Abstract
Caspases are an evolutionary conserved family of cysteine proteases that are centrally involved in cell death and inflammation responses. A wealth of foundational insight into the molecular mechanisms that control caspase activation has emerged in recent years. Important advancements include the identification of additional inflammasome platforms and pathways that regulate activation of inflammatory caspases; the discovery of gasdermin D as the effector of pyroptosis and interleukin (IL)-1 and IL-18 secretion; and the existence of substantial crosstalk between inflammatory and apoptotic initiator caspases. A better understanding of the mechanisms regulating caspase activation has supported initial efforts to modulate dysfunctional cell death and inflammation pathways in a suite of communicable, inflammatory, malignant, metabolic and neurodegenerative diseases. Here, we review current understanding of caspase biology with a prime focus on the inflammatory caspases, and outline important topics for future experimentation.
Keywords: caspase, inflammasome, gasdermin, interleukin, apoptosis, pyroptosis
Introduction
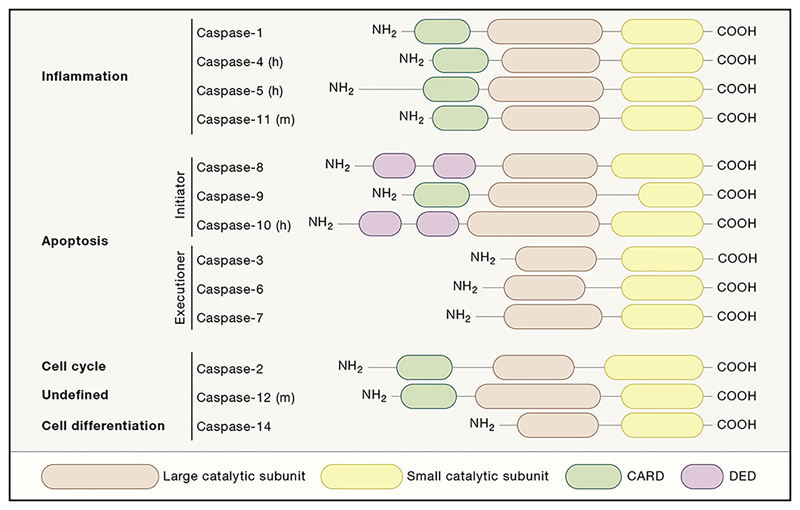
Caspases are a family of evolutionary conserved cysteine-dependent endoproteases that hydrolyse their substrates after specific aspartic acid residues (Lamkanfi et al., 2002). They consist of an amino-terminal domain of variable size sequentially followed by large and small catalytic subunits of respectively ~20 kDa and ~10 kDa that together form the protease domain (Figure 1). The amino-terminal regions of initiator caspases contain a caspase recruitment domain (CARD; caspases 1, 2, 4, 5, 9, 11) or death effector domains (DED; caspases 8, 10) that promote their recruitment and activation in multiprotein complexes. The process of proximity-induced autoactivation of the caspase zymogen into the active protease is driven by dimerization-induced conformational changes that lead to proteolytic excision of the flexible linker regions separating the prodomain and the large and small catalytic subunits (Lamkanfi et al., 2002). In contrast, executioner caspases lack an extended amino-terminal prodomain and require cleavage by initiator caspases for their activation (Ramirez and Salvesen, 2018).
Figure 1. Functional classification and domain architecture of murine and human caspases.
The members of the caspase-family are classified as inflammatory or apoptotic based on the described functions and domain architecture. The caspases-1, 4, -5 and -11 are grouped as inflammatory caspases and share a CARD-domain at the N-terminal end. The apoptotic caspases can be further sub-categorized in initiator- and executioner caspases. The caspases-8, -9 and -10 have domain architecture akin to the inflammatory caspases, however, their function is to initiate apoptosis through the activation of the executioner caspases-3, -6 and -7. In addition, caspase-2 shares domain structures with the inflammatory caspases, however, its function is described to be cell cycle related. Structurally, murine caspase-12 clusters with the inflammatory caspases, however, until today its function remains undefined. Alternatively, for human caspase-12, it is generally accepted that 75% of the population is knock-out, while the remaining 25% carry a proteolytically inactive pseudoprotease (h*). The function of caspase-14 is linked to cell differentiation. m, murine and h, human.
Historically, caspases have been associated with the induction of apoptosis, a homeostatic and non-lytic regulated cell death mode that supports the coordinated dismantling and removal of old and injured cells (Ramirez and Salvesen, 2018). More recently, caspase-mediated cleavage events have been shown to suppress necroptosis, a lytic regulated cell death mode that is driven by receptor-interacting protein (RIP) kinases (Pasparakis and Vandenabeele, 2015). Additionally, the mechanisms have been uncovered in recent years by which inflammatory caspases promote pyroptosis, another major lytic cell death mode that is associated with the secretion of the inflammatory cytokines interleukin (IL)-1β and IL-18 (Vande Walle and Lamkanfi, 2016). An extensive body of evidence from the past three decades has implicated dysregulated caspase activation as a causal disease mechanism in tumorigenesis, autoimmunity, autoinflammation and infectious pathologies (Halaby, 2012; Van Gorp et al., 2019). Here, we will succinctly review the roles and signalling mechanisms of apoptotic caspases to then focus the discussion on recent insights on activation mechanisms of inflammatory caspases and the diverse roles of caspases in cell death and inflammation. Additionally, we will highlight emerging evidence of extensive molecular crosstalk between inflammatory and apoptotic caspases and review emerging strategies for therapeutic modulation of inflammatory caspase activation in a suite of communicable, inflammatory, malignant, metabolic and neurodegenerative diseases.
Caspase signalling in apoptosis
Apoptotic caspases are functionally subdivided into initiator (caspases 8, 9 and 10) and effector (caspases 3, 6 and 7) caspases (Figure 1). In the ‘extrinsic apoptosis’ pathway, the Death-Inducing Signalling Complex (DISC) that is assembled at the cytosolic face of several members of the TNF receptor family supports caspase dimerization as a critical and sufficient step for activation of initiator caspases 8 and 10 (Ramirez and Salvesen, 2018). Caspase-8 is constitutively and widely expressed in most rodent and human cells. In species that encode caspase-10 - i.e. all primates and a subset of rodents (guinea pigs and squirrel but not mouse and rat – this caspase appears to temper autophagic and apoptotic responses to promote NF-κB activation and cell survival (Horn et al., 2017; Lamy et al., 2013), although pro-apoptotic functions have also been proposed (Ramirez and Salvesen, 2018). Notably, the gene encoding human caspase-10 clusters together with c-FLIP, suggesting that the two caspase-8 paralogues arose from a gene duplication event. Whereas caspase-10 is a proteolytic enzyme, however, cFLIP is a catalytically inactive pseudoprotease that heterodimerizes with caspases 8 and 10, and functions as a rheostat that regulates apoptosis, NF-κB signaling and survival pathways (Horn et al., 2017; Lamy et al., 2013). Akin to caspases 8 and 10, initiator caspase-9 undergoes proximity-induced autoactivation in the so-called ‘intrinsic apoptosis’ pathway upon its CARD-assisted recruitment into the apoptosome. In addition to caspase-9, this cytosolic wheel-shaped multi-protein complex is composed of 7-8 Apoptotic peptidase activating factor 1 (Apaf-1) units that bind the nucleotide dATP and mitochondrial cytochrome c, the cytosolic leakage of which serves as a marker of extensive mitochondrial damage and mitochondrial outer membrane permeabilization (Dorstyn et al., 2018). The zymogens of apoptotic effector caspases 3 and 7 are inactive homodimers that gain proteolytic activity when initiator and activated executioner caspases cut the linker that separates their large and small catalytic subunits (Figure 1). The apoptotic executioner caspases are responsible for the characteristic morphological changes of apoptosis that include membrane blebbing, cell shrinkage, the formation of ‘apoptotic bodies’ and chromosomal DNA fragmentation (Ramirez and Salvesen, 2018). These hallmark features of apoptosis are accompanied by the cleavage of several hundred substrates, few of which have demonstrated roles in apoptotic cell dismantling and externalization of ‘find-me’ and ‘eat-me’ signals that guide efferocytosis, the process of removal of the cellular corpses by professional phagocytes (Julien and Wells, 2017).
