Abstract
We hypothesize that, ancestrally, sex-specific immune modulation evolved to facilitate survival of the pregnant person in the presence of an invasive placenta and an immunologically challenging pregnancy; an idea we term the Pregnancy Compensation Hypothesis. Further, we propose sex differences in immune function are mediated, at least in part, by the evolution of gene content and dosage on the sex chromosomes, and regulated by reproductive hormones. Finally, we propose that changes in reproductive ecology in industrialized environments exacerbate these evolved sex differences resulting in the increasing risk of autoimmune disease observed in females, and a counteracting reduction in diseases, such as cancer, that can be caught by heightened immune surveillance. The Pregnancy Compensation Hypothesis generates a series of expectations that can be tested empirically and may help identify mechanisms of sex differences in modern human diseases.
Keywords: X inactivation, sex differences, placentation, autoimmunity and cancer, dosage compensation, pregnancy compensation hypothesis
Pregnancy Compensation Hypothesis: an explanation for sex differences in human disease risk
Sex differences exist across a range of human diseases, and have, to date, been understudied and largely unexplained [1]. For example, females in industrialized (see Glossary) populations exhibit a higher prevalence of most autoimmune diseases than do males (Table 1;[2]). By contrast, females have a lower risk of developing cancer, with nearly all non-reproductive cancers showing a higher incidence in males (Table 1;[3]). Here we present the Pregnancy Compensation Hypothesis (PCH, see Glossary), which explains both the proximate and ultimate (evolutionary) mechanisms responsible for sexual dimorphism observed in human disease, as mediated by selection on the immune system due to pregnancy and placentation (Figure 1 (Key Figure) and Figure 2). We propose that sex differences in diseases are a consequence of evolution shaping the human immune system differently in males and females, and this- in conjunction with changing conditions that accompanied industrialization-explains why sexual dimorphism in certain diseases is more pronounced in urban, industrialized contexts.
Table 1. The occurrence of common autoimmune diseases and cancers of non-reproductive tissues.
Disease occurrence and the sex ratio within any category vary across human populations. Here we report overall female to male (F:M) ratios in disease occurrence from the literature for immune diseases. F:M ratios of cancer types were estimated from on the numbers of new cases across all ethnicities in 2018 in the NIH SEER program statistics.
| Disease | F:M | Tissue | Reference |
|---|---|---|---|
| Immune diseases | |||
| Sjögren’s syndrome | 16:1 | Systemic | [69] |
| Hashimoto’s thyroiditis | 9:1 | Thyroid | [2] |
| Primary biliary cirrhosis | 8:1 | Bile ducts | [70] |
| Systemic lupus erythematosus | 8:1 | Systemic, connective tissue | [2] |
| Grave’s disease | 7:1 | Thyroid | [2] |
| Rheumatoid arthritis | 7:1 | Spine, joints | [2] |
| Multiple sclerosis | 3:1 | Myelin | [71] |
| Addison’s disease | 2.5:1 | Adrenal glands | [72] |
| Alzheimer’s disease | 2.34:1 | Neurons | [73] |
| Celiac disease | 1.8:1 | Small intestine | [74] |
| Myasthenia gravis | 1.5:1 | Neuromuscular receptors | [75] |
| Crohn’s disease | ~1:1 | Digestive tract | [2] |
| Guillain-Barre syndrome | ~1:1 | Myelin | [2] |
| Psoriasis | ~1:1 | Skin | [76] |
| Psoriatic arthritis | ~1:1 | Joints | [77] |
| Type 1 diabetes | ~1:1 | Pancreas | [78] |
| Ulcerative colitis | ~1:1 | Digestive tract | [2] |
| Ankylosing spondylitis | 0.8:1 | Spine, joints | [79] |
| Cancers | |||
| Thyroid cancer | 2.9:1 | Thyroid | NIH SEER 2018 |
| Colorectal cancer | 1:1.3 | Colon, rectum | NIH SEER 2018 |
| Lung and bronchus cancer | 1:1.3 | Lung, bronchus | NIH SEER 2018 |
| Myeloma | 1:1.6 | Plasma cells | NIH SEER 2018 |
| Melanoma | 1:1.7 | Melanocytes | NIH SEER 2018 |
| Kidney and renal pelvis cancer | 1:2 | Kidney | NIH SEER 2018 |
| Liver and intrahepatic bile duct cancer | 1:2.9 | Liver | NIH SEER 2018 |
| Bladder cancer | 1:4.1 | Bladder | NIH SEER 2018 |
| Esophageal cancer | 1:4.2 | Esophagus | NIH SEER 2018 |
Figure 1. Key Figure. Pregnancy Compensation Hypothesis (PCH): Pregnancy, Pathogens, and Parity.
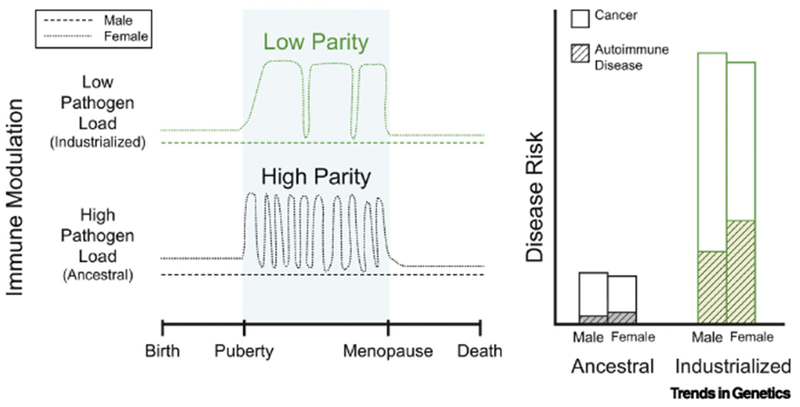
Here we lay out the expected immune differences to explain the differences we expect between high and low parity (layered on top of high/low pathogen load). In particular, we think that low pathogen load (Hygiene hypothesis) will affect immune function in both men and women (green lines in attached figure), making everyone more susceptible to more autoimmune disease, while low parity, will only affect the immune system in women, exacerbating the immune compensation that evolved in response to tolerating an internal pregnancy, further increasing the immune risk for women in industrialized regions.
