Abstract
Our understanding of the plasma membrane has markedly increased since Singer and Nicolson proposed the fluid mosaic model in 1972. While their revolutionary theory of the lipid bilayer remains largely valid, it is now known that lipids and proteins are not randomly dispersed throughout the plasma membrane but instead may be organized within membrane microdomains, commonly referred to as lipid rafts. Lipid rafts are highly dynamic, detergent resistant, and enriched with both cholesterol and glycosphingolipids. The two main types are flotillin-rich planar lipid rafts and caveolin-rich caveolae. It is proposed that flotillin and caveolin proteins regulate cell communication by compartmentalizing and interacting with signal transduction proteins within their respective lipid microdomains. Consequently, membrane rafts play an important role in vital cellular functions including migration, invasion, and signaling; thus, alterations in their microenvironment can initiate signaling pathways that affect cellular function and behavior. Therefore, the identification of lipid rafts and their associated proteins is integral to the study of transmembrane signaling. Here, we review the current standard protocols and biochemical approaches used to isolate and define raft proteins from epithelial cells and tissues. Furthermore, in Section 3 of this chapter, detailed protocols are offered for isolating lipid rafts by subjection to detergent and sucrose density centrifugation, as well as an approach for selectively isolating caveolae. Methods to manipulate rafts with treatments such as methyl-β-cyclodextrin and flotillin III are also described.
Keywords: Lipid raft, Detergent resistant, Membrane microdomain, Caveolae, Caveolin, Methyl-β-cyclodextrin
1. Introduction
In 1972, the fluid mosaic model of the cell membrane was proposed by Singer and Nicolson (1). They hypothesized that lipid and protein molecules are randomly distributed throughout the lipid bilayer; four decades later, a more dynamic model has evolved, revealing organized membrane microdomains, referred to as lipid rafts (2, 3). Lipid rafts contain a high concentration of both cholesterol and glycosphingolipids and are able to float freely within the plasma membrane, thus permitting aggregation and formation of larger, more stable platform domains (4). In addition, protein–protein and lipid–protein interactions, especially by those involving cyto-skeletal proteins, increase the stability and regulatory functions of membrane rafts (5). There are two types of lipid rafts, planar and caveolae (Fig. 1). Caveolae are distinguishable as flask-shaped invaginations (50–100 nm) of the membrane formed by the integral membrane scaffolding protein, caveolin. In the absence of caveolins, planar lipid rafts are sustained by the integral membrane protein flotillin. Lipid rafts are emerging as key players in many biological functions including protein trafficking, endocytosis, neurotransmission, and cell communication by serving as organization centers for signaling molecules (6, 7).
Fig. 1.
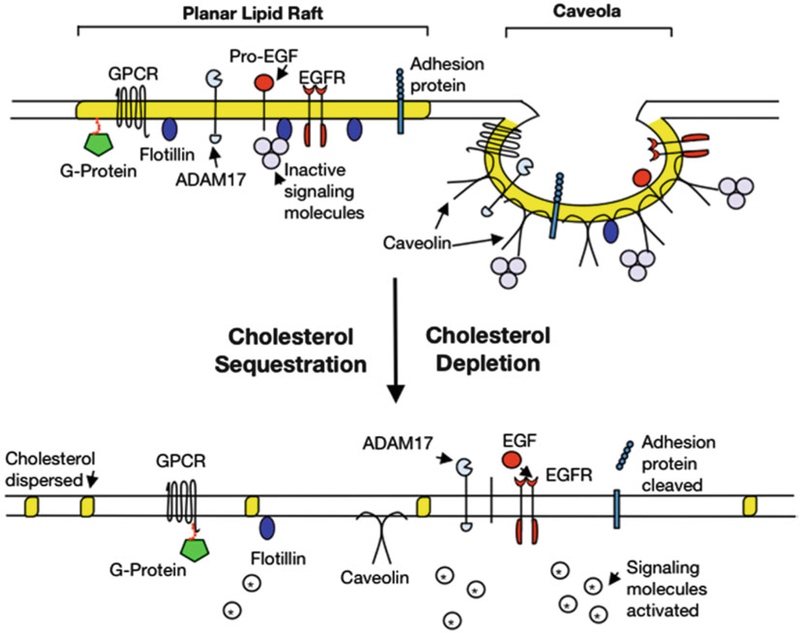
