Abstract
Plasmodium infections in endemic areas are often asymptomatic, can be caused by different species and contribute significantly to transmission. We performed a cross-sectional study in February/March 2016 including 840 individuals ≥ 1 year living in rural Gabon (Ngounié and Moyen-Ogooué). Plasmodium parasitemia was measured by high-sensitive, real-time quantitative PCR. In a randomly chosen subset of P. falciparum infections, gametocyte carriage and prevalence of chloroquine-resistant genotypes were analysed. 618/834 (74%) individuals were positive for Plasmodium 18S-rRNA gene amplification, of these 553 (66.3%) carried P. falciparum, 193 (23%) P. malariae, 74 (8.9%) P. ovale curtisi and 38 (4.6%) P.ovale wallikeri. Non-falciparum infections mostly presented as mixed infections. P. malariae monoinfected individuals were significantly older (median age: 60 years) than coinfected (20 years) or P. falciparum monoinfected individuals (23 years). P. falciparum gametocyte carriage was confirmed in 109/223 (48.9%) individuals, prevalence of chloroquine-resistant genotypes was high (298/336, 89%), including four infections with a new SVMNK genotype. In rural Gabon, Plasmodium infections with all endemic species are frequent, emphasizing that malaria control efforts shall cover asymptomatic infections also including non-falciparum infections when aiming for eradication.
Subject terms: Microbiology, Haplotypes
Introduction
Due to huge efforts in elimination and eradication of malaria the numbers of malaria cases and deaths reduced substantially according to WHO in recent years1. Despite this, between 2014 and 2016 there was a considerable increase in number of malaria cases, showing that it needs continuous efforts to have sustainable success. To reach the ambitious aim of eradication, control measures have to remain constantly high also including asymptomatic and submicroscopic carriers to eliminate this constant reservoir. Malaria control efforts should additionally include remote areas, where maintaining health care is difficult. This investigation analyzed by molecular methods the silent reservoir of malaria parasites in a rural area of Gabon, Central Africa.
We did a cross-sectional survey to investigate and further characterize the different Plasmodium species prevalent in a rural population in the area of Fougamou, Gabon. P. malariae and P. ovale infections (P. ovale wallikeri and P. ovale curtisi) are commonly underreported as parasitemia is often low and severe disease is uncommon2,3. However, infections by these species can be chronic and contribute significantly to morbidity by e.g. causing anemia4. A previous study in the same area on symptomatic patients with malaria caused by non-falciparum/mixed species infections revealed an unexpected complexity of infections when analysis was done by deep sequencing technology5. Within the current study, the submicroscopic and microscopic prevalence of the different Plasmodium species in the resident population was investigated before we analysed in more depth the most virulent and abundant parasite P. falciparum by evaluating prevalence of gametocytes and the presence of chloroquine resistant genotypes.
Recently recognized as an important malaria transmission reservoir, asymptomatic infections and submicroscopic gametocytemia are major hurdles for malaria elimination6. Control and elimination strategies of malaria therefore need to employ highly sensitive techniques to identify such reservoirs. One approach is to assess P. falciparum gametocyte carriage by quantitative real-time PCR (qPCR) based on the marker Pfs25. In addition, molecular markers for drug-resistance and other polymorphisms can be analyzed to inform the best intervention strategies. E.g., in many African countries chloroquine sensitivity of P. falciparum returned after change of treatment policy and subsequent withdrawal of chloroquine from the market7, but this was not yet observed in Gabon8,9 despite discontinuation of chloroquine for malaria treatment in Gabon since 200310.
With this study, we aimed at characterizing the whole Plasmodium population infecting humans in Fougamou, Gabon. This is done by assessing prevalence of different Plasmodium species, P. falciparum gametocyte carriage and prevalence of P. falciparum chloroquine resistance by qPCR in a cross-sectional survey.
Results
We included 840 individuals and obtained blood of 834 persons, description of the study population is given in Fig. 1. 311/834 (37%) individuals were positive for Plasmodium parasites by Giemsa stained thick blood smear, 72 (8.6%) of them were considered as being mixed or non-falciparum infections. Microscopic results have been published before11. Body temperature was recorded for 771 individuals, mean temperature was 37.1 °C; 24 individuals presented with a body temperature ≥ 38 °C, 14 of those 24 had a parasitemia that could be detected by microscopy.
Figure 1.
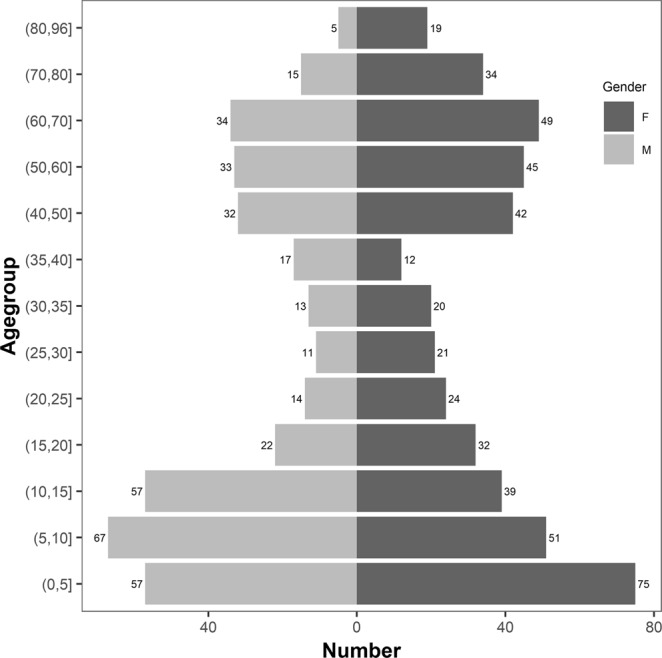
Demographic characteristics of study population.
