1. Background
Nuclear medicine complements more conventional radiographic techniques and plays a vital role in the evaluation of orthopedic infections. In this review, we will analyze scintigraphic imaging techniques such as the Nuclear Medicine (NM) bone scan, Gallium 67, radiolabeled white blood cells (WBC)/sulfur colloid and 18FDG PET/CT in evaluation of osteomyelitis, cellulitis, septic vs. aseptic prosthetic joints, and septic arthritis. The addition of hybrid imaging using SPECT/CT will be addressed. We also provide a brief overview of novel scintigraphic techniques including imaging with radiolabeled antibodies,99mTc-Ciprofloxacin, 99mTc-antimicrobial peptides, radiolabeled receptor-specific proteins, 99mTc-nanocolloid studies and experimental PET-CT tracers.
In order to comprehend the role Nuclear Medicine plays in the evaluation of infections, we must first understand the role of conventional imaging. Standard radiographic imaging evaluates anatomy whereas Nuclear Medicine evaluates function and physiology. Standard radiographs should be used in the initial assessment of infections but have limited sensitivity in the early stages (10–21 days after onset of osteomyelitis) as demonstrable morphologic changes may have not yet occurred.1, 2, 3 The triple phase Nuclear Medicine bone scan can demonstrate the presence of osteomyelitis within 2–3 days of the onset of infection. CT scan is not typically used in the evaluation of osteomyelitis or soft tissue infection except when determining the extent of osseous destruction, where biopsy guidance is required, or if patients have a contraindication to MRI.4,5 Regarding conventional imaging techniques, MRI is the modality of choice to evaluate osteomyelitis or soft tissue infections. In cases of infection, MRI typically demonstrates soft tissue edema and hyperemia and has excellent soft tissue contrast.6,7 Though MRI demonstrates high sensitivity and specificity for osteomyelitis (90%), surgical hardware can induce artifacts and obscure evaluation of periprosthetic soft tissues. Additionally, in evaluation of suspected infection in the setting of recent trauma, soft tissue changes secondary to trauma versus those secondary to infection can be difficult to differentiate on MRI; this is where functional imaging can be helpful in diagnosis. Nuclear medicine interpretation is not typically limited by internal hardware and does not rely on morphological changes for diagnosis. When standard radiographic imaging is inconclusive or contraindicated, Nuclear Medicine allows diagnostic imaging of infection from a different vantage point.
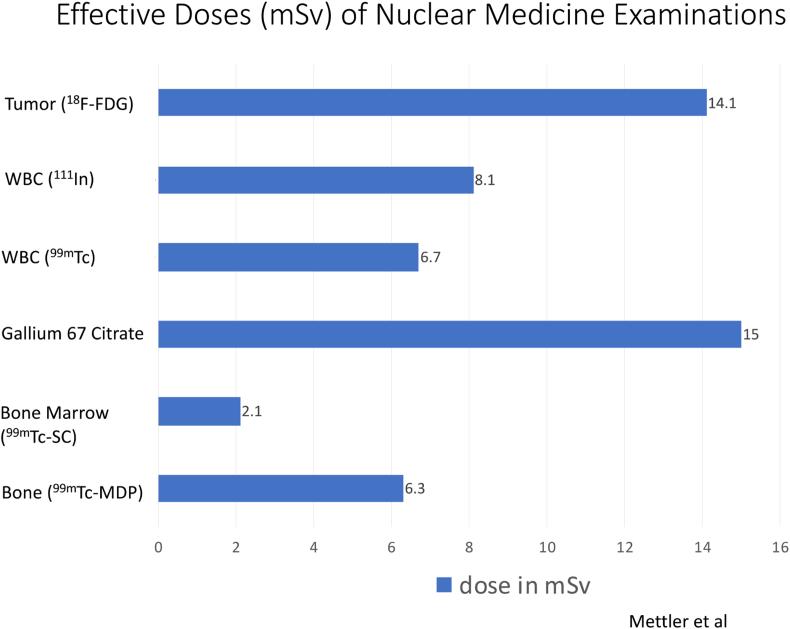
We will also briefly address the various radiation exposure rates of the Nuclear Medicine modalities described in this review (Fig. 1). The level of radiation exposure of nuclear medicine examinations is similar to that of CT scans, ranging from 2 to 15 milliSieverts (mSv).8 The International Commission on Radiologic protection recommends that artificial radiation exposure be limited to no more than 50 mSv per year.9
Fig. 1.
Effective doses of Nuclear Medicine examinations.
2. Osteomyelitis
Our first point of discussion is osteomyelitis, a broad group of infectious diseases that involve the cortical bone and bone marrow (myeloid). Classification of acute versus chronic osteomyelitis is based on histopathologic diagnosis rather than duration of disease. Osteomyelitis can arise via hematogenous or non-hematogenous routes. It can also be classified based on the underlying etiology such as in diabetic feet, in which osteomyelitis results from contiguous spreading through overlying infected foot ulcers.
In regard to nuclear medicine scintigraphy, the three-phase bone scan is routinely performed first. In the setting of chronic bone conditions or pathology, the nuclear medicine bone scan is frequently non-diagnostic and may be skipped entirely if clinically appropriate. However, the bone scan may be necessary if Gallium scintigraphy is the infection agent of choice and if Indium WBC scan is requested to image very small areas like the hands and feet (described below). Abnormal bone scan results in the setting of acute, uncomplicated osteomyelitis can establish a diagnosis of osteomyelitis. If results on bone scan are equivocal, further nuclear medicine imaging is required and routinely performed. Bone scan images reflect perfusion, chemisorption, and osteoblastic bone activity. Abnormalities on bone scan represent increased bone mineral turnover rather than being specific for infection.2,10 In acute osteomyelitis, a three-phase bone scan normally demonstrates focal hyperperfusion, focal hyperemia, and focal increased uptake in delayed imaging (2–4 h). The absence of increased blood flow and blood pool on the first two phases with focal uptake on delayed images creates significant doubt for a diagnosis of osteomyelitis. Some investigators suggest 24-h bone scan images to improve specificity; however, this is not standard protocol and not routinely done, unless requested by the interpreter.11 While sensitive, the three-phase bone scan is not specific for osteomyelitis. Bone scans are most useful when the scan is negative, ruling out osteomyelitis with a high likelihood.7 Further evaluation is advised if clinical suspicion is high for a diagnosis of osteomyelitis and bone scan is negative. False negative cases can be related to decreased blood supply to the bone.