Planar and caveolae lipid rafts within the plasma membrane. Yellow-highlighted regions represent areas of high concentration of cholesterol and sphingolipids. Caveolin proteins, essential to caveolae formation, and flotillin proteins are able to bind and compartmentalize signaling molecules and regulate their activity. Lipid raft disruption by treatment with MβCD, filipin III, or shear stress results in dispersion of cholesterol molecules and leveling of caveolae. Subsequently, several signaling molecules are activated potentiating signal transduction events. Abbreviations: MβCD methyl-β-cyclodextrin, GPCR G-protein-coupled receptor, ADAM17 a disintegrin and metalloprotease domain 17 or TACE, Pro-EGF pro-epidermal growth factor, EGFR epidermal growth factor receptor
Discerning the roles of lipid rafts and their constitutive proteins has proven to be a challenging task and is not without controversy with regards to both methods and results. An attractive approach for tackling this endeavor has been to investigate the effects of disrupting lipid rafts with methyl-β-cyclodextrin (MβCD), a cyclic oligosaccharide that forms soluble complexes with cholesterol and depletes them from the membrane. In keratinocytes, raft disruption results in upregulation of many signal transduction proteins such as IL8, MMPs, EGFR, ERK, Akt, p38 MAPK, and ERK1/2 (8–11). Interestingly, an increase in substrates cleaved by tumor necrosis factor-alpha (TNF-α) converting enzyme (ADAM17 or TACE) occurs with MβCD treatment, leading investigators to postulate that lipid rafts also regulate enzymatic activity by limiting substrate entry into rafts (12). Furthermore, lipid raft disruption by ultraviolet (UV) irradiation results in a decrease in raft cholesterol levels and activation of pro-apoptotic pathways by Fas-receptor protein and ceramide (13). Treatment with sterols, which lessen cholesterol loss, partly decreased this response (14). In summary, results from these studies suggest that lipid rafts are integral to homeostatic cell-to-cell interactions and that their alteration leads to activation of pathways, which affect vital cellular processes.
A point of fact, alterations in lipid rafts have been found to have pathological implications. For instance, an inverse relationship between caveolin expression and severity of the skin disease, psoriasis has been reported (15). Similarly, atopic dermatitis, an inflammatory skin disease, has changes in gene expression that are comparable to those resulting from lipid raft disruption in keratinocytes (9). In cancer, caveolin-1 has been found to have a dual role. Studies in keratinocytes showed that caveolins suppress growth factor signaling pathways (16). This finding is supported by findings in Cavl null mice, which lack caveolae, that showed increased epidermal proliferation and susceptibility to premalignant lesions in response to the chemical carcinogen DMBA/TPA (17). Conversely, in anchorage-independent cancer cells, such as melanoma, caveolin expression is associated with increased malignancy and metastasis (18,19). It is postulated that the association of lipid raft proteins, such as caveolins, with cytoskeletal proteins and their role in cell adhesion processes may partially explain the mechanism for malignant invasion (20). Lipid rafts have been shown to be associated with a number of adhesion junction proteins (Table 1); however, more studies are needed to determine the implications of such interactions. Indeed, the Mahoney lab recently discovered that caveolin-1 associates with desmoglein-2, a desmosomal adhesion protein that modulates mitogenic signaling suspected to be involved in oncogenesis (21).
Table 1.
