Abstract
During the in vitro culture of plants some physiological disorders caused major problems that have been associated with culture media composition. The objective of this study was to better understand the abnormal physiological response of two pistachio rootstocks to changes in culture media ingredients. On this purpose, two computer-based tools were employed: design of experiment (DOE) and neurofuzzy logic. DOE was employed to generate a five-dimensional IV-design spaces allowing to reduce the number of treatments from 6,250 to 61. The second one, an artificial intelligence (AI) tool, neurofuzzy logic, was used to understand the cause-effect relationships between the factors studied (25) and seven physiological disorders including shoot-tip necrosis (STN), leaf necrosis (LN), leaf color (LC), basal callus (BC) formation, shoot fasciation (SF), hyperhydricity and epinasty, typically described during pistachio in vitro culture. Four out of the seven disorders were successfully modeled, being significantly affected by a limited number of factors. STN and BC were significantly affected by the concentration of EDTA−. However, while a low concentration of EDTA− reduces the STN, promotes BC. LN and LC were strongly alleviated by high amounts of thiamine-HCl. Undoubtedly, the results demonstrate the importance of recording and using data related to physiological disorders along with growth parameters when developing suitable culture media for plant tissues. The computer-based tools have been useful to: i) well sample experimental design; ii) reduce the final number of treatments and the experimental work; iii) identify the key factors affecting each disorder; iv) get insight about the causes that promote the appearance of physiological disorders. Our findings demonstrate that the recently AI designed POM media, although not optimal, is the most suitable (favouring growth and limiting physiological abnormalities) media for in vitro culture of pistachio compared to those media, currently used.
Subject terms: Agricultural genetics, Computational models
Introduction
During the in vitro culture of plants is frequent that physiological disorders appear such as shoot-tip necrosis (STN), callus formation at the base of shoots (BC), hyperhydricity, shoot fasciation (SF), epinasty, leaf necrosis (LN) or leaf color (LC), which reduce the yield and the quality of the production.
The necrosis of shoot-tips (STN) was first illustrated by McCown and Sellmer1 as a progressive discoloration of the apical meristems that lead the death (necrosis) of the shoot-tip. Among the causes of STN, the growth media type, the cytokinin or the micro-environment of culture vessels have been well-documented2–5.
Leaf necrosis (LN) has been characterized by dark or progressively discolored spots. Leaf edge necrosis is frequently reported during in vitro culture of variety of species6–8. Reed and co-workers9 indicated that imbalanced mineral nutrition of commonly utilized culture media such as MS10 was associated to the appearance of the disorder in diverse pear germplasm, giving promising improvements on reducing the disorder by increasing CaCl2.2H2O, KH2PO4 and MgSO4.7H2O up to certain concentrations.
The lack of certain nutrients in the culture media also lead variations in leaf color (LC) from green to red in micropropagated shoots of different species6–9,11.
The formation of basal callus (BC) is particularly important in the commercial shoot micro-propagation of diverse species, since its appearance probably slows down or even inhibits the absorption of nutrients by the shoots, especially alongside the callus senesces12. In melon cultivars, BC has been attributed to the accumulation of calcium in that area of the plant, which can lead to deficiencies of Ca2+ in the upper parts of the shoots13.
Hyperhydricity has been associated to hypolignification and poor cell wall development14,15. Hyperhydric shoots become translucent and water soaked. Leaves become brittle, shiny, dark green and glassy16. Moreover, the malformed plantlets do not survive when they are transferred to soil17. The type of culture medium or the gelling agent, the mineral nutrients, the plant growth regulators (PGRs), the micro-environment conditions or the containers have been pointed out as factors leading to hyperhydricity in different plant species15,18–22. Particularly, in Pistacia cultures it has been associated to the type of growth medium23 and the cytokinins type and/or concentrations24–26.
Shoot fasciation (SF), also named as cristation, is a disorder associated with hyperhydricity and characterized by the development of flatted and abnormal apical meristem, suggesting many stems have fused together15. Recently, the causes of the disorder in different plant species have been associated to inadequate type and/or concentration cytokinins as well as a reduced amount of total nitrogen of standard MS medium27.
Epinasty is a physiological disorder attributed to the accumulation of produced gases e.g. ethylene in air tight vessels28 or insufficient content of mineral nutrients of culture media e.g. calcium7. Typical macroscopic symptoms appear in reduced leaf expansion together with promoting downward leaves during micropropagation in a range of species such as Rosa hybrid and Musa sp.29,30.
