Abstract
South Asia specific reviews on the role of physical activity (PA) domains on chronic disease prevention are lacking. This study aimed to systematically review published literature to identify the association between PA domains and chronic diseases and to provide summary estimates of the strength of association. Nine electronic databases were searched using the predefined inclusion criteria which included population (South Asian adults 40 years or older), exposure (PA or sedentary behaviour) and outcome (type 2 diabetes mellitus, breast cancer, colorectal cancer, coronary heart disease, stroke, vascular disease and musculoskeletal diseases and their markers). A random-effects meta-analysis was carried out for cardiometabolic outcomes whereas narrative synthesis was completed for other outcome variables. Inactive or less active South Asian adults were at 31% higher risk of being hypertensive. Likewise, the risk of cardiometabolic outcomes was 1.34 times higher among inactive adults. Household PA was found to have a protective effect on breast cancer risk. Total and leisure time PA had a protective effect on osteoporosis among males and females respectively. Contemporary studies with a longitudinal design, representative samples, valid and reliable assessment of different domains are needed to establish the role of PA in chronic disease prevention in the region.
Subject terms: Risk factors, Epidemiology
Introduction
Chronic diseases are emerging as a public health challenge in South Asia1,2. Genetic predisposition, increasing life expectancy, urbanisation, mechanisation, inadequate health services and rapid economic development fuelling sedentariness and changing dietary patterns are contributing to rising chronic disease burden in the region3–5. Further, compared to the rest of the world, South Asians develop these diseases at a lower body mass index (BMI)6 and earlier in adulthood, leading to higher cost and loss of productive years7. Lack of awareness, poor access to health services and multiple chronic conditions further exacerbate the problem8.
Physical inactivity is a well-established risk factor for chronic diseases9. It is related to an increased incidence of coronary heart disease (CHD), type 2 diabetes mellitus (T2DM), breast cancer, colon cancer and reduced life-expectancy10. Compared to those who are inactive, those less active (600–3999 Metabolic equivalent (MET)-minutes) have a 14%, 16% and 3% reduced risk of T2DM, CHD and breast cancer, respectively11. The risk of all these diseases further decrease with higher levels of PA11. Overall, every 10% decrease in population-level inactivity is expected to avoid half a million global deaths annually12. The World Health Organization (WHO) recommends at least 150 minutes of moderate- physical activity (MPA) or equivalent per week. Increased duration to 300 minutes/week is recommended for additional health benefits9.
Total physical activity (PA) constitutes activities carried out across various domains of daily life, including leisure time PA (LTPA), occupational PA (OPA), household PA (HPA) and transport-related PA (TPA). Understanding all these domains is crucial as the nature of activities vary between countries13, and strategies to change PA will vary between the domains. LTPA is the predominant form in the high-income countries while the other three domains are more prevalent in the low and middle-income countries14. Consistent with other developing countries in the Asia-pacific region13, work and transport related activities are the most common forms of PA in South Asia15–18. Available data show a low prevalence of LTPA in the South Asian region: 5% among 45–59 year olds in Sri Lanka17, 14% among 40–69 year olds in Bhutan16 and 20% among 45–54 year olds in Bangladesh18. Variations exist both between and within the countries, with a higher prevalence of inactivity among females and urban dwellers19,20.
Most of the evidence regarding the effect of PA on chronic disease prevention comes from studies of leisure time activities in developed countries21–24. However, this domain of PA constitutes a small portion of total daily activity among South Asian adults15–18 and hence cannot provide an overall picture of their daily PA. Though a previous review of studies in low-and-middle-income countries by Milton et al.25 has concluded similar benefits of PA to that in high-income countries, it has also emphasised the importance of context specific research. Further, it has reinforced the importance of localised evidence to generate political support and assist in physical activity related policy development and programs25.
Though the demographic, epidemiological and economic transition, along with genetic susceptibility to some diseases such as T2DM, makes South Asians a priority group for chronic disease research, reviews examining the relationship between the range of PA domains and chronic diseases in the region are limited. Understanding whether the dominant forms of PA are associated with higher or lower levels of disease risk in the region is relevant to public health policy and programs. This knowledge can also provide some directions concerning the population groups and types of PA that should be the focus of determinants research. Hence, this study aimed to (1) systematically review published, peer-reviewed literature to identify the association between PA domains (total, transport, household, occupational and leisure) and selected chronic diseases and their markers, and (2) provide summary estimates of the strength of associations among South Asian adults 40 years or older.
Methods
This systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; Registration no. CRD42018096505; available from https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=96505) and is guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement26,27. The review focuses on PA or sedentary behaviour in routine circumstances among South Asian adults 40 years or older. The published protocol paper further provides details of the review methodology28.
Outcome measure
Chronic diseases (T2DM, breast cancer, colorectal cancer, cardiovascular disease (CHD, stroke, vascular disease) and musculoskeletal diseases (osteoarthritis, osteoporosis, back and neck pain) are the primary outcome variables while risk markers (body weight, BMI, blood sugar, blood pressure, lipids, cholesterol, bone mass density (BMD), hypertension (HTN) and the metabolic syndrome (MetS)) are the secondary outcome variables of interest. Study outcomes are classified as cardiometabolic conditions (HTN, T2DM, MetS or CHD), breast cancer and musculoskeletal conditions for reporting the review results.
Search strategy and inclusion criteria
Nine electronic databases: MEDLINE, EMBASE, PSYCINFO, CENTRAL, CINAHL PLUS, SPORTDiscus, AgeLine, Scopus and the Web of Science were systematically searched for English language, peer-reviewed papers published between January 2000 and March 2018. The MEDLINE search strategy was developed through a review of published literature and in consultation with a medical librarian experienced in systematic reviews. It was then adapted to other databases with an additional limit of excluding MEDLINE records whenever the databases provided that option. The MEDLINE search strategy is presented in Supplementary File: Table 1. A manual search of references and forward citations of relevant systematic reviews and relevant articles was also carried out to ensure all potential studies were captured.
The search was limited to quantitative studies examining the association between chronic disease or their risk markers and PA in routine circumstances among South Asian adults 40 years or older. In this review, routine PA refers to activities of varied intensities carried out as part of a regular daily routine and can relate to regular work, household, transport or leisure-time activities. Structured activities carried out in a controlled, and supervised environment for research purposes were excluded. No limits were applied for study design, but non-peer reviewed literature was excluded.
Study selection
All the identified articles were initially imported into Endnote X8 software29 and duplicate records were removed. These articles were then uploaded to Covidence systematic review software (available at www.covidence.org) where SP screened the titles. Two reviewers (EOA and SP) then independently screened each abstract and full text article against the predefined inclusion/exclusion criteria. Only those records which were included by both the reviewers passed on to the final review stage. Discrepancies were resolved by discussion and consensus among the authors. Reference lists of these eligible studies were manually checked to ensure no potentially relevant articles were missed.