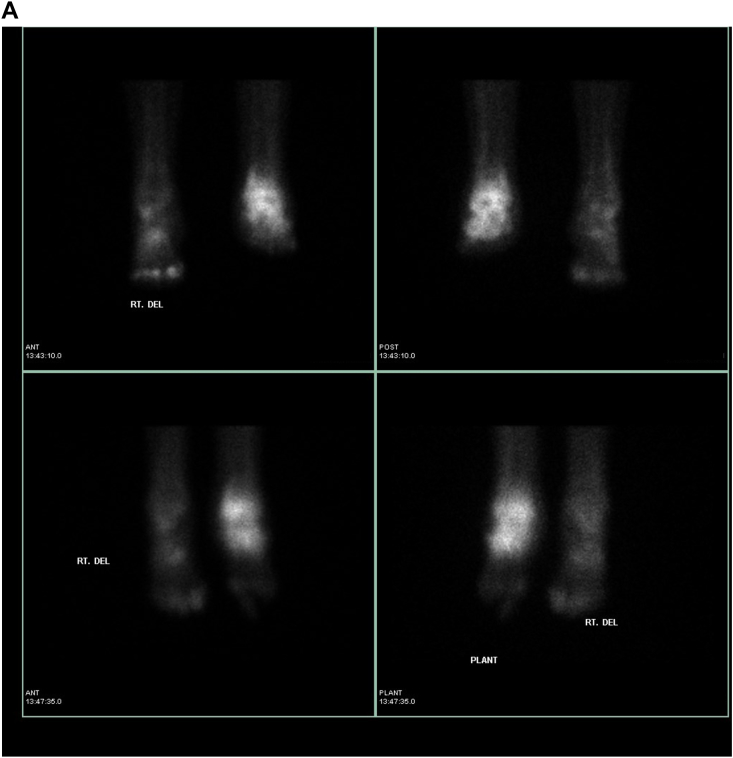
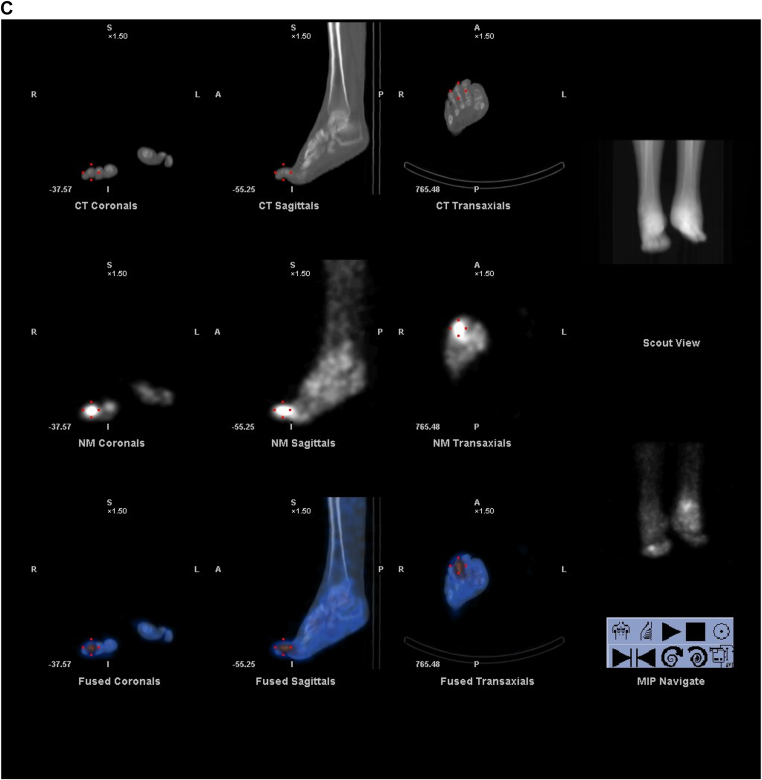
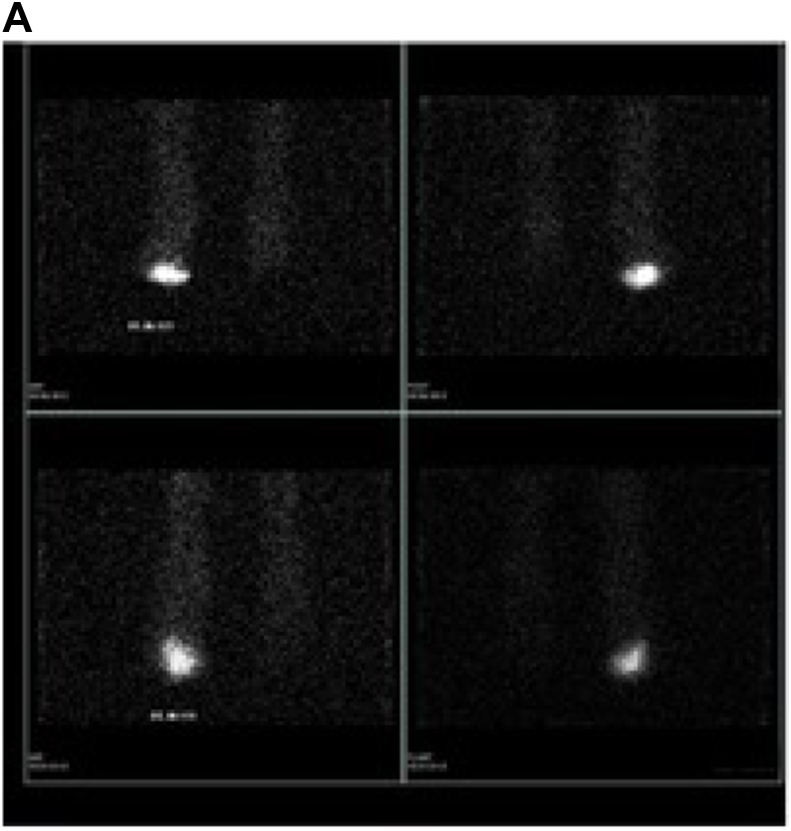
Once bone scan is completed, the next step in the infectious workup includes a Gallium or combined 111 In-oxine WBC/99m Tc- sulfur colloid study. Gallium-67 is an iron analogue known to have a high binding affinity for siderophores. Gallium-67 binds to transferrin in the blood and then goes to the source of inflammation and binds lactoferrin. The combined bone scan and Gallium studies are compared and determined to be positive, negative, or equivocal for infection (Fig. 2a, 2b, 2c). Limitations of this approach include the low clinical utility of an equivocal result and Gallium's 70% accuracy for evaluation of infection.12 Definitively positive or negative studies occur in only 1/3 of cases. In addition, this prolonged imaging technique can delay a diagnosis. Gallium scans for the evaluation of osteomyelitis are less sensitive and less specific than combined 111 Indium-oxine WBC/99mTc Sulfur colloid examination. However, Gallium may be preferred if a patient is neutropenic or osteomyelitis is suspected in the spine.13
Fig. 2.