Microscopic reading versus PCR
PCR and microscopy reading results were well correlated (r = 0.7403, p < 0.0001). All PCR samples with a Cq < 17.7 (n = 85) were microscopically positive, and all samples with a Cq > 29 were microscopically negative (n = 76), hence below the limit of detection for microscopy. 214/217 (98.6%) PCR negative samples were also read negative by microscopy, three were read false positive with a low parasitemia (below 25 parasites/µl) confirming the accuracy of our microscopy results. Non-falciparum species were not further distinguished by microscopy, but reported as such, so that only differentiation of P. falciparum versus non-falciparum species was performed.
PCR results for Plasmodium species
Further analysis by qPCR revealed an unexpected complexity of infections and high prevalence of non-falciparum coinfections. In total 618/834 (74%) individuals were positive for 18 S plasmodial DNA, of these 553 (66.3%) were positive for P. falciparum, 193 (23%) for P. malariae, 74 (8.9%) for P. ovale curtisi and 38 (4.6%) for P. ovale wallikeri. Parasitemia of individuals decreased with age (p < 0.0001 Fig. 2). Number of parasites per microliter was calculated with the help of a standard curve. The parasitemia of P. malariae positive individuals also decreased with age (p < 0.0001, Fig. 3). However, these data should not be over-interpreted as species-specific PCR was done after preamplification.
Figure 2.
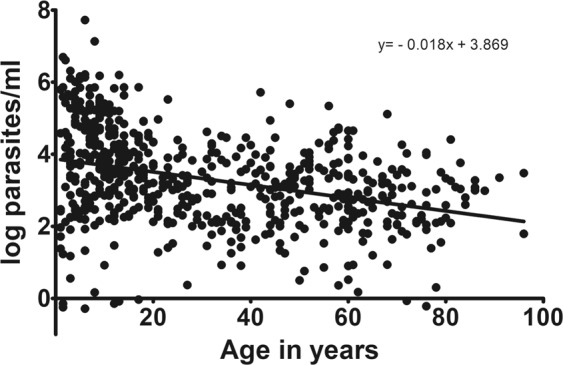
Age versus Plasmodium parasitemia. Age of participants versus log number of parasites (pan-Plasmodium RNA and DNA) per ml of blood (derived of qPCR data). Results show that the parasitemia declines with age. Parasitemia has been extrapolated from qPCR data with the help of a standard curve.
Figure 3.
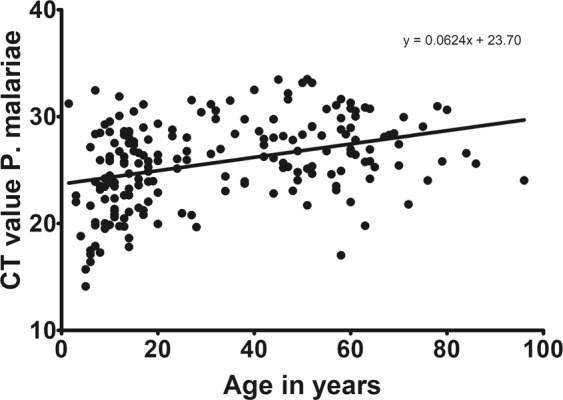
Age versus P. malariae parasitemia. Age of participants versus Cq values for P. malariae. Results show that also for P. malariae there is a decrease of parasitemia (indicated by raising Cq) with age.
Most non-falciparum infections presented as mixed infections with P. falciparum. In five individuals, all four prevalent species were found at the same time. The different combinations of infections can be seen in Table 1. P. malariae was the most abundant non-falciparum parasite, 90.6% (175/193) infections presented as coinfections with another species. We did not find any P. vivax infection in our samples.
Table 1.
Number of mono and multiple infections of the different Plasmodium species.
| Plasmodium species infection | ||
|---|---|---|
| Multiplicity of infection | Number of individuals and % | |
| PCR | Microscopy** | |
| Pf monoinfection | 357 (43) | 239 (28.7) |
| Pm monoinfection | 18 (2) | NA |
| Poc monoinfection | 7 (0.8) | NA |
| Pow monoinfection | 1 (0.1) | NA |
| Mixed infection (Pf + non-falciparum) | 592 (71) | 72 (8.6) |
| Pf + Pm | 123 (14.7) | NA |
| Pf + Poc | 21 (2.5) | NA |
| Pf + Pow | 18 (2.1) | NA |
| Pm + Poc | 1 (0.1) | NA |
| Pf + Pm + Poc | 36 (4.3) | NA |
| Pf + Pm + Pow | 10 (1.1) | NA |
| Pf + Poc + Pow | 4 (0.5) | NA |
| Pf + Pm + Poc + Pow | 5 (0.6) | NA |
| Pan-Plasmodium positive but species negative | 17 (2) | NA |
| Total positive | 618 (74) | 311 (37.3) |
| Total analysed | 834* | 834* |
NA: not applicable (Microscopic result was limited to separating P. falciparum from non-falciparum, therefore each species was not determined by microscopy).
*‘Total analysed’ (N = 834) is the denominator for calculating ‘percentage of individuals infected’.