Data extraction and quality assessment
A Microsoft Excel data extraction template was developed, pretested and approved by the review team before data extraction. SP then extracted the data using this template while EOA verified the accuracy and completeness of the extracted data. Information on authors, publication date, country of origin, study population, sample size, outcome measures, exposure variables, types of PA, measures of association and the study findings were extracted from all eligible studies. Several study authors were contacted to clarify study details. The review utilised information reported in the articles when the study authors did not respond.
The National Institute of Health (NIH) quality assessment checklist was used to assess the quality of the included studies with separate checklists for case-control and cross-sectional studies30. Included studies were assessed against several quality criteria such as research question, study population, participation rate, inclusion criteria, sample size, exposure prior to outcome, sufficiency of time frame, different levels of exposure, exposure measures, multiple exposure assessment, outcome measures, blinding, follow-up rate and statistical analyses30. SP and EOA independently assessed the quality of the papers. Final quality scores were assigned to the studies in consultation with BJS and AJO. A score higher than 75% was considered good quality, 50–75% was deemed as fair quality and less than 50% as poor quality as used in previous studies31. The review team was not blinded to the authors or journals during the review process.
The quality of evidence for each outcome measure was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework32. GRADE provides four categories: high, moderate, low and very low for evidence grading. Higher quality indicates greater confidence that future research is unlikely to change the effect estimate while lower quality indicates higher likelihood that future research will change the effect estimate and the level of confidence in the estimates. The framework allows for upgrading or downgrading the quality of evidence depending upon the risk of bias, imprecision, indirectness or inconsistency32. SP evaluated the overall quality of evidence for each study design which was then verified by the review team.
Statistical analysis
A General variance-based random effects modelling was used to calculate summary Odds Ratios (OR) and 95% Confidence Intervals (CI) for PA and cardio-metabolic outcomes. When a single study reported more than one outcome33,34, only the primary outcome variable was used to avoid the issues of non-independent data35. In this analysis, the highest PA category was compared with the lowest PA category, using the highest category as the reference group. When PA was categorised into more than two categories, the highest and lowest categories were compared. Adjusted estimates were used wherever available. Out of 9 studies, reported ORs were used for pooling in 6 instances33,36–40, three of which required the reference category to be reversed38–40, with ORs calculated from reported raw data in the remaining three studies34,41,42. We did not convert the correlation coefficients from cross-sectional studies to ORs due to the substantial difference in the nature of studies reporting these two different measures of association35.
Sub-group analysis was performed across cardio-metabolic outcomes (HTN, T2DM, CHD and MetS), study design (cross-sectional vs case-control studies) and country (India vs non-India based papers). Sensitivity analysis was conducted by removing one study at a time to determine the impact this had upon pooled results. I2 and Chi2 values were calculated to test the magnitude of heterogeneity. Publication bias was assessed using a funnel plot of standard error versus effect size and Egger’s test. All statistical analyses were performed using Review Manager version 5 software (RevMan 5)43. Whenever a meta-analysis was not feasible because of a limited number of studies or heterogeneity across the studies, a narrative summary was produced.
Results
Study selection
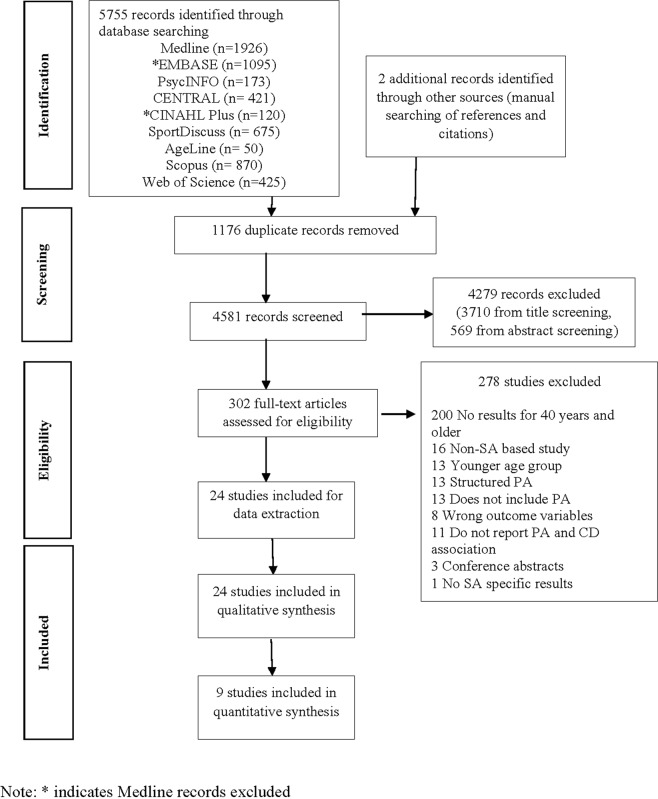
A systematic search of nine electronic databases identified 5755 records while two additional articles were located from manual searching of references and citations. The 4581 non-duplicate records were then screened for title and abstracts which further excluded 4279 records: 3710 from title screening and 569 from abstract screening. Full texts of the remaining 302 articles were retrieved and assessed in detail using predefined criteria, and an additional 278 records were excluded in this stage. The majority (72%) of the articles were excluded because the studies did not report associations between PA and chronic diseases/risk factors for people 40 years and older. Figure 1 presents the study selection process using the PRISMA flow chart.
Figure 1.
Flowchart of study selection.
There were 24 studies included in the narrative synthesis, 9 of which were also included in the quantitative synthesis of the relationship between PA and cardio-metabolic outcomes. Seven of the 16 studies examining cardio-metabolic outcomes could not be included in the pooled analysis for one or more of the following reasons: they did not report any effect estimate or provide convertible raw data44,45; the effect estimate was not given in a form that could be transformed and included (such as hazard ratio46 or correlation coefficient47); or they reported PA on a scale that did not allow us to combine the results with other studies48,49. A case-control study by Kumar50 provided raw data for the relationship between PA and a secondary outcome variable, but the calculated OR was markedly higher than those reported in other studies and hence was not used. Meta-analysis of other outcome variables (i.e. musculoskeletal conditions, breast cancer) was not feasible because of a limited number of studies and heterogeneity regarding PA types and their categorisation.
Study characteristics
Of the 24 studies included in this review, more than half (54%) were conducted in India36,38,40,42,44,45,47–49,51–54, two each in Pakistan34,46 and Nepal33,41 and one each in Sri Lanka55, Bangladesh56 and Afghanistan37. One study57 reported results for five South Asian countries while two studies39,50 were based in two South Asian countries. More than half of the studies (14 out of 24) were published between 2001 and 2010. Eighteen of the 24 studies were cross-sectional in design33,37–42,44–49,53–57 while the remaining six were matched case-control studies34,36,50–52,58. Age was one of the matching variables in all these six studies. Six studies examined women only49,51,52,54,56,58, four examined men only36,48,53,55 and two reported results separately for men and women46,57. Supplementary File: Table 2 summarises the descriptive characteristics of the included studies.