Suspected osteomyelitis of the right foot. a: Bone scan: Delayed 2-h images on planar bone scan images demonstrate increased uptake involving the left ankle and areas of focal osseous uptake in the bilateral feet. b: Gallium scan: Corresponding Gallium planar images demonstrate congruent uptake in the left ankle and foot, therefore determined to be a non-infectious process compatible with known degenerative changes. However there is uptake on Gallium within the right foot (3rd phalanx) greater than seen on bone scan, compatible with osteomyelitis. c: SPECT/CT Gallium scan: Images confirm osteomyelitis of the right foot (3rd phalanx) as well as congruent uptake in the left ankle and hind foot compatible with known degenerative changes.
Radiolabeled WBC scintigraphy is considered to be the gold standard for evaluation of acute osteomyelitis.3 Indium labeled leukocyte scintigraphy is considered more accurate and consistent than Gallium scans and has replaced Gallium scans in most practices. Some authors have suggested WBC scintigraphy may not be as sensitive in cases of chronic osteomyelitis, infections involving parasites, and in patients who are neutropenic or have altered leukocyte migration (i.e. diabetic patients who are hyperglycemic).14,15 However, chronic infection impacting results of radiolabeled WBC scintigraphy is considered to be controversial. Most investigators have not found a significant difference in sensitivity in detecting acute versus chronic infections. 111 In-oxine WBC is routinely used by most clinics in the United States. 99mTc-HMPAO leukocyte scintigraphy can also be used to evaluate infection; however this test is less utilized by providers given the short half-life of the agent, label instability, and requirement to delay complementary marrow imaging 48–72 h if dual isotope imaging is desired.16 Labeling WBC's is a time-consuming procedure and requires highly skilled technologists.
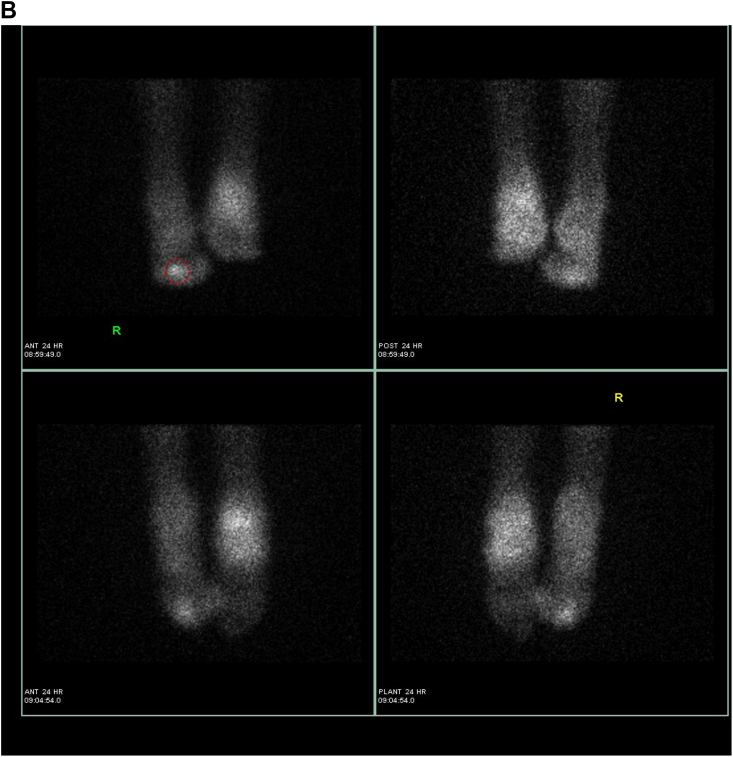
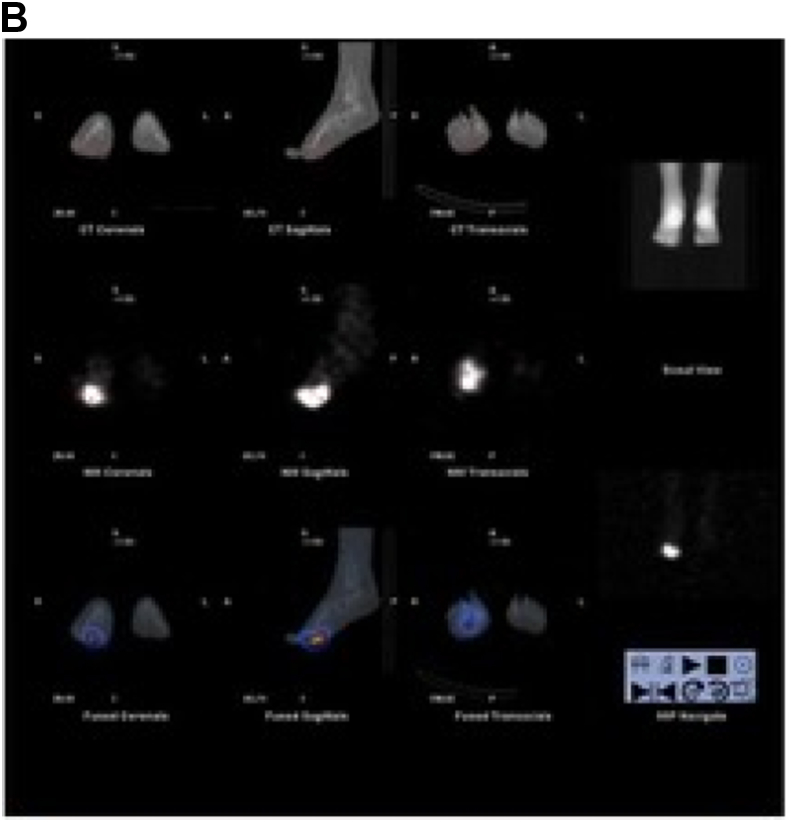
Indium WBC scintigraphy has poor spatial resolution and limited anatomic detail so bone scan may be necessary in imaging certain small areas such as the hands and feet. If SPECT-CT was to be combined with the WBC scan, this would likely obviate the need for a combined bone scan. Radiolabeled WBC's target sites of inflammation by a process known as chemotaxis. Labeled WBC's highlight bacteria and also the normal bone marrow. Therefore, it is helpful for the interpreting physician to simultaneously image the patient's bone marrow distribution to determine if there is an infection versus normal hematopoietic marrow distribution, especially in previously violated bone. This is why 99m Tc- sulfur colloid images are often required to better delineate the patient's hematopoietic marrow distribution. Once the studies are complete, Indium WBC images are compared to the sulfur colloid images and determined to be either positive or negative for infection (Fig. 3a, 3b, 3c). Indium-labeled WBC results, like Gallium, are also prolonged and typically require an 18–24 h set of images. 