**Microscopy data already published11.
Individuals with a monoinfection of a non-falciparum species
There were only 26/834 individuals who had a monoinfection of a Plasmodium species that was non-falciparum. Median age of the P. malariae monoinfected individuals (n = 18) was 60 years (interquartile range 57.25–69.75 years) and therefore significantly older than for the individuals having a mixed infection of P. malariae with P. falciparum (n = 174, median age = 20 years, IQR: 11.75–49 years; p < 0.0001, U test, see Fig. 4). P. falciparum monoinfected individuals had a median age of 23 years (IQR: 8–52 years, n = 357). For the other non-falciparum infections sample size was small, for P. ovale curtisi monoinfected individuals (n = 7) the median age was 42 years (IQR: 10–60 years) versus 14 years (IQR: 9–46 years) in individuals having a mixed infection of P. ovale curtisi with P. falciparum (n = 43). Only one individual aged 8 years had a P. ovale wallikeri monoinfection. In general, a trend towards higher age in mono-infected subjects is present.
Figure 4.
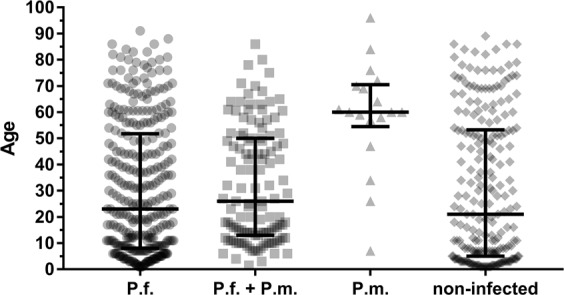
Age of P. malariae monoinfected individuals. Median age and interquartile range of P. falciparum monoinfected individuals (P.f.), versus age of coinfected individuals with P. falciparum + P. malariae (P.f. + P.m.) versus P. malariae monoinfected (P.m.) versus non-infected individuals. P. malariae monoinfected individuals are significantly older than Pf + Pm coinfected individuals (p < 0.0001).
Age of Plasmodium species positives
When dividing the population in age groups and looking at the pan-Plasmodium positive samples, one can see that in all age groups an evenly high proportion of individuals was infected (around 70%) (Table 2). Only the very young were less infected as only 13/33 (39.4%) children with 12–23 months were infected, and 10/20 (50%) of the 24–25 months old children were infected. Parasitemia was highest in the young children and declined with age (Fig. 2).
Table 2.
Individuals positive for the different Plasmodium species per age group.
| Age group (years) | N screened | Pan-Plasmodium n (%) | Pf n (%) | Pm n (%) | Pow n (%) | Poc n (%) |
|---|---|---|---|---|---|---|
| 1–5 | 132 (100%) | 74 (56.1) | 70 (53) | 6 (4.5) | 4 (3.0) | 5 (3.8) |
| 6–10 | 118 (100%) | 91 (77.1) | 88 (74.6) | 30 (25.4) | 8 (6.8) | 13 (11.0) |
| 11–15 | 96 (100%) | 80 (83.3) | 78 (81.3) | 34 (35.4) | 11 (11.5) | 20 (20.8) |
| 16–20 | 54 (100%) | 45 (83.3) | 44 (81.5) | 22 (40.7) | 3 (5.6) | 6 (11.1) |
| 21–25 | 38 (100%) | 28 (73.7) | 28 (73.7) | 5 1(3.2) | 0 | 3 (7.9) |
| 26–30 | 32 (100%) | 24 (75) | 23 (71.9)) | 7 (21.9) | 2 (6.3) | 2 (6.3) |
| 31–40 | 63 (100%) | 51 (81) | 48 (76.2) | 13 (20.6) | 2 (3.2) | 3 (4.8) |
| 41–50 | 73 (100%) | 58 (79.5) | 55 (75.3) | 22 (30.1) | 2 (2.7) | 5 (6.8) |
| 51–60 | 94 (100%) | 70 (74.5) | 60 (63.8) | 27 (28.7) | 3 (3.2) | 11 (11.7) |
| 61–70 | 67 (100%) | 49 (73.1) | 41 (61.2) | 17 (25.4) | 0 | 5 (7.5) |
| 71–80 | 49 (100%) | 33 (67.3) | 27 (55.1) | 7 (14.3) | 1 (2.0) | 1 (2.0) |
| 81–96 | 24 (100%) | 15 (62.5) | 12 (50.0) | 3 (12.5) | 0 | 0 |
| Sum | 840 | 618 | 574 | 193 | 36 | 74 |
Number and percentage of individuals screened and positive for the different Plasmodium species in the different age groups. One individual can be positive for different species. Age groups from 30 years on comprise 10 years.
When having a closer look at the non-falciparum infections, a similar pattern to the P. falciparum infection status can be seen. All age groups were similarly infected, with a peak in the adolescents and less infections in the very young.
P. falciparum analysis
P. falciparum gametocyte results
109 of the subset of 223 individuals were positive for Pfs25 by qPCR, of these 70 were also positive by microscopy. Age of Pfs25 positive individuals was significantly lower (median age = 13 years) than age of non-carriers (median age = 35 years), (p = 0.0002, U-test, Fig. 5). No age dependency in number of gametocytes was found.
Figure 5.
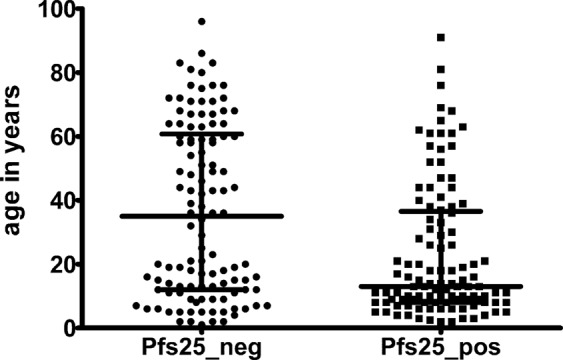
Age of gametocyte carriers. Median age and interquartile range of the subset of individuals analysed for Pfs25 positivity and negativity. Pfs25 positive individuals were significantly younger than Pfs25 negative individuals (p = 0.0002).