Figure 2. Explanations for sex differences in autoimmune disease and cancer.
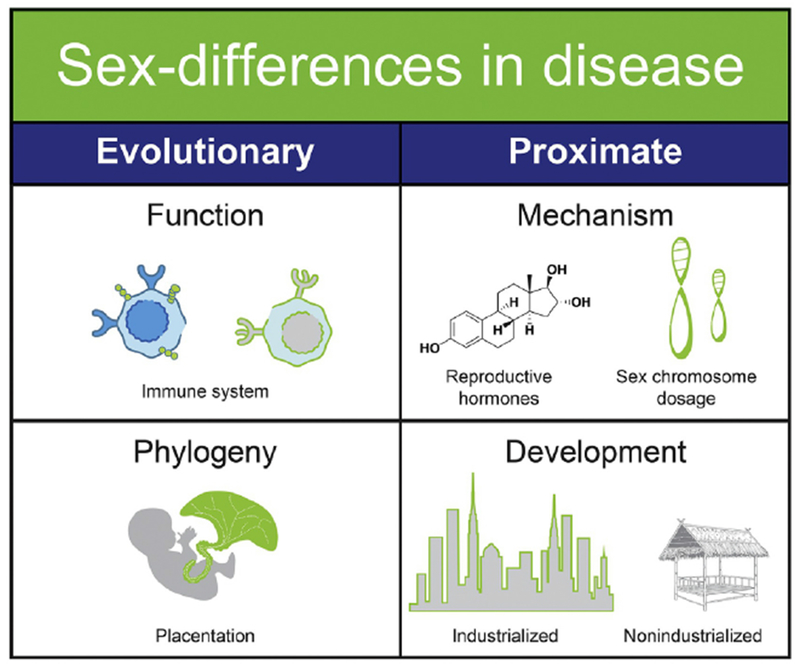
There are longer-term evolutionary explanations and the more immediate proximate explanations for sexual dimorphism in immune function. The female immune system must compensate for unique DNA from the placenta and pregnancy. The placenta is phylogenetically shared, but with additional unique evolutionary pressures including invasive hemochorial placentas in eutherian mammals. Dimorphism in immune function in response to placentation and pregnancy occurs via the direct impact of reproductive hormones on immune function, and through heritable variation in sex chromosome dosage. While evolution has shaped these sex differences over millions of years, industrialized urban populations experience exacerbated sex differences in the hormonal mechanisms as well as reduced pregnancies compared to non-industrialized populations.
Specifically, under the PCH, we propose that the evolution of eutherian (see Glossary) placentation exerted significant sex-specific selection on immune function to tolerate fetal antigens while still defending the pregnant individual against parasites and pathogens [4]. We theorize that this process is regulated proximately via hormones and mediated genetically by dosage on the sex chromosomes, and that today, the mismatch between an ancestral environment (being pregnant or lactating for the majority of adult reproductive years) and urban industrial environment (where common contraceptive use results in reduced pregnancies) interacts with this evolved compensatory immune regulation and results in the observed sex differences in disease risk (Figure 2). Finally, a sedentary lifestyle that affects reproductive hormone levels exacerbates these differences.
In this paper, as an example set, we focus on two disease classes with documented immune components that also show sex differences in incidence and etiology: autoimmune diseases and cancer. We anticipate that the sex differences in immune function will, to some extent, contribute to sex differences in all human diseases. However, our rationale for focusing on these particular classes of disease for this paper is two-fold: first, our hypothesis intersects factors related to shifting reproductive states, parasite loads, and energetic availability, which are particularly relevant for these disease classes; and second, we believe it is important to narrow the scope, to effectively use empirical studies to illustrate evidence for specific aspects of our hypothesis. Below, we will unpack the interrelated components of the PCH, and how each relates to either proximate or evolutionary underpinnings of differences in disease risk (Figure 2). We will, for the purposes of this manuscript, refer to karyotypic (see Glossary) males and females, both across species and when discussing humans unless otherwise noted. Although not often explicitly stated in the manuscripts, where possible, we will emphasize differences between genetic sex, gonadal/reproductive hormones, and gender in the research discussed here.
There are sex differences in autoimmune disease and cancer incidence
Sexual dimorphism in immune function appears as a general feature of many species, with differences documented across vertebrates and invertebrates, though at varying magnitudes[5]. Across a number of vertebrate species, there is evidence of female bias in the peripheral abundance of markers of innate and adaptive immunity [5,6]. Autoimmunity is characterized by the presence of an increased level of autoantibodies, inflammatory and mediatory cells, resulting in chronic inflammation [7] that affects females more than males (Table 1). In mammals in particular, both sex chromosome complement, as well as hormone levels have been implicated in the female bias in autoimmune disease prevalence, including in systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes [8]. In contrast, females have a reduced rate of nearly all non-reproductive tissue cancers relative to males (Table 1). Sex differences in environmental exposure alone do not explain the sex differences in cancer [3]. Further, there are sex differences in response to cancer immunotherapy [9], suggesting that perhaps the sex differences in immune function that underlie autoimmune disease may also contribute to the sex difference in cancer incidence and response to therapy. Consistent with this, clinical and epidemiological studies have demonstrated more robust innate and adaptive immune responses in females compared to males in humans; females exhibit better outcomes and survival from infections, injuries, and sepsis [10,11]. Females also develop higher antibody responses to vaccinations than males [12].
Industrialization explains increases in autoimmunity and cancer, but not sex differences.