111 In-oxine WBC is reported as 90% sensitive and 90% specific for an infection in the acute phase. As described above in cases of vertebral osteomyelitis, if MRI cannot be performed 67Ga is preferred over 111 In-oxine WBC scintigraphy.17,18 Radiolabeled WBCs have a low sensitivity for spinal infections; more than half of spine infections demonstrate a cold region in the affected area. In addition, the use of 18FDG PET/CT, described below, has been validated in the setting of vertebral osteomyelitis.
Fig. 3.
Suspected osteomyelitis of the right foot. a: Indium WBC scan: Intense abnormal update is seen in the right forefoot. b: Indium WBC scan with SPECT/CT images: Intense abnormal activity is identified in the right forefoot and involves phalanges 1–4. c: Sulfur Colloid bone marrow scan: There is diffuse mildly increased marrow distribution within the right foot. Given positive findings on indium scan and minimal findings on sulfur colloid scan: results are compatible with osteomyelitis involving the forefoot and phalanges 1–4.
Radiolabeled WBC's and Gallium 67 scintigraphy can be helpful in evaluating response to therapy. Positive scintigraphic findings of infection should revert to normal 2–8 weeks after appropriate antibiotic therapy is given.19
SPECT/CT hybrid imaging is used routinely in most US practices and can be combined with Technetium, Gallium and Indium to provide improved sensitivity, accuracy, and localization of lesions.7,20 18FDG PET/CT, although more commonly used for oncologic purposes, can also be used for imaging of areas of suspected infection or osteomyelitis. 18FDG is a radioactive glucose analogue which undergoes decay by emitting a positron. This emitted positron then annihilates into two 511 Kev photons that are subsequently imaged by a PET/CT scanner. 18FDG PET/CT provides higher resolution and better anatomic localization than 99mTc-MDP, Gallium and Indium agents. Increased metabolism within the PET portion of the study is easily correlated to the CT imaging to demonstrate exact localization within the bone or soft tissue. Sensitivity of 18FDG PET/CT in osteomyelitis ranges from 94 to 100% and specificity from 87 to 100%.3,15 Leukocytes demonstrate intense metabolism of radioactive glucose concentrated at sites of infection. However, tumors can also be intensely metabolically avid on PET/CT so if clinical suspicion includes malignancy, a biopsy or culture may be required to differentiate between the diagnostic possibilities. 