrong J., Szöor B., Fordham-Skelton A. P., Tabernero L., MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem. J. 406, 13–18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazo J. S., McQueeney K. E., Sharlow E. R., New approaches to difficult drug targets: The phosphatase story. SLAS Discov. Adv. Life Sci. R&D. 22, 1071–1083 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Hoff R. H., Wu L., Zhou B., Zhang Z.-Y., Hengge A. C., Does positive charge at the active sites of phosphatases cause a change in mechanism? The effect of the conserved arginine on the transition state for phosphoryl transfer in the protein-tyrosine phosphatase fromYersinia. J. Am. Chem. Soc. 121, 9514–9521 (1999). [Google Scholar]
- 35.Guchhait S., Madaan C., Thakkar B., A highly flexible and efficient Ugi-type multicomponent synthesis of versatile N-fused aminoimidazoles. Synthesis 2009, 3293–3300 (2009). [Google Scholar]
- 36.Lian R.-C., Lin M. H., Liao P. H., Fu J. J., Wu M. J., Wu Y. C., Chang F. R., Wu C. C., Pan P. S., Direct synthesis of the arylboronic acid analogues of phenylglycine via microwave-assisted four-component Ugi reaction. Tetrahedron 70, 1800–1804 (2014). [Google Scholar]
- 37.Sanghai N., Jain V., Preet R., Kandekar S., Das S., Trivedi N., Mohapatra P., Priyadarshani G., Kashyap M., Das D., Satapathy S. R., Siddharth S., Guchhait S. K., Kundu C. N., Bharatam P. V., Combretastatin A-4 inspired novel 2-aryl-3-arylamino-imidazo-pyridines/pyrazines as tubulin polymerization inhibitors, antimitotic and anticancer agents. Med. Chem. Commun. 5, 766–782 (2014). [Google Scholar]
- 38.Gravel M., Thompson K. A., Zak M., Bérubé C., Hall D. G., Universal solid-phase approach for the immobilization, derivatization, and resin-to-resin transfer reactions of boronic acids. J. Org. Chem. 67, 3–15 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Zajdlik A., Wang Z., Hickey J. L., Aman A., Schimmer A. D., Yudin A. K., α-Boryl isocyanides enable facile preparation of bioactive boropeptides. Angew. Chem. Int. Ed. 125, 8569–8573 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Kaldas S. J., Rogova T., Nenajdenko V. G., Yudin A. K., Modular synthesis of β-amino boronate peptidomimetics. J. Org. Chem. 83, 7296–7302 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Yu P., Li S. Y., Sun H., Xiang S. H., Wang J. J., Houk K. N., Tan B., Asymmetric phosphoric acid–catalyzed four-component Ugi reaction. Science 361, eaas8707 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Obrecht R., Herrmann R., Ugi I., Isocyanide synthesis with phosphoryl chloride and diisopropylamine. Synthesis 1985, 400–402 (1985). [Google Scholar]
- 43.Ugi I., Isocyanide synthesis with diphosgene. Agnew. Chem., Int. Ed. 16, 259–260 (1977). [Google Scholar]
- 44.Ugi I., Fetzer U., Eholzer U., Knupfer H., Offermann K., Isonitrile syntheses. Agnew. Chem., Int. Ed. 4, 472–484 (1965). [Google Scholar]
- 45.Ugi I., Meyr R., o-Tolyl Isocyanide. Organic Synth. 1, 101–101 (1961). [Google Scholar]
- 46.Gokel G. W., Widera R. P., Weber W. P., Phase-transfer Hofmann carbylamine reaction: tert-butyl isocyanide. Organic Synth. 55, 96–96 (2003). [Google Scholar]
- 47.Weber W. P., Gokel G. W., Ugi I. K., Phase transfer catalysis in the Hofmann carbylamine reaction. Agnew. Chem., Int. Ed. 11, 530–531 (1972). [Google Scholar]
- 48.Neochoritis C. G., Zarganes-Tzitzikas T., Stotani S., Dömling A., Herdtweck E., Khoury K., Dömling A., Leuckart–Wallach route toward isocyanides and some applications. ACS Comb. Sci. 17, 493–499 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Huang Y., Wolf S., Bista M., Meireles L., Camacho C., Holak T. A., Dömling A., 1,4-Thienodiazepine-2,5-diones via MCR (I): Synthesis, virtual space and p53-Mdm2 activity. Chem. Biol. Drug Des. 76, 116–129 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L., Dai C., Burroughs S. K., Wang S. L., Wang B., Arylboronic acid chemistry under electrospray conditions. Chem. A Eur. J. 19, 7587–7594 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Madhavachary R., Abdelraheem E. M. M., Rossetti A., Twarda-Clapa A., Musielak B., Kurpiewska K., Kalinowska-Tłuścik J., Holak T. A., Dömling A., Two-step synthesis of complex artificial macrocyclic compounds. Angew. Chem. Int. Ed. 129, 10865–10869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantoliano M. W., Petrella E. C., Kwasnoski J. D., Lobanov V. S., Myslik J., Graf E., Carver T., Asel E., Springer B. A., Lane P., Salemme F. R., High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 6, 429–440 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Reinhard L., Mayerhofer H., Geerlof A., Mueller-Dieckmann J., Weiss M. S., Optimization of protein buffer cocktails using Thermofluor. Acta Crystallogr., Sect. F: Struct. Biol. Commun. 