While most research on cancer and autoimmunity are conducted in urban populations, most of human evolution occurred in small-scale subsistence populations; sedentary industrialized life has resulted in major changes to human reproductive ecology and lifetime fertility[13]. There is a documented increase in the incidence of autoimmune disorders over the last several decades in urban and industrialized populations [14]. The “old friends” or Hygiene Hypothesis (see Glossary) suggests that because humans co-evolved with helminths and other pathogens, the human immune system has an ‘evolved dependency’ on parasites, and is specifically tailored to expect these infections for effective immunomodulation [15,16][17,18]. The absence of these parasites and pathogens is cited as an explanation for the increasing rates of asthma and immune-related diseases in industrialized populations, but cannot explain why there is sexual dimorphism in disease risk.
Curiously, the shift in the immunological profile that occurs during pregnancy is not unlike that which occurs as a result of chronic infection by a helminthic parasite, like the hookworm. While no single extant nonindustrialized (see Glossary) population is an exemplar of the mosaic of environments in which humans evolved, evidence from existing hunter-gatherer and forager-horticultural populations suggest that most of the female reproductive career throughout human history was spent either pregnant or lactating [19,20]. This sustained reproductive state would have led to chronic readjustments of components of the maternal immune system. For the pregnant person, maintaining a pathogen competent immune system while regulating the immune response to an invasive pregnancy (which may be considered ‘foreign’ to the maternal immune system) is a balancing act involving numerous immunoregulatory mechanisms driven by both maternal and fetal signals. To accommodate these dual needs, the maternal immune system is not uniformly down-regulated during pregnancy. Rather it is differentially modulated throughout the gestational period. Described as ‘maternal-fetal immune tolerance’, the maternal immune system is primed to up-regulate specific immunological pathways to maintain a sufficient immune response to survive pathogens and parasites, while also down-regulating other pathways to tolerate the genetically-distinct pregnancy [21].
The Pregnancy Compensation Hypothesis proposes that the genetically distinct placenta and fetus exert pressure on the maternal immune system that modulates the ‘host’ immune response, protecting itself from elimination and minimizing damage to the host environment (Figure 1). In both circumstances, the immune response shifts towards an anti-inflammatory bias [22,23]. However, in contrast with the Hygiene Hypothesis, the Pregnancy Compensation Hypothesis postulates that the maternal immune system has to compensate to tolerate the pregnancy, while still protecting the maternal body against parasites and pathogens. The PCH, therefore, predicts sex-specific immune modulation.
In urban industrialized contexts, pregnancy compensation explains dimorphism in disease risk
Despite the caloric availability to maintain a high number of pregnancies, fertility in modern urban environments has decreased [19]. In the contemporary industrialized context, the absence of repeat pregnancies may leave the immune system of an urban industrial female prone to dysregulation. Given the role of the immune system in surveying and preventing cancer [24,25], this “un-dampened” immune response should facilitate increased immune surveillance, which may be protective against some cancers in females; however, it may also lead to correspondent triggering of autoimmune diseases. Further, energetic constraints that limited reproduction throughout human evolutionary history, including reduced caloric availability and high immune burden, no longer limit investment in reproductive hormone levels. As a result, people in industrialized populations can attain high, if not evolutionarily novel, levels of testosterone, estrogens, and progesterone compared to extant subsistence populations [26–29]. These high hormone levels increase the prevalence of reproductive-related conditions including benign prostatic hyperplasia and prostate cancer [29,30], as well as breast and endometrial cancers [13]. Under the Pregnancy Compensation Hypothesis, these high hormone levels in industrialized populations are expected to heighten sex differences in non-reproductive cancers and contribute to the increased prevalence of autoimmune disease. Consistent with this, both the incidence and prevalence of autoimmune diseases are increasing [31]. The diminished immunomodulation consequent from reduced pregnancy and lactation further leaves the immune system primed for over-activation.
Pregnancy, and a related hormonal shift, are directly linked with changes in the maternal immune system and incidence/onset of autoimmune disease
The maternal immune system is alternately dampened and recalibrated throughout gestation and lactation to allow for a successful pregnancy. Such immuno-modulation is facilitated by the highly invasive nature of the eutherian placenta, composed of primarily fetal tissue, and is accomplished through coordinated signaling between the fetus and the mother. Early implantation by the fetal trophoblast is facilitated by innate inflammatory processes at the maternal-fetal interface [52], followed by a progressive shift in maternal immunity from a pro-inflammatory bias towards a systemic anti-inflammatory phenotype during the last two trimesters of pregnancy [32]. These anti-inflammatory processes, facilitated by actions by the fetal-unit, modulate the maternal immune response, decreasing some aspects of both humoral and cell-mediated immunity, while increasing levels of regulatory immune cells, which foster a stable tolerant immune profile (reviewed by[33])[34][35]. As the fetus develops, tolerance is further established through biosynthesis of the maternal hormone estriol, and it’s positive effect the continued production of regulatory immunity. This ‘tit-for-tat’ maternal-fetal conflict would have imposed additional selective pressures for a highly plastic immune response at different periods over the life course (e.g. pregnant versus cycling females) that is distinct from males, and is manifest in distinct immune regulation in males, cycling females, and pregnant females (Figure 1; Table 2).
Table 2. Immunological differences between human males, and cycling females, and pregnant females.