Eight of the studies reported response rates, which was more than 80% in all cases38,39,45,46,49,51,52,56. A sample size justification or a power calculation was provided only in one-third of the included studies33,37,39,41,44,49,53,54. Altogether, this review includes results from 26,092 participants with the sample size in the cross-sectional studies ranging from 9048 to 723838 and between 33050 to 165952 in the case-control studies. One study45 did not report a sample size for the target age-group and hence was not included in total sample size calculation for this review.
The majority of the studies (75%) analysed the association between PA and chronic diseases or their risk factors using regression analysis, although three reported p-values from a chi-square test41,42,55, one reported a p-value from a t-test50, and two reported correlation coefficients47,48. The presence of chronic diseases or their risk factors were established through medical records, biochemical tests (such as an oral glucose tolerance test (OGTT), blood tests), anthropometric measurements and bone scans. Two studies relied on patients self-report of their conditions42,57.
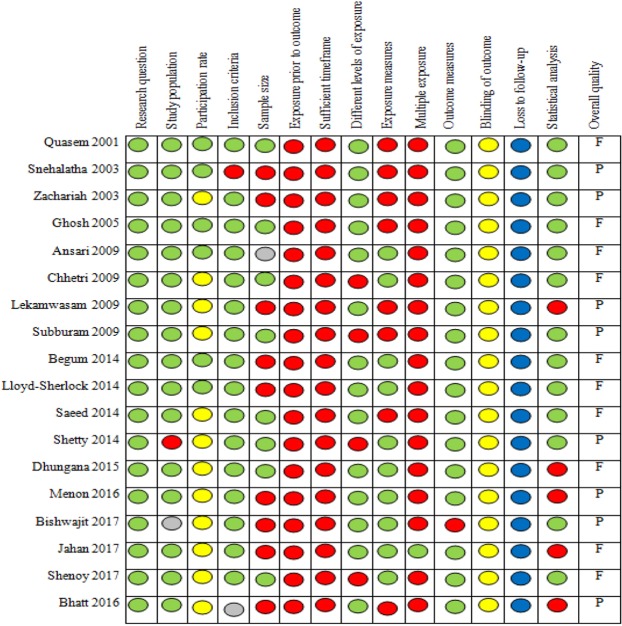
Application of the quality assessment checklists showed that the quality scores ranged between 32% and 62% for cross-sectional studies and between 42% and 58% for case-control studies. None of the 24 studies was ranked as high quality, 11 were of fair quality, and the remaining 13 were of poor quality. Figure 2 shows the quality ranking of cross-sectional studies across different criteria with green circles for “yes”, red for “no” and yellow, grey and blue circles for “not reported”, “cannot determine” and “not applicable” respectively. The quality of evidence from all studies for all outcome measures was rated “very low” based on the GRADE framework (Table 1). The quality was downgraded from “low” to “very low” because of serious risk of bias (such as questionable validity and reliability of the measurement instrument, no sample size justification or recruitment of cases and controls from different populations) and/or serious imprecision which implies that future studies are highly likely to change the estimate and/or the level of confidence in the estimates.
Figure 2.
Quality assessment of cross-sectional studies using the NIH checklist.
Table 1.
Overall summary of findings by outcome measures.
| Outcome measures | Number of studies | Quality of evidencea | Summary of findings (number of studies reporting direction of association across the PA domains)b |
|---|---|---|---|
| Cardiometabolic outcomes | |||
| HTN | 5 | Very low |
Total PA: null (1), mixed (1) OPA: null (1), mixed (1) Walking: null (1) |
| T2DM | 3 | Very low |
Total PA: null (1), mixed (1) LTPA: mixed (2) Walking, cycling: null (1) |
| CHD/CVD risk | 3 | Very low | Total PA: null (2), negative (1) |
| Obesity measures | 5 | Very low |
Total PA: negative (2) Walking: null (2), negative (1) |
| Breast cancer | 2 | Very low |
HPA (5–6 hr/day): negative (2) Walking: mixed (1) Watching television: null (1) |
| Musculoskeletal conditions | |||
| Osteoporosis | 5 | Very low |
Total PA: null (1), negative (2) LTPA: null (1), negative (1) Walking: null (1) |
| Back pain | 1 | Very low | Walking, moderate and vigorous total PA: mixed (1) |
aThe quality of evidence is assessed using the GRADE criteria and has been downgraded from “low” to “very low” for all the studies because of the serious risk of bias and/or serious imprecision indicating that future studies are highly likely to change the estimate and/or the level of confidence in the estimates.
bThe number of studies reporting null/mixed associations will not add up to the total number of studies for an outcome measure as some studies have reported more than one domain of PA.
PA domains and assessment
There was considerable variation in the domains of PA studied. Most of the included studies reported associations between total PA and the outcome variables34,36,38,39,41,42,45–47,53,55–57. Nine studies reported associations for walking37,46–50,52,57,58, four reported LTPA33,46,54,58, three reported HPA46,51,52 and two reported OPA40,44. All studies, except one47, used questionnaires to measure PA. The study by Jahan and Shenoy47 used both the International Physical Activity Questionnaire (IPAQ) and pedometer counts to determine participants’ PA scores47. None of the case-control studies used a standardised instrument to measure PA or reported any form of reliability and validity estimates for the measurement tool used. On the other hand, 10 of the 18 cross-sectional studies either used previously validated questionnaires such as IPAQ47,54,56, the Global Physical Activity Questionnaire (GPAQ)38,41,42, or another instrument for which validity and/or reliability was reported33,46,53,57. The majority of the studies collected information regarding activities carried out in the last seven days or an average day/week36,39–41,44,47,49,51,52,54–57 while 10 studies (42%) did not provide any information on the recall period33,34,37,38,42,45,48,50,53,58. A study by Ansari46 collected information regarding engagement in exercises and sports during the last two years while another study concerning breast cancer asked participants to report the activities carried out one year before the disease was diagnosed52.
Association between PA and chronic diseases
Cardio-metabolic outcomes
The associations between PA and cardio-metabolic outcomes were examined in 16 of the 24 studies. Of these 16 studies, 5 reported HTN37–40,44, 3 each reported T2DM33,45,46 and CHD or CVD risk36,41,42 and 1 reported metabolic syndrome34. The remaining studies examined body composition measures (such as body weight, fat mass index (FMI), fat-free mass index (FFMI), waist circumference (WC), waist-hip ratio (WHR)) or total cholesterol as the outcome variables. Among these studies, two were specific to males36,48, one was specific to females49 while one reported associations separately for males and females46. Several studies were limited to one type of PA while some examined associations across multiple PA types. All studies, except two36,50, were cross-sectional in design.