69, 209–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shelley J. C., Cholleti A., Frye L. L., Greenwood J. R., Timlin M. R., Uchimaya M., Epik: A software program for pK(a) prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 21, 681–691 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Grundner C., Perrin D., Hooft van Huijsduijnen R., Swinnen D., Gonzalez J., Gee C. L., Wells T. N., Alber T., Structural basis for selective inhibition of Mycobacterium tuberculosis protein tyrosine phosphatase PtpB. Structure 15, 499–509 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sastry G. M., Adzhigirey M., Day T., Annabhimoju R., Sherman W., Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27, 221–234 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Zhu K., Borrelli K. W., Greenwood J. R., Day T., Abel R., Farid R. S., Harder E., Docking covalent inhibitors: A parameter free approach to pose prediction and scoring. J. Chem. Inf. Model. 54, 1932–1940 (2014). [DOI] [PubMed] [Google Scholar]
- 58.F. Fuller, S. Gul, R. Chatterjee, J. Kern, V. Yachandra, J. Yano, Community Contributed Protocol Exchange (2017); doi: 10.1038/protex.2017.017. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/7/eaaw4607/DC1
Supplementary Materials and Methods
Fig. S1. Isocyanide syntheses.
Fig. S2. Reactions in destination plate I.
Fig. S3. Reactions in destination plate II.
Fig. S4. Reactions in destination plate III.
Fig. S5. Reactions in destination plate IV.
Fig. S6. Labcyte Echo plate reformat software.
Fig. S7. Heat plots with product structures, green for major product formation, yellow for medium product formation, and blue for no product formation.
Fig. S8. Stabilization effect of 18a as proof of interaction with MptpB as assessed by DSF.
Fig. S9. Binding curve of 18a to the fluorescently labeled MptpB sample as assessed by MST.
Fig. S10. Three-dimensional structure of the target phosphatase.
Fig. S11. Proposed docking model for 18a covalently bound to Cys160 (PDB ID: 2OZ5).
Fig. S12. Proposed docking model for 18a covalently bound to Ser57 (PDB ID: 2OZ5).
Fig. S13. Proposed docking model for 18a covalently bound to Thr223 (PDB ID: 2OZ5).
Fig. S14. ADE technology.
Table S1. Summary table of the docking scores for Covdock and Scorpion.
Scheme S1. Quality control results for destination plate I.
Scheme S2. Performance of formylphenyl boronic acids in destination plate I.
Scheme S3. Performance of isocyanides in GBB-3CR reaction in destination plate I.
Scheme S4. Performance of isocyanides in Ugi-based macrocycles in destination plate I.
Scheme S5. Performance of isocyanides in U-4CR in destination plate I.
Scheme S6. Performance of isocyanides in UT-4CR in destination plate I.
Scheme S7. Performance of carboxylic acids in U-4CR in destination plate I.
Scheme S8. Performance of amines in U-4CR in destination plate I.
Scheme S9. Performance of amines in UT-4CR in destination plate I.
Scheme S10. Performance of amidines in GBB-3CR reaction in destination plate I.
Scheme S11. Performance of α,ω-amino carboxylic acids in Ugi-based macrocycles in destination plate I.
Scheme S12. Quality control results for destination plate II.
Scheme S13. Performance of aminophenyl boronic acids in destination plate II.
Scheme S14. Performance of isocyanides in U-4CR in destination plate II.
Scheme S15. Performance of isocyanides in UT-4CR in destination plate II.
Scheme S16. Performance of oxo components in U-4CR in destination plate II.
Scheme S17. Performance of oxo components in UT-4CR in destination plate II.
Scheme S18. Performance of carboxylic acids in U-4CR in destination plate II.
Scheme S19. Quality control results for destination plate III.
Scheme S20. Performance of carboxyphenyl boronic acids in destination plate III.
Scheme S21. Performance of isocyanides in U-4CR in destination plate III.
Scheme S22. Performance of isocyanides in U-4CR with CH2O in destination plate III.
Scheme S23. Performance of oxo component in U-4CR in destination plate III.
Scheme S24. Performance of amines in U-4CR in destination plate III.
Scheme S25. Performance of amines in U-4CR with CH2O in destination plate III.
Scheme S26. Quality control results for destination plate IV.
Scheme S27. Performance of MCR boronic acid building blocks in destination plate IV.
Scheme S28. Performance of aryl halides in destination plate IV.