In pistachio, physiological disorders has been described to occur quite frequently during in vitro culture26. Those abnormalities have been associated to an imbalance of mineral nutrients in the culture media employed: MS10, DKW31 or WPM32. Several solutions such as readjusting components of media e.g. increasing boron or calcium content or using high (up to 4 mg L−1) BAP concentrations12,24,26,33–38 have been proposed, but a final solution is far from being found.
The study of the causes of the appearance of physiological disorders in plant tissue cultures has not been addressed very efficiently, mainly due to two reasons. Firstly, plant tissue culture combine a large amount of factors (mineral components, PGRs, vitamins, organic compounds and growth culture conditions) which makes difficult to find the key factor/s causing those physiological abnormalities using factorial designs. Recently, the use of computer-based design of experiment (DOE) has permitted researchers to simultaneously study the effects of multiple factors on a process, with the advantage of a considerable reduction in the number of treatments to carry out39. In addition, it allows to obtain general conclusions compared to traditional approaches such as one-factor-at-a-time (OFAT)40. Using this methodology, Reed and coworkers41 have pointed out the crucial influence of unbalances mineral nutrients on physiological disorders for diverse genotypes of pear. They pointed out that a low concentration of salts categorized as nitrogen (NH4NO3 or KNO3) or mesos (CaCl2.2H2O, MgSO4.7H2O and KH2PO4) gives rise to STN. Many other authors have reached similar conclusions using this type of methodology7,8,11,42,43.
Secondly, establishing the effect of a large number of mineral nutrients, vitamins and PGRs on the appearance of physiological disorders, would involve modeling an extremely complex database, which would be difficult using traditional statistical methods44,45 but can be easily achieved using artificial intelligence tools46. In recent years, the combination of artificial neural networks (ANNs) with fuzzy logic, named as neurofuzzy logic, has been presented as a powerful data mining strategy, which allows the modeling of complex databases and the identification of the key factors to improve a specific response47. Neurofuzzy logic systems have two strengths: i) they are able to model very complex databases, and ii) the models are presented as a set of ‘IF- THEN’ rules, which allows researchers to understand the analyzed process and make appropriate decisions to implement optimal culture conditions48. This tool has been successfully applied to in vitro plant tissue culture in order to model germination rates, shoot multiplication rhizogenesis and acclimatization45,49,50. As far as we know, only two physiological disorders, caused by in vitro plant tissue culture, were included in neurofuzzy logic models until now51.
On this basis, the goal of the present study was to establish the cause of the appearance of the most common physiological disorders in Pistacia shoots, as consequence of the use of different genotypes and media formulations (mineral composition, vitamins, glycine and PGRs). To that end, we have combined in one very large database the results obtained from two independent experiments, both developed using of computer-based design of experiment (DOE) to simultaneously study the effects of multiple factors on pistachio tissue culture. Later, we have employed neurofuzzy logic, to model the database and find the key factors involved in the appearance of the physiological disorders.
Results
Several physiological disorders were detected after the micropropagation of pistachio as STN & LN (Fig. 1A), LC (Fig. 1B), BC (Fig. 1C), SF (Fig. 1D), hyperhydricity (Fig. 1E) and epinasty (Fig. 1F).
Figure 1.
Physiological response of UCB 1 pistachio shoots to compositions of culture media. (A) STN and LN; (B) LC, (C) BC, (D) SF, (E) hyperhydricity and (F) epinasty.