Adhesion proteins and lipid rafts
| Protein | Junction type | Lipid raft association | Reference |
|---|---|---|---|
| Nectin-1 | Adherens | No | (45) |
| Cadherin 13 | Adherens | Yes | (46) |
| Afadin | Adherens | Minimal | (45) |
| Filamin | Adherens | Yes | (6) |
| Eplin | Adherens | TBD | |
| Alpha-catenin | Adherens | Yes | (47) |
| Beta-catenin | Adherens | Yes | (37, 48,49) |
| Plekha7 | Adherens | TBD | |
| Nezha | Adherens | TBD | |
| E-cadherin | Adherens | Yes | (37, 47) |
| Claudin 1–5 | Tight | Yes | (50) |
| Claudin 14 | Tight | Yes | (51) |
| Occludin | Tight | Yes | (52) |
| Jam-1 | Tight | No | (53) |
| Zo-1 | Tight | Yes | (54) |
| Connexin 43, 32, 36, 46 | Gap | Yes | (40, 55) |
| Connexin 26, 50 | Gap | No | (55) |
| Desmoglein 2 | Desmosome | Yes | (21, 46, 56) |
| Desmoglein 3 | Desmosome | Yes | (57) |
| Desmocollin 2 | Desmosome | Yes | (58) |
| Plakoglobin | Desmosome | Yes | (46, 56) |
| Desmoplakin | Desmosome | Yes | (58) |
| Actin | Cytoskeletal | Yes | (46) |
| Integrin, beta 1 | Focal adhesion | Yes | (46) |
TBD to be determined
Major adhesion junction proteins of epithelial cells and their known association with lipid rafts are listed
To date, most of our knowledge of lipid rafts is a result of the hypothesis that the high glycolipoprotein content of the lipid raft renders it insoluble in nonionic detergents; hence, lipid rafts are also known as detergent-resistant membrane domains (22). The method of lipid raft isolation by detergent is controversial in that results may differ depending on the conditions, including temperature, detergent concentration, and type of detergent utilized (23). Also, biochemical methods are unable to isolate lipid rafts in their innate structure (24). In fact, for some time there was uncertainty regarding the existence of lipid rafts at all, as the nanometric size of lipid rafts is below the diffraction limit of standard confocal laser scanning microscopy, and, thus, rafts were not detectable in vivo. Any uncertainty has been disbanded by novel techniques, such as Forster resonance energy transfer, fluorescence polarization anisotropy, total internal reflection fluorescence microscopy, and single-molecule spectroscopy (25). These techniques have provided confirmation of the existence of cholesterol and sphingolipid microdomains. Furthermore, diffusion of cholesterol-dependent GPI-anchored proteins in the apical plasma membrane has been observed (25, 26). Although promising, these innovative methods still have their own individual challenges, which may affect the intrinsic state of the cell (25).
Similarly, biochemical techniques for lipid raft isolation and pro-teomic analysis are not without criticism, but they do have the advantage of being well studied. The detergent Triton X-100 (TX-100) at 4 °C is most commonly used for lipid raft purification, but other detergents have been utilized with results perhaps reflecting the nature of the raft domain isolated (27). Other detergents studied include Brij 58, Brij 96, Brij 98, Lubrol WX, CHAPS, and Triton X-114 (28). Notably, Brij 98 may be used at physiologic temperature, 37 °C, thus avoiding any effect temperature may have, as it has been suggested that lipid rafts aggregate upon treatment at 4 °C, therefore not allowing identification of proteins in distinct rafts (27). Ideally, the least amount of detergent that will dissolve the non-raft membrane proteins, such as transferrin receptor, should be used (27). The reproducible solubility of these proteins is the advantage of using TX-100. Inconsistent reports of whether Lubrol WX sufficiently dissolves non-raft membrane proteins have been published, and while Brij 96 and Brij 98 are efficient at solubilizing the non-raft membrane when compared to Lubrol WX, the detergent-resistant, light density fraction still contains non-raft proteins (28). Furthermore, Schuck et al. compared the lipid component of insoluble fractions from various detergents and found that the lipid component of TX-100 contained a lipid ratio comparable to that expected in lipid rafts. Conversely, Lubrol WX and Brij 98 contained a ratio more comparable to the total membrane, suggesting that these detergents are not as accurate in preferentially isolating lipid rafts. On the other hand, TX-100 solubilizes some lipid raft proteins including insulin receptors, which are known to interact with caveolins (29). Manipulation of lipid rafts can be performed biochemically as well. Removal of cholesterol may be achieved with MβCD, and inhibition of cholesterol synthesis may be accomplished with HMG-CoA reductase inhibitors (2). Additionally, rafts may be sequestered with antimicrobial agents such as nystatin A or filipin III (30). Cholera toxin, which targets gangliosides, can be used to stain for lipid rafts (31).