Markers of immune function show differences in overall levels in gonadal males and females. Notably, however, there are many immunological differences between gonadal females who are cycling versus those who are pregnant. Here we catalog a variety of cell types and reported results of differences in each of these cell types between healthy human males, cycling females, and pregnant females.
| Main Cell Types | Finding | Direction | Study |
|---|---|---|---|
| Eosinophils; lymphocytes; monocytes | Cycling females have higher levels of all cells compared to males. | F > M | [5] |
| CD4+ cells | Cycling females have higher levels of CD4+ than males | F > M | [6] |
| CRP | Females have generally higher levels than males | F > M | [80] |
| TLR pathways; TLR6; Dendritic Cells; B-cells; Immunoglobulins | Cycling females have higher levels than men | F > M | [8] |
| Tregs; NK cells; CD8+ cells | Males have higher levels than cycling females | M > F | [8] |
| Macrophage; monocytes; dendritic cells | Females have higher expression than males | F > M | [81] |
| NK cells | Males have higher levels than females | M > F | [82] |
| Tregs | Compared with cycling females, pregnant females have higher levels of Tregs | PF > CF | [6] |
| Eosinophils | Cycling females have higher levels of eosinophils than pregnant females | CF > PF | [83] |
| Neutrophils; Lymphocytes | Pregnant females have higher levels of neutrophils and lymphocytes than cycling | PF > CF | [83] |
| TNF-alpha; IL-6; IL-2 | Compared with cycling females, pregnant females have higher levels of IL-2, IL-6, TNF-alpha | PF > CF | [84] |
Consistent with the Pregnancy Compensation Hypothesis, estrogen metabolites and their receptors also have distinct effects on immune function in relation to autoimmunity and cancer. Importantly, estriol—which is placentally-derived and produced almost exclusively during pregnancy— may have a role in generating immune tolerance during pregnancy through the production of Tregs [36]. Estriol and the presence of its preferred receptor, ERβ, has been found to ameliorate a number of autoimmune diseases in general [37–39], and is considered to be protective against cancer[40].
In particular, an estriol spike during pregnancy has also been found to ameliorate a number of autoimmune diseases throughout gestation [37–39]. Notably, in patients with rheumatoid arthritis who become pregnant, 75% of pregnancies are characterized by an improvement in symptoms, particularly during the second and the third trimesters [41]. Similarly, multiple sclerosis relapse rates decline during pregnancy from 0.7 relapses per person per year prepregnancy to 0.2 relapses per person per year during the third trimester of pregnancy [42]. In contrast to rheumatoid arthritis and multiple sclerosis, which are mainly mediated by T-cell activation [43], systemic lupus erythematosus is associated with more complex and heterogeneous immunological abnormalities. Curiously, patients with systemic lupus erythematosus experience typically exacerbation or no changes in symptoms during pregnancy [44,45]. Under the Pregnancy Compensation Hypothesis we predict that these hormonal regulators of immune function during pregnancy are encoded by genetic pathways that evolved in response to placentation, starting millions of years ago.
The evolution of the mammalian sex chromosomes and placentation lead to differences in immune function for males and females
Although eutherian mammals are commonly called placental mammals to contrast them with marsupials, it is now understood that the development of a placenta, if even for a short time, occurs in marsupial mammals and is important for successful pregnancy [46]. Thus, while all mammals share lactation as a trait [47], placentation evolved in the common ancestor of therian mammals, after they diverged from the egg-laying monotreme mammals (Figure 4). Further, phylogenetic comparisons have been used to suggest that the early eutherian placenta was highly invasive (hemochorial), and critical for sustaining a pregnancy, initially exerting a huge selective pressure for immune compensation at the offset, and only later placentas diverged in invasiveness across individual eutherian clades [48].
Figure 4. Evolution of placentation and X chromosome.
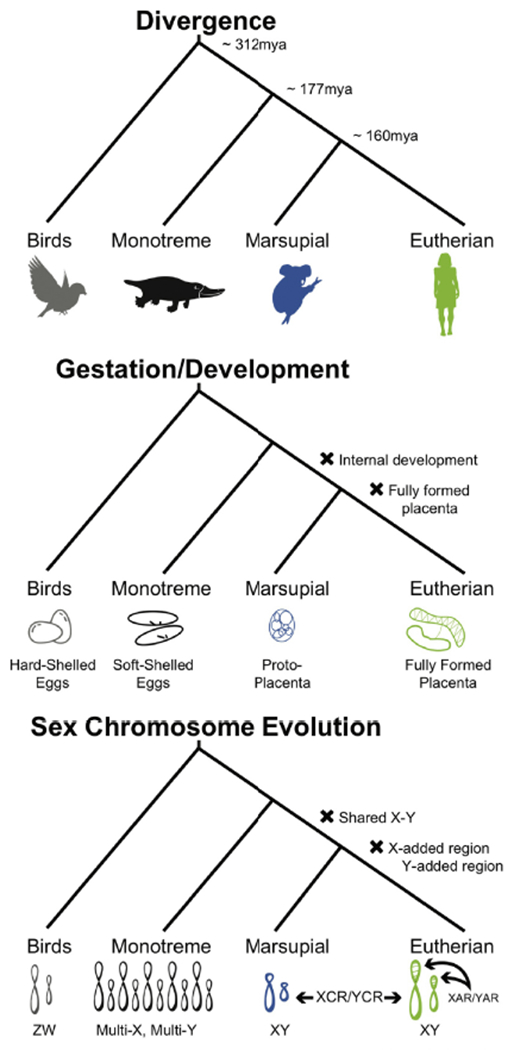
We observe that the evolution of placentation and lactation in mammals correlates with the evolution of different regions of the mammalian X chromosome. Notably, there is a shift the mechanism of dosage compensation and structure of the mammalian X chromosomes that correlates with the evolution of fully formed placentas.
We propose that the evolution of mammalian sex chromosome is one genetic alteration that could have been involved in the physiological shift of placentation that prompted significant sex-specific selection on immune function, and set the scene for future dimorphism in disease risk. Human sex chromosomes originated from a pair of autosomes in the common ancestor or marsupial and eutherian mammals [49], concurrently with the initial evolution of the placenta in the therian common ancestor (Figure 4). Moreover, at the same time as the second major shift in placenta physiology occurred in the common ancestor of eutherian mammals, the eutherian X and Y chromosomes experienced the addition of an X-added and Y-added region that comprises approximately 30% of the modern human sex chromosomes ([50]; Figure 4).