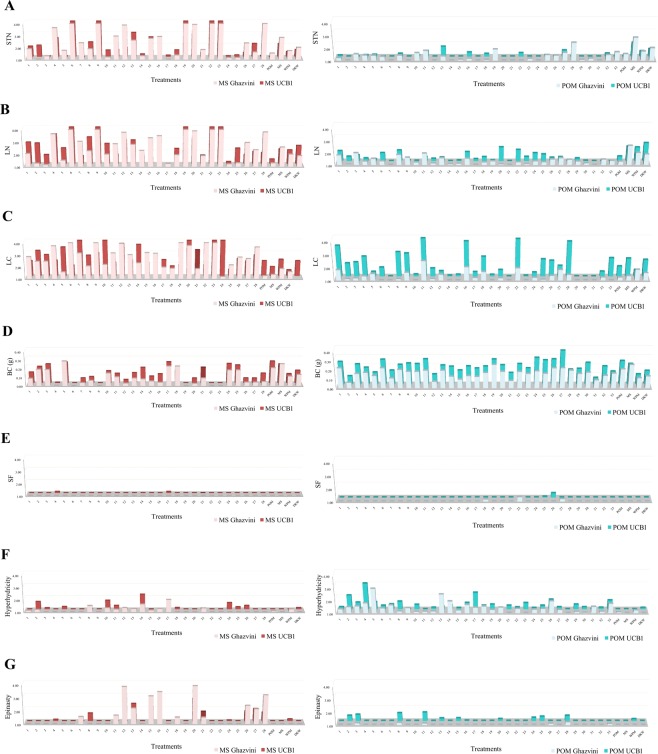
Although all data were modeled and analyzed using neurofuzzy logic as described below, we also included here some simple graphs of the results obtained in order to show how difficult is to interpret a cause-effect of factors on the parameters measured using them (Fig. 2). The graphs represent the physiological abnormalities ranged from 1 (no abnormality) to 4 (maximum disorder), except BC which was expressed in grams, being 0 no callus formation. As it can be observed in Fig. 2: (i) all treatments produce physiological abnormalities to some degree, including POM, MS, WPM and DKW media used as controls; (ii) some abnormalities such as STN, LN, LC or BC are frequently (detected in most treatments), while others are rare (only in few treatments) such as SF, hyperhydricity or epinasty; (iii) treatments based on MS media caused higher STN, LN, LC and epinasty than those based in POM media, but contrary, POM based treatments caused higher BC and hyperhydricity compared to MS medium; (iv) some treatments based on MS (6, 9, 19, 22 and 23) caused the maximum disorder (categorized as 4) for STN, LN and LC in both genotypes. On the contrary, those treatments caused the lower BC content; (v) finally, if compared the four basal media used as controls, clearly the pistachio optimized medium (POM) reduced the STN, LN and LC compared to MS, WPM and DKW media. However, the composition of POM or MS promotes more BC than WPM and DKW.
Figure 2.
Graphical representation of physiological response of the pistachio shoots to different treatments based on MS and POM together with original mineral nutrients of POM, MS, WPM and DKW; (A) STN, (B) LN, (C) LC, (D) BC, (E) SF, (F) hyperhydricity, and (G) epinasty.
The previous results showed the great complexity of the micropropagation process and the enormous difficulty for its optimization, since none of the proven treatments was capable of producing healthy shoots without physiological disorders. The establishment of optimal conditions for micropropagation will undoubtedly require the adoption of a compromise solution between the different factors that allow maximizing the growth parameters, simultaneously minimizing the appearance of all abnormalities. In these circumstances, the difficulty in achieving this goal with traditional statistical tools is evident. Nor does the statistics allow us to easily indicate which of the factors determines the appearance of each physiological disorder. The neurofuzzy logic tool was used in order to model the complete data set (Table S1). The results obtained show that this technology has allowed the successful modeling of four of seven analyzed physiological disorders, for which a high determination coefficient (R2) between the experimental values and those predicted by the model (STN, LN, LC and BC, Fig. 3) was found. On this basis, the components of the studied culture media can be considered as causal agents of the appearance of these four physiological disorders.
Figure 3.