18FDG PET/CT can also be positive in non-infected areas of inflammation.20,21 18FDG PET/CT has been successfully used in the evaluation of vertebral osteomyelitis and may provide utility in cases of chronic osteomyelitis, as activated macrophages in an infection would also demonstrate intense FDG avidity.15,21,22 18FDG PET/CT can be used to monitor response to antimicrobial therapy and to help decide when therapy can be safely discontinued.23
Some articles have mentioned the disadvantageous cost of 18FDG PET/CT. Notably, use of 18FDG PET/CT to diagnose infection is not reimbursable by Medicare and Medicaid services.13 However, the overall expense of a combined approach including bone scan, Indium WBC scan, and marrow imaging studies may overshadow the cost of one 18FDG PET/CT scan. Additionally results of 18FDG PET/CT scans are accessible the day of procedure while Gallium or Indium scans require 24–72 h before results are obtained.4
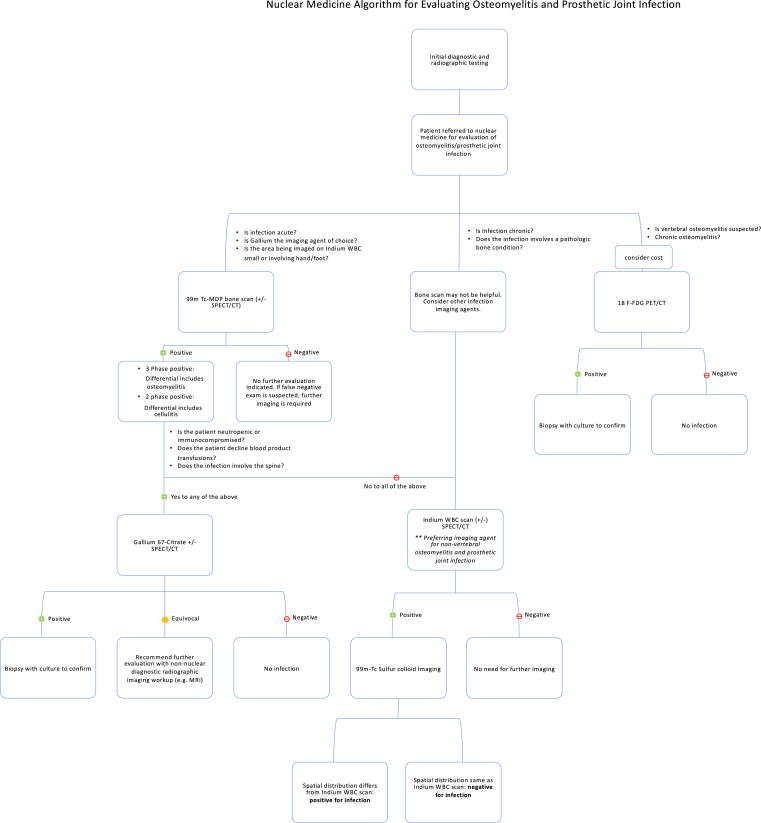
A flow chart (Fig. 5) has been provided which describes the nuclear medicine algorithm for evaluating osteomyelitis and prosthetic joint infection (described below).
Fig. 5.
Nuclear Medicine algorithm for evaluating osteomyelitis and prosthetic joint infection.
3. Cellulitis
The next disease state we will address is cellulitis, a bacterial infection involving the dermis and subcutaneous tissue. Cellulitis can spread to lymph nodes and the blood stream if untreated. Cellulitis can further spread to the osseous structures underneath and lead to osteomyelitis. Orthopedic Surgeons also must distinguish between the two etiologies in order to determine appropriate treatment management.
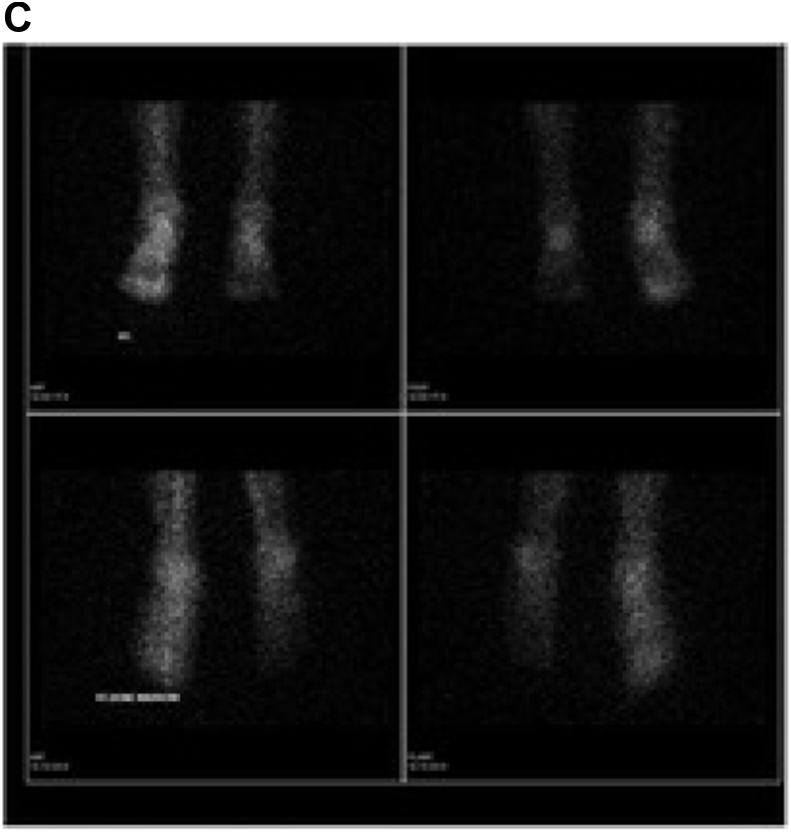
When plain radiographs are unremarkable, a three-phase bone scan can distinguish osteomyelitis from cellulitis with high sensitivity and specificity.13 In cellulitis, the first two phases of bone scan would demonstrate increased blood flow and blood pool while delayed 2 h images are normal13,18,24 (Fig. 4a, 4b, 4c). In cases of cellulitis, 111 In-oxine WBC scintigraphy demonstrates increased activity in the soft tissues rather than the surrounding osseous structures. In the peripheral extremities, given decreased anatomic resolution of 111 In-oxine WBC images, a bone scan should be performed for anatomic comparison in order to distinguish between soft tissue and bony involvement. If 67Gallium scintigraphy was performed to evaluate for infection, it should also be done in conjunction with a bone scan to better differentiate bone and soft tissue involvement1 (Fig. 4d). Additionally, SPECT/CT could provide better anatomic and spatial resolution in localizing the infection to either soft tissue or bone (see Fig. 5).
Fig. 4.