In summary, there is no single, ideal method for isolating lipid rafts, and thus, an integrated approach utilizing biochemical, imaging, and novel techniques needs to be established. As biochemical techniques for lipid raft isolation are currently the most widely used and cost-effective methodology available, this chapter focuses on and provides validated biochemical methods as well as practical notes for the isolation and analysis of lipid rafts.
2. Materials
2.1. Major Equipment
SW60 swing bucket rotor (Beckman Coulter, Brea, CA, USA); corresponding ultracentrifuge tubes (Cat# 326819; 5.0 mL thinwall, polyallomer tubes for SW60 rotor; Beckman Coulter).
SW41Ti swing bucket rotor (Cat# 333790, Beckman Coulter); corresponding ultracentrifuge tubes (Cat# 331372; 13.2 mL thinwall, polyallomer tubes for SW41 rotor; Beckman Coulter).
Ultracentrifuge (Beckman Coulter).
2.2. Reagents
Unless otherwise stated, most reagents are available from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin (P/S) (Life Technologies, Grand Island, NY); CnT-57 medium and supplements (CELLnTEC, Bern, Switzerland); EpiLife medium supplemented with Human Keratinocyte Growth Supplement (Life Technologies); 5-cholestene-5-β-ol (Steraloids, Newport, RI); Caveolin-1 competing peptide-a fusion of the caveolin-1 scaffolding domain peptide (aa 82–101) with the cell permeable Antennapedia sequence (aa 43–58) (Cat# 219482) and scrambled caveolin-1 negative control peptide (Cat# 219483) (Millipore, La Jolla, CA); Trypan Blue Exclusion Assay (Cat# 15250061, Life Technologies).
2.3. Buffers
Phosphate-buffered saline (PBS; Cat# BP665–1; Fisher Scientific, Pittsburgh, PA).
TNE buffer: 25 mM Tris–HCl (pH 7.5) (Cat# BP1757–500; Fisher Scientific), 150 mM NaCl, and 5 mM EDTA.
Complete cell lysis TNE buffer: 25 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 % (v/v) Triton X-100 (TX-100; Cat# BP151–500; Fisher Scientific), 1 mM phenylmethanesulfonylfluoride or phenylmethylsulfonyl fluoride (PMSF; Cat# 93482), complete protease inhibitor cocktail (Cat# 11697–498-001, Roche Diagnostics, Indianapolis, IN), and phosphatase inhibitor cocktail 2 (Cat# P8340).
90, 35, or 5 % (w/v) Sucrose (Cat# 84097) in TNE buffer: Dissolve 90, 35, or 5 g of sucrose with TNE buffer to bring the volume to 100 mL. Warm with stirring until sucrose is dissolved.
2.4. Antibodies
Antibodies to lipid raft proteins include anti-caveolin-1 (Cat#sc-894; Santa Cruz Biotechnology, Santa Cruz, CA); anti-caveolin-2 (Cat# 8522; Cell Signaling Technology, Danvers, MA); anti-caveolin-2 (Cat# ab2912; Abcam, Cambridge, MA); anti-flotillin-1 (Cat# 610820; BD Transduction Labs, Franklin Lakes, NJ); and anti-flotillin-2 (Cat# 610383; BD Transduction Labs).
2.5. Cells
HaCaT, a spontaneously immortalized, nontumorigenic cell line derived from human keratinocytes (32, 33).
A431, an epidermoid carcinoma cell line (Cat# CRL-1555; ATCC, Bethesda, MD).
Alternatively, primary human epidermal keratinocytes can be used and are available from several commercial sources: PHEK (CELLnTEC) and HEK (Life Technologies).
3. Methods
3.1. Cell Culture
HaCaT and A431 cells are maintained in DMEM supplemented with 10 % FBS, 2 mM glutamine, and 1 % P/S (33, 34). PHEK and HEK cultures are grown in complete CnT-57 or EpiLife supplemented with human keratinocyte growth supplement, respectively. All cells are maintained in a humidified incubator with 5 % CO2 at 37 °C. Cells are plated at a density of approximately 2 × 106 in 100 mm culture dishes to obtain approximately 9 × 106 cells at confluence. If grown in FBS-containing medium, cells are serum-starved from 1 to 24 h prior to experimentation. Cells should be approximately 70–80 % confluent when used.