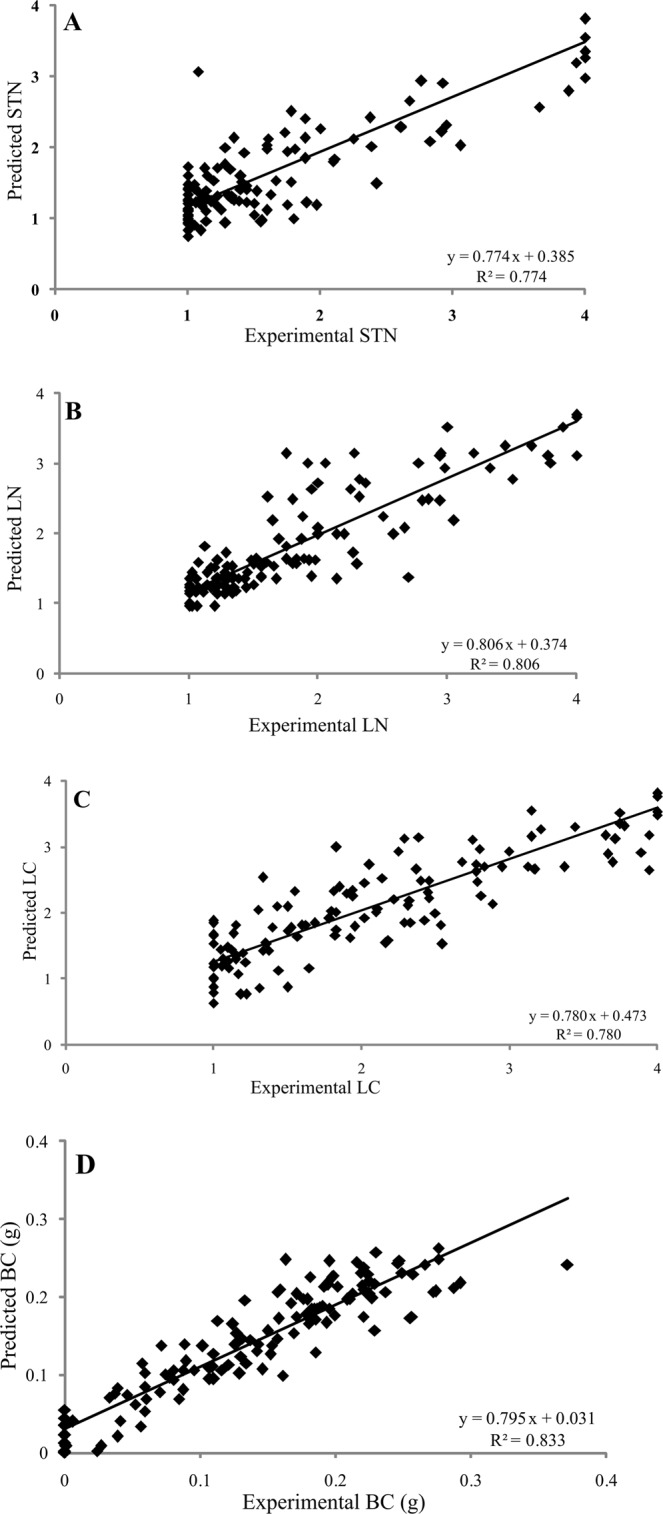
Determination coefficient (R2) of experimental vs. predicted values achieved by neurofuzzy logic models for the different parameters or outputs studied: (A) STN, (B) LN, (C) LC, (D) BC.
The results of STN, LN, BC and LC were explained as a function of independent or interaction of ions, vitamins or PGRs (Table 1), while SF, hyperhydricity and epinasty were not further studied, due to insufficient predictabilities of their models (Train set R2 < 70%). Furthermore, the ANOVA F ratio for those models was always greater than the f critical values together with, assessing the good performance and quality of neurofuzzy logic models.
Table 1.
Critical factors for each output and quality parameters of the neurofuzzy logic models. The inputs with stronger effect on each output have been highlighted.
| Outputs | Submodel | Significant inputs | Train Set R2 | F ratio | df1, df2 | f critical (α < 0.01) |
|---|---|---|---|---|---|---|
| STN | 1 | EDTA− × K+ | 77.44 | 36.82 | 11, 118 | 2.40 |
| 2 | BAP | |||||
| 3 | Cl− | |||||
| 4 | Genotype | |||||
| 5 | Na+ | |||||
| LN | 1 | Na+ × Thiamine-HCl | 80.63 | 62.94 | 8, 121 | 2.66 |
| 2 | Cl− | |||||
| 3 | K+ | |||||
| LC | 1 | Genotype × Thiamine-HCl | 78.07 | 27.05 | 15, 114 | 2.19 |
| 2 | Fe2+ × Mn2+ | |||||
| 3 | Cl− | |||||
| 4 | Glycine | |||||
| 5 | K+ × SO42− | |||||
| BC (g) | 1 | EDTA− × Mn2+ | 82.28 | 49.82 | 11, 128 | 2.40 |
| 2 | Glycine | |||||
| 3 | Cl− | |||||
| 4 | Genotype | |||||
| 5 | NH4+ | |||||
| SF | — | — | 4.39 | 2.92 | 2, 127 | 4.77 |
| Hyperhydricity | — | — | 11.51 | 8.26 | 2, 127 | 4.77 |
| Epinasty | — | — | 21.02 | 6.60 | 5, 124 | 3.16 |
Neurofuzzy logic model also give information about the key factors involved in each abnormality (Table 1). Just 12 out of 25 inputs studied (genotype, NH4+, K+, Na+, Fe2+, Cl−, Mn2+, EDTA−, SO42−, thiamine-HCl, glycine and BAP) affected significantly the disorders.