Because apoptotic bodies are efficiently cleared in vivo, apoptosis is usually not associated with the release of danger-associated molecular patterns (DAMPs) that may recruit inflammatory cells and cause damage to the surrounding tissue. Hence, apoptosis is widely viewed as a homeostatic non-inflammatory process that discards of damaged, infected and aging cells without alarming the immune system. However, an extensive body of literature has unveiled critical pathological mechanisms by which apoptosis contributes to the ethology of infectious and inflammatory diseases. Lymphocytopenia, immunosuppression and organ disfunction in sepsis have been associated with apoptosis of lymphocytes and some parenchymal tissues (Girardot et al., 2017). Extensive apoptosis and active caspase-3 staining have been documented in the splenic white pulp regions of critically ill patients, and caspase inhibition significantly improves survival rates in animal models of sepsis (Girardot et al., 2017). Extensive apoptosis also contributes to hepatocellular pathology in a mouse model of anti-Fas antibody-induced fulminant hepatitis by inducing activation of caspase-8 and truncated Bid-mediated activation of caspase-9 (Brenner et al., 2013). Defective uptake of apoptotic corpses by macrophages may result in secondary necrosis, in which rupture of the plasma membrane triggers release of damage associated molecular patterns (DAMPs) that stimulate inflammatory and immunogenic reactions. Indeed, defective efferocytosis promotes non-resolving inflammation and plaque instability in advanced atherosclerosis (Yurdagul et al., 2017). Inefficient degradation of DNA-containing neutrophil extracellular traps (NETs) and apoptotic cells has also been associated with systemic autoimmunity in patients with systemic lupus erythematosus (Mahajan et al., 2016). Taken together, these findings demonstrate that preventing accumulation of apoptotic cells is critically important for avoiding DAMP release and maintaining homeostasis.
Inflammatory caspases drive pyroptosis and inflammatory cytokine secretion
Caspase-1 was cloned nearly three decades ago as the Interleukin-1β Converting Enzyme (ICE) in a hunt for the enzyme that is responsible for the proteolytic maturation of the pro-inflammatory cytokine IL-1β in human monocytes (Lamkanfi and Dixit, 2014, 2017). The subsequent generation of caspase-1-deficient mice confirmed the essential role of caspase-1 in IL-1β secretion and demonstrated that caspase-1 is largely dispensable for apoptosis. Other work establishes caspase-1 as the protease that drives maturation and secretion of IL-18, which at that time was known as the ‘interferon-γ inducing factor’. More recently, the discovery that caspase-1-mediated cleavage of the cytosolic protein gasdermin D (GSDMD) promotes the formation of GSDMD membrane pores and cell lysis, which sheds much needed light on the necrotic execution mechanism of pyroptosis (de Vasconcelos et al., 2019a; Kayagaki et al., 2015; Shi et al., 2015). The gasdermin family consists of several members that exert the ability to form large oligomeric membrane pores with an inner diameter of 12-20 nm in the inner layer of the plasma membrane and intracellular organelles (Ding et al., 2016; Mulvihill et al., 2018; Rogers et al., 2017; Sborgi et al., 2016; Wang et al., 2017), but in vitro studies suggest that GSDMD is the only member that is a substrate of inflammatory caspases (Shi et al., 2015). The term pyroptosis was first coined by Brennan and Cookson to contrast the intrinsically non-inflammatory nature of apoptosis (Cookson and Brennan, 2001). This caspase-1-regulated cell death mode frequently coincides with the secretion of IL-1β and IL-18, and the release of DAMPs (Vande Walle and Lamkanfi, 2016).
In addition to caspase-1, the inflammatory caspase subfamily in humans consists of caspases 4 and 5 (Figure 1). Caspase-11 in rodents is the ortholog of human caspases 4 and 5 (Lamkanfi et al., 2002). Although these additional inflammatory caspases are incapable of cleaving proIL-1β and proIL-18 into bioactive cytokines, they each have been shown to cleave GSDMD and induce pyroptosis (Kayagaki et al., 2015; Schmid-Burgk et al., 2015; Shi et al., 2015). These observations have elicited proposals to redefine pyroptosis as programmed necrosis driven by inflammatory caspases and gasdermin family members (Shi et al., 2017; Vande Walle and Lamkanfi, 2016). The requirement for inflammatory caspases in executing pyroptosis distinguishes it from necroptosis, a regulated necrotic cell death mode that relies on RIP kinase 3 (RIPK3) and its pseudokinase substrate mixed lineage kinase domain like pseudokinase (MLKL) (Pasparakis and Vandenabeele, 2015).
Although several mechanisms of IL-1β secretion from viable cells have recently been reported (Evavold et al., 2018; Gaidt et al., 2016), pyroptosis and necroptosis are rapidly emerging as the prime mechanisms for secretion of IL-1β and IL-18, as well as DAMPs such as ATP, HMGB1, S100 proteins and IL-1α (Kayagaki et al., 2011; Lamkanfi et al., 2010). These molecules each lack secretion signals and it has been debated for over two decades how they reach the extracellular environment where binding to their cognate receptors may contribute to the acute phase response, inflammatory tissue damage, fever, cytokine release syndrome, neurotoxicity and potentially systemic organ failure in sepsis and other diseases (Lamkanfi, 2011). Firstly, ex vivo studies in inflammasome-stimulated Gsdmd-/- BMDMs have shown that fully matured IL-1β and IL-18 as well as IL-1α and HMGB1 are retained intracellularly, supporting the notion that pyroptosis mediates extracellular release of these inflammasome-dependent cytokines and DAMPs (Kayagaki et al., 2015; Shi et al., 2015). Consistently, a single cell imaging analysis of transgenic macrophages expressing a caspase-1 fluorescence resonance energy transfer (FRET) sensor shows that IL-1β secretion fully coincides with pyroptosis induction in the same cells (Liu et al., 2014). Much progress has been made in recent years in understanding the critical mechanisms by which pyroptosis contributes to host defense and lethal shock in in vivo. Defective pyroptosis induction renders Gsdmd-deficient mice highly susceptible to Francisella novicida infection (Zhu et al., 2018). It has also been established that inflammasomes drive LPS-induced lethal shock in vivo. Caspase-1-mediated IL-1β and IL-18 signaling contributes to lethality by lower LPS doses, but this protective effect is not sustained at higher LPS doses (Berghe et al., 2014; Lamkanfi et al., 2010). However, caspase-11-deficient and Gsdmd-/- mice also resist high dose LPS-induced lethality, indicating that caspase-11–dependent pyroptosis rather than caspase-1–dependent secretion of IL-1β and IL-18 is the prominent mechanism that drives LPS-induced lethal shock (Berghe et al., 2014; Kayagaki et al., 2015; Kayagaki et al., 2011; Lamkanfi et al., 2010). A recent report further clarifies the mechanism by which pyroptosis promotes lethality in LPS-challenged mice by showing that pyroptotic myeloid cells shed tissue factor-containing microvesicles, which results in systemic intravascular blood clotting and lethality (Wu et al., 2019). Together, these studies highlight the key role of inflammasome activation in host defence against infectious agents, and in eliciting lethal shock.
Caspase-1 activation by canonical inflammasome pathways
Akin to apoptotic initiator caspase-9, the prototypical inflammatory caspase-1 harbours a CARD in its amino-terminal propeptide through which it gets recruited into inflammasomes, cytosolic multi-protein complexes that support caspase-1 dimerization and drive its proximity-induced auto-activation. A suite of pattern recognition receptors (PRRs) that responds to microbial and pathogen-associated molecular patterns (MAMPs and PAMPs), and in some cases to environmental and host-derived DAMPs assemble canonical inflammasomes that engage caspase-1 (Figure 2). The list of genetically validated inflammasomes with defined stimuli comprises at least five distinct complexes that are typically named after the PRR that assembles the inflammasome (Lamkanfi and Dixit, 2014, 2017). We distinguish the canonical inflammasomes formed by several members of the intracellular nucleotide-binding domain and leucine-rich repeat containing (NLR) family, the HIN200 family member AIM2 and the TRIM family member Pyrin.
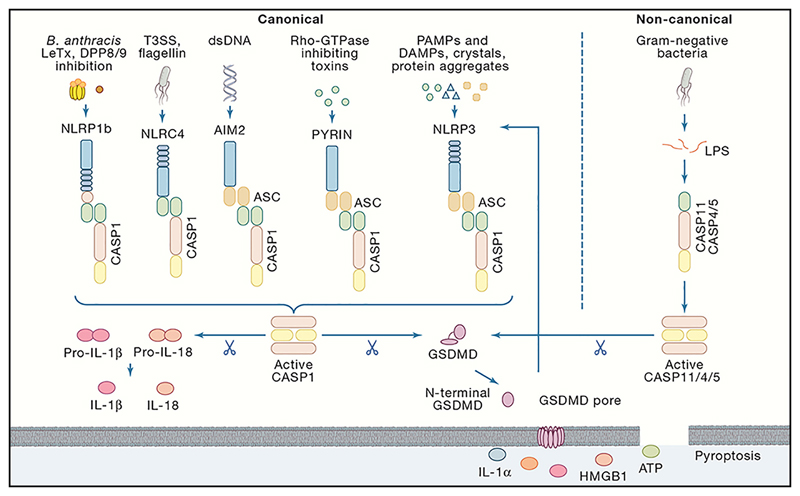
Figure 2. Overview of canonical and non-canonical inflammasomes driving pyroptosis and release of IL-1β and IL-18.
Recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) by their respective inflammasome-sensors NLRP1b, NLRP3, NLRC4, AIM2 and Pyrin leads to the assembly of a multi-protein complex termed the inflammasome. Consequently, caspase-1 undergoes autoproteolytic processing to lock the protease in its active form. Caspase-1 directly cleaves its substrates gasdermin D and the pro-inflammatory cytokines pro-IL-1β and pro-IL-18. The 31kDa N-terminal cleavage fragment of Gsdmd forms pores in the host cell membrane thereby mediating the release of cytoplasmic content, the mature IL-1β and IL-18 and the other DAMPs IL-1α, HMGB1 and ATP. Alternatively, detection of cytosolic LPS coming from Gram-negative bacteria by the murine caspase-11 or its human orthologues caspases-4 and -5 initiates activation of the proteolytic activity thereby cleaving gasdermin D resulting in pyroptosis. Consequently, the NLRP3 inflammasome is activated leading to the caspase-1-mediated cleavage of the pro-inflammatory cytokines pro-IL-1β and pro-IL-18. T3SS, Type III Secretion System.
In addition to the prototypical PYD, NACHT and Leucine-rich repeat (LRR) domains found in other NLRP family members, the human inflammasome sensor NLRP1 contains a unique carboxy-terminus extension that harbours a Function-to-find (FIIND) domain and a CARD (Figure 2). The FIIND domain is an autoproteolytic domain that is uniquely shared between NLRP1 and CARD8 and that undergoes post-translational autocleavage as a prerequisite for ligand-induced activation (Lamkanfi and Dixit, 2014, 2017). Mice lack a CARD8 homolog, but encode three orthologous Nlrp1 genes, namely Nlrp1a, Nlrp1b and Nlrp1c that each lack the PYD domain in the amino-terminus of human NLRP1. Murine Nlrp1c is considered a pseudogene, and evidence that Nlrp1a assembles a functional inflammasome is based on a reported activating Nlrp1aQ593P mutation that drives IL-1-dependent leukopenia in mice linked to excessive inflammasome activation and pyroptosis in hematopoietic progenitor cells (Masters et al., 2012). However, it is currently unclear which of the murine orthologs functionally correspond to human NLRP1 because well-defined (patho)physiologic/biochemical agents that selectively activate the murine Nlrp1a or human NLRP1 inflammasomes are yet to be discovered. Nlrp1b is highly polymorphic and Bacillus anthracis lethal toxin (LeTx) activates the murine Nlrp1b inflammasome in macrophages of sensitive strains (Lamkanfi and Dixit, 2014). However, human NLRP1 is unresponsive to LeTx. Recent studies have shown that pharmacological inhibitors of the cytosolic post-proline dipeptidyl peptidases (DPP)8 and DPP9 activate NLRP1 and CARD8 to induce pyroptosis in human keratinocytes, the human monocytic-like cell line THP-1 and in primary peripheral blood mononuclear cells, respectively (Johnson et al., 2018; Zhong et al., 2018). Moreover, pharmacological inhibition of DPP8 and DPP9 protease activity has been shown to induce pyroptosis without eliciting other inflammasome hallmark responses such as caspase-1 autocleavage, and secretion of IL-1β in murine C57BL/6J macrophages (Okondo et al., 2017; Okondo et al., 2018). Contrastingly, DPP8 and DPP9 inhibition in the BALB/c-derived monocyte cell line J774.A1 and in primary bone marrow-derived macrophages (BMDM) of mice that express a LeTx-responsive Nlrp1b allele elicited a rapid pyroptotic response concomitant with caspase-1 maturation, ASC speck assembly and secretion of mature IL-1β and IL-18 (de Vasconcelos et al., 2019b). The fact that C57BL/6J mice lack a LeTx-responsive Nlrp1b allele may contribute to a differential inflammasome response in macrophages in this genetic background.
Unlike NLRP1, a broad suite of environmental crystals and pollutants and host-derived DAMPs and protein aggregates activate the NLRP3 inflammasome (Lamkanfi and Dixit, 2014, 2017). Examples of clinically relevant DAMPs that engage NLRP3 include uric acid and cholesterol crystals that cause gout and atherosclerosis, amyloid-β fibrils that are neurotoxic in Alzheimer’s disease and asbestos particles that cause mesothelioma. Additionally, NLRP3 is activated by infectious agents such as Vibrio cholerae; fungal pathogens such as Aspergillus fumigatus and Candida albicans; adenoviruses and influenza A virus. Recent work establishes that activation of the NLRP3 inflammasome in influenza A-infected cells is relayed by Z-DNA binding protein 1 (ZBP1) and RIPK1 together with the induction of necroptosis and apoptosis (Kesavardhana et al., 2017; Kuriakose et al., 2016a; Thapa et al., 2016) (Figure 3). Given the wealth and structural diversity in NLRP3 stimuli, it is thought that NLRP3 senses a secondary messenger or cellular state that is commonly induced by these stimuli. Many NLRP3-activating agents trigger damage to membrane-bound organelles. Hence, membrane damage and K+ efflux have emerged as upstream mechanisms that may regulate NLRP3 activation (Di et al., 2018; Munoz-Planillo et al., 2013). The K+ efflux channel TWIK2 has been recently shown to promote ATP-induced NLRP3 inflammasome activation in cultured macrophages, and its genetic deletion dampens polymicrobial sepsis- and LPS-induced endotoxemia-induced inflammatory lung injury in mice (Di et al., 2018). However, unlike NLRP3 activation induced by ATP, pore-forming toxins and crystals, the Toll-like receptor 7 (TLR7) agonist imiquimod and related molecules have been shown to activate NLRP3 through a K+ efflux-independent mechanism (Gross et al., 2016; Kanneganti et al., 2006). The mechanism of imiquimod-induced NLRP3 activation has primarily been interrogated in murine bone-marrow derived dendritic cells (BMDCs), but imiquimod also engages the NLRP3 inflammasome in BMDMs (Gross et al., 2016; Kanneganti et al., 2006). Moreover, a recent examination of granulocyte–macrophage colony-stimulating factor (GM-CSF)-derived BMDCs reveals that a subpopulation of monocyte-derived macrophages, rather than dendritic cells, are responsible for NLRP3-mediated caspase-1 activation and IL-1β secretion (Erlich et al., 2019). An alternative NLRP3 inflammasome pathway has been described that promotes IL-1β secretion from human monocytes stimulated with the TLR4 agonist LPS without eliciting K+ efflux and classical inflammasome hallmarks such as ASC speck formation and pyroptosis (Gaidt et al., 2016). Collectively, these findings suggest that although K+ efflux frequently accompanies NLRP3 activation, it may not be the much sought-after universal secondary messenger that engages NLRP3 (Lamkanfi and Dixit, 2014).
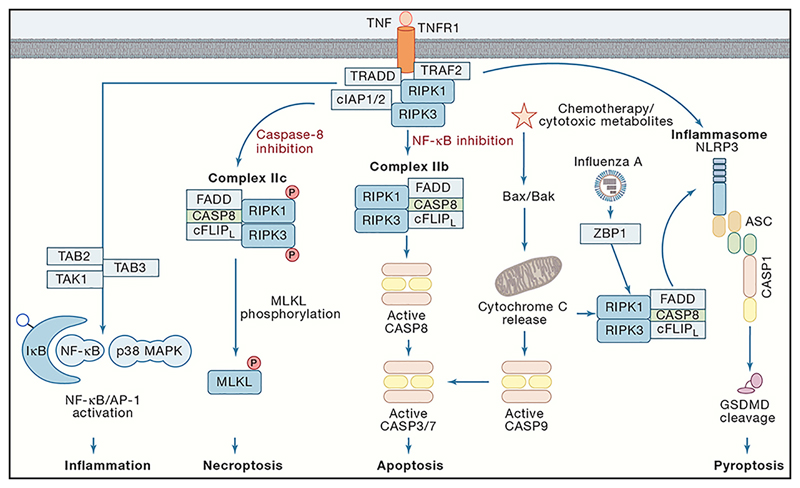
Figure 3. Caspase crosstalk pathways.