Most Y-linked genes have been pseudogenized or deleted[51], resulting in unequal gene content between the X and Y, and a potential mechanism for at least some sex-specific gene regulation. To balance the dosage of X-linked genes between males and females, one of the X-copies in females is largely epigenetically silenced during embryogenesis, a process termed X-inactivation (see Glossary), [52]). Interestingly, the additions to the sex chromosomes that coincide with invasive placentation also correspond with an apparent shift in the mechanism of dosage compensation, from paternally-silenced X-inactivation in marsupials to random X-inactivation, including cell-by-cell heterogeneity and gene-specific silencing, observed in eutherian mammals, specifically humans [53,54]. In humans, notably, dosage compensation is incomplete[55]; as many as 30% of the genes on the inactive X are known to escape inactivation [54], or may never have been silenced. While some “escapees” show uniformity, most of these genes exhibit heterogeneity in escape from X-inactivation between individuals, tissues, and cells, and may contribute to heterogeneity in disease phenotypes ([53,54]; Figure 3). We propose that the addition of these genes to the sex chromosomes, and evolution of a new dosage compensation mechanism, is one mechanism to achieve sex-specific immuno-modulation in response to invasive placentation.
Figure 3. Inactivation status of X-chromosomal genes.
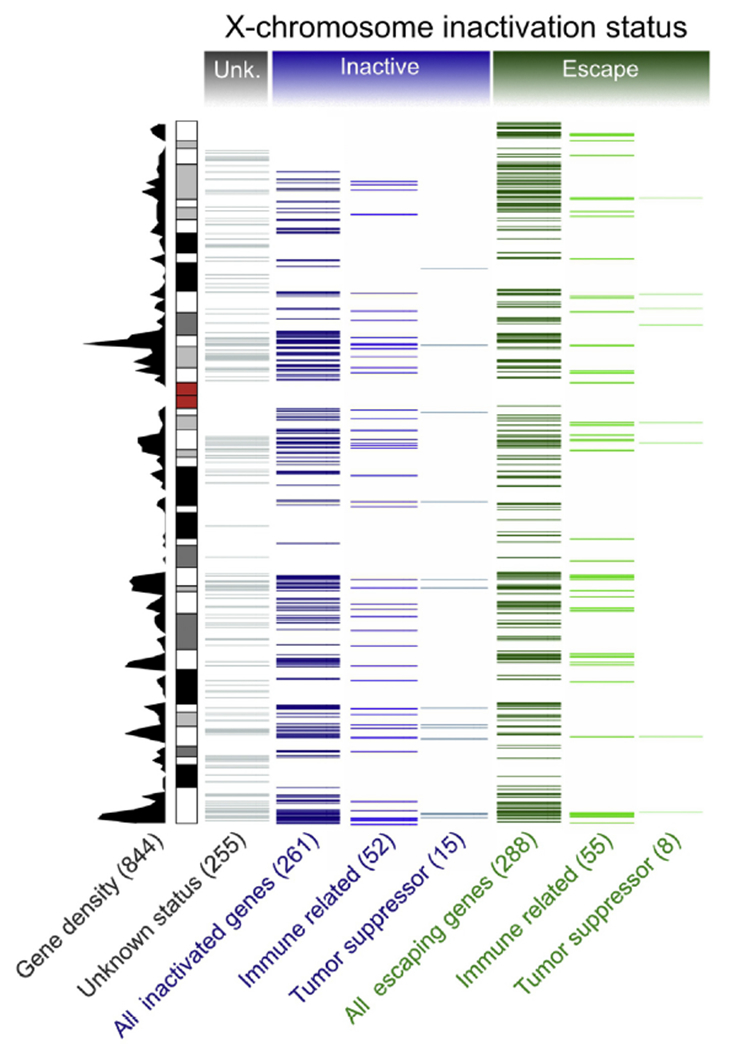
All protein-coding X-linked genes (Gencode release 29) are presented as a density plot, and the inactivation status, as reported in recent studies [53,54], is summarized. Genes with unknown status (Unk.) are shown in grey. Genes reported to be subject to X-inactivation in all tissues and cell lines surveyed are labeled as inactive (blue). Genes found to escape X-inactivation in any of the individuals, tissues, or cell lines in any individual are labeled escaping (green). Numbers of genes belonging to each category are shown in parenthesis. The information on putative tumor suppressor genes was obtained from the Tumor Suppressor Gene database [67]. Immune-related genes contain genes that were obtained from ImmPort [68] or belong to the Gene Ontology class “immune response”.
Gene dosage on the X chromosome has a compounding effect on immunity, in combination with reproductive hormones (see Glossary), as illustrated by people with Klinefelter syndrome and Turner syndrome, both of which are characterized by numeric and structural variations of the X chromosome. Klinefelter syndrome occurs in males who carry two X-copies (47,XXY). Klinefelter males have higher immunoglobulin concentrations and adaptive immune cell levels compared with XY males [56], and are at an increased risk of developing autoimmune diseases, particularly systemic lupus erythematosus than XY males [57,58]. In contrast, Turner syndrome is characterized by a single X chromosome in affected females (45,X0), sometimes with a partial X or Y. Female patients with Turner syndrome have lower immunoglobulin concentrations and adaptive immune cell levels compared to XX females [59]. Turner syndrome is associated with T-cell immune alterations, suggestive of a possible immune deficiency [60]. Interestingly, while females with Turner syndrome have higher reported rates of autoimmune disease than XX females, they have particularly higher rates of autoimmune diseases typically characterized by male-predominance [61]. Hormone therapy may reverse some of the immunological effects of Klinefelter [56] and Turner syndrome [62], elucidating the complex interactions of endocrinological functions and the X-chromosome in regulating immunity.