STN can be explained by five submodels: the interaction of EDTA− and K+ as submodel 1 (stronger effect), followed by independent influence of BAP, Cl−, genotype and Na+ as submodels 2 to 5, respectively. While LN can be explained simply by only three inputs: the interaction of Na+ and thiamine-HCl (stronger effect), and independent influence of Cl− and K+; LC depends on a complex action of eight inputs: three interactions (genotype and thiamine-HCl (stronger effect), Fe2+ and Mn2+ and K+ and SO42−) and two independent inputs (Cl− and glycine). Finally, EDTA− also has had an impact on BC in interaction with Mn2+ as submodel 1, followed by independent effects of glycine, Cl−, genotype and NH4+ on the disorder as submodels 2 to 5, respectively Table 1).
Table 2 presents the whole set of ‘IF-THEN’ rules generated by the neurofuzzy logic software for the four abnormalities. To better visualize and interpret those ‘IF-THEN’ rules, both Fig. S1 and Table 3 can be used, which show the ranges corresponding to each word for each variable.
Table 2.
Rules selection generated by neurofuzzy logic showing the best combination of inputs to obtain the highest results for each output. The inputs with stronger effects on each output indicated by the model have been highlighted.
| Rules | Genotype | NH4+ | K+ | Na+ | Cl− | SO42− | Mn2+ | Fe2+ | EDTA− | Thiamine-HCl | Glycine | BAP | STN | LN | LC | BC(g) | Membership Function | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IF | Low | Low | THEN | Low | 1.00 | |||||||||||||
| 2 | High | Low | Low | 1.00 | |||||||||||||||
| 3 | Low | Mid | Low | 1.00 | |||||||||||||||
| 4 | High | Mid | Low | 1.00 | |||||||||||||||
| 5 | Low | High | High | 1.00 | |||||||||||||||
| 6 | High | High | High | 1.00 | |||||||||||||||
| 7 | Low | High | 1.00 | ||||||||||||||||
| 8 | High | Low | 1.00 | ||||||||||||||||
| 9 | Low | High | 1.00 | ||||||||||||||||
| 10 | High | Low | 1.00 | ||||||||||||||||
| 11 | Ghazvini | High | 0.55 | ||||||||||||||||
| 12 | UCB1 | Low | 0.93 | ||||||||||||||||
| 13 | Low | Low | 0.78 | ||||||||||||||||
| 14 | Mid | High | 0.94 | ||||||||||||||||
| 15 | High | Low | 1.00 | ||||||||||||||||
| 16 | IF | Low | Low | THEN | Low | 0.73 | |||||||||||||
| 17 | High | Low | High | 1.00 | |||||||||||||||
| 18 | Low | High | Low | 1.00 | |||||||||||||||
| 19 | High | High | Low | 1.00 | |||||||||||||||
| 20 | Low | High | 0.88 | ||||||||||||||||
| 21 | Mid | Low | 1.00 | ||||||||||||||||
| 22 | High | Low | 0.85 | ||||||||||||||||
| 23 | Low | Low | 0.82 | ||||||||||||||||
| 24 | Mid | Low | 1.00 | ||||||||||||||||
| 25 | High | High | 0.68 | ||||||||||||||||
| 26 | IF | Ghazvini | Low | THEN | High | 1.00 | |||||||||||||
| 27 | UCB1 | Low | High | 1.00 | |||||||||||||||
| 28 | Ghazvini | High | Low | 1.00 | |||||||||||||||
| 29 | UCB1 | High | Low | 1.00 | |||||||||||||||
| 30 | Low | Low | Low | 1.00 | |||||||||||||||
| 31 | High | Low | High | 1.00 | |||||||||||||||
| 32 | Low | Mid | Low | 1.00 | |||||||||||||||
| 33 | High | Mid | Low | 1.00 | |||||||||||||||
| 34 | Low | High | High | 1.00 | |||||||||||||||
| 35 | High | High | High | 0.99 | |||||||||||||||
| 36 | Low | High | 1.00 | ||||||||||||||||
| 37 | Mid | Low | 1.00 | ||||||||||||||||
| 38 | High | Low | 1.00 | ||||||||||||||||
| 39 | Low | High | 1.00 | ||||||||||||||||