Among the five studies examining the association between PA and HTN, 1 reported negative association39, 2 reported null associations37,44 and 2 reported mixed associations38,40. The mixed associations were reported in 2 studies which examined occupational PA and HTN40,44. Zachariah et al.40 found a decreased likelihood of HTN among individuals whose occupation involved MPA (AOR: 0.35 (0.13–0.94)) compared to those with sedentary occupations while there was no significant effect of mild PA. On the other hand, Subburam et al. reported no significant difference in the risk of HTN among individuals whose occupations involved a varying degree of PA44.
Of the three studies reporting T2DM, one each found a negative33, null45 and mixed associations46, respectively. Both the studies reporting associations between LTPA and T2DM found an increased risk of diabetes among individuals with low activity levels33,46. Stair climbing and cycling were also inversely associated with the risk of diabetes, but there was no effect of HPA46.
Two of the three studies reporting associations between total PA and CHD or CVD risk found null association36,41 while the other reported negative association42. Mixed associations were reported for blood pressure37,47,50 and fat mass48,49 and a negative association was reported with cholesterol50.
Overall, among 16 studies reporting cardiometabolic outcomes, null associations were reported in 5 of the 16 studies36,37,41,44,45, negative associations in another 5 studies34,39,42,49,50 and mixed associations were reported in the remaining 6 of the16 studies33,38,40,46–48. One-quarter of the included studies (6 of the 24) reported associations between walking and cardio-metabolic conditions. No association existed between walking and T2DM46, waist circumference47,48 and BMI48. Supplementary File: Table 3 provides study specific associations.
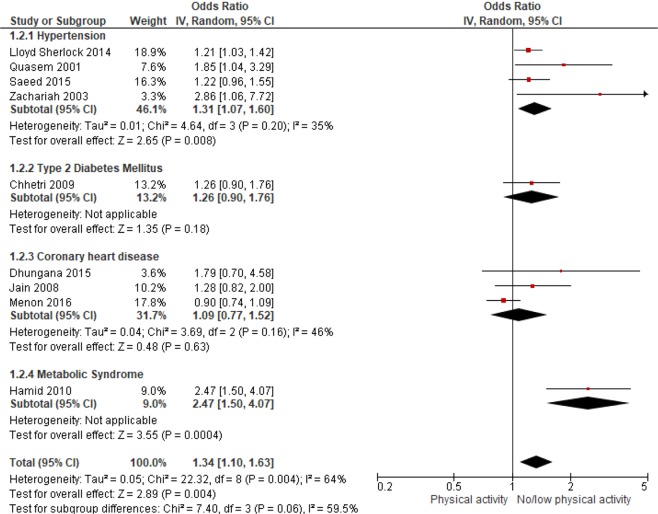
The results from the meta-analysis of 9 studies showed an inverse association between PA and cardiometabolic outcomes. The pooled OR for HTN was 1.31 (1.07–1.60) indicating that the inactive or those with low levels of PA were 31% more likely to be hypertensive (Fig. 3). No statistically significant association was found between PA and CHD or CVD risk (pooled OR: 1.09 (0.77–1.52), I2 = 46%). Overall, South Asian adults with no or low PA were 1.34 times more likely to suffer from cardio-metabolic conditions than active adults (pooled OR: 1.34 (1.10–1.63), I2 = 64%) ((Fig. 3). The pooled result for cross-sectional studies resulted in an OR of 1.23 (95% CI: 1.02 to 1.48) while pooled OR of case-control studies was 1.76 (95% CI: 0.92–3.35) (figure not shown). Pooled OR for studies conducted in South Asian countries other than India indicated a 49% higher risk (pooled OR: 1.20 (1.06–1.59), I2 = 65%) of cardiometabolic outcomes among inactive individuals, however, the pooled OR was not significant for studies conducted in India (figure not shown). Sensitivity analysis was performed by removing one study or one disease group at a time, but this had no substantial effect on the pooled effect size (results not shown). The funnel plot showed some evidence of publication bias (Fig. 4). This is further confirmed by Egger’s test (p-value: 0.032).
Figure 3.
ORs of cardiometabolic outcomes for physically active versus inactive individuals. Horizontal bars represent confidence intervals and small squares represent relative contribution of each study in pooling. An OR > 1.00 indicates higher odds of cardiometabolic outcomes among inactive individuals.
Figure 4.
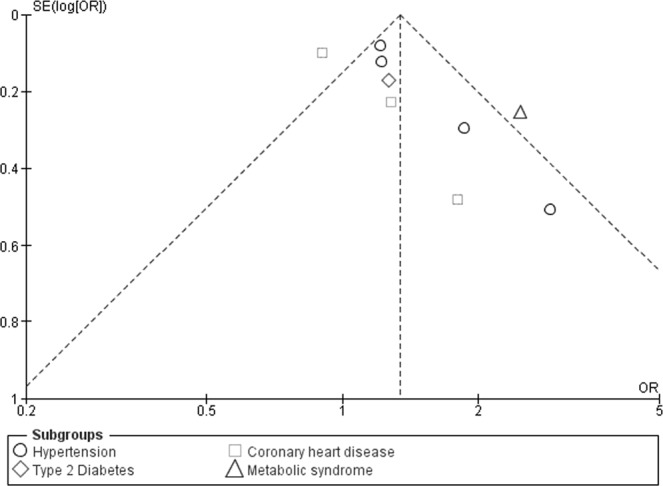
Funnel plot for PA and cardiometabolic outcomes.
Breast cancer
The relationship between breast cancer and PA was examined in 2 of the 24 studies51,52. Both studies were age and residence status (urban/rural) matched case-control studies among post-menopausal Indian women. Cases were histologically confirmed incident primary breast cancer cases while controls were cancer-free women who accompanied another type of cancer patient to the same hospital. The study by Dey further classified the cases by estrogen receptor (ER) status and reported associations separately for ER + and ER- cases51.
Both studies reported decreased breast cancer risk among postmenopausal women engaged in HPA51,52 (Supplementary File: Table 4). Dey et al. found a protective effect of increased duration of HPA for both ER + (p-value for trend = 0.003) and ER- cases (p-value for trend = 0.009)51. Postmenopausal women engaging in HPA for 5–6 hours/day were 40% less likely to have ER + breast cancer (AOR: 0.60 (0.36–0.98) than women undertaking <3 hours/day of HPA. The study found no protective effect of engaging in HPA for 3–4 hours/day and >6 hours per day for both ER + and ER- cases51. Mathew et al. reported 51% and 49% decreased risk of breast cancer among women engaging in 5–6 hours and more than 6 hours/day of HPA, respectively52. Watching television during the weekdays or weekends was not associated with breast cancer risk among postmenopausal women52. Study-specific results are summarised in Supplementary File: Table 4: Characteristics and results of studies: Breast Cancer.