There was a significant but weak correlation of asexual infection with gametocyte carriage (rho = 0.194, p < 0.05). Though not significant, the odds of carrying gametocytes in infected individuals was 1.5 (95% CI: 0.9, 2.5) times higher in microscopy positive than submicroscopic participants showing the influence of asexual infection on gametocyte carriage.
P. malariae coinfection neither affected gametocyte carriage rate for P. falciparum (35 P.malariae positive individuals were positive for Pfs25, while 32 were negative for Pfs25), nor gametocytemia (median Pfs25 Cq of P.malariae carriers = 30.47 and non-carriers = 30.45).
Prevalence of chloroquine resistant Plasmodium falciparum
Of 336 samples screened for the three pfcrt haplotypes, 223 (66.4%) had CVIET haplotype only, 38 (11.3%) had CVMNK only, and 1 (0.3%) harbored a SVMNK monoinfection. The rest carried double and triple infections of wild and mutant haplotypes as; CVMNK and CVIET in 71 (21.1%), CVMNK and SVMNK in 2 (0.6%), and 1 triple infection (0.3%) as shown in Fig. 6. Overall, the prevalence of CQ resistance remained at 89% sixteen years after the withdrawal of the drug from national treatment guidelines.
Figure 6.
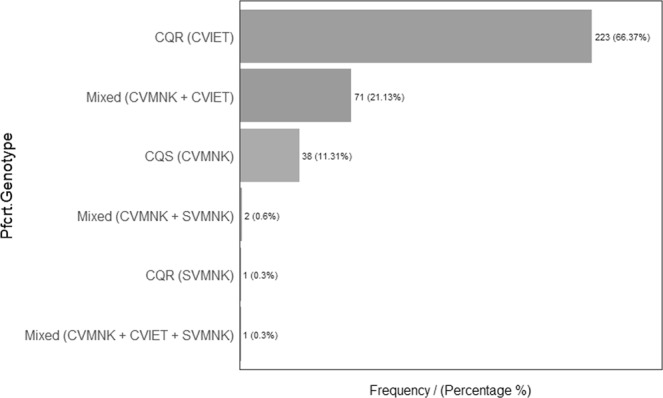
Distribution of PfCRT genotypes.
Discussion
Analysis by highly sensitive qPCR of blood samples of a rural population in Gabon revealed an unexpected breath of infection with 74% of individuals being infected with any Plasmodium species. Infection was evenly distributed over all age groups, with the exception that very young children were less infected maybe be due to increased care by the parents. Highest prevalence was found in children/adolescents between 5–20 years as typical for endemic areas12,13. There is no indication that immunity against malaria wanes in very old age in our study population. Results are in line with other studies from rural areas in Gabon showing no decline in malaria transmission in recent years14 but in contrast with findings from urban areas15,16 and results from the RTSS vaccine trial17 where a much lower prevalence of Plasmodium infections was detected.
Parasitemia was higher in very young children and was decreasing with age as expected for a population in a highly endemic area. Prevalence of non-falciparum infections was higher than expected. Nearly 50% of all infections presented as coinfections with a non-falciparum species, with P. malariae being the most prevalent. Frequent multi-species infections have been shown for certain communities harboring, low-density parasitemias of several species3. We could not find a single P. vivax infection in the investigated population neither as mono nor as co-infections. In fact, P. vivax has never been reported from Gabon. This is in line with the dogma that P. vivax is not present in Central/Western African populations as they are largely Duffy negative and P. vivax usually cannot infect Duffy negative individuals. However, our finding contradicts with recent findings reporting P. vivax presence in many regions across malaria-endemic Africa including countries bordering Gabon even though only at very low frequencies18,19. Reports show that P. vivax is regularly infecting Duffy-negative individuals in Madagascar20, and is present at low frequencies across most malaria endemic regions in Africa, infecting Duffy positive and Duffy negative individuals21,22. One hypothesis is that African wild living apes are infected with closely related P. vivax strains, that could in rare cases infect humans23. We could not see this in our samples, even though in national parks close to our sampling area wild apes are present. However, maybe the distance to this region is too big, or our sample size was too small. Further studies on P. vivax in Africa as well as in Gabon are needed to further elucidate this question. P. malariae is the second most abundant human malaria parasite after P. falciparum in many African countries2,24,25. Generally, quartan malaria caused by P. malariae is regarded as benign malaria even though it has been associated with nephrotic syndromes especially in young children26 and more long-lasting and chronic illness associated with anaemia3. A recent hospital-based survey studied systematically clinical symptoms due to P. malariae in Papua New Guinea discovering that even though a rare disease, children hospitalized with P. malariae also developed severe symptoms and even died4. The authors concluded that P. malariae is associated with anemia and a similar risk of mortality when hospitalized compared to children hospitalized with malaria caused by another Plasmodium (P. falciparum, P. vivax) species.
The burden of disease caused by P. malariae and P. ovalespecies is not well investigated. Hypnozoites of P. ovale. species infections in this area are usually not treated with primaquine/tafenoquine as glucose 6 phosphate dehydrogenase (G6PD) deficiency is prevalent in this populations, testing of G6PD-deficiency is currently not reliably possible in field settings and treatment without prior testing is prohibited27,28. A study in Gabon investigating the efficacy of artemether-lumefantrine for the treatment of non-falciparum and mixed species infections found good efficacy for this standard drug combination in this indication28. However, others found that the standard treatment for P. falciparum might not be ideal to clear non-falciparum parasites completely2,29–31.