Complaint of left hip pain. a: Early dynamic bone scan: Images demonstrate increased blood flow to the left hip and buttock. b: Early blood pool bone scan: Images demonstrate increased blood pool within the left hip and buttock. c: Delayed bone scan: Images demonstrate very mild uptake in the proximal left femur, and the soft tissue uptake in the left hip and thigh has resolved. Given Gallium findings, as below this is compatible with soft tissue infection (e.g. cellulitis). Increased uptake within the left shoulder is noted as well. d: 24-h Gallium scan: Images demonstrate increased uptake within the soft tissues of the left thigh and buttock without evidence of osseous involvement. This is compatible with soft tissue cellulitis and inflammation. Increased uptake is noted in the left shoulder, in a pattern which is different than on bone scan; a component of infection cannot be excluded within that region.
4. Prosthetic joint infections
The evaluation of prosthetic joint infections provides a common but challenging scenario to the Orthopedic Surgeons. Is a patient with a painful prosthetic joint presenting with a loosening prosthesis or an infected joint replacement? Although radiographs have low sensitivity and specificity in evaluation of the prosthetic joint, they are routinely performed as part of the initial work up. Degradation of images from metallic prosthetic artifacts limits the utility of MRI evaluation.25 Therefore, Nuclear Medicine imaging studies such as 99mTc MDP and combined 111Indium/marrow imaging play a significant role in the evaluation of septic versus aseptic prosthetic joints.
In an infected prosthetic joint, a typical three phase bone scan is positive in all three phases. Bone scan sensitivity is high (90–100%) but specificity has been found to be poor (about 18–35%).6,25 The overall accuracy of a bone scan in the evaluation of prosthetic joint ranges from 50 to 70%.26 There have been attempts to study uptake of 99m Tc-MDP around prosthetic hardware in order to determine if particular patterns were characteristic of infection versus aseptic loosening. Originally, it was believed that diffuse uptake around a prosthetic device on a bone scan indicated infection while a more focal pattern of uptake around the tip, greater and lesser trochanters indicated loosening. Loosening has also been described in the literature as diffusely increased uptake around the acetabular component. This is no longer believed to be the case as diffuse uptake around aseptic prosthetic devices has been observed.25,27 Bone scans have also been found to be positive the first year after surgery due to osseous remodeling relating to post-surgical changes.
The NM bone scan has been determined to be unreliable in distinguishing between a loosened prosthetic device and infection. The differentiation between loosened prosthetic device and infection is better evaluated with radiolabeled WBC scintigraphy. However, a negative bone scan helps rule out both osteomyelitis and prosthesis loosening.
Combined 111 In-oxine WBC and 99mTc-sulfur colloid scintigraphy is considered the gold standard in Nuclear Medicine to evaluate septic prosthetic joint versus aseptic loosening. If images are congruent, meaning the distribution of activity on WBC imaging is similar to sulfur colloid, then the device is not infected. Uptake of radiolabeled WBC's surrounding the device in distribution different than seen on the sulfur colloid exam is considered to be positive for septic prosthetic joint.
Utility of Gallium scans alone in infected prosthesis is limited and not recommended due to its low accuracy. The combination of bone scan with gallium imaging increases accuracy to 65–80%.6,27
In evaluating 18FDG PET/CT scans for prosthetic joint infections, the interpreter must be mindful to review the uncorrected PET images rather than corrected images. Extensive reconstruction artifacts present on PET/CT secondary to metallic hardware on the corrected images. FDG has reported to perform better in septic hip rather than septic knee, nevertheless, it should be noted that 18FDG PET/CT does not appear to have a significant role in evaluation of infected prosthesis.25,27
5. Septic arthritis
Lastly, infectious (septic) arthritis is another area in which Orthopedic Surgeons must attempt to distinguish between pyogenic (septic) or non-pyogenic (aseptic) etiologies. Septic arthritis can rapidly destroy a native joint and requires urgent treatment while aseptic arthritis (e.g. osteoarthritis) is a chronic problem that is managed in the outpatient setting.28 Aspiration of the area of interest and an MRI with and without contrast are considered appropriate diagnostic pathways for the evaluation of the septic arthritis. The reference standard used to diagnosis septic joint is a positive culture from a joint aspirate. However, a negative culture does not exclude the diagnosis.7
Nuclear Medicine studies have limited utility in distinguishing septic from aseptic arthritis. Bone scans will be three-phase positive in both septic and aseptic arthritis.13 Gallium and Indium WBC scintigraphy cannot reliably distinguish aseptic from septic arthritis. 67Gallium has been found to be falsely positive in cases of inflammatory joints that are not infected.2 Radiolabeled WBC uptake can be seen in acute gouty arthritis, rheumatoid arthritis, and pseudogout.10 There is no current role of 18FDG PET/CT in septic joint as the radioactive glucose demonstrates uptake in both inflammatory and infectious arthritis.7
6. Novel nuclear medicine imaging modalities
Nuclear Medicine imaging agents currently being investigated for evaluation of infection which will be discussed in brief. The agents being investigated include: (1) radiolabeled antibodies (2) 99mTc-nanocolloid studies (3) 99mTc-Ciprofloxacin, (4) 99mTc-antimicrobial peptides, (5) radiolabeled receptor-specific proteins and (6) experimental PET tracers. These agents are not currently available for commercial use in the United States.
Radiolabeled antigranulocyte antibodies are directed against leukocyte antigens or receptors, thereby localizing infections or inflammation. Agents developed include murine monoclonal immunoglobulin G (Granuloscint) which binds to a cross-reactive antigen on neutrophils, and a monoclonal immunoglobulin G1 agent, an Fab’ fragment (leukoScan) which binds to a cross reactive antigen on leukocytes.18 Fab’ fragments demonstrate less immunoreactivity than whole antibodies and have a better target-to-background ratio resulting in superior images as a result of rapid renal clearance. Leukoscan has been permanently withdrawn from use in the European market. There is minimal interest in Granuloscint as the agent can be used one time only to avoid HAMA (Human anti-mouse antibody) response.