Musculoskeletal conditions
Six of the included 24 studies (25%) examined the association between PA and musculoskeletal conditions (Supplementary File: Table 5). Four cross-sectional studies53–56 and one case-control study58 reported associations between PA and osteoporosis while another cross-sectional study57 reported associations for self-reported back pain. Three studies were limited to females54,56,58 and the remaining two were specific to males 50 years or older53,55.
Osteoporosis
Both studies which examined associations between total PA and osteoporosis among males found a protective effect53,55. Shetty et al. reported 40% less risk (adjusted OR: 0.4 (0.12–0.9), p < 0.0001) of osteoporosis among physically active males compared to their inactive counterparts53.
Among females, one study reported no association between osteoporosis and total PA56 while two studies found a protective effect of LTPA54,58. There was 32% less risk of osteoporosis with every additional 10 metabolic equivalents (METs) of LTPA (adjusted OR: 0.68 (0.66–0.71))54. Only one study examined the association between regular walking and BMD and found a null association58.
Back pain
The association between back pain and PA was examined in one study that was conducted across five South Asian countries57. Bishwajit et al. analysed the data from the World Health Survey 2002 among 8502 men and women aged 50 years and above from Bangladesh, India, Nepal, Pakistan and Sri Lanka57. Separate associations were reported for walking, MPA, and vigorous PA (VPA).
Walking was not found to have a significant association with back-pain among women except in India. Indian women who walked a few days/week or who never walked had 26% (AOR: 1.26 (1.00–1.58)) and 33% higher odds (AOR: 1.33 (1.00–1.75)) respectively of reporting back pain compared to women who walked daily. In the case of men, similar associations were found in Nepal and Pakistan but only for those who never walked57.
Indian men and women who did not engage in daily MPA were more likely to report back pain. For those who did not undertake any MPA, the odds of reporting back pain were 29% (AOR: 1.29 (1.04–1.59)) and 56% (AOR: 1.56 (1.00–2.44) higher for women and men respectively, compared to those undertaking daily MPA. Likewise, Indian men and women engaging in MPA for a few days a week were 38% and 36% more likely to report back-pain than those undertaking daily MPA. No significant associations were found for Bangladeshi and Nepali men and women and Sri Lankan men. In case of Pakistani and Sri Lankan women, a higher risk was found among those undertaking no MPA. The study did not find any significant association between VPA and self-reported back pain among men across all five countries. Significant associations were found among women in Pakistan and Sri Lanka57. Supplementary File: Table 5 presents country-specific findings.
Discussion
This study systematically reviewed 24 peer-reviewed studies to determine the association between PA and chronic diseases among South Asian adults aged 40 years and older. Total PA was the most reported exposure variable, with few studies reporting other PA forms such as walking, HPA or LTPA. Cardiometabolic outcomes were the most studied outcome variables, followed by musculoskeletal conditions. No clear dose-response relationship was evident because of differences in PA classifications and domains, and mixed associations across levels of PA categories.
PA and cardiometabolic outcomes
The results from the meta-analysis indicate an increased risk of cardio-metabolic outcomes (HTN, T2DM, MetS or CHD) among inactive South Asian adults. The risk was 34% (range, 10–63%) higher among inactive people compared to those with moderate or higher levels of total PA. Some of the studies could not be pooled because they did not report ORs, provide raw data or other convertible effect estimates, or they used PA categorisations that were not comparable to other studies. This reflects the heterogeneity across the studies and highlights the need to use comparable PA measures and classifications in future studies.
Inactive or less active South Asian adults were at 31% (range, 7–60%) higher risk of being hypertensive. The results are consistent with the existing literature, which has found that PA is a preventive, as well as a treatment strategy for managing HTN59. A meta-analysis of non-South Asia based cohort studies has reported a 41% increased risk (range, 15–72%) of HTN among individuals with low total PA compared with those with high total PA60. Another meta-analysis reported no association between OPA and HTN61, however, our review found mixed results across the two studies reporting OPA. Hypertension is an escalating public health problem among South Asian adults with the prevalence ranging from 20% in Bhutan (40–69 years)16 to 24% in the Maldives (45–64 years)62 and 47% in Nepal (45–69 years)15. The findings of this meta-analysis indicate the potential value of public health interventions promoting total PA to reduce the burden of HTN in South Asia.
Engagement in LTPA was found to have a protective effect on T2DM risk, as reported in 2 studies, however, this review concluded that there is limited evidence on the role of PA on T2DM risk among South Asian adults because of the small number of studies reporting the association. A meta-analysis of cohort studies including at least 2 of the 4 PA domains has reported a decreased risk of T2DM incidence by 26% among individuals with at least 150 min/week of MPA63. This protective effect of PA on T2DM has been reported in other studies25,64. Further studies are required to establish the role of PA in tackling the T2DM burden in the South Asian region.
There was no statistically significant association between CHD or CVD risk and total PA in this review. This finding contrasts with other reviews that have reported a protective effect11,25,65. The Global Burden of Disease Study 2013 found a reduced CHD risk of 25% and 23% among highly and moderately active individuals respectively, compared to those insufficiently active11. The plausible explanation for the differences in findings could be the nature of the studies included in our meta-analysis. Of the three studies used for pooling, one was a case-control study among CHD cases36 while the other two papers used different CVD risk scores41,42. Future studies using standard outcome measure (CHD incidence) and PA assessment criteria are recommended to determine the role of PA in CHD prevention in the region.
While non-South Asia based studies show a negative association between PA and obesity66–68, the only study included in this review found no significant correlation between PA and BMI, WC, WHR and MetS47. Walking was also not found to be associated with decreased waist circumference or BMI. These findings need to be interpreted with caution as they are derived from a few studies only. Additionally, lack of objective assessment of PA in most of the studies might have resulted in an under or over reporting of levels of activity and underestimation of its association with weight and metabolic variables69.
PA and breast cancer
Both the studies reporting an association for breast cancer found a protective effect of HPA among Indian women, with 5–6 hours/day of HPA being the optimum amount. This finding is consistent with systematic reviews on breast cancer and HPA. A meta-analysis that pooled 21 HPA comparisons from 15 studies found that the risk of breast cancer was reduced by 22% among those with the highest HPA level compared to the lowest70. Another meta-analysis has revealed a risk reduction of 11% (95% CI: 5% to 17%) for HPA71. The findings of the current review are particularly crucial in the South Asian context where HPA is the dominant PA form among women15,16,19,62. However, the findings need to be validated with larger longitudinal studies.
PA and musculoskeletal conditions
Adults are at an increased risk of osteoporosis because of physical inactivity, morbidities, hormonal changes and decreased intake of calcium and vitamin D72. It is an increasing problem in South Asia, where there is often late diagnosis, and is reported to be exacerbated by vitamin D deficiency73–75. In India, it has been reported that hip fractures due to low BMD occur almost a decade earlier compared to the western nations74. Our review found that total PA had a protective effect on osteoporosis among males only.