Correlation of qPCR parasitemia data with age of P. malariae infected individuals indicated that immunity against P. malariae is also – as for P. falciparum – build up with age, as parasite load was higher in younger individuals than in older ones. However, these data have to be interpreted with a caveat because preamplification was done before the species-specific PCR. Monoinfections with non-falciparum species only rarely appeared and most P. malariae infections presented as coinfections with P. falciparum. Monoinfections of P. malariae (18/193) were mainly found in older individuals. We have no explanation for this finding but one hypothesis could be that even though immunity to P. malariae is built up with age similarly to P. falciparum, but this immunity with old age is less complete for P. malariae than for P. falciparum as there is less exposure due to lower parasite load/infection intensity/immune stimulation. Further studies are needed to elucidate this further.
In malaria endemic regions, asymptomatic P. falciparum infections are commonly present and reported from different transmission settings32–34 showing some level of dependency with age34 seasonality35 and geographic area34. These asymptomatic infections are mostly submicroscopic (detected by molecular methods) and in some cases carry the transmission stages, gametocytes36,37. Half of the analyzed samples in our study cohort were positive for P. falciparum gametocytes showing a potential of high transmission intensity in the area. Similar to our finding, P. falciparum PCR-positive individuals had a 45% and 44% gametocyte carriage rate in a study conducted in Malawi35 and Kenya36, respectively. In several studies, gametocyte carriage was inversely associated with age of the infected person38; revealing that under-five year olds39 and school-age children35 were carrying more gametocytes than adults. We also observed that gametocyte carriage is more frequent in young children than in adults. Gametocyte density was similar in children and adults in our cohorts, though Coalson et. al reported a trend of lower gametocyte density in school-age children than other age groups35.
The chloroquine resistant genotype remains high in this P. falciparum parasite population. Similar results were found in previous studies showing that the dominant haplotype remains the resistant haplotype CVIET9,40,41. This is unexpected as CQ is no longer commonly available in the market for malaria treatment in the study area. However, the closely related drug (amodiaquine) that is given as P. falciparum first line treatment in combination with artesunate might play a role in selection of chloroquine resistant strains. While pfcrt CVIET is the most prevalent genotype in Africa, another resistance-associated genotype pfcrt SVMNT is rarely reported from Africa but more commonly found in South-America and some Asian countries42. The presence of SVMNT genotype was first reported in Tanzania in 2004 and later in Angola43,44. To our knowledge, this is the first time the presence of SVMNK genotype is detected in Gabon. SVMNT has been associated with the emergence of amodiaquine resistance45 and this finding highlights the need for continuous monitoring and re-evaluation of current therapy where amodiaquine, as in Gabon, is used in combination with artemisinin.
We did not evaluate the presence of CQ resistance in non-falciparum species in our study area where high level of P. falciparum resistant alleles is consistently reported after the withdrawal of the treatment. In general, except in the case of P. vivax infection, CQ-resistance is rarely reported in non-falciparum infection. Delayed parasite clearance and treatment failures of non-falciparum species are usually attributed to the special biology of these parasites (hypnozoites in case of P. ovale species, or longer life cycle, i.e. 72 hours in case of P. malariae), also considering stage specific activities of the drugs46. One study from Indonesia showed CQ resistance in P. malariae infections47, but this was not more repeatedly reported. As chloroquine resistance was never widely reported from other areas in non-falciparum species (except P. vivax), we would speculate that chloroquine remains efficacious against non-falciparum parasites in this area.
Conclusion
Analysis by qPCR reveals the complexity of Plasmodium infection in this rural population in Gabon. For the final aim of elimination and eradication, also submicroscopic infections as well as non-falciparum species have to be considered to be able to sustain malaria control efforts. Otherwise, rural areas will always convey a reservoir for future infections.
Methods
Ethical approvals
Ethics approval was obtained from the responsible ethics committee Comité d´Ethique Institutionel of the Centre de Recherches Médicales de Lambaréné. Signed informed consent was obtained from adults ≥ 18 years or the legal guardian in case of minors, assent was additionally obtained from adolescents ≥ 12 years. All methods were performed in accordance with relevant guidelines and regulations.
Study area and sampling
We performed a cross-sectional study in February/March 2016 to assess the prevalence of Plasmodium infections in 840 individuals aged from 1–96 years in a rural area of Gabon (Fougamou and villages in the surroundings). The area is characterized by close proximity to primary rain forest and small-scale farming. We invited everybody who lived in the chosen area and was older than 1 year to take part in the study. Blood was collected by venipuncture in an EDTA blood tube (Sarstedt), and temperature was taken by axillary measurement. A Giemsa-stained thin and a thick blood smear was performed and analyzed by Lambaréné method reading 100 microscopic fields on the same day by microscopy. All malaria cases (all species) that were diagnosed positive by thick blood smear were treated according to the national guidelines (three days treatment of artemether-lumefantrine or artesunate-amodiaquine irrespective of the Plasmodium species, no treatment for hypnozoites was given). Whole blood samples (500 µL) were collected mixed with 1,300 µL RNALater (Thermo Fisher Scientific) and stored at −20 °C until nucleic acid extraction was performed.
Nucleic acids extraction
RNAlater stabilized blood specimen were used for two types of extractions: either total nucleic acids (DNA and RNA) or total RNA automated in the QIAsymphony® SP system (Qiagen). Before total nucleic acids extraction with QIAsymphony DSP DNA kit, RNAlater solution was removed by centrifugation and packed erythrocytes were resuspended with 1X PBS to a final volume of 420 μL and extracted by the QIAsymphony® SP as per the manufacturer’s instructions.