| 40 | High | Low | 1.00 | ||||||||||||||||
| 41 | Low | Low | High | 0.81 | |||||||||||||||
| 42 | Low | High | Low | 1.00 | |||||||||||||||
| 43 | High | Low | Low | 0.64 | |||||||||||||||
| 44 | High | High | High | 1.00 | |||||||||||||||
| 45 | IF | Low | Low | THEN | High | 0.79 | |||||||||||||
| 46 | Mid | Low | High | 1.00 | |||||||||||||||
| 47 | High | Low | High | 1.00 | |||||||||||||||
| 48 | Low | High | Low | 1.00 | |||||||||||||||
| 49 | Mid | High | Low | 1.00 | |||||||||||||||
| 50 | High | High | Low | 0.85 | |||||||||||||||
| 51 | Low | High | 1.00 | ||||||||||||||||
| 52 | High | Low | 1.00 | ||||||||||||||||
| 53 | Low | Low | 1.00 | ||||||||||||||||
| 54 | Mid | High | 1.00 | ||||||||||||||||
| 55 | High | High | 0.64 | ||||||||||||||||
| 56 | Ghazvini | Low | 0.64 | ||||||||||||||||
| 57 | UCB1 | High | 0.80 | ||||||||||||||||
| 58 | Low | Low | 0.60 | ||||||||||||||||
| 59 | High | High | 0.76 |
Table 3.
Meaning of the levels of each inputs after the fuzzyfication process developed by neurofuzzy logic software after modelling graphically represented in Fig. S1.
| Inputs | Level | Physiological disorders | |||
|---|---|---|---|---|---|
| STN | LN | LC | BC (g) | ||
| Cl− | Low | 0.24 < x < 9.10 mM | 0.24 < x < 4.67 mM | 0.24 < x < 4.67 mM | 0.24 < x < 4.67 mM |
| Mid | — | 4.67 < x < 13.53 mM | 4.67 < x < 13.53 mM | 4.67 < x < 13.53 mM | |
| High | 9.10 < x < 17.96 mM | 13.53 < x < 17.96 mM | 13.53 < x < 17.96 mM | 13.53 < x < 17.96 mM | |
| K+ | Low | 0.31 < x < 11.44 mM | 0.31 < x < 5.88 mM | 0.31 < x < 11.44 mM | |
| Mid | — | 5.88 < x < 17.00 mM | — | ||
| High | 11.44 < x < 22.56 mM | 17.00 < x 22.60 mM | 11.44 < x < 22.56 mM | ||
| Na+ | Low | 0.20 < x < 0.40 mM | 0.20 < x < 0.61 mM | ||
| Mid | 0.40 < x < 0.80 mM | — | |||
| High | 0.80 < x < 1.00 mM | 0.61 < x < 1.01 mM | |||
| EDTA− | Low | 0.06 < x < 0.17 mM | 0.06 < x < 0.28 mM | ||
| Mid | 0.17 < x < 0.39 mM | — | |||
| High | 0.39 < x < 0.50 mM | 0.28 < x < 0.50 mM | |||
| NH4+ | Low | 4.12 < x < 16.42 mM | |||
| High | 16.42 < x < 28.71 mM | ||||
| SO42− | Low | 0.49 < x < 6.41 mM | |||
| High | 6.41 < x < 12.33 mM | ||||
| Mn2+ | Low | 0.01 < x < 0.28 mM | 0.01 < x < 0.15 mM | ||
| Mid | — | 0.15 < x < 0.42 mM | |||
| High | 0.28 < x < 0.56 mM | 0.42 < x < 0.56 mM | |||
| Fe2+ | Low | 0.06 < x < 0.17 mM | |||
| Mid | 0.17 < x < 0.39 mM | ||||
| High | 0.39 < x < 0.50 mM | ||||
| Thiamine-HCl | Low | 0.10 < x < 2.70 mgL−1 | 0.10 < x < 2.70 mgL−1 | ||
| High | 2.70 < x < 5.30 mgL−1 | 2.70 < x < 5.30 mgL−1 | |||
| Glycine | Low | 0.25 < x < 1.13 mgL−1 | 0.25 < x < 1.13 mgL−1 | ||
| High | 1.13 < x < 2.00 mgL−1 | 1.13 < x < 2.00 mgL−1 | |||
| BAP | Low | 1.10 < x < 1.30 mg L−1 | |||
| High | 1.30 < x < 1.50 mg L−1 | ||||
The ‘IF-THEN’ rules for STN model indicate that the appearance of this disorder in the pistachio shoots is strongly associated to High concentrations of EDTA−, regardless K+ content (rules 5–6). Additionally, the lowest STN values are also achieved on media including High concentration of BAP and Cl− (rules 8 and 10). ‘UCB1’ shoots showed more resistance than ‘Ghazvini’ rootstock with regard to STN (rules 11–12). Finally, the appearance of STN on the pistachio shoots was affected by Na+, being the lowest STN when High amount of sodium is added to the media (rule 15).