On the other hand, LTPA had a protective association among females, but no association was found for walking. A review of intervention studies has found that PA prevents bone loss and has a protective effect on BMD among postmenopausal women, but was unable to conclude the type, intensity, duration and frequency of PA that is beneficial72. Because of a limited number of studies eligible in our review, it is difficult to reach to a definite conclusion about the role of PA in the prevention of osteoporosis in South Asia.
The only study that examined the association between self-reported back pain and PA, across five South Asian countries, found mixed associations across PA type and dosage between males and females. Previous systematic reviews have also found an inconsistent association between low back pain and PA76,77. A review of systematic reviews concluded limited evidence on the causal relationship between walking and low back pain78. Given the limited research on back pain and different PA domains, and the inconsistency across the available evidence, further research is needed to reach to a definite conclusion in the South Asian context.
Methodological limitations of the included studies
The lack of age-specific results was the primary reason for exclusion of more than 70% of screened papers for this review. All the studies were either ranked as fair or poor quality using the NIH checklist. Common weaknesses were the lack of temporal difference in exposure and outcome measurement, insufficient timeframe for the outcome to manifest, not having repeated exposure measurement, and not using validated measurement instruments. All studies, except one, used questionnaires to assess the participant’s PA. While questionnaires are the measures of choice in population-level surveys, particularly in developing countries to capture activities across the range of PA domains79,80, it cannot be denied that they are likely to under or overestimate correct exposure because of recall or social desirability bias or the lack of common understanding between respondents and researchers81.
Meta-analysis was only possible for nine studies reporting cardio-metabolic outcomes because of the heterogeneity of outcome variables, PA domains, and availability of raw data or convertible measures of association. The recall period was not mentioned in 10 of the 24 studies while one study asked participants to recall the activities carried out during the last two years46. Only one-third of the studies reported a response rate and sample size calculation, which made it difficult to ascertain the representativeness of the sample. Poor reporting of research methods was common in the included articles.
The studies included in this review have shown either a null association, negative association or a mix of null and negative association across the PA domains/categories. While some of these differences might be real, the variations in the type of PA questionnaires used, types of PA domains studied and the categorisation of PA scores for reporting of the results might have affected the study results. Comparing the results across the studies was difficult because of the variations in the categorisation of PA between the studies. Using the standard categorisation of PA based on the cut-offs suggested in the GPAQ and IPAQ analysis guides82,83 would help to maintain uniformity and facilitate comparison between studies. Longitudinal studies with relatively larger sample size, use of validated tools for PA assessment and objective assessment of chronic diseases are recommended to ensure the production of high-quality evidence to inform policy and practice.
Strengths and limitations of the review
To our knowledge, this is the first study to systematically review the association between PA and multiple chronic disease outcomes and risk markers and to quantitatively summarise the association between cardiometabolic outcomes and PA among South Asian adults. Routine PA was the primary focus of the review and studies which only examined structured PA were excluded, which was a strength given that routine HPA and TPA contribute to the significant portion of PA among adults in South Asian nations15–18.
Several limitations also need to be considered when interpreting the study findings. More than half of the included studies were from India while none were conducted in the Maldives or Bhutan. Two-thirds of the studies were cross-sectional which restricts the ascertainment of causality. Meta-analysis dichotomised PA as yes/no or low/high because of the lack of estimates for the middle category in some studies, which decreased the sample size in the pooled analysis and increased the confidence intervals for the odds ratios. Further, only statistical significance, not clinical significance, was considered while interpreting the study findings. Limiting the search only to published peer-reviewed English language studies could have missed some information.
Conclusion
The rapidly changing demographics, haphazard urbanisation and economic development, along with genetic susceptibility to diseases such as T2DM, make South Asians a priority group for non-communicable disease risk factor research. The pooled results from the meta-analysis of observational studies included in this review suggest that physical inactivity is associated with the higher risk of cardiometabolic conditions, particularly, hypertension among South Asian adults. Public health interventions addressing PA could potentially contribute to addressing the surging cardiometabolic disease burden in the region. The limited number of studies included in this review restricted drawing conclusions on the association of PA with other outcome variables such as T2DM, osteoporosis, CHD, obesity and breast cancer. Based on the global evidence, this review also recommends incorporating PA in interventions targeting these conditions along with conducting high-quality studies, with a longitudinal design, representative samples, and objective assessment of PA and disease outcomes, to generate local evidence. Scientific evaluation of existing interventions can also provide useful information on the role of PA in chronic disease prevention. Further, most of the studies included in this review have examined total PA with very few that investigated associations for different PA domains: including occupational, household and transport related PA. Future studies should focus on all the PA domains and use standard categorisation of PA to allow for comparisons across the studies.
Supplementary information
Acknowledgements
We would like to thank Ms. Lorena Romero, Senior Medical Librarian at the Ian Potter Library, Alfred Hospital, Melbourne for her guidance in finalising the search strategy. SP has received Monash International Postgraduate Research Scholarship and Monash Graduate Scholarship for her PhD from Monash University. EOA is funded through the Australian Government Research Training Program Award and Monash University’s Sir James McNeill Scholarship.
Author Contributions
S.P. was responsible for searching the literature, screening the papers, working on the design, quality assessment, analysing the data and drafting the manuscript. E.O.A. was involved in screening the papers and quality assessment, reviewed and edited the manuscript. A.J.O. and B.J.S. provided inputs in drafting the search strategy, screening of papers, quality assessment, data analysis, reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Data Availability
This review uses data and findings from already published studies that are publicly available.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46154-3.
References
- 1.World Health Organization (WHO). Noncommunicable Diseases Country Profiles 2011. (WHO, Geneva, 2011).
- 2.World Health Organization (WHO). Noncommunicable Diseases Country Profiles 2014. (WHO, Geneva, 2014).
- 3.Siegel KR, Patel SA, Ali MK. Non-communicable diseases in South Asia: contemporary perspectives. British Medical Bulletin. 2014;111:31–44. doi: 10.1093/bmb/ldu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. British Medical Journal. 2004;328:807. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metabolic Syndrome & Related Disorders. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 6.Vikram NK, et al. Non-obese (body mass index <25 kg/m2) Asian Indians with normal waist circumference have high cardiovascular risk. Nutrition. 2003;19:503–509. doi: 10.1016/S0899-9007(02)01083-3. [DOI] [PubMed] [Google Scholar]
- 7.Khuwaja AK, Qureshi R, Fatmi Z. Noncommunicable diseases and injuries: action needed in South Asia too. PLoS Medicine. 2007;4:e38. doi: 10.1371/journal.pmed.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arokiasamy, P. J. T. L. G. H. India’s escalating burden of non-communicable diseases. 6, e1262–e1263 (2018). [DOI] [PubMed]
- 9.World Health Organization (WHO). Global recommendations on physical activity for health. (World Health Organization, Switzerland, 2010). [PubMed]
- 10. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. (World Health Organization, Geneva, 2009).