Total RNA extraction for P. falciparum gametocyte detection by RT-qPCR was done as reported earlier48 with some modifications. Briefly, frozen samples were thawed and half the volume of the blood-RNALater mix (900 µL) was separated, transferred to a 2-mL sample tube (Sarstedt, Numbrecht, Germany) and centrifuged for 3 min at 16,000 g. The supernatant was discarded and 300 µL buffer RTL Plus (Qiagen) supplemented with 1% (v/v) β-mercaptoethanol was added to the pellet and vortexed for 5 min before loading onto the QIAsymphony SP (Qiagen). RNA purification was automated using the QIAsymphony RNA Kit (Qiagen) according to the RNA CT 400 protocol provided with the instrument.
Statistical analysis
All data was entered and reviewed using Excel, further analyses were done with GraphPad Prism version 5 and R v3.5.0. R packages tidyverse and funModeling were used for data processing and generation of graphics. Correlation of data was analyzed by Spearman test, data were compared by Mann Whitney U test, lines were fitted by linear regression. A p-value smaller than 0.05 was considered statistically significant.
Ultra-sensitive RT-qPCR for parasite detection
Screening for Plasmodium infections was performed by pan-Plasmodium reverse transcription quantitative PCR (RT-qPCR) assay as described earlier28, with a lower limit of detection of 6 parasites/mL. The small subunit ribosomal RNA gene (18 S rDNA) as well as the expressed transcripts were co-amplified in a single step for high sensitivity detection of Plasmodium parasites. The amplicon was selected from a gene domain that is 100% conserved across human malaria species.
A result was interpreted as positive if the quantification threshold cycle of the amplification curve was below 40 (cutoff Cq < 40). The assay setup was performed in a sterile PCR workstation and the pipetting robot (QIAgility, Qiagen) was used for sample and mastermix dispensing into the wells of a 384 plate. The qPCR assay was carried out in the LightCycler 480 II (Roche Applied Science).
Nested qPCR for species identification
Pan-Plasmodium assay positives were first subjected to conventional PCR amplification using primers by Snounou49 before further utilized as template for the qPCR. A limited cycle PCR was performed in a 50 µL reaction volume containing 7.5 µL of nucleic acids extract, 300 µM of each primer (PLU5 and PLU6), 1X Qiagen PCR buffer with 1.5 mM MgCl2, 250 µM dNTPs each, and 1U Taq DNA Polymerase (Qiagen). The cycling conditions were: Initial denaturation at 95 °C for 5 min, followed by 20 cycles of [95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1:20 min] and a final extension at 72 °C for 5 min.
Single-plex qPCR assay was performed for five human malaria species (P. falciparum, P. malariae, P. ovale curtisi, P. ovale wallikeri, and P. vivax) using primers and probes described previously28. Briefly, each of the species-specific qPCR assays consisted of 2.5 µl of the pre-amplified product, 1X SensiMix II Probe No-ROX (Bioline), 300 µM of each primer pair and 150 µM of each probe (Supplementary Table S1) per 10 µL final reaction volume. Cycling conditions were: polymerase activation at 94 °C for 10 min, followed by 45 cycles of [95 °C for 10 s and 60 °C for 60 s]. Samples were tested in duplicate and all assays included a non-template control and positive control in duplicates. Quantification cycle values (Cq) were calculated by default using the second derivative maximum method integrated in the LightCycler 480 software (version 1.5.1.62). Positivity was considered after visual assessment of the amplification curves for variability between each sample replicates (standard deviation ≤ 1 cycle) and the quantification threshold value less than 40 (Cq < 40). Pan-Plasmodium PCR positive but species PCR negative samples were repeated by a standard PCR using primers specific for cytochrome B50 (n = 16) or 18 S rRNA genes5 (n = 6) and subsequent Sanger’s sequencing for samples with a high enough parasitemia (Cq below 36). All but one (P. ovale) of the sequencing results revealed a P. falciparum infection.
Generation of standard curve for quantification of parasite load by PCR
A standard curve of highly synchronized ring-stage P. falciparum 3D7 laboratory strain was prepared by using the extracted total nucleic acid to estimate parasitemia. Quantification of parasites was done by extrapolating Cq values to the standard curve based on linear regression analysis. Therefore, quantification is not fully equivalent to microscopic reading as in clinical infections in addition to ring stage parasites, gametocytes as well as later asexual stages might be present, especially in non-falciparum species infections. For the species-specific PCR, quantification is only approximate as evaluation was done after preamplification, which allows at most ordinal comparison between infections.
Detection of Plasmodium falciparum gametocytes in blood by RT-qPCR
Among samples positive for P. falciparum by qPCR, a subset (n = 223) of samples was selected and analyzed by RT-qPCR for the presence of gametocytes in the infected participants. Gametocyte specific qPCR was performed to measure the transcript levels of Pfs25 mRNA using previously published targets, with modifications. A Taqman RNA-to-CT™ 1-Step kit (Thermo Fisher Scientific) was used for the quantitation of the gene expression levels. The reaction contains 1 × Taqman enzyme mix, 150 nM probe, 1 × Taqman RTqPCR mix, 400 nM of each primer and 2.5 µL of RNA extract.
The reaction conditions consisted of reverse transcription (48 °C/20 min), enzyme activation (96 °C/10 min), and two-temperature cycling steps (45 cycles, 95 °C for 15 s and 62 °C for 1 min). The Cq value was determined as above for the species qPCR. All samples were tested in triplicates together with the non-RT and non-template controls. Samples were considered positive when a Cq value below 40 was present in at least two technical replicates. The used primer and probe sequences were published previously48 and are given in S1. Gametocyte carriage was only evaluated for P. falciparum and not for the other Plasmodium species.
P. falciparum gametocyte detection by microscopy
The samples positive by qPCR for Pfs25 were re-read by microscopy to check for P. falciparum gametocytes. 300 microscopic fields were read using the Lambaréné method by two independent experienced microscopists.