It is interesting to note that ‘IF-THEN’ rules pinpointed the beneficial effect of a High content of thiamin-HCl independently of Na+ concentration to reduce LN disorder. However, in culture media with a Low content of thiamine-HCl, the inclusion of sodium at High concentration strongly promotes LN disorder (rules 18–19). Chloride and potassium ions also impacted LN but with different thresholds, the lowest LN where obtained at Mid-High concentration of Cl− (rules 21–22) and Low-Mid levels of K+ (rules 23–24).
The LC variability is explained by eight out of the 25 inputs, with dominant influence of interaction genotype and thiamine-HCl as submodel 1 (Table 1). In both genotypes, the inclusion of a high concentration of thiamine-HCl reduced this disorder, achieving green and healthy leaves (rules 26–29, Table 2). A complex interaction between Fe2+ and Mn2+ from submodel 2 (Table 1) and LC was found: i) if the culture media had Low concentration of Fe2+ and Mn2+ promotes low LC and ii) also, when using Mid content of Fe2+ was used, independently of Mn2+ level (Low or High), low LC was achieved, too (rules 30, 32–33; Table 2). Additionally, the inclusion of Mid-High concentration of chloride or High glycine also alleviates LC disorder (rules 37, 38, 40; Table 2). Finally, submodel 5 (Table 1) shows the complex interaction between K+ and SO42− on LC, that can be summarized as both ions should be in opposite concentration (e.g. Low K+ with High SO42−) to produce the lowest LC disorder (rules 42–43; Table 2).
Callus formation (BC) variability is explained by complex interaction of EDTA− and Mn2+ (the strongest effect) and the independent role of glycine, chloride, genotype and ammonium (Table 1). High amount of EDTA− in the media (rules 48–50; Table 2) caused always Low BC, especially if Mn2+ is at Mid level (stronger effect; rule 49; Table 2). Pistachio formed low BC on media supplemented with High amount glycine (rule 52; Table 2), Low content of chloride and NH4+ (rules 53 and 58; Table 2) and ‘Ghazvini’ as genotype (rule 56; Table 2).
Discussion
In a previous study, POM medium was developed through the use of artificial intelligence tools as fuzzy logic, artificial neural networks and genetic algorithms. Despite of this medium was developed on the basis of a poorly sampled design space, its improvement of growth parameters in P. vera rootstock micropropagation compared to other general media such as MS, DKW and GNH was demonstrated52.
In the present study, media based on MS showed higher STN, LN and LC than those based on POM (Fig. 2A–C), but also lower BC formation (Fig. 2D). These results suggest that although POM is an excellent media for pistachio micropropagation can even be improved by including all those physiological disorders in a future modeling and optimization.
The occurrence of physiological disorders during micropropagation of certain species of Pistacia has been attributed to several causes as the culture media composition, the PGRs or the culture system26,34,37,53–55, but none of the studies focus on determining the causes of those disorders. We have paid attention to the physiological abnormalities that occurred during two independent micropropagation experiments of Pistacia carried out in our laboratory, recording and categorizing data related to several disorders to get insight about the causality of their appearance.
The use an IV-optimal design in both experiments, based on the MS and on the POM medium guaranteed a well sampled design space39,51. As controls, both studies included WPM and DKW culture media, as they have been described as causing disorders during pistachio micropropagation23. The resulting database covers a wide range of concentrations of each culture media ingredient and permits investigating simultaneously the effects of all media minerals, vitamins, PGRs and genotype on the appearance of abnormalities, an objective that, taking into account the complexity of the generated database, can only be addressed through the AI tools.
Neurofuzzy logic has previously been used as a data mining technique that allow to model and produce intelligent rules to discover key parameters influencing a biological process46,56, facilitating the decision making.