- 11.Kyu HH, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Love, R., Adams, J., van Sluijs, E., Foster, C. & Humphreys, D. A cumulative meta‐analysis of the effects of individual physical activity interventions targeting healthy adults. Obesity Reviews (2018). [DOI] [PMC free article] [PubMed]
- 13.Macniven, R., Bauman, A. & Abouzeid, M. J. B. p. h. A review of population-based prevalence studies of physical activity in adults in the Asia-Pacific region. 12, 41 (2012). [DOI] [PMC free article] [PubMed]
- 14.Bauman AE, et al. Correlates of physical activity: why are some people physically active and others not? The lancet. 2012;380:258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 15.Aryal, K. K. et al. Non communicable diseases risk factors: STEPS Survey Nepal 2013. (Nepal Health Research Council (NHRC), 2014). [PubMed]
- 16.World Health Organization (WHO). National survey for noncommunicable disease risk factors and mental health using STEPS approach in Bhutan – 2014. (WHO, India, 2014).
- 17.World Health Organization (WHO). Non Communicable Disease Risk Factor Survey Sri Lanka 2015. (WHO, 2015).
- 18.World Health Organization (WHO). Global Recommendation on Physical Activity for Health. (WHO Press, Geneva, 2010).
- 19.Moniruzzaman M, et al. Physical activity levels in Bangladeshi adults: results from STEPS survey 2010. Public Health. 2016;137:131–138. doi: 10.1016/j.puhe.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anjana RM, et al. Physical activity and inactivity patterns in India–results from the ICMR-INDIAB study (Phase-1)[ICMR-INDIAB-5] International Journal of Behavioral Nutrition and Physical Activity. 2014;11:26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzmarzyk P, Craig C, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007;50:538–544. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- 22.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 23.Nocon M, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 24.Batty GD. Physical activity and coronary heart disease in older adults: a systematic review of epidemiological studies. The European Journal of Public Health. 2002;12:171–176. doi: 10.1093/eurpub/12.3.171. [DOI] [PubMed] [Google Scholar]
- 25.Milton K, Macniven R, Bauman A. Review of the epidemiological evidence for physical activity and health from low-and middle-income countries. Global public health. 2014;9:369–381. doi: 10.1080/17441692.2014.894548. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudel, S., Owen, A. J., Owusu-Addo, E. & Smith, B. J. Physical activity participation and the risk of chronic diseases among South Asian adults: protocol for a systematic review and meta-analysis. Systematic Reviews7, 10.1186/s13643-018-0848-9 (2018). [DOI] [PMC free article] [PubMed]
- 29.Rheuters, T. (Thomson Rheuters, 2011).
- 30.National Institute of Health. Study Quality Assessment Tools, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (2014).
- 31.Vuong HG, et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget. 2017;8:10637. doi: 10.18632/oncotarget.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Chhetri M, Chapman R. Prevalence and determinants of diabetes among the elderly population in the Kathmandu Valley of Nepal. Nepal Med Coll J. 2009;11:34–38. [PubMed] [Google Scholar]
- 34.Hamid N, et al. Metabolic Syndrome and its Relationship with associated risk factors. J Med Sci. 2010;18:186–190. [Google Scholar]
- 35.Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to meta-analysis. (John Wiley & Sons, 2011).
- 36.Jain P, Jain P, Bhandari S, Siddhu A. A case-control study of risk factors for coronary heart disease in urban Indian middle-aged males. Indian Heart J. 2008;60:233–240. [PubMed] [Google Scholar]
- 37.Saeed KMI, Rasooly MH, Brown NJ. Prevalence and predictors of adult hypertension in Kabul, Afghanistan. BMC Public Health. 2014;14:386. doi: 10.1186/1471-2458-14-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low-and middle-income countries: prevalence, awareness and control. International Journal of Epidemiology. 2014;43:116–128. doi: 10.1093/ije/dyt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quasem I, et al. Prevalence, awareness, treatment and control of hypertension among the elderly in Bangladesh and India: a multicentre study. Bulletin of the World health Organization. 2001;79:490. [PMC free article] [PubMed] [Google Scholar]
- 40.Zachariah MG, Thankappan K, Alex SC, Sarma P, Vasan R. Prevalence, correlates, awareness, treatment, and control of hypertension in a middle-aged urban population in Kerala. Indian Heart Journal. 2003;55:245–251. [PubMed] [Google Scholar]
- 41.Dhungana R, et al. Assessment of Short Term Cardiovascular Risk Among 40 Years and Above Population in a Selected Community of Kathmandu, Nepal. Journal of Nepal Health Research Council. 2015;13:66–72. [PubMed] [Google Scholar]
- 42.Menon Vidya P., Edathadathil Fabia, Sathyapalan Dipu, Moni Merlin, Don Ann, Balachandran Sabarish, Pushpa Binny, Prasanna Preetha, Sivaram Nithu, Nair Anupama, Vinod Nithu, Jayaprasad Rekha, Menon Veena. Assessment of 2013 AHA/ACC ASCVD risk scores with behavioral characteristics of an urban cohort in India. Medicine. 2016;95(49):e5542. doi: 10.1097/MD.0000000000005542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Review Manager (RevMan). [Computer program]. Version 5.3., https://community.cochrane.org/help/tools-and-software/revman-5 (2014).