3.2. Lipid Raft Disruption by Cholesterol Depletion
To disrupt lipid rafts by depleting or sequestering membrane cholesterol, cultured cells in serum-free medium are treated with MβCD (10 mM or 1 %) or filipin III (2 μg/mL) for 1 h. Alternatively, cells can be treated with 5-cholestene-5-β-ol (5 μM) for 2 h or simvastatin (5 mg/mL) for 24 h. Repletion is performed by adding cholesterol (5 μM or 10 μg/mL) or cholesterol-loaded MβCD (5 μM or 10 μg/mL) (35–38). Trypan blue exclusion assay can be used to measure the level of cell death after drug treatment.
3.3. Disruption of Caveolin Association by Scaffolding Domain Peptide
To displace proteins from binding to caveolin-1, treat cells with a cell-permeable caveolin-1 scaffolding domain peptide (3 μM) for up to 24 h (16). As negative control, treat cells with a scrambled peptide (3 μM) of the caveolin-1 scaffolding domain.
3.4. Preparation of Lipid Raft Fraction
The following ultracentrifugation method relies on two unique properties of lipid rafts: (1) cold detergent resistance and (2) low buoyant density.
Pre-chill all equipment and solutions on ice.
Wash cells three times with ice-cold PBS, and scrape cells in 2 mL complete TNE (see Note 1) buffer lysis buffer containing 1 % TX-100 (see Notes 2 and 3) using a cell scraper (Cat# 08–771-1A; Fisher Scientific) (21, 39).
Disrupt cell by passing through a tight-fitting glass Dounce Homogenizer (Cat# NC0253759; Fisher Scientific) 20 times or by repeated aspiration through a 23-gauge needle (Cat# 305120; BD Biosciences, Waltham, MA) using a 5 mL syringe (Cat# 309646; BD Biosciences) 20 times.
Vortex and transfer 2 mL into a 13-mL ultracentrifuge tube. Save the remaining sample for total cell lysate.
Add 2 mL of 90 % sucrose in TNE to bring the sucrose concentration to 45 %.
Carefully overlay with 4 mL each of 35 and 5 % sucrose in TNE by gently adding the solutions down the side of the tube (1 mL at a time; Fig. 2).
Centrifuge the gradients at 38,000–40,000 rpm (maximum force of approximately 273,865 × g) for 16–20 h in an SW41Ti rotor at 4 °C (see Notes 4 and 5).
Collect twelve 1-mL fractions from the top while keeping all samples on ice (Fig. 2).
Boil aliquots (10–40 μL) of each fraction in Laemmli buffer in preparation for SDS-PAGE and immunoblotting. Store the remaining samples at –70 ° C.
Fig. 2.
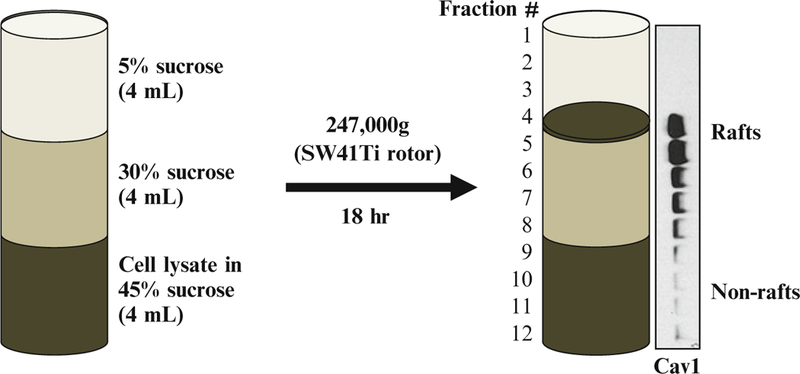
Schematic diagram showing isolation of lipid rafts by discontinuous sucrose gradient centrifugation. Cell lysate is prepared in a lysis buffer containing 45 % sucrose and transferred to an ultracentrifuge tube. Two, 4 mL each, of 30 and 5 % sucrose are layered over the sample as shown. The gradient is subjected to centrifugation for 18 h at approx. 247,000 × g allowing the buoyant lipid rafts to float to the 30 and 5 % interface. Twelve 1-mL fractions are collected from the top and aliquots prepared for Western blotting showing the presence of caveolin-1 (Cav1) in the light density fractions (#4 and #5) (21)
3.5. Characterization of Lipid Raft-Associated Proteins
Western blotting: Equal amount of each fraction (Section 3.4) is resolved over SDS-PAGE (12.5 % acrylamide) and electro-transferred onto a nitrocellulose or a PVDF membrane for immunoblotting as previously described (21) for caveolin-1, caveolin-2, flotillin-1, and flotillin-2 (all antibodies at 1:1,000 dilution).