Concluding Remarks: In urban industrialized environments there is an imbalance between the pressures of parasites and pregnancy that exacerbates sexual dimorphism in immune function
Here we present the Pregnancy Compensation Hypothesis to explain how evolution may have shaped gene dosage on the X chromosome and reproductive hormones in response to placentation and pregnancy to mechanistically explain sex differences in disease as an ancestral mismatch with modern environments. There are both general trends and specific exceptions in sex differences in disease that can be explained by the Pregnancy Compensation Hypothesis. For example, it is notable that a key exception to the reduced female incidence in cancers is thyroid cancer [63]. This is provocative because the thyroid is involved in reproduction and critical during pregnancy [64]. The thyroid increases in size during pregnancy and plays a critical role in iodine sufficiency, which is required to pass through the placenta during pregnancy [65]. Of concern and relevance here is that papillary thyroid cancer is increasing in incidence in females across all race/ethnic groups in industrialized populations, and cannot be explained by increases in surveillance alone [66]. However, under the Pregnancy Compensation hypothesis, one would expect the thyroid to be primed to be active for a woman’s reproductive career. In industrialized populations and the absence of routine pregnancy, the thyroid may be primed for constant usage and cell proliferation but experience underutilization, resulting in increased susceptibility to thyroid cancer in females but not in males.
Under the synthesis presented here, a suite of new questions and testable hypotheses emerge (Outstanding Questions) that we anticipate will push both evolutionary and clinical research forward. We propose that there is a combinatorial effect of the way evolution has differentially shaped immune function between males and females, the reduced amount of time spent pregnant and lactating, and the environmental effects on hormone levels lead to the observed sexual dimorphism in disease risk in urban, industrialized societies (Figure 5). We further propose that the evolution of gene dosage on the human sex chromosomes, and primarily on the X chromosome, is the heritable mechanism by which the maternal immune system ancestrally compensated to tolerate placentation, and now may provide the explanation for the sexual dimorphism in autoimmune disease and cancer in industrialized populations.
Figure 5. Comparison of environmental differences between humans living in active subsistence populations versus sedentary industrial populations.
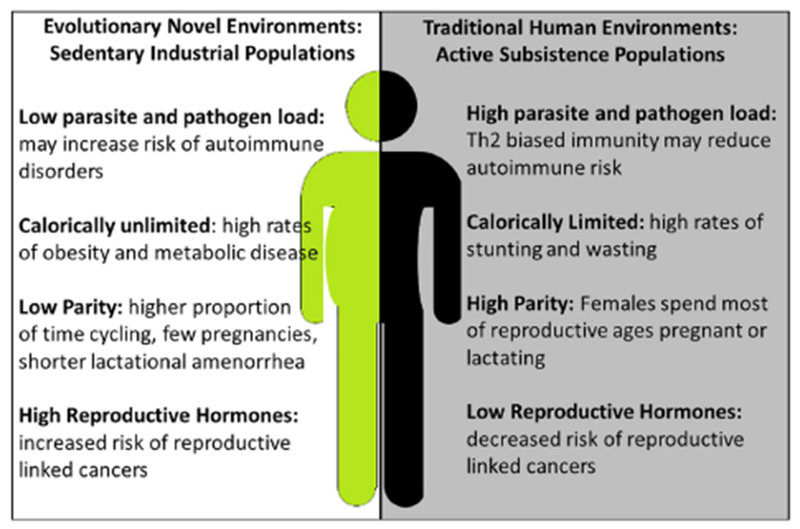
While humans evolved in a mosaic of different environments, sedentary industrialized urban environments are evolutionarily novel. We predict that these shifts in the environment may contribute to mismatches between how our systems (e.g., the immune system) have been shaped by natural selection to respond to the environment and how they are now responding, resulting in human disease.
Highlights.
There are major sex differences in human disease that cannot be explained by reproductive hormones or environmental exposures alone.
Genes on the sex chromosomes exhibit differences in expression independent of reproductive hormones that could contribute to sex differences in disease.
We propose that the ancestral immune system was strongly shaped by the requirement to compensate for unique immune regulation due to pregnancy.
Dimorphism in immune function in response to placentation and pregnancy occurs via direct impact of reproductive hormones on immune function, and through heritable variation in sex chromosome dosage.
While evolution has shaped sex differences in immune function over millions of years, industrialized urban populations experience exacerbated sex differences in hormonal composition as well as reduced pregnancies compared to non-industrialized populations.
Acknowledgments
This study was supported by ASU Center for Evolution and Medicine postdoctoral fellowship for HMN and ARG, the Marcia and Frank Carlucci Charitable Foundation postdoctoral award from the Prevent Cancer Foundation for HMN, startup funds from the School of Life Sciences and the Biodesign Institute at Arizona State University to MAW, partial support from NIH/NIA RF1AG054442-01 for BCT. This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM124827 to MAW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- Eutherian mammal
A classification of mammals that include those that have fully-formed placentas, often referred to as “placental” mammals.
- Hygiene hypothesis
This decreasing incidence of infections in western countries is at the origin of the increasing incidence of both autoimmune and allergic diseases, due to insufficient immunomodulation caused by the lack of early exposure to parasites and pathogens.
- Industrialized population
Middle to high development index countries with labor- and/or technology-driven economy that enables mass food production, with a high capacity for the division of labor, and advanced infrastructure, sanitation systems, and access to national or global economies. These populations tend to be more sedentary, have access to modern medical care and vaccines, low parasite and pathogen loads, and have a higher-calorie diet, rich with high-carbohydrate, low-protein, and processed foods.
- Karyotypic
A karyotype is a characterization of the chromosomal complement and structure of an individual. We use karyotypic to refer to the typical karyotype observed in genetic females (46, XX) and genetic males (46, XY).
- Nonindustrialized population
Low development index populations that rely on traditional subsistence activities or a mix of subsistence and wage-labor to acquire food and material goods, and where there tends to be less infrastructure for basic sanitation (leading to a higher prevalence of parasites and pathogens), and little access to medical care. Individuals in nonindustrial populations tend to lead more active lifestyles, necessitated by subsistence activities, tend to have lower-calorie diets with little or no industrial-processed foods.
- Pregnancy Compensation Hypothesis
The maternal immune system is evolved to compensate to both tolerate the pregnancy, and yet still protect the maternal body against parasites and pathogens, and the mismatch with industrialized environments results in an increased incidence of autoimmune disorders and decreased risk of cancer in females relative to males.