Radiolabeled human nonspecific IgG has also been used to image infection and inflammation. Accumulation of this tracer is reportedly related to capillary permeability, locally increased extracellular space, and macromolecular entrapment in the site of infection.29,30 It provides greatest diagnostic utility in detection of bone, joint and joint prosthesis infections. Currently, there is very little interest in the commercial use of this agent as results have been found to be inconsistent.
99mTc-Nanocolloid is a highly sensitive bone marrow imaging agent used for diagnosing bone and joint infections. Uptake occurs from extravasation through the capillary basement membrane, followed by phagocytosis or adsorption of particles by granulocytes and macrophages. The radiopharmaceutical can accumulate in both sterile and infected inflammatory sites.29,31 There is currently very little interest in this agent for commercial use.
The use of radiolabeled antibiotics such as 99mTc-Ciprofloxacin (Infecton) are also under investigation. Studies report its utility in detecting bacterial infections and imaging lesions in the spine. The broad-spectrum antibiotic Ciprofloxacin is linked to 99m Technetium which allows for the scintigraphic imaging of infection.32 Initial in vitro and animal studies have demonstrated that 99m Tc- Ciprofloxacin localizes in abscesses that are infected with both gram positive and gram negative bacteria.31 It is moderately sensitive in infection (70–85%) but highly specific (91–96%).27,33 There is currently less interest in radiolabeled antibiotics for commercial use given variable results.
99mTc-antimicrobial peptides are also under investigation. Reportedly, these agents demonstrate greater specificity for infection than conventional agents.31 Radiolabeled synthetic fragments of Ubiquicidin present in murine macrophages have been extensively studied and appear to be both sensitive and specific for infections involving the musculoskeletal system.10 There is significant commercial interest in this technique for infection scintigraphy.
Radiolabeled receptor-specific proteins are being investigated for scintigraphic imaging of infection. There has been movement away from imaging using larger, more nonspecific radiolabeled proteins (such as anti-granulocyte antibodies) to smaller, receptor-specific proteins and peptides (such as cytokines, chemotactic peptides and chemokines).31 These proteins are involved in initiating, amplifying and terminating the inflammatory response. They can localize rapidly in an abscess due to the small size of these radiolabeled agents. There is limited data available for this agent as large clinical trials have not been performed.
There is significant interest for novel PET tracers in infection imaging. 68Gallium Citrate PET/CT has been described as a possible agent for imaging suspected bone infections with reliable sensitivity, specificity, and accuracy.33 68Gallium demonstrates vastly superior image quality compared to 67Gallium; the procedure is simple compared to current techniques used in daily practice and results are available the same day.33 6″-18F-fluoromaltotriose is also being investigated. It is a derivative of maltose which is radiolabeled with fluorine-18. It has been described as a promising tracer for diagnosing both gram positive and gram-negative bacterial infections. [18F] F-PABA is also being investigated to distinguish bacterial infection from sterile inflammation. Unlike FDG, [18F] F-PABA is reported to be a highly specific agent that is only taken up by bacterial cells.
7. Conclusions
In summation, Nuclear Medicine imaging provides a useful complement to standard radiologic imaging techniques in the evaluation of orthopedic infections. Nuclear Medicine can differentiate osteomyelitis from inflammation in the setting of patients with previously traumatized bone. Nuclear Medicine bone scans are helpful in distinguishing cellulitis from osteomyelitis, especially in non-violated bone. Bone scans are most useful when the scan is negative, ruling out osteomyelitis with a high likelihood. In the setting of chronic bone conditions or pathology, the nuclear medicine bone scan is frequently non-diagnostic and may be skipped entirely if clinically appropriate. However, the bone scan may be necessary if Gallium scintigraphy is the infection agent of choice and if Indium WBC scan is requested to image very small areas like the hands and feet. Indium WBC scan/Sulfur colloid is more sensitive and specific than a Gallium scan and is often the preferred nuclear medicine imaging agent of choice in bone and joint infections including evaluation of the painful prosthetic joint. However, Indium WBC scintigraphy has been reported to be less sensitive in cases of spinal and parasitic infections, and in patients who are neutropenic or have altered leukocyte migration. Chronic infection impacting results of radiolabeled WBC scintigraphy is considered to be controversial. In the evaluation of vertebral osteomyelitis, if MRI cannot be performed, 18FDG PET/CT is preferred over Gallium; however, either may be utilized successfully. Nuclear medicine imaging demonstrates little diagnostic value in differentiating septic from aseptic arthritis. 18FDG PET/CT does not typically have a significant role in the setting of orthopedic infection, especially in the setting of suspected infected prosthesis as hardware artifacts often interfere with interpretation. 18FDG PET/CT performs better in the evaluation of osteomyelitis including vertebral osteomyelitis. However, PET/CT is costly and currently not reimbursable by Medicare or Medicaid in the evaluation of infection. Novel scintigraphic methods under investigation but not available for commercial use include radiolabeled antibodies, 99mTc-Ciprofloxacin, 99mTc-antimicrobial peptides, radiolabeled receptor-specific proteins and 99mTc-nanocolloid studies and PET tracers such as 68 Gallium, 6″- 18F-fluoromaltotriose and [18F]F-PABA, 68Gallium Citrate.
Conflicts of interest
The authors have no disclosures.