Immunoprecipitation: Combine fractions 4 and 5 (Section 3.4) for immunoprecipitation using anti-caveolin-1 or -caveolin-2 antibodies as previously described (40).
4. Notes
- Alternative to TNE, the following buffers can also be used:
-
(a)MBS buffer: 25 mM 2-(N-morpholino)ethanesulfonic acid (MES, pH 6.6, Cat# M2933) and 150 mM NaCl.
-
(b)RIPA buffer: PBS, 1 % Nonidet P-40 (Cat# 98379), and 0.5 % sodium deoxycholate (Cat #30970).
-
(a)
Alternative to TX-100, other nonionic detergents can be used including Lubrol WX (1 %), CHAPS (1 %), Brij 98 (1 %), or 500 mM sodium carbonate (41, 42). Note that cholesterol resides in both lipid rafts and non-lipid rafts, and this may alter results from membrane extractions in the presence of detergents (43).
Acute depletion of membrane cholesterol also disrupts other lipids including PI(4, 5)P2, and thus not all cellular changes result solely from disruption of lipid rafts (44).
Membrane preparations must be subjected to ultracentrifugation immediately and not frozen.
If using an SW60 rotor, mix 0.4 mL cell lysate in complete TNE with 1 % TX-100 with 0.4 mL of 90 % sucrose in TNE. Layer with 2.2 mL 30 % sucrose in TNE and then 1.2 mL of 5 % sucrose in TNE. Centrifuge at 49,000 rpm for 18 h, and collect 0.4 mL fractions.
Acknowledgments
We thank Dr. Michael DiPersio (Center for Cell Biology & Cancer Research, Albany Medical College), Dr. Mon-Li Chu, Donna Brennan, and Andrew Overmiller for critically reading the paper and for their insightful discussions. We thank Jordan Wesolowski and Dr. Fabienne Paumet for the protocol using the SW60 rotor. This work was supported by grants from the National Institutes of Health (Mahoney, R01AR056067).
References
- 1.Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731 [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39 [DOI] [PubMed] [Google Scholar]
- 3.Suomalainen M (2002) Lipid rafts and assembly of enveloped viruses. Traffic 3:705–709 [DOI] [PubMed] [Google Scholar]
- 4.Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327:46–50 [DOI] [PubMed] [Google Scholar]
- 5.Head BP, Patel HH, Insel PA (2014) Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838:532–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA (2006) Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281:26391–26399 [DOI] [PubMed] [Google Scholar]
- 7.Stuermer CA (2010) The reggie/flotillin connection to growth. Trends Cell Biol 20: 6–13 [DOI] [PubMed] [Google Scholar]
- 8.Giltaire S, Lambert S, Poumay Y (2011) HB-EGF synthesis and release induced by cholesterol depletion of human epidermal keratinocytes is controlled by extracellular atp and involves both p38 and ERK½ signaling pathways. J Cell Physiol 226:1651–1659 [DOI] [PubMed] [Google Scholar]
- 9.Mathay C, Pierre M, Pittelkow MR, Depiereux E, Nikkels AF, Colige A, Poumay Y (2010) Transcriptional profiling after lipid raft disruption in keratinocytes identifies critical mediators of atopic dermatitis pathways. J Invest Dermatol 131:46–58 [DOI] [PubMed] [Google Scholar]
- 10.Bang B, Gniadecki R, Gajkowska B (2005) Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp Dermatol 14:266–272 [DOI] [PubMed] [Google Scholar]
- 11.Gniadecki R (2004) Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun 320:165–169 [DOI] [PubMed] [Google Scholar]
- 12.Tellier E, Canault M, Rebsomen L, Bonardo B, Juhan-Vague I, Nalbone G, Peiretti F (2006) The shedding activity of ADAM17 is sequestered in lipid rafts. Exp Cell Res 312: 3969–3980 [DOI] [PubMed] [Google Scholar]
- 13.Grether-Beck S, Salahshour-Fard M, Timmer A, Brenden H, Felsner I, Walli R, Füllekrug J, Krutmann J (2008) Ceramide and raft signaling are linked with each other in UVA radiation-induced gene expression. Oncogene 27:4768–4778 [DOI] [PubMed] [Google Scholar]
- 14.Bayer M, Proksch P, Felsner I, Brenden H, Kohne Z, Walli R, Duong TN, Götz C, Krut-mann J, Grether-Beck S (2011) Photoprotection against UVAR: effective triterpenoids require a lipid raft stabilizing chemical structure. Exp Dermatol 20:955–958 [DOI] [PubMed] [Google Scholar]