PfCRT genotyping
A multiplex qPCR assay was used to genotype Pfcrt gene spanning codons 72–76 using previously described hydrolysis probes and primers with modifications51,52. To enhance sensitivity, a preamplification followed by qPCR was done with primers listed in S1. In brief, a conventional PCR was carried out using PfCRT_Preamp1 & PfCRT_Preamp2 for 20 cycles in 25 μL reaction volume. Limited PCR-cycled products were used as templates in a qPCR multiplex reaction containing three hydrolysis probes. Each probe representing one of the three Pfcrt haplotypes; the CQ sensitive haplotype (CVMNK), and the two CQ resistance-associated haplotypes (CVIET, SVMNT). Different fluorophores were tagged to each probe enabling detection of the haplotypes in parallel from each sample. DNA extracted from P. falciparum strains 3D7, Dd2, and 7G8 were used as positive controls for haplotypes CVMNK, CVIET and SVMNT, respectively.
Supplementary information
Acknowledgements
We thank all participants who took part in the study. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen. This work was supported by the University of Tübingen.
Author Contributions
B.M., T.G.W., J.H., A.L. conceived the idea of the project. T.G.W., M.G., L.V., G.B.T., P.B.M. did the data collection as well as microscopic analysis. A.L., T.T.N., T.G.W. did the experiments. T.G.W., A.L., T.T.N. and J.H. interpreted the data. All authors revised and approved the manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamirat Gebru Woldearegai and Albert Lalremruata contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46194-9.
References
- 1.WHO. World malaria report, 2017, http://www.who.int/malaria/publications/world-malaria-report-2016/en (2017).
- 2.Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasitol Drugs Drug Resist. 2013;3:45–50. doi: 10.1016/j.ijpddr.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland CJ. Persistent Parasitism: The Adaptive Biology of Malariae and Ovale Malaria. Trends Parasitol. 2016;32:808–819. doi: 10.1016/j.pt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Langford S, et al. Plasmodium malariae Infection Associated with a High Burden of Anemia: A Hospital-Based Surveillance Study. PLoS Negl Trop Dis. 2015;9:e0004195. doi: 10.1371/journal.pntd.0004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalremruata A, et al. Species and genotype diversity of Plasmodium in malaria patients from Gabon analysed by next generation sequencing. Malar J. 2017;16:398. doi: 10.1186/s12936-017-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider P, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 2007;76:470–474. doi: 10.4269/ajtmh.2007.76.470. [DOI] [PubMed] [Google Scholar]
- 7.Frosch AE, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116. doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voumbo-Matoumona DF, et al. Prevalence of Plasmodium falciparum antimalarial drug resistance genes in Southeastern Gabon from 2011 to 2014. Infect Drug Resist. 2018;11:1329–1338. doi: 10.2147/IDR.S160164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank M, et al. A thirteen-year analysis of Plasmodium falciparum populations reveals high conservation of the mutant pfcrt haplotype despite the withdrawal of chloroquine from national treatment guidelines in Gabon. Malar J. 2011;10:304. doi: 10.1186/1475-2875-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nsimba B, et al. Sulphadoxine/pyrimethamine versus amodiaquine for treating uncomplicated childhood malaria in Gabon: a randomized trial to guide national policy. Malar J. 2008;7:31. doi: 10.1186/1475-2875-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manego RZ, et al. Demography, maternal health and the epidemiology of malaria and other major infectious diseases in the rural department Tsamba-Magotsi, Ngounie Province, in central African Gabon. BMC Public Health. 2017;17:130. doi: 10.1186/s12889-017-4045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Barraquer, I. et al. Quantification of anti-parasite and anti-disease immunity to malaria as a function of age and exposure. Elife7, 10.7554/eLife.35832 (2018). [DOI] [PMC free article] [PubMed]
- 13.Rodriguez-Barraquer I, et al. Quantifying Heterogeneous Malaria Exposure and Clinical Protection in a Cohort of Ugandan Children. J. Infect. Dis. 2016;214:1072–1080. doi: 10.1093/infdis/jiw301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assele V, Ndoh GE, Nkoghe D, Fandeur T. No evidence of decline in malaria burden from 2006 to 2013 in a rural Province of Gabon: implications for public health policy. BMC Public Health. 2015;15:81. doi: 10.1186/s12889-015-1456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouyou-Akotet MK, et al. Evidence of decline of malaria in the general hospital of Libreville, Gabon from 2000 to 2008. Malar J. 2009;8:300. doi: 10.1186/1475-2875-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mawili-Mboumba DP, et al. Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malar J. 2013;12:3. doi: 10.1186/1475-2875-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drakeley C, et al. Longitudinal estimation of Plasmodium falciparum prevalence in relation to malaria prevention measures in six sub-Saharan African countries. Malar J. 2017;16:433. doi: 10.1186/s12936-017-2078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culleton R, Carter R. African Plasmodium vivax: distribution and origins. Int. J. Parasitol. 2012;42:1091–1097. doi: 10.1016/j.ijpara.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Twohig KA, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019;13:e0007140. doi: 10.1371/journal.pntd.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard D, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc. Natl. Acad. Sci. USA. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo G, et al. Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J. 2017;16:74. doi: 10.1186/s12936-017-1722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazeau NF, et al. Plasmodium vivax Infections in Duffy-Negative Individuals in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2018;99:1128–1133. doi: 10.4269/ajtmh.18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loy DE, et al. Evolutionary history of human Plasmodium vivax revealed by genome-wide analyses of related ape parasites. Proc. Natl. Acad. Sci. USA. 2018;115:E8450–E8459. doi: 10.1073/pnas.1810053115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce MC, et al. Effect of transmission setting and mixed species infections on clinical measures of malaria in Malawi. PloS one. 2008;3:e2775. doi: 10.1371/journal.pone.0002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutledge GG, et al. Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution. Nature. 2017;542:101–104. doi: 10.1038/nature21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrich JH, Eke FU. Malaria-induced renal damage: facts and myths. Pediatric nephrology. 2007;22:626–637. doi: 10.1007/s00467-006-0332-y. [DOI] [PubMed] [Google Scholar]