Acknowledgements
Charito Love, MD; Christopher J. Palestro, MD.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2019.04.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Palestro C.J., Torres M.A. Radionuclide imaging in orthopedic infections. Semin Nucl Med. 1997;27(4):334–345. doi: 10.1016/s0001-2998(97)80006-2. [DOI] [PubMed] [Google Scholar]
- 2.Johnson J.E. Prospective study of bone, indium-111-labeled white blood cell, and gallium-67 scanning for the evaluation of osteomyelitis in the diabetic foot. Foot Ankle Int. 1996;17(1):10–16. doi: 10.1177/107110079601700103. [DOI] [PubMed] [Google Scholar]
- 3.van der Bruggen W. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med. 2010;40(1):3–15. doi: 10.1053/j.semnuclmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Hatzenbuehler J., Pulling T.J. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84(9):1027–1033. [PubMed] [Google Scholar]
- 5.Papanas N., Zissimopoulos A., Maltezos E. (18)F-FDG PET and PET/CT for the diagnosis of diabetic foot osteomyelitis. Hippokratia. 2013;17(1):4–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A., Al-Nahhas A., Win Z. Current practice in pancreatic imaging: the role of nuclear medicine. Nucl Med Commun. 2006;27(6):477–480. doi: 10.1097/00006231-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Beaman F.D. ACR appropriateness criteria((R)) suspected osteomyelitis, septic arthritis, or soft tissue infection (excluding spine and diabetic foot) J Am Coll Radiol. 2017;14(5S):S326–S337. doi: 10.1016/j.jacr.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Mettler F.A., Jr. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 9.Censullo A., Vijayan T. Using nuclear medicine imaging wisely in diagnosing infectious diseases. Open Forum Infect Dis. 2017;4(1) doi: 10.1093/ofid/ofx011. p. ofx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palestro C.J. Radionuclide imaging of musculoskeletal infection: a review. J Nucl Med. 2016;57(9):1406–1412. doi: 10.2967/jnumed.115.157297. [DOI] [PubMed] [Google Scholar]
- 11.Alazraki N. Value of a 24-hour image (four-phase bone scan) in assessing osteomyelitis in patients with peripheral vascular disease. J Nucl Med. 1985;26(7):711–717. [PubMed] [Google Scholar]
- 12.Hakki S. Comparative study of monoclonal antibody scan in diagnosing orthopaedic infection. Clin Orthop Relat Res. 1997;(335):275–285. [PubMed] [Google Scholar]
- 13.Connolly C.M., Donohoe K.J. Nuclear medicine imaging of infection. Semin Roentgenol. 2017;52(2):114–119. doi: 10.1053/j.ro.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Corstens F.H., van der Meer J.W. Nuclear medicine's role in infection and inflammation. Lancet. 1999;354(9180):765–770. doi: 10.1016/S0140-6736(99)06070-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang H., Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32(1):47–59. doi: 10.1053/snuc.2002.29278. [DOI] [PubMed] [Google Scholar]
- 16.Love C., Palestro C.J. Nuclear medicine imaging of bone infections. Clin Radiol. 2016;71(7):632–646. doi: 10.1016/j.crad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Love C. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25(12):963–977. doi: 10.1097/00003072-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Mettler FA G.J. Saunders; 2011. Essentials of Nuclear Medicine Imaging 6ed. [Google Scholar]
- 19.Ziessman HA O.M.J., Thrall J.H. third ed. Elsevier Health Sciences; 1994. Nuclear Medicine: The Requisites. [Google Scholar]
- 20.Rosenberg R. vol 26. 2011. Nuclear Medicine Procedures in the Diagnosis of Orthopedic Infections Techniques in Orthopedics; pp. 271–289. (4) [Google Scholar]
- 21.Palestro C.J. FDG-PET in musculoskeletal infections. Semin Nucl Med. 2013;43(5):367–376. doi: 10.1053/j.semnuclmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht R., Hertel E. [Clinical experiences in the antibiotic treatment of secondary chronic osteomyelitis] Med Klin. 1968;63(24):968–972. [PubMed] [Google Scholar]
- 23.Glaudemans A.W., Signore A. FDG-PET/CT in infections: the imaging method of choice? Eur J Nucl Med Mol Imaging. 2010;37(10):1986–1991. doi: 10.1007/s00259-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugawara Y. Rapid detection of human infections with fluorine-18 fluorodeoxyglucose and positron emission tomography: preliminary results. Eur J Nucl Med. 1998;25(9):1238–1243. doi: 10.1007/s002590050290. [DOI] [PubMed] [Google Scholar]
- 25.Cyteval C., Bourdon A. Imaging orthopedic implant infections. Diagn Interv Imaging. 2012;93(6):547–557. doi: 10.1016/j.diii.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Love C. Role of nuclear medicine in diagnosis of the infected joint replacement. Radiographics. 2001;21(5):1229–1238. doi: 10.1148/radiographics.21.5.g01se191229. [DOI] [PubMed] [Google Scholar]
- 27.Palestro C.J. Nuclear medicine, the painful prosthetic joint, and orthopedic infection. J Nucl Med. 2003;44(6):927–929. [PubMed] [Google Scholar]
- 28.Nunez-Atahualpa L. 2016. Septic Arthritis Imaging 2016 May 12.https://emedicine.medscape.com/article/395381-overview February 20, 2019]; Available from: [Google Scholar]
- 29.Palestro C.J., Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med. 2009;39(1):52–65. doi: 10.1053/j.semnuclmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Love C., Palestro C.J. Radionuclide imaging of infection. J Nucl Med Technol. 2004;32(2):47–57. quiz 58-9. [PubMed] [Google Scholar]
- 31.El-Maghraby T.A., Moustafa H.M., Pauwels E.K. Nuclear medicine methods for evaluation of skeletal infection among other diagnostic modalities. Q J Nucl Med Mol Imaging. 2006;50(3):167–192. [PubMed] [Google Scholar]
- 32.Amaral H. Cold-hot mismatch between Tc-99m HMPAO-labeled leukocytes and Tc-99m ciprofloxacin in axial skeleton infections: a report of three cases. Clin Nucl Med. 1999;24(11):855–858. doi: 10.1097/00003072-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Kumar V., Boddeti D.K. (68)Ga-radiopharmaceuticals for PET imaging of infection and inflammation. Recent Results Canc Res. 2013;194:189–219. doi: 10.1007/978-3-642-27994-2_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.