Schematic representation of the death receptor signalling pathways. Upon ligation of TNF ligand to its receptor (TNFR1), several molecules are required to the cytoplasmic domain of the receptor (TRAF, TRADD, RIPK1 and IAP). The cIAPs recruit the LUBAC complex causing ubiquitination of RIPK1 ultimately leading to the activation of NF-κB and induction of inflammation. Dysregulation of this pathway leads to the formation of complex IIa by recruitment of caspase-8 resulting in the induction of apoptosis. Under the conditions that also the activation of caspase-8 is hampered (complex IIb), RIPK1 will recruit RIPK3 resulting in the phosphorylation of MLKL and the induction of necroptosis. Next to the extrinsic apoptosis pathway, the intrinsic apoptosis pathway is described as the activation of the Bax-Bak-dependent mitochondrial outer membrane permeabilization (MOMP). Consequently, cytochrome C is released from the mitochondria thereby stimulating the activation of caspase-9 and downstream executioners caspases-3 and -7 to initiate apoptosis. Chemotherapy or cytotoxic metabolites enable, through MOMP, activation of a RIPK1-caspase-8-driven NLRP3 activation. Also, ZBP1 is a cytoplasmic sensor for viral RNA of Influenza A virus resulting in the activation of the same RIPK1/caspase-8 pathway. Moreover, caspase-8 by itself can cleave pro-IL-1β. Additionally, caspase-8 and FADD have been found to associate with the NRLP3 inflammasome resulting in the gasdermin D-mediated pyroptotic form of cell death.
NLR family, apoptosis inhibitory proteins (NAIPs) physically bind bacterial flagellin and components of some bacterial type III secretion systems to activate the NLRC4 inflammasome (Figure 2). By sensing these conserved bacterial structures in the cytosol of infected monocytes and macrophages, the NLRC4 inflammasome contributes to host defense against facultative intracellular pathogens such as Salmonella Typhimurium, Shigella flexneri, Pseudomonas aeruginosa, Burkholderia thailandensis, and Legionella pneumophila (Lamkanfi and Dixit, 2014). A recent study unveils that interferon regulatory factor 8 (IRF8) is required for constitutive expression of NAIP family members, and thus for optimal activation of the NLRC4 inflammasome (Karki et al., 2018).
Absent in melanoma 2 (AIM2), a cytosolic member of the HIN200 PRRs, is equipped with a dsDNA-sensing HIN200 domain and supports assembly of an inflammasome that contributes to host defence against Francisella tularensis, Listeria monocytogenes, vaccinia virus and cytomegalovirus infections (Lamkanfi and Dixit, 2014). Interferon-inducibe GTPases of the guanylate-binding proteins (GBP) family have been shown to be critical for activation of the AIM2 inflammasome triggered by F. tularensis and other bacterial pathogens (Man et al., 2015; Meunier et al., 2015). The AIM2 inflammasome has also been reported to provoke a detrimental inflammatory response to genomic dsDNA breaks in mice subjected to subtotal body irradiation-induced gastrointestinal syndrome and total body irradiation-induced hematopoietic failure (Hu et al., 2016). These findings suggest that AIM2 inhibition might represent a novel approach to alleviate this common and severe complication of radio- and chemotherapy in cancer patients.
The Pyrin inflammasome indirectly senses the activity of bacterial toxins such as Clostridium botulinum C3 toxin, toxins A and B of Clostridium difficile and Burkholderia cenocepacia TecA that all covalently inactivate the host RhoA small GTPase (Aubert et al., 2016; Gao et al., 2016; Van Gorp et al., 2016; Xu et al., 2014). Additionally, the RhoA-inhibiting effector YopE of Yersinia pestis and Y. pseudotuberculosis activate the Pyrin inflammasome, while Y. pestis and Y. pseudotuberculosis YopM suppress Pyrin activation (Chung et al., 2016; Ratner et al., 2016). In naïve macrophages, Pyrin phosphorylation (at S208/S242 in human Pyrin; S205/S241 in murine Pyrin) keeps the protein autoinhibited by recruiting 14-3-3 proteins (Masters et al., 2016). The above-mentioned toxins relieve inhibition by stimulating Pyrin de-phosphorylation and disrupting 14-3-3 binding. A functional microtubule network is required downstream of Pyrin dephosphorylation in toxin-stimulated macrophages for assembly of ASC specks and caspase-1-mediated cytokine secretion, although future experimentation should address the mechanism involved (Gao et al., 2016; Van Gorp et al., 2016).
Activation of human caspases 4 and 5, and murine caspase-11 by the non-canonical inflammasome
Since the discovery in 2011 that initially reported caspase-1-deficient mouse strains also lacked expression of caspase-11 (Kayagaki et al., 2011), the non-canonical inflammasome pathway has gained considerable traction as a key mechanism in Gram-negative infections and sepsis (Aglietti et al., 2016; Hagar et al., 2013; Kayagaki et al., 2015; Knodler et al., 2014; Lagrange et al., 2018; Mandal et al., 2018; Schmid-Burgk et al., 2015). In this pathway, internalized lipopolysaccharide (LPS) from Gram-negative bacteria interacts with the CARD domain and activates human caspases 4 and 5 and murine caspase-11 (Baker et al., 2015; Hagar et al., 2013; Kayagaki et al., 2013; Schmid-Burgk et al., 2015; Shi et al., 2014). Murine caspase-11 autonomously cleaves GSDMD to induce pyroptosis as highlighted by the observation that extracellular release of the DAMPs IL-1α and HMGB1 from macrophages infected with Escherichia coli, Citrobacter rodentium and Vibrio cholerae was fully caspase-11-dependent (Kayagaki et al., 2011). GSDMD cleavage parallelly connects caspase-11 with K+ efflux and activation of the NLRP3 inflammasome to drive caspase-1-dependent maturation and secretion of IL-1β and IL-18 (Aglietti et al., 2016; Kayagaki et al., 2015; Ruhl and Broz, 2015; Shi et al., 2015). Akin to their role in the AIM2 inflammasome, bacterial vacuoles in infected host cells that shield Gram-negative pathogens from innate immune detection are lysed by GBPs to promote caspase-11 activation by leaked Escherichia coli and Citrobacter rodentium LPS in the cytoplasm (Meunier et al., 2014; Pilla et al., 2014). In both pathways, GBPs recruit the murine interferon-inducible protein IRGB10 to intracellular bacteria to facilitate activation of the AIM2 inflammasome by Francisella novicida, and activation of the non-canonical inflammasome by E. coli and C. rodentium (Man et al., 2016). It remains to be determined whether LPS-induced oligomerization of human caspases 4 and 5, and murine caspase-11 is facilitated by currently unknown factors, but a recent study suggests that LPS internalization and caspase-11 activation in LPS-challenged mice is mediated by hepatocyte-released HMGB1, which physically binds LPS in circulation and facilitates its access into the cytosol of myeloid and endothelial cells by binding the receptor for advanced glycation end-products (RAGE) followed by permeabilization of the lysosomal membrane (Deng et al., 2018). Notably, a recent report presented genetic evidence that caspase-11 self-cleavage at the linker peptide that separates the large and small catalytic subunits is indispensable for caspase-11 activation (Lee et al., 2018b). This is unlike caspase-1, which based on observations in ASC-deficient macrophages retains the ability to induce pyroptosis in response to stimuli of the NLRC4 and NLRP1b inflammasomes in the apparent absence of caspase-1 autocleavage (Broz et al., 2010; Guey et al., 2014; Van Opdenbosch et al., 2014). Together, these findings suggest that the mechanism of murine caspase-11 activation resembles more that of apoptotic executioner caspases, whereas caspase-1 activation by canonical inflammasomes aligns with the proximity-induced autoactivation process of apoptotic initiator caspases.
Caspases 2, 12 and 14 – the odd men out
The functions of both caspases 2 and 14 are not fully understood but based on recent developments they appear to exert roles beyond apoptosis and inflammation (Figure 1). Caspase-2 protease activity has been recently shown to counteract genomic instability and tumorigenesis upon cytokinesis failure and in cells with extra centrosomes by cleaving the p53 repressor MDM2, which induces cell cycle arrest (Fava et al., 2017). Studies in genetically ablated mice have established a role for caspase-14 in keratinocyte differentiation and the maintenance of a normal stratum corneum, but further analysis is required to fully understand the underlying mechanisms (Denecker et al., 2007; Hoste et al., 2013).