- 44.Subburam R, Sankarapandian M, Gopinath D, Selvarajan S, Kabilan L. Prevalence of hypertension and correlates among adults of 45–60 years in a rural area of Tamil Nadu. Indian Journal of Public Health. 2009;53:37–40. [PubMed] [Google Scholar]
- 45.Snehalatha C, Ramchandran A, Kapur A, Vijay V. Age-specific prevalence and risk associations for impaired glucose tolerance in urban southern Indian population. Journal of Association of Physicians of India. 2003;51:766–770. [PubMed] [Google Scholar]
- 46.Ansari, R. M. Effect of physical activity and obesity on type 2 diabetes in a middle-aged population. Journal of Environmental and Public Health2009 (2009). [DOI] [PMC free article] [PubMed]
- 47.Jahan N, Shenoy S. Relation of pedometer steps count & self reported physical activity with health indices in middle aged adults. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2017;11:S1017–S1023. doi: 10.1016/j.dsx.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt N, Bains K, Aggarwal R. Association of selected anthropometric and body composition measures with diet and life style related factors among Punjabi adult males of India. Applied Biological Research. 2016;18:155–162. doi: 10.5958/0974-4517.2016.00024.0. [DOI] [Google Scholar]
- 49.Ghosh A, Das AC. Explaining body composition by some covariate factors among the elderly Bengalee Hindu women of Calcutta, India. The. Journal of Nutrition, Health & Aging. 2005;9:403–406. [PubMed] [Google Scholar]
- 50.Kumar, A., Sivakanesan, R. & Nagtilak, S. Behavioral pattern, life style and socio economic status in elderly normolipidemic acute myocardial infarct subjects-a case control study from South Asia. Internet J Cardiovasc Res6 (2009). [PubMed]
- 51.Dey S, et al. Risk factors according to estrogen receptor status of breast cancer patients in Trivandrum, South India. International Journal of Cancer. 2009;125:1663–1670. doi: 10.1002/ijc.24460. [DOI] [PubMed] [Google Scholar]
- 52.Mathew A, et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: a case–control study. European Journal of Cancer Prevention. 2009;18:368–376. doi: 10.1097/CEJ.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]
- 53.Shetty Sahana, Kapoor Nitin, Naik Dukhabandhu, Asha Hesarghatta Shyamasunder, Prabu Suresh, Thomas Nihal, Seshadri Mandalam Subramaniam, Paul Thomas Vizhalil. Osteoporosis in Healthy South Indian Males and the Influence of Life Style Factors and Vitamin D Status on Bone Mineral Density. Journal of Osteoporosis. 2014;2014:1–5. doi: 10.1155/2014/723238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shenoy S, Chawla JK, Gupta S, Sandhu JS. Prevalence of low bone health using quantitative ultrasound in Indian women aged 41–60 years: Its association with nutrition and other related risk factors. Journal of Women & Aging. 2017;29:334–347. doi: 10.1080/08952841.2016.1188620. [DOI] [PubMed] [Google Scholar]
- 55.Lekamwasam S, Wijayaratne L, Rodrigo M, Hewage U. Prevalence and determinants of osteoporosis among men aged 50 years or more in Sri Lanka: a community-based cross-sectional study. Archives of Osteoporosis. 2009;4:79–84. doi: 10.1007/s11657-009-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Begum RA, et al. Osteopenia and osteoporosis among 16–65 year old women attending outpatient clinics. Journal of Community Health. 2014;39:1071–1076. doi: 10.1007/s10900-014-9853-7. [DOI] [PubMed] [Google Scholar]
- 57.Bishwajit G, Tang S, Sanni Yaya ZF. Participation in physical activity and back pain among an elderly population in South Asia. Journal of Pain Research. 2017;10:905. doi: 10.2147/JPR.S133013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keramat A, et al. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskeletal Disorders. 2008;9:28. doi: 10.1186/1471-2474-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancia G, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Pressure. 2013;22(278):193. doi: 10.3109/08037051.2013.812549. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, et al. Dose–response association between physical activity and incident hypertension: a systematic review and meta-analysis of cohort studies. Hypertension. 2017;69:813–820. doi: 10.1161/HYPERTENSIONAHA.116.08994. [DOI] [PubMed] [Google Scholar]
- 61.Huai P, et al. Physical activity and risk of hypertension: a meta-analysis of prospective cohort studies. Hypertension. 2013;62:1021–1026. doi: 10.1161/HYPERTENSIONAHA.113.01965. [DOI] [PubMed] [Google Scholar]
- 62.WHO. WHO STEPS survey on risk factors for noncommunicable diseases Maldives, 2011. (World Health Organization, 2011).
- 63.Wahid A, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta‐analysis. Journal of the American Heart Association. 2016;5:e002495. doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeon CY, Lokken RP, Hu FB, Van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Siegrist J. Physical activity and risk of cardiovascular disease—a meta-analysis of prospective cohort studies. International Journal of Environmental Research and Public Health. 2012;9:391–407. doi: 10.3390/ijerph9020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity–a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dwyer T, et al. The inverse relationship between number of steps per day and obesity in a population-based sample–the AusDiab study. International Journal of Obesity. 2007;31:797. doi: 10.1038/sj.ijo.0803472. [DOI] [PubMed] [Google Scholar]
- 68.Kwon S, Wang M, Hawkins M. Association between self-reported physical activity and obesity among White, Black, Hispanic, and Asian Americans: 2007 and 2009 brfss. Ethnicity & Disease. 2013;23:129–135. [PubMed] [Google Scholar]
- 69.Prince SA, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi Y, et al. Household physical activity and cancer risk: a systematic review and dose-response meta-analysis of epidemiological studies. Scientific Reports. 2015;5:14901. doi: 10.1038/srep14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Research and Treatment. 2013;137:869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 72.Segev D, Hellerstein D, Dunsky A. Physical activity-does it really increase bone density in postmenopausal women? A Review of articles published between 2001-2016. Current Aging Science. 2018;11:4–9. doi: 10.2174/1874609810666170918170744. [DOI] [PubMed] [Google Scholar]
- 73.Mithal A, Bansal B, Kyer CS, Ebeling P. The Asia-pacific regional audit-epidemiology, costs, and burden of osteoporosis in India 2013: a report of international osteoporosis foundation. Indian Journal of Endocrinology and Metabolism. 2014;18:449. doi: 10.4103/2230-8210.137485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malhotra, N. & Mithal, A. Osteoporosis in Indians. Indian Journal of Medical Research127 (2008). [PubMed]
- 75.LeKhi A, LeKhi M, Sathian B, Mittal A. The role of biochemical markers in the early detection of osteoporosis in women: A comparative study from the Western Region of Nepal. J Clin Diagnos Res. 2012;6:274–277. [Google Scholar]
- 76.Sitthipornvorakul E, Janwantanakul P, Purepong N, Pensri P, van der Beek AJ. The association between physical activity and neck and low back pain: a systematic review. European Spine Journal. 2011;20:677–689. doi: 10.1007/s00586-010-1630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heneweer H, Staes F, Aufdemkampe G, van Rijn M, Vanhees L. Physical activity and low back pain: a systematic review of recent literature. European Spine Journal. 2011;20:826–845. doi: 10.1007/s00586-010-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon B, Roffey D, Bishop P, Dagenais S, Wai E. Systematic review: occupational physical activity and low back pain. Occupational Medicine. 2011;61:541–548. doi: 10.1093/occmed/kqr092. [DOI] [PubMed] [Google Scholar]
- 79.Trinh, O. T., Nguyen, N. D., Dibley, M. J., Phongsavan, P. & Bauman, A. E. J. B. p. h. The prevalence and correlates of physical inactivity among adults in Ho Chi Minh City. 8, 204 (2008). [DOI] [PMC free article] [PubMed]
- 80.Hallal, P. C. et al. Physical inactivity: prevalence and associated variables in Brazilian adults. 35, 1894–1900 (2003). [DOI] [PubMed]
- 81.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research Quarterly for Exercise and Sport. 2000;71:1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. (World Health Organization, Geneva).
- 83.The IPAQ Group. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire., http://www.ipaq.ki.se (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This review uses data and findings from already published studies that are publicly available.