- Reproductive hormones
Hormones typically produced in the reproductive organs: ovaries (e.g. estrogens and progesterone) and testes (e.g. testosterone). Reproductive hormones are known to have impacts on immune function and contribute to the development of reproductive cancers.
- X-inactivation
The process by which one of the two X chromosomes (or indeed every X chromosome in excess of one) is inactivated and most of the genes on that X chromosome are silenced.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Khramtsova EA et al. (2019) The role of sex in the genomics of human complex traits. Nat. Rev. Genet 20, 173–190 [DOI] [PubMed] [Google Scholar]
- 2.Ngo ST et al. (2014) Gender differences in autoimmune disease. Front. Neuroendocrinol 35, 347–369 [DOI] [PubMed] [Google Scholar]
- 3.Dorak MT and Karpuzoglu E (2012) Gender differences in cancer susceptibility: an inadequately addressed issue. Front. Genet 3, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gobert M and Lafaille JJ (2012) Maternal-fetal immune tolerance, block by block. Cell 150, 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunn CL et al. (2009) On sexual dimorphism in immune function. Philos. Trans. R. Soc. Lond. B Biol. Sci 364, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fish EN (2008) The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol 8, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos PS et al. (2015) Genetics of autoimmune diseases: insights from population genetics. J. Hum. Genet 60, 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein SL and Flanagan KL (2016) Sex differences in immune responses. Nat. Rev. Immunol 16, 626–638 [DOI] [PubMed] [Google Scholar]
- 9.Carrera C et al. (2018) Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 19, e375. [DOI] [PubMed] [Google Scholar]
- 10.Choudhry MA et al. (2006) Gender and susceptibility to sepsis following trauma. Endocr. Metab. Immune Disord. Drug Targets 6, 127–135 [DOI] [PubMed] [Google Scholar]
- 11.Gannon CJ et al. (2004) Male gender is associated with increased risk for postinjury pneumonia. Shock 21, 410–414 [DOI] [PubMed] [Google Scholar]
- 12.Klein SL et al. (2015) Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg 109, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasienska G et al. (2017) Human reproduction and health: an evolutionary perspective. Lancet 390, 510–520 [DOI] [PubMed] [Google Scholar]
- 14.Bach J-F (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med 347, 911–920 [DOI] [PubMed] [Google Scholar]
- 15.Rook GAW (2012) Hygiene hypothesis and autoimmune diseases. Clin. Rev. Allergy Immunol 42, 5–15 [DOI] [PubMed] [Google Scholar]
- 16.Gurven MD et al. (2016) Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evol Med Public Health DOI: 10.1093/emph/eow028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill K (2017) Ache Life History, The Ecology and Demography of a Foraging People Routledge; (2017). [Google Scholar]
- 18.Bennett FJ et al. (1970) Helminth and protozoal parasites of the Hadza of Tanzania. Trans. R. Soc. Trop. Med. Hyg 64, 857–880 [DOI] [PubMed] [Google Scholar]
- 19.Mace R (2008) Reproducing in Cities. Science 319, 764–766 [DOI] [PubMed] [Google Scholar]
- 20.Strassmann BI (1997) The Biology of Menstruation in Homo Sapiens: Total Lifetime Menses, Fecundity, and Nonsynchrony in a Natural-Fertility Population. Curr. Anthropol 38, 123–129 [Google Scholar]
- 21.Williams Z (2012) Inducing tolerance to pregnancy. N. Engl. J. Med 367, 1159–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson DP and Klein SL (2012) Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav 62, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotez PJ et al. (2008) Helminth infections: the great neglected tropical diseases. J. Clin. Invest 118, 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palucka AK and Coussens LM (2016) The Basis of Oncoimmunology. Cell 164, 1233–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison PT (2002) Population variation in age-related decline in male salivary testosterone. Hum. Reprod 17, 3251–3253 [DOI] [PubMed] [Google Scholar]
- 27.Núñez-de la Mora A et al. (2007) Childhood conditions influence adult progesterone levels. PLoS Med. 4, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor KA et al. (2003) Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin. Chem 49, 1139–1148 [DOI] [PubMed] [Google Scholar]
- 29.Trumble BC et al. (2015) Challenging the Inevitability of Prostate Enlargement: Low Levels of Benign Prostatic Hyperplasia Among Tsimane Forager-Horticulturalists. J. Gerontol. A Biol. Sci. Med. Sci 70, 1262–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calistro Alvarado L and Alvarado LC (2010) Population differences in the testosterone levels of young men are associated with prostate cancer disparities in older men. Am. J. Hum. Biol 22, 449–455 [DOI] [PubMed] [Google Scholar]
- 31.Lerner A et al. (2015) The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis 3, 151–155 [Google Scholar]
- 32.Graham C et al. (2017) In vivo immune signatures of healthy human pregnancy: Inherently inflammatory or anti-inflammatory? PLoS One 12, e0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warning JC et al. (2011) A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 141, 715–724 [DOI] [PubMed] [Google Scholar]
- 34.Guzman-Genuino RM and Diener KR (2017) Regulatory B Cells in Pregnancy: Lessons from Autoimmunity, Graft Tolerance, and Cancer. Front. Immunol 8, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Rocca C et al. (2014) The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett 162, 41–48 [DOI] [PubMed] [Google Scholar]
- 36.Thomas MP and Potter BVL (2013) The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol 137, 27–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palaszynski KM et al. (2004) Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol 149, 84–89 [DOI] [PubMed] [Google Scholar]
- 38.Papenfuss TL et al. (2011) Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol 186, 3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sicotte NL et al. (2002) Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol 52, 421–428 [DOI] [PubMed] [Google Scholar]
- 40.Skov BG et al. (2008) Oestrogen receptor β over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 59, 88–94 [DOI] [PubMed] [Google Scholar]
- 41.Hazes JMW et al. (2011) Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology 50, 1955–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Confavreux C et al. (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med 339, 285–291 [DOI] [PubMed] [Google Scholar]