Caspase-12 phylogenetically clusters with the inflammatory caspase subfamily (Lamkanfi et al., 2002) (Figure 1). Although physiologically relevant substrates have yet to be identified, it is evident that murine caspase-12 is a functional protease that undergoes self-cleavage in the linker peptide that separates the large and small catalytic subunits at ATAD318 (Fujita et al., 2002). Early studies suggest caspase-12 to promote endoplasmic reticulum stress-induced apoptosis, but the current consensus is that caspase-12 is dispensable for apoptosis. A subsequent hypothesis proposed that caspase-12 acts as a dominant-negative regulator that represses caspase-1 activation and inhibits secretion of IL-1β and IL-18. However, a recent systematic analysis of canonical and non-canonical inflammasome responses in caspase-12-deficient macrophages and mice has refuted also this hypothesis (Vande Walle et al., 2016). Future experimentation should focus on characterization of the recently reported selectively-targeted caspase-12-deficient mice to clarify the physiologic roles of this enigmatic caspase. Unlike its rodent ortholog, human caspase-12 is thought to represent a proteolytically inactive pseudoprotease based on the substitution of critical residues that surround the active site. Whereas in murine caspase-12 and other caspases the catalytic histidine residue is invariantly followed by an evolutionary conserved glycine, this amino acid is mutated to serine in human caspase-12 (Fischer et al., 2002; Lamkanfi et al., 2002). Consistently, recombinantly purified human caspase-12 fails to cleave the synthetic peptide substrate Ac-ATAD-amc that is readily processed by mouse caspase-12 (Demon D and Lamkanfi M, unpublished results). Notably, all Caucasians and large parts of the human populations of Africa, the Americas and Asia likely are ablated for caspase-12 protein expression.This is because of a C>T single nucleotide polymorphism (SNP; rs497116) in the caspase-12 open reading frame that arose 100,000-500,000 years ago, and likely causes nonsense-mediated decay of the corresponding transcripts. Interestingly, 20-30% of the population of North and Sub-Saharan Africa, lack or are heterozygous for the rs497116 SNP, and express full-length capase-12 (Fischer et al., 2002; Kachapati et al., 2006). The read-through mutation is also present in peoples of the Middle East and South-East Asia, albeit at lower frequencies (Kachapati et al., 2006). Because the pocket bordering the catalytic histidine is still altered, caspase-12 may function as a pseudoprotease in individuals expressing this read-through isoform (Reynolds and Fischer, 2015). As such, human caspase-12 may resemble c-FLIPL, a non-catalytic pseudoprotease that heterodimerizes with caspase-8 and modulates its cellular functions (Tummers and Green, 2017). The precise environmental factors that drove the rs497116 SNP to near-fixation during human evolution are unclear (Xue et al., 2006), but it is tempting to speculate that caspase-12 acted as a pseudoprotease that contributed to increased risk for severe infections or other debilitating conditions. To conclude, whereas murine caspase-12 is an active caspase, most humans likely lack caspase-12 expression and others may express a pseudoprotease of unknown role.
Crosstalk between apoptotic and inflammatory caspases
As research into signalling pathways of inflammatory caspases progressed, an unexpected amount of crosstalk with apoptotic caspases has emerged over the past years. Firstly, reports of proIL-1β maturation that is mediated directly by caspase-8 point to an inflammatory role for this apoptotic initiator caspase (Bossaller et al., 2012; Gringhuis et al., 2012; Maelfait et al., 2008; Vince et al., 2012). Caspase-8 also regulates IL-1β secretion by regulating priming and activation of the canonical and non-canonical inflammasome pathways (Gurung et al., 2014; Man et al., 2014), as well as activation of the alternative NLRP3 inflammasome pathway in human monocytes (Gaidt et al., 2016). A mechanistic model is emerging in which the necrosome that contains caspase-8, RIPK1 and RIPK3 acts as a central hub for the integration of inflammasome and cell death responses that are triggered by a broad suite of agents that alter MAP kinase and NF-κB signalling cascades in macrophages, for instance by changing cellular amounts of Inhibitor of Apoptosis (IAP) proteins and targeting Transforming growth factor beta-activated kinase 1 (TAK1) (Malireddi et al., 2018; Philip et al., 2014; Sarhan et al., 2018; Vince et al., 2012). Agents that rely on caspase-8 and RIPK1 for cell death and inflammasome responses include influenza A virus (Kesavardhana et al., 2017; Kuriakose et al., 2016a; Kuriakose et al., 2016b; Thapa et al., 2016), Y. pseudotuberculosis and Y. enterocolitica (Orning et al., 2018; Philip et al., 2014; Sarhan et al., 2018; Weng et al., 2014), Candida albicans (Ganesan et al., 2014), as well as chemotherapeutic drugs and cytotoxic metabolites that engage the intrinsic apoptosis pathway in macrophages (Antonopoulos et al., 2013; Surup et al., 2018; Vince et al., 2018). Additionally, recent work reveals that LPS-induced shock and E. coli sepsis in mice is driven by the collaborative pro-apoptotic and pyroptotic signalling activities of caspases 8 and 11 respectively, which is induced by TNF and type 1 interferon cytokines in target tissues and amplifies inflammatory signals associated with tissue damage in the small intestine, spleen and thymus to induce lethality (Mandal et al., 2018). Mice with a myeloid-selective deletion of the NF-κB regulator A20 present with severe inflammatory arthritis that develops independently of TNF but is mediated by NLRP3 inflammasome-induced IL-1 signalling (Matmati et al., 2011; Vande Walle et al., 2014). A recent study reveals a remarkable amount of crosstalk between the NLRP3 inflammasome and the necrosome by demonstrating that RIPK1-dependent necroptosis drives inflammasome activation and inflammatory pathology in this mouse model of inflammatory arthritis (Polykratis et al., 2019).
Other evidence of caspase crosstalk is based on the observation that caspase-1-deficient macrophages induce caspase-8-mediated apoptosis in response to NLRP3 and AIM2 agonists (Pierini et al., 2012; Sagulenko et al., 2013). Subsequent studies have focussed on the CARD-based inflammasome sensors NLRP1b and NLRC4 to demonstrate that caspase-8 activation occurs in cytosolic ASC specks during apoptosis in caspase-1-deficient macrophages, and that this mechanism also is present in intestinal epithelial cells (Lee et al., 2018a; Mascarenhas et al., 2017; Rauch et al., 2017; Van Opdenbosch et al., 2017). Moreover, TLR agonists upregulate c-FLIP expression in primed macrophages, which suppresses caspase-8-mediated apoptosis downstream of ASC speck assembly (Van Opdenbosch et al., 2017). This is a surprising observation given that ASC expression and TLR priming have been previously shown to be dispensable for pyroptosis induction by the NLRP1b and NLRC4 inflammasomes (Broz et al., 2010; Guey et al., 2014; Van Opdenbosch et al., 2014). Akin to caspase-1-deficient macrophages, deletion of GSDMD similarly reroutes the inflammasome-induced cell death response to apoptosis (de Vasconcelos et al., 2019a; He et al., 2015). This suggests the existence of a caspase-1-driven apoptosis pathway. In agreement, a recent report shows that caspase-1 induced apoptosis in GSDMD-deficient macrophages by engaging caspase-3 downstream of BID cleavage and the apoptosome through the intrinsic apoptosis pathway (Tsuchiya et al., 2019). Caspase-1 has also been shown to activate the apoptotic executioner caspase-7 in S. Typhimurium-infected macrophages and in response to the NLRP3 agonists ATP and nigericin (Lamkanfi et al., 2008). Deletion of caspase-7 does not protect cells from pyroptosis, but it would be interesting to determine whether this mechanism contributes to inflammasome-induced apoptosis in GSDMD-deficient macrophages. In this context, differential tissue- and cell type-specific expression profiles of caspase signalling factors may determine the cellular response to cell death-inducing agonists. Indeed, C. difficile infection of macrophages triggers pyroptosis upon Pyrin inflammasome activation by the enterotoxins TcdA and TcdB (Gao et al., 2016; Van Gorp et al., 2016; Xu et al., 2014). However, intestinal epithelial cells - the physiologic targets of TcdA and TcdB - lack Pyrin expression and the intrinsic apoptosis pathway instead is engaged by these enterotoxins in a process that contributes to in vivo host defence against C. difficile infection (Saavedra et al., 2018).
Caspases in human monogenic diseases
Monogenic diseases that are caused by nonsense and missense mutations in caspase genes and components of caspase signalling pathways highlight the clinical implications of dysregulated caspase activation. Autoimmune lymphoproliferative syndrome (ALPS) is a recessive primary immunodeficiency that manifests in early childhood and is caused by dysfunctional Fas (CD95) signalling. Symptoms include lymphadenopathy and splenomegaly, defective Fas-induced extrinsic apoptosis and T-, B-, and natural killer (NK)-cell activation, accumulation of autoreactive lymphocytes and recurrent bacterial and viral infections. Most patients with this syndrome carry mutations in the Fas receptor or it ligand (Meynier and Rieux-Laucat, 2019). Moreover, patients with ALPS-related syndromes are reported to be deficient for caspases 8 or 10 (Chun et al., 2002; Meynier and Rieux-Laucat, 2019; Wang et al., 1999).
The importance of a tight regulation of caspase-1 activation is reflected by the variety of autoinflammatory diseases linked to activating mutations in several key components of the inflammasome (Van Gorp et al., 2019). Autoinflammatory diseases (AID) are defined as a dysfunction of the innate immune system and are often referred to as ‘periodic fever syndromes’ because many of these syndromes are characterized by recurrent fever and organ-specific inflammation. Since many syndromes share symptoms, it is often challenging to diagnose the patients correctly in a timely manner. To date, AID patients with mutations in all canonical inflammasome sensors but AIM2 have been identified, possibly because the human dsDNA-sensing inflammasome is linked to cGAS-STING-mediated lysosomal damage and activation of NLRP3 parallelly to the established role of cytosolic DNA recognition by the cGAS-STING immune axis that drives antiviral immunity by inducing type I interferons (Ablasser and Chen, 2019; Gaidt et al., 2017).