- 15.Ma WY, Zhuang L, Cai DX, Zhong H, Zhao C, Sun Q (2012) Inverse correlation between caveolin-1 expression and clinical severity in psoriasis vulgaris. J Int Med Res 40: 1745–1751 [DOI] [PubMed] [Google Scholar]
- 16.Qin H, Bollag WB (2013) The caveolin-1 scaffolding domain peptide decreases phosphati-dylglycerol levels and inhibits calcium-induced differentiation in mouse keratinocytes. PLoS One 8:e80946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trimmer C, Sotgia F, Lisanti MP, Capozza F (2013) Cav1 inhibits benign skin tumor development in a two-stage carcinogenesis model by suppressing epidermal proliferation. Am J Transl Res 5:80–91 [PMC free article] [PubMed] [Google Scholar]
- 18.Staubach S, Hanisch FG (2011) Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics 8:263–277 [DOI] [PubMed] [Google Scholar]
- 19.Fecchi K, Travaglione S, Spadaro F, Quattrini A, Parolini I, Piccaro G, Raggi C, Fabbri A, Felicetti F, Carè A (2012) Human melanoma cells express FGFR/Src/Rho signaling that entails an adhesion-independent caveolin-1 membrane association. Int J Cancer 130:1273–1283 [DOI] [PubMed] [Google Scholar]
- 20.Parton RG, del Pozo MA (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14:98–112 [DOI] [PubMed] [Google Scholar]
- 21.Brennan D, Peltonen S, Dowling A, Medhat W, Green KJ, Wahl JK, Del Galdo F, Mahoney MG (2011) A role for caveolin-1 in desmoglein binding and desmosome dynamics. Oncogene 31:1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572 [DOI] [PubMed] [Google Scholar]
- 23.Babiychuk EB, Draeger A (2006) Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J 397:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heerklotz H (2002) Triton promotes domain formation in lipid raft mixtures. Biophys J 83:2693–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11:688–699 [DOI] [PubMed] [Google Scholar]
- 26.Pinaud F, Michalet X, Iyer G, Margeat E, Moore HP, Weiss S (2009) Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking. Traffic 10:691–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain LH (2004) Detergents as tools for the purification and classification of lipid rafts. FEBS Lett 559:1–5 [DOI] [PubMed] [Google Scholar]
- 28.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A 100:5795–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Strålfors P (1999) Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 13: 1961–1971 [PubMed] [Google Scholar]
- 30.Brown DA (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21:430–439 [DOI] [PubMed] [Google Scholar]
- 31.Vind-Kezunovic D, Nielsen CH, Wojewodzka U, Gniadecki R (2008) Line tension at lipid phase boundaries regulates formation of membrane vesicles in living cells. Biochim Biophys Acta 1778:2480–2486 [DOI] [PubMed] [Google Scholar]
- 32.Schoop VM, Mirancea N, Fusenig NE (1999) Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol 112:343–353 [DOI] [PubMed] [Google Scholar]
- 33.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP (1973) In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 51:1417–1423 [DOI] [PubMed] [Google Scholar]
- 35.Klein U, Gimpl G, Fahenholz F (1995) Alteration ofthe myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34:13784–13793 [DOI] [PubMed] [Google Scholar]
- 36.Powers KA, Szászi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD (2006) Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med 203:1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roitbak T, Surviladze Z, Tikkanen R, Wandinger-Ness A (2005) A polycystin multiprotein complex constitutes a cholesterol-containing signalling microdomain in human kidney epithelia. Biochem J 392:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss JI, Garrett TJ, Hansen PJ (2012) Involvement of free cholesterol and high-density lipoprotein in development and resistance of the preimplantation bovine embryo to heat shock. J Anim Sci 90:3762–3769 [DOI] [PubMed] [Google Scholar]