- 27.Hounkpatin AB, Kreidenweiss A, Held J. Clinical utility of tafenoquine in the prevention of relapse of Plasmodium vivax malaria: a review on the mode of action and emerging trial data. Infect Drug Resist. 2019;12:553–570. doi: 10.2147/IDR.S151031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groger, M. et al. Prospective Clinical Trial Assessing Species-Specific Efficacy of Artemether-Lumefantrine for the Treatment of Plasmodium malariae, Plasmodium ovale, and Mixed Plasmodium Malaria in Gabon. Antimicrob. Agents Chemother62, 10.1128/AAC.01758-17 (2018). [DOI] [PMC free article] [PubMed]
- 29.Betson M, et al. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology. 2014;141:1880–1890. doi: 10.1017/S003118201400033X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutledge Gavin G., Marr Ian, Huang G. Khai Lin, Auburn Sarah, Marfurt Jutta, Sanders Mandy, White Nicholas J., Berriman Matthew, Newbold Chris I., Anstey Nicholas M., Otto Thomas D., Price Ric N. Genomic Characterization of Recrudescent Plasmodium malariae after Treatment with Artemether/Lumefantrine. Emerging Infectious Diseases. 2017;23(8):1300–1307. doi: 10.3201/eid2308.161582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calleri G, Balbiano R, Caramello P. Are artemisinin-based combination therapies effective against Plasmodium malariae? The Journal of antimicrobial chemotherapy. 2013;68:1447–1448. doi: 10.1093/jac/dkt005. [DOI] [PubMed] [Google Scholar]
- 32.Niang M, et al. Substantial asymptomatic submicroscopic Plasmodium carriage during dry season in low transmission areas in Senegal: Implications for malaria control and elimination. PLoS ONE. 2017;12:e0182189. doi: 10.1371/journal.pone.0182189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mvumbi DM, et al. High Prevalence of Plasmodium falciparum Infection in Asymptomatic Individuals from the Democratic Republic of the Congo. Malar Res Treat. 2016;2016:5405802. doi: 10.1155/2016/5405802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baliraine FN, et al. High prevalence of asymptomatic plasmodium falciparum infections in a highland area of western Kenya: a cohort study. J. Infect. Dis. 2009;200:66–74. doi: 10.1086/599317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coalson JE, et al. High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J. 2016;15:527. doi: 10.1186/s12936-016-1587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J. 2016;15:421. doi: 10.1186/s12936-016-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shekalaghe SA, et al. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop. Med. Int. Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 38.Koepfli C, et al. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. PLoS ONE. 2015;10:e0126747. doi: 10.1371/journal.pone.0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bousema JT, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mawili-Mboumba DP, et al. Pfcrt 76T and pfmdr1 86Y allele frequency in Plasmodium falciparum isolates and use of self-medication in a rural area of Gabon. Trans. R. Soc. Trop. Med. Hyg. 2014;108:729–734. doi: 10.1093/trstmh/tru147. [DOI] [PubMed] [Google Scholar]
- 41.Maghendji-Nzondo S, et al. Malaria in urban, semi-urban and rural areas of southern of Gabon: comparison of the Pfmdr 1 and Pfcrt genotypes from symptomatic children. Malar J. 2016;15:420. doi: 10.1186/s12936-016-1469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sa JM, Twu O. Protecting the malaria drug arsenal: halting the rise and spread of amodiaquine resistance by monitoring the PfCRT SVMNT type. Malar J. 2010;9:374. doi: 10.1186/1475-2875-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alifrangis M, et al. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J. Infect. Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 44.Gama BE, et al. Plasmodium falciparum isolates from Angola show the StctVMNT haplotype in the pfcrt gene. Malar J. 2010;9:174. doi: 10.1186/1475-2875-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beshir K, et al. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siswantoro H, et al. In vivo and in vitro efficacy of chloroquine against Plasmodium malariae and P. ovale in Papua, Indonesia. Antimicrob. Agents Chemother. 2011;55:197–202. doi: 10.1128/AAC.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maguire JD, et al. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet. 2002;360:58–60. doi: 10.1016/S0140-6736(02)09336-4. [DOI] [PubMed] [Google Scholar]
- 48.Gebru T, Lalremruata A, Kremsner PG, Mordmuller B, Held J. Life-span of in vitro differentiated Plasmodium falciparum gametocytes. Malar J. 2017;16:330. doi: 10.1186/s12936-017-1986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snounou G, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 50.Sundararaman SA, et al. Plasmodium falciparum-like parasites infecting wild apes in southern Cameroon do not represent a recurrent source of human malaria. Proc. Natl. Acad. Sci. USA. 2013;110:7020–7025. doi: 10.1073/pnas.1305201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushman M, et al. Within-host competition and drug resistance in the human malaria parasite Plasmodium falciparum. Proc Biol Sci. 2016;283:20153038. doi: 10.1098/rspb.2015.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson PE, et al. Prevalence of pfcrt mutations in Congolese and Malawian Plasmodium falciparum isolates as determined by a new Taqman assay. Acta Trop. 2005;93:97–106. doi: 10.1016/j.actatropica.2004.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