NLRP1 is predominantly expressed in keratinocytes, and gain-of-function mutations in NLRP1 predispose patients to skin inflammation and skin cancer (Zhong et al., 2016). Primary keratinocytes from these patients show spontaneous inflammasome activation and elevated IL-1β amounts. Additionally, NLRP1 mutations that result in elevated systemic amounts of caspase-1 and IL-18 have been identified in patients that present with skin dyskeratosis, arthritis and periodic fever (Grandemange et al., 2017). Cryopyrin-associated periodic syndromes (CAPS) is a collective term that captures three autosomal dominant disorders that are caused by gain-of-function mutations in NLRP3. From the mildest to the most severe pathologies, these are familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID) syndrome (Harapas et al., 2018). In addition to the critical role of IL-1β in the ethiology of CAPS, studies in an FCAS mouse model pinpoint caspase-1-mediated cell death, IL-18 and TNF secretion as additional pathophysiological mechanisms (Brydges et al., 2013; McGeough et al., 2017).
Familial Mediterranean Fever (FMF) is the most prevalent monogenic AID and is caused by missense mutations in Mefv that trigger unchecked Pyrin inflammasome signaling (Van Gorp et al., 2019). Recent studies show that deletion of GSDMD fully rescues systemic inflammatory pathology in mouse models of FMF and CAPS (Kanneganti et al., 2018; Xiao et al., 2018), suggesting that pyroptosis represents a key in vivo mechanism of inflammasome-driven autoinflammatory pathology. Gain-of-function mutations in NLRC4 have been associated with periodic fever syndromes that induce high circulating IL-18 amounts and increased risk for the development of macrophage activation syndrome (MAS) (Canna et al., 2014; Romberg et al., 2014). Indeed, an infant with life-threatening NLRC4-associated hyperinflammation that was diagnosed with MAS has been effectively treated with recombinant IL-18 binding protein whereas anti-IL-1β and anti-TNF therapies had been unsuccessful (Canna et al., 2017). Strikingly, patients with inactivating SNPs in human CASP1 also presented with autoinflammatory disease. While the genetic variants tempered or blocked caspase-1 protease activity and IL-1β secretion, the patients present with severe inflammation, which has been proposed to be a consequence of a more robust interaction between catalytically inactive caspase-1 and the NF-κB-activating kinase RIPK2 (Kersse et al., 2011; Lamkanfi et al., 2004; Luksch et al., 2013; Luksch et al., 2015). Deficiency in IL-1Ra (DIRA) leads to uncontrolled IL-1 signalling, which - when left unattended - can be life-threatening due to development of systemic inflammation and multiorgan failure (Aksentijevich et al., 2009). However, these patients respond well to lifelong treatment with IL-1neutralizing biologics.
Therapeutic targeting of caspase pathways
The central role of caspases in cell death and inflammation signaling makes them attractive targets for therapeutic intervention in many human diseases across therapeutic areas. Caspase inhibitors are widely available as research tools and have helped to define the roles of specific caspases in cell death and inflammatory processes. However, the development of selective caspase inhibitors for therapeutic use has proven challenging, and none have successfully reached the clinic despite tremendous efforts by the pharmaceutical industry (Cornelis et al., 2007; Kudelova et al., 2015). Most recently, the pan-caspase inhibitor emricasan (IDN-6556) has been evaluated in phase 2 clinical trials in patients suffering from nonalcoholic steatohepatitis (NASH), liver fibrosis or acutely decompensated cirrhosis. The drug was safe and well-tolerated in patients with advanced liver disease, but it overall failed to provide proof-of-concept support for caspase inhibition as a treatment for NASH and cirrhosis patients (Garcia-Tsao et al., 2019; Mehta et al., 2018). However, tremendous progress over the past three decades in understanding the molecular mechanisms that regulate caspase activation has revealed new approaches for therapeutic modulation of caspase activity. Examples of strategies that are being evaluated in clinical trials to re-engage apoptosis in cancer therapy include antagonists of anti-apoptotic BCL-2 family members and Smac mimetics that target Inhibitor of Apoptosis (IAP) proteins (Fulda, 2017; Opferman, 2016).
Although currently still in the preclinical phase, there is extensive interest to therapeutically target inflammasome pathways. The currently approved therapies anakinra (IL-1 receptor antagonist), canakinumab (IL-1β neutralizing antibody) and rilonacept (soluble decoy receptor for IL-1β and IL-1α) focus on neutralizing IL-1β or neutralizing its signaling receptor on effector cells (Van Gorp et al., 2019). While valuable in treating autoinflammatory diseases, these biologics do not halt other aspects of detrimental inflammasome responses, such as pyroptosis, the release of DAMPs and IL-18-driven immune activation. Moreover, constitutive neutralization of IL-1β comes with increased risk for potentially life-threatening infections, as documented in rheumatoid arthritis patients taking anakinra (Galloway et al., 2011), and in a large cohort of atherosclerosis patients on canakinumab in the recently reported CANTOS study (Ridker et al, 2017). The need for subcutaneous administration of these biological agents represents another drawback to patients, especially when requiring daily dosing. Small molecule inhibitors that work upstream in a specific inflammasome pathway would act more broadly on the inflammasome response while at the same time alleviating several of the concerns discussed above. Particularly the NLRP3 inflammasome is gaining traction as a drug target given its association with a broad range of inflammatory, metabolic and neurodegenerative diseases (Mangan et al., 2018). Additionally, compounds that target GSDMD and inhibit pyroptosis have recently been reported (Rathkey et al., 2018) (Figure 4).
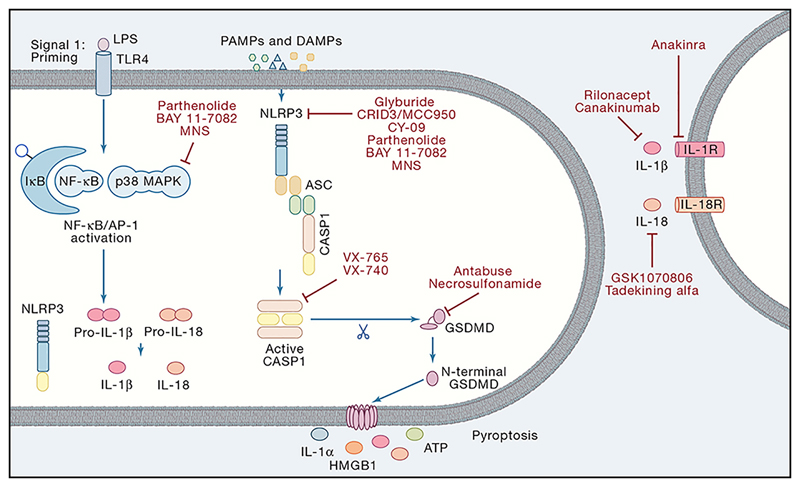
Figure 4. Therapeutic targets in the inflammasome pathways.
To date, several inhibitors have been described for the NLRP3 inflammasome. Parthenolide, BAY 11-7082 and 3,4-methylenedioxy-β-nitrostyrene (MNS) are known to inhibit both NF-κB signalling and the NLRP3 inflammasome. Additionally, specific inhibition of NLRP3 has been reported by studies using glyburide and its derivative CRID3/MCC950. Also, CY-09 has been described as a NLRP3 specific inhibitor. More downstream, both caspase-1 and gasdermin D have been targeted by several compounds. Belnacasan (VX-765) and Pralnacasan (VX-740) are bioavailable prodrugs of a potent inhibitor for caspase-1. Alternatively, Antabuse and necrosulfonamide (NSA) were reported to block cleavage and/or oligomerisation of Gsdmd thereby preventing pyroptosis. Once released, the mature IL-1β and IL-18 signal through its appropriate receptors to initiate inflammation in neighbouring cells. Also, anti-cytokine therapies have been generated. Anakinra is recombinant IL-1 receptor antagonist (IL-1Ra) thereby preventing IL-1 signalling. Canakinumab is a fully human monoclonal anti-IL-1β antibody directed against human IL-1β. Finally, the extracellular domains of both receptors (IL-1R1 and IL-1RAcP) were made into a fusion protein as a soluble decoy receptor (Rilonacept). Alternatively, two IL-18 targeted therapeutics have been described. GSK1070806 is a neutralizing humanized monoclonal antibody and Tadekinig alfa is recombinant human IL-18BP both involved in captured bio-active IL-18 away from its receptor.