- 39.Galbiati F, Volonte D, Brown AM, Weinstein DE, Ben-Ze’ev A, Pestell RG, Lisanti MP (2000) Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem 275:23368–23377 [DOI] [PubMed] [Google Scholar]
- 40.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2008) Caveolin-1 and −2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19:912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pike LJ, Han X, Gross RW (2005) Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem 280:26796–26804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddell DR, Chistie G, Hussain I, Dingwall C (2001) Compartmentalization of β-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol 11:1288–1293 [DOI] [PubMed] [Google Scholar]
- 43.Zidovetzki R, Levitan I (2007) Use of cyclo-dextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 1768:1311–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pike LJ, Miller JM (1998) Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem 273:22298–22304 [DOI] [PubMed] [Google Scholar]
- 45.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH (2003) Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol 77:9542–9552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blonder J, Terunuma A, Conrads TP, Chan KC, Yee C, Lucas DA, Schaefer CF, Yu LR, Issaq HJ, Veenstra TD, Vogel JC (2004) A proteomic characterization of the plasma membrane of human epidermis by high-throughput mass spectrometry. J Invest Dermatol 123:691–699 [DOI] [PubMed] [Google Scholar]
- 47.Seveau S, Bierne H, Giroux S, Prévost MC, Cossart P (2004) Role of lipid rafts in E-cad-herin-and HGF-R/Met-mediated entry of Listeria monocytogenes into host cells. J Cell Biol 166:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Causeret M, Taulet N, Comunale F, Favard C, Gauthier-Rouvière C (2005) N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol Biol Cell 16:2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patra SK, Bettuzzi S (2007) Epigenetic DNA-methylation regulation of genes coding for lipid raft-associated components: a role for raft proteins in cell transformation and cancer progression (review). Oncol Rep 17: 1279–1290 [PubMed] [Google Scholar]
- 50.Sugibayashi K, Onuki Y, Takayama K (2009) Displacement of tight junction proteins from detergent-resistant membrane domains by treatment with sodium caprate. Eur J Pharm Sci 36:246–253 [DOI] [PubMed] [Google Scholar]
- 51.Lambert D, O’Neill CA, Padfield PJ (2005) Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J 387:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthöfer H (2001) Involvement of lipid rafts in nephin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 159:1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL (2004) RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol 287:C327–C335 [DOI] [PubMed] [Google Scholar]
- 54.Bowie RV, Donatello S, Lyes C, Owens MB, Babina IS, Hudson L, Walsh SV, O’Donoghue DP, Amu S, Barry SP, Fallon PG, Hopkins AM (2012) Lipid rafts are disrupted in mildly inflamed intestinal microenvironments without overt disruption of the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 302: G781–G793 [DOI] [PubMed] [Google Scholar]
- 55.Schubert AL, Schubert W, Spray DC, Lisanti MP (2002) Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41:5754–5764 [DOI] [PubMed] [Google Scholar]
- 56.Nava P, Laukoetter MG, Hopkins AM, Laur O, Gerner-Smidt K, Green KJ, Parkos CA, Nusrat A (2007) Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium. Mol Biol Cell 18:4565–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delva E, Jennings JM, Calkins CC, Kottke MD, Faundez V, Kowalczyk AP (2008) Pemphigus vulgaris IgG-induceddesmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin-and dynamin-independent mechanism. J Biol Chem 283:18303–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resnik N, Sepcic K, Plemenitas A, Windoffer R, Leube R, Veranic P (2011) Desmosome assembly and cell-cell adhesion are membrane raft-dependent processes. J Biol Chem 286:1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]