- 43.Cope AP et al. (2007) The central role of T cells in rheumatoid arthritis. Clin. Exp. Rheumatol 25, S4–11 [PubMed] [Google Scholar]
- 44.Petri M et al. (1991) Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 34, 1538–1545 [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Irastorza G et al. (1996) Increased rate of lupus flare during pregnancy and the puerperium: a prospective study of 78 pregnancies. Br. J. Rheumatol 35, 133–138 [DOI] [PubMed] [Google Scholar]
- 46.Renfree MB (2010) Review: Marsupials: placental mammals with a difference. Placenta 31 Suppl, S21–6 [DOI] [PubMed] [Google Scholar]
- 47.Power ML and Schulkin J (2013) Maternal regulation of offspring development in mammals is an ancient adaptation tied to lactation. Appl Transl Genom 2, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildman DE et al. (2006) Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl. Acad. Sci. U. S. A 103, 3203–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbott JK et al. (2017) Sex chromosome evolution: historical insights and future perspectives. Proc. Biol. Sci, 284 (2017), Article 20162806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross MT et al. (2005) The DNA sequence of the human X chromosome. Nature 434, 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson Sayres MA and Makova KD (2012) Gene Survival and Death on the Human Y Chromosome. Mol. Biol. Evol 30, 781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payer B and Lee JT (2008) X chromosome dosage compensation: how mammals keep the balance. Annu. Rev. Genet 42, 733–772 [DOI] [PubMed] [Google Scholar]
- 53.Balaton BP et al. (2015) Derivation of consensus inactivation status forX-linked genes from genome-wide studies. Biol. Sex Differ 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tukiainen T et al. (2017) Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrel L and Willard HF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 [DOI] [PubMed] [Google Scholar]
- 56.Koçar IH et al. (2000) The effect of testosterone replacement treatment on immunological features of patients with Klinefelter’s syndrome. Clin. Exp. Immunol 121, 448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawalha AH et al. (2009) Autoimmunity and Klinefelter’s syndrome: when men have two X chromosomes. J. Autoimmun 33, 31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seminog OO et al. (2015) Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 48, 125–128 [DOI] [PubMed] [Google Scholar]
- 59.Bianchi I et al. (2012) The X chromosome and immune associated genes. J. Autoimmun 38, J187–92 [DOI] [PubMed] [Google Scholar]
- 60.Thrasher BJ et al. (2016) Epigenetic Dysfunction in Turner Syndrome Immune Cells. Curr. Allergy Asthma Rep 16, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jørgensen KT et al. (2010) Autoimmune diseases in women with Turner’s syndrome. Arthritis Rheum. 62, 658–666 [DOI] [PubMed] [Google Scholar]
- 62.Rongen-Westerlaken C et al. (1991) Immunologic studies in Turner syndrome before and during treatment with growth hormone. J. Pediatr 119, 268–272 [DOI] [PubMed] [Google Scholar]
- 63.Yao R et al. (2011) Gender differences in thyroid cancer: a critical review. Expert Rev. Endocrinol. Metab 6, 215–243 [DOI] [PubMed] [Google Scholar]
- 64.Krassas GE et al. (2010) Thyroid function and human reproductive health. Endocr. Rev 31, 702–755 [DOI] [PubMed] [Google Scholar]
- 65.Burrow GN et al. (1994) Maternal and fetal thyroid function. N. Engl. J. Med 331, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 66.Petrick JL et al. (2016) International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. International journal of cancer 139, 1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao M et al. (2016) TSGene 2.0: an updated literature-based knowledgebase for tumor suppressor genes. Nucleic Acids Res. 44, D1023–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharya S et al. (2018) ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci Data 5, 180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandt JE et al. (2015) Sex differences in Sjögren’s syndrome: a comprehensive review of immune mechanisms. Biol. Sex Differ 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boonstra K et al. (2012) Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J. Hepatol 56, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 71.Harbo HF et al. (2013) Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord 6, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitchell AL and Pearce SHS (2012) Autoimmune Addison disease: pathophysiology and genetic complexity. Nat. Rev. Endocrinol 8, 306–316 [DOI] [PubMed] [Google Scholar]
- 73.Mouton A et al. (2018) Sex ratio in dementia with Lewy bodies balanced between Alzheimer’s disease and Parkinson’s disease dementia: a cross-sectional study. Alzheimers. Res. Ther 10, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Megiorni F et al. (2008) HLA-DQ and susceptibility to celiac disease: evidence for gender differences and parent-of-origin effects. Am. J. Gastroenterol 103, 997–1003 [DOI] [PubMed] [Google Scholar]
- 75.Abukhalil F et al. (2015) Gender and Ethnicity Based Differences in Clinical and Laboratory Features of Myasthenia Gravis. Autoimmune Dis. 2015, 197893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hägg D et al. (2017) Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am. J. Clin. Dermatol 18, 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scotti L et al. (2018) Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum 48, 28–34 [DOI] [PubMed] [Google Scholar]
- 78.Soltesz G et al. (2007) Worldwide childhood type 1 diabetes incidence-what can we learn from epidemiology? Pediatr. Diabetes 8 Suppl 6, 6–14 [DOI] [PubMed] [Google Scholar]
- 79.Haroon NN et al. (2014) Increasing proportion of female patients with ankylosing spondylitis: a population-based study of trends in the incidence and prevalence of AS. BMJ Open 4, e006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sørensen CJ et al. (2014) Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLoS One 9, e88196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melgert BN et al. (2010) Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol 42, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yovel G et al. (2001) The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol. Oncol 81, 254–262 [DOI] [PubMed] [Google Scholar]
- 83.Pitkin RM and Witte DL (1979) Platelet and leukocyte counts in pregnancy. JAMA 242, 2696–2698 [PubMed] [Google Scholar]
- 84.Christian LM and Porter K (2014) Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine 70, 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]