Concluding remarks
Caspases are centrally involved in cell death and inflammation, rendering their signalling pathways attractive targets for therapeutic intervention. Progress in understanding caspase biology has continued at a tremendous pace in recent years with the discovery of the non-canonical inflammasome, the identification of GSDMD as the executioner of pyroptosis and the characterization of novel inflammasome pathways. In-depth understanding of caspase pathways has also revealed an unexpected amount of crosstalk between apoptosis, necroptosis and pyroptosis that requires further experimentation before potential clinical implications can be fully appreciated. Further probing is required to understand the physiologic roles of murine caspase-12 as this may shed light on the enigmatic reasons that converted human caspase-12 into a putative pseudoprotease during early human evolution. Studies are also needed to explore potential functions for its pseudoprotease as a scaffold, inhibitor or regulator of cell death and inflammation mechanisms. Further understanding of the physiological roles of caspases 2, 6, 10 and 14 could further expand the significance of caspases in regulation of apoptosis, inflammation, cell cycle and cell differentiation signalling. Undoubtedly, the quest for novel inflammasomes, regulators and upstream signalling components of the NLRP3 and Pyrin inflammasomes will continue to bear fruit and provide unexpected insights. In-depth profiling of caspase cleavage events that occur during activation of the canonical and non-canonical inflammasomes might reveal whether there are differences between these pathways and clarify why nature has evolved several inflammatory caspases with the ability to induce seemingly identical pyroptosis responses. Finally, the field is likely to gain from an expansive focus on human caspase biology to expedite translational work and to complement mechanistic models that are drawn on experimentation in rodents. An important aspect in this regard is to define the molecular machinery and regulation mechanisms of IL-1β secretion from viable monocytes.
Acknowledgements
We apologize to colleagues whose work was not cited because of space constraints. This work was supported by European Research Council Grant 683144 (PyroPop) to M.L.
Footnotes
Conflict of Interest Statement
NVO and ML are employees of Janssen Pharmaceutica. The authors declare that they have no conflict of interest.
References
- Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363 doi: 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. Journal of immunology. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert DF, Xu H, Yang J, Shi X, Gao W, Li L, Bisaro F, Chen S, Valvano MA, Shao F. A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation. Cell Host Microbe. 2016;19:664–674. doi: 10.1016/j.chom.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D'Silva DB, Tanzer MC, Monteleone M, Robertson AA, Cooper MA, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- Berghe TV, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J, et al. Simultaneous Targeting of IL-1 and IL-18 Is Required for Protection against Inflammatory and Septic Shock. American journal of respiratory and critical care medicine. 2014;189:282–291. doi: 10.1164/rccm.201308-1535OC. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–1701. doi: 10.1016/j.jaci.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Chung LK, Park YH, Zheng Y, Brodsky IE, Hearing P, Kastner DL, Chae JJ, Bliska JB. The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome. Cell Host Microbe. 2016;20:296–306. doi: 10.1016/j.chom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:367–385. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2019a;26:146–161. doi: 10.1038/s41418-018-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos NM, Vliegen G, Goncalves A, De Hert E, Martin-Perez R, Van Opdenbosch N, Jallapally A, Geiss-Friedlander R, Lambeir AM, Augustyns K, et al. DPP8/DPP9 inhibition elicits canonical Nlrp1b inflammasome hallmarks in murine macrophages. Life science alliance. 2019b;2 doi: 10.26508/lsa.201900313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D'Herde K, Hachem JP, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity. 2018;49:740–753 e747. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, Malik AB. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49:56–65 e54. doi: 10.1016/j.immuni.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Akey CW, Kumar S. New insights into apoptosome structure and function. Cell Death Differ. 2018;25:1194–1208. doi: 10.1038/s41418-017-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, Lew AM, Lawlor KE, Zhan Y, Vince JE, Gerlic M. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol. 2019 doi: 10.1038/s41590-019-0313-5. [DOI] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity. 2018;48:35–44 e36. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava LL, Schuler F, Sladky V, Haschka MD, Soratroi C, Eiterer L, Demetz E, Weiss G, Geley S, Nigg EA, Villunger A. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes & development. 2017;31:34–45. doi: 10.1101/gad.289728.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Jimbo A, Isoai A, Maruyama K, Momoi T. Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ. 2002;9:1108–1114. doi: 10.1038/sj.cdd.4401080. [DOI] [PubMed] [Google Scholar]
- Fulda S. Smac Mimetics to Therapeutically Target IAP Proteins in Cancer. Int Rev Cell Mol Biol. 2017;330:157–169. doi: 10.1016/bs.ircmb.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O'Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell. 2017;171:1110–1124 e1118. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Watson KD, Lunt M, Consortium BCC, Symmons DP British Society for Rheumatology Biologics, R. The risk of serious infections in patients receiving anakinra for rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2011;50:1341–1342. doi: 10.1093/rheumatology/ker146. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Rathinam VAK, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM, et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. Journal of immunology. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:E4857–4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tsao G, Fuchs M, Shiffman M, Borg BB, Pyrsopoulos N, Shetty K, Gallegos-Orozco JF, Reddy KR, Feyssa E, Chan JL, et al. Emricasan (IDN-6556) Lowers Portal Pressure in Patients With Compensated Cirrhosis and Severe Portal Hypertension. Hepatology. 2019;69:717–728. doi: 10.1002/hep.30199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardot T, Rimmele T, Venet F, Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22:295–305. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, Frouin E, Seyger M, Girard M, Puechberty J, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis) Ann Rheum Dis. 2017;76:1191–1198. doi: 10.1136/annrheumdis-2016-210021. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, et al. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity. 2016;45:761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci U S A. 2014;111:17254–17259. doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of immunology. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaby R. Apoptosis and Autoimmune Disorders. In: Chan J, editor. Autoimmune Diseases. 2012. [Google Scholar]
- Harapas CR, Steiner A, Davidson S, Masters SL. An Update on Autoinflammatory Diseases: Inflammasomopathies. Curr Rheumatol Rep. 2018;20:40. doi: 10.1007/s11926-018-0750-4. [DOI] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S, Hughes MA, Schilling R, Sticht C, Tenev T, Ploesser M, Meier P, Sprick MR, MacFarlane M, Leverkus M. Caspase-10 Negatively Regulates Caspase-8-Mediated Cell Death, Switching the Response to CD95L in Favor of NF-kappaB Activation and Cell Survival. Cell Rep. 2017;19:785–797. doi: 10.1016/j.celrep.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste E, Denecker G, Gilbert B, Van Nieuwerburgh F, van der Fits L, Asselbergh B, De Rycke R, Hachem JP, Deforce D, Prens EP, et al. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742–750. doi: 10.1038/jid.2012.350. [DOI] [PubMed] [Google Scholar]
- Hu B, Jin C, Li HB, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A, Bachovchin DA. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med. 2018;24:1151–1156. doi: 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien O, Wells JA. Caspases and their substrates. Cell Death Differ. 2017;24:1380–1389. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachapati K, O'Brien TR, Bergeron J, Zhang M, Dean M. Population distribution of the functional caspase-12 allele. Hum Mutat. 2006;27:975. doi: 10.1002/humu.9448. [DOI] [PubMed] [Google Scholar]
- Kanneganti A, Malireddi RKS, Saavedra PHV, Vande Walle L, Van Gorp H, Kambara H, Tillman H, Vogel P, Luo HR, Xavier RJ, et al. GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J Exp Med. 2018;215:1519–1529. doi: 10.1084/jem.20172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Karki R, Lee E, Place D, Samir P, Mavuluri J, Sharma BR, Balakrishnan A, Malireddi RKS, Geiger R, Zhu Q, et al. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell. 2018;173:920–933 e913. doi: 10.1016/j.cell.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Kersse K, Lamkanfi M, Bertrand MJ, Vanden Berghe T, Vandenabeele P. Interaction patches of procaspase-1 caspase recruitment domains (CARDs) are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor kappaB signaling. J Biol Chem. 2011;286:35874–35882. doi: 10.1074/jbc.M111.242321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti TD. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med. 2017;214:2217–2229. doi: 10.1084/jem.20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe. 2014;16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudelova J, Fleischmannova J, Adamova E, Matalova E. Pharmacological caspase inhibitors: research towards therapeutic perspectives. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2015;66:473–482. [PubMed] [Google Scholar]
- Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016a;1 doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T, Man SM, Subbarao Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016b;1 doi: 10.1126/sciimmunol.aag2045. aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, Martin A, Di Lorenzo F, Py BF, Molinaro A, Henry T. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun. 2018;9:242. doi: 10.1038/s41467-017-02682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. In Retrospect: The inflammasome turns 15. Nature. 2017;548:534–535. doi: 10.1038/548534a. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279:24785–24793. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. Journal of immunology. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy L, Ngo VN, Emre NC, Shaffer AL, 3rd, Yang Y, Tian E, Nair V, Kruhlak MJ, Zingone A, Landgren O, Staudt LM. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer cell. 2013;23:435–449. doi: 10.1016/j.ccr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Mirrashidi KM, Stowe IB, Kummerfeld SK, Watanabe C, Haley B, Cuellar TL, Reichelt M, Kayagaki N. ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci Rep. 2018a;8:3788. doi: 10.1038/s41598-018-21998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Stowe IB, Gupta A, Kornfeld OS, Roose-Girma M, Anderson K, Warming S, Zhang J, Lee WP, Kayagaki N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med. 2018b;215:2279–2288. doi: 10.1084/jem.20180589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K, Kaisho T, Takemoto K, Suzuki T, Kuranaga E, et al. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep. 2014;8:974–982. doi: 10.1016/j.celrep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Luksch H, Romanowski MJ, Chara O, Tungler V, Caffarena ER, Heymann MC, Lohse P, Aksentijevich I, Remmers EF, Flecks S, et al. Naturally occurring genetic variants of human caspase-1 differ considerably in structure and the ability to activate interleukin-1beta. Hum Mutat. 2013;34:122–131. doi: 10.1002/humu.22169. [DOI] [PubMed] [Google Scholar]
- Luksch H, Winkler S, Heymann MC, Schulze F, Hofmann SR, Roesler J, Rosen-Wolff A. Current knowledge on procaspase-1 variants with reduced or abrogated enzymatic activity in autoinflammatory disease. Curr Rheumatol Rep. 2015;17:45. doi: 10.1007/s11926-015-0520-5. [DOI] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, Herrmann M, Munoz LE. Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Front Immunol. 2016;7:35. doi: 10.3389/fimmu.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, Kanneganti TD. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med. 2018;215:1023–1034. doi: 10.1084/jem.20171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16:467–475. doi: 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T, et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016;167:382–396 e317. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Feng Y, Lyons JD, Berger SB, Otani S, DeLaney A, Tharp GK, Maner-Smith K, Burd EM, Schaeffer M, et al. Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock. Immunity. 2018;49:42–55 e46. doi: 10.1016/j.immuni.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nature reviews. Drug discovery. 2018;17:688. doi: 10.1038/nrd.2018.149. [DOI] [PubMed] [Google Scholar]
- Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 2017;13:e1006502. doi: 10.1371/journal.ppat.1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, Lagou V, Jeru I, Baker PJ, Van Eyck L, Parry DA, Lawless D, De Nardo D, Garcia-Perez JE, Dagley LF, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Science translational medicine. 2016;8 doi: 10.1126/scitranslmed.aaf1471. 332ra345. [DOI] [PubMed] [Google Scholar]
- Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43:908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- McGeough MD, Wree A, Inzaugarat ME, Haimovich A, Johnson CD, Pena CA, Goldbach-Mansky R, Broderick L, Feldstein AE, Hoffman HM. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest. 2017;127:4488–4497. doi: 10.1172/JCI90699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G, Rousell S, Burgess G, Morris M, Wright G, McPherson S, Frenette C, Cave M, Hagerty DT, Spada A, Jalan R. A Placebo-Controlled, Multicenter, Double-Blind, Phase 2 Randomized Trial of the Pan-Caspase Inhibitor Emricasan in Patients with Acutely Decompensated Cirrhosis. J Clin Exp Hepatol. 2018;8:224–234. doi: 10.1016/j.jceh.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16:476–484. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynier S, Rieux-Laucat F. FAS and RAS related Apoptosis defects: From autoimmunity to leukemia. Immunol Rev. 2019;287:50–61. doi: 10.1111/imr.12720. [DOI] [PubMed] [Google Scholar]
- Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Muller DJ. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 2018;37 doi: 10.15252/embj.201798321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okondo MC, Johnson DC, Sridharan R, Go EB, Chui AJ, Wang MS, Poplawski SE, Wu W, Liu Y, Lai JH, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13:46–53. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okondo MC, Rao SD, Taabazuing CY, Chui AJ, Poplawski SE, Johnson DC, Bachovchin DA. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol. 2018;25:262–267 e265. doi: 10.1016/j.chembiol.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT. Attacking cancer's Achilles heel: antagonism of anti-apoptotic BCL-2 family members. The FEBS journal. 2016;283:2661–2675. doi: 10.1111/febs.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A. 2014;111:6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polykratis A, Martens A, Eren RO, Shirasaki Y, Yamagishi M, Yamaguchi Y, Uemura S, Miura M, Holzmann B, Kollias G, et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol. 2019 doi: 10.1038/s41556-019-0324-3. [DOI] [PubMed] [Google Scholar]
- Ramirez MLG, Salvesen GS. A primer on caspase mechanisms. Semin Cell Dev Biol. 2018;82:79–85. doi: 10.1016/j.semcdb.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D, Orning MP, Proulx MK, Wang D, Gavrilin MA, Wewers MD, Alnemri ES, Johnson PF, Lee B, Mecsas J, et al. The Yersinia pestis Effector YopM Inhibits Pyrin Inflammasome Activation. PLoS Pathog. 2016;12:e1006035. doi: 10.1371/journal.ppat.1006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and -8. Immunity. 2017;46:649–659. doi: 10.1016/j.immuni.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SL, Fischer K. Pseudoproteases: mechanisms and function. The Biochemical journal. 2015;468:17–24. doi: 10.1042/BJ20141506. [DOI] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- Saavedra PHV, Huang L, Ghazavi F, Kourula S, Vanden Berghe T, Takahashi N, Vandenabeele P, Lamkanfi M. Apoptosis of intestinal epithelial cells restricts Clostridium difficile infection in a model of pseudomembranous colitis. Nat Commun. 2018;9 doi: 10.1038/s41467-018-07386-5. 4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Surup F, Chauhan D, Niggemann J, Bartok E, Herrmann J, Keck M, Zander W, Stadler M, Hornung V, Muller R. Activation of the NLRP3 Inflammasome by Hyaboron, a New Asymmetric Boron-Containing Macrodiolide from the Myxobacterium Hyalangium minutum. ACS chemical biology. 2018;13:2981–2988. doi: 10.1021/acschembio.8b00659. [DOI] [PubMed] [Google Scholar]
- Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y, Miura M, et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09753-2. 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev. 2017;277:76–89. doi: 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp H, Saavedra PH, de Vasconcelos NM, Van Opdenbosch N, Vande Walle L, Matusiak M, Prencipe G, Insalaco A, Van Hauwermeiren F, Demon D, et al. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:14384–14389. doi: 10.1073/pnas.1613156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp H, Van Opdenbosch N, Lamkanfi M. Inflammasome-Dependent Cytokines at the Crossroads of Health and Autoinflammatory Disease. Cold Spring Harb Perspect Biol. 2019;11 doi: 10.1101/cshperspect.a028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opdenbosch N, Van Gorp H, Verdonckt M, Saavedra PHV, de Vasconcelos NM, Goncalves A, Vande Walle L, Demon D, Matusiak M, Van Hauwermeiren F, et al. Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4. Cell Rep. 2017;21:3427–3444. doi: 10.1016/j.celrep.2017.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Walle L, Jimenez Fernandez D, Demon D, Van Laethem N, Van Hauwermeiren F, Van Gorp H, Van Opdenbosch N, Kayagaki N, Lamkanfi M. Does caspase-12 suppress inflammasome activation? Nature. 2016;534:E1–4. doi: 10.1038/nature17649. [DOI] [PubMed] [Google Scholar]
- Vande Walle L, Lamkanfi M. Pyroptosis. Current biology: CB. 2016;26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512:69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, Vijayaraj S, Lindqvist LM, Bouillet P, et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1beta Activation. Cell Rep. 2018;25:2339–2353 e2334. doi: 10.1016/j.celrep.2018.10.103. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, Yao X, Puck JM, Straus SE, Lenardo MJ. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity. 2019 doi: 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, Civitelli R, Abu-Amer Y, Kanneganti TD, Link DC, Mbalaviele G. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018;16:e3000047. doi: 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- Xue Y, Daly A, Yngvadottir B, Liu M, Coop G, Kim Y, Sabeti P, Chen Y, Stalker J, Huckle E, et al. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–670. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdagul A, Jr, Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med. 2017;4:86. doi: 10.3389/fcvm.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverenyi I, Takeichi T, Balaji R, Lau A, et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167:187–202 e117. doi: 10.1016/j.cell.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Zhong FL, Robinson K, Teo DET, Tan KY, Lim C, Harapas CR, Yu CH, Xie WH, Sobota RM, Au VB, et al. Human DPP9 represses NLRP1 inflammasome and protects against auto-inflammatory diseases via both peptidase activity and FIIND domain binding. J Biol Chem. 2018 doi: 10.1074/jbc.RA118.004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti TD. Gasdermin D Promotes AIM2 Inflammasome Activation and Is Required for Host Protection against Francisella novicida. Journal of immunology. 2018;201:3662–3668. doi: 10.4049/jimmunol.1800788. [DOI] [PMC free article] [PubMed] [Google Scholar]