In this work, the use of this tool allows us to establish which are the critical factors for each registered physiological disorder, particularly those with the greatest effect (Table 1) and, through the interpretation of the ‘IF-THEN’ rules (Table 2), to understand how these factors modulate the results. The rules are constructed with words whose meaning for each variable is presented in Table 3. Note that the ranges corresponding to each word of each variable may differ in relation to the categorized physiological disorder. This is because the fuzzification process, necessary for the generation of each model, can differ among the disorders.
EDTA− does not fall into mineral nutrients group, but it is an inseparable part of today culture media ingredients and is commonly preferred to other alternative Fe-chelating agents57. Murashige and Skoog10 used EDTA− at 0.05–0.5 mM in equimolar concentration with iron (Na-Fe-EDTA) to study their effect. An increase in tobacco suspension cultures yield on the media containing up to 0.25 mM of EDTA was found−, although they recommended 0.1 mM in the MS media. In agreement with them, pistachio optimized media developed in our laboratory (POM) also includes 0.112 mM of EDTA52. The ‘IF-THEN’ rules point out the independent influence of EDTA− on two out of the four disorders (STN and BC). While Low-Mid concentration of EDTA− reduces STN (0.06 <EDTA− < 0.39 mM; Table 3), BC is simultaneously promoted by Low concentrations (0.06 <EDTA−< 0.28 mM; Table 3). It is noteworthy to point out that the inclusion of High amount of EDTA− has a negative influence on pistachio proliferation rate in complex interaction with potassium and sulfate51. Therefore, it would be postulated that an excess of EDTA− increases STN and decreases of BC by the inhibitory effect on shoot growth that causes in agreement with previous results51. Moreover, an excessive level of EDTA− in the culture media is toxic for some plants or chelates other metals, leading to certain mineral deficiency in shoots during their in vitro multiplication57. Then we recommend using EDTA− at 0.1 mM as suggested in MS and POM.
Potassium can be found in high amounts in plant tissues and has several physiological and biochemical roles. Taiz and Zeiger58 pointed out the function of potassium in maintaining turgor and electroneutrality of cells, having also a role as cofactor for more than 40 enzymes involved in cell growth and development. Here, a wide range (0.31–22.56 mM) of potassium was studied. Variations on three physiological disorders (STN, LN and LC) can be explained as a consequence of changes in potassium concentration in the media. Firstly, it causes some significant effect on STN in combination with EDTA−, no conclusion can be drawn from the rules obtained. Secondly, Low potassium concentration (0.31 < K+ <5 .88 mM; Table 3) reduces the LN disorder. POM media included the lowest concentration of this ion (10.85 mM) in comparison to all control media (ranged from 12.6 in WPM to 20.05 mM in MS), and all media based on POM showed also lower LN (Fig. 2B).
The same comes true in the case of LC, as Low content of potassium together with a high content of SO42− improved LC on the pistachio. Wada and coworkers7 using RSM reported the requirement for high concentration of MgSO4 to improve LC of diverse pear genotypes. In fact, six out of ten pear genotypes in their study demanded high concentration of MgSO4 compared to the MS as control, although ion confounding problem did not allow them to clarify the roles of both ion. Akin and co-workers59, using Chi-squared automatic interaction detection data mining algorithm, reported the requirement of a High content of SO42− (8 mM) to increase growth parameters hazelnuts shoots culture, e.g. number of shoots. In agreement with those findings, among the disorders recorded here, LC is affected by SO42−, preventing the disorder when a High concentration (6.41 < SO42− < 12.33 mM) of this ion combined with a Low amount of potassium (0.31 < K+ < 11.44 mM; Table 3) is used. POM presents a high concentration of SO42− (4.075 mM) compared to MS (1.732 mM) and causes lower LC disorder.
Manganese and iron are two microelements with impact on two out of the four disorders. The interaction between them affects the appearance of LC. The deficiencies in manganese and iron have commonly been associated to appearance of leaf chlorosis60–62. Moreover, manganese interacts with EDTA−, having an effect on BC. Noticeably, the results indicated that the use of unbalanced amounts of those ions should promote the disorders (rules 30–35 and 45–50; Table 2). By contrast, the inclusion of Fe/EDTA and MnSO4, each separately, at a range of 0–1 mM, influenced differently growth parameters of Gerbera hybrid cultures, giving 0.1 mM (MS) of the Fe/EDTA as optimum point for the studied parameters63.
The vitamins-mixtures of original MS10, Gamborg B564 or DKW31 are commonly added to the components of culture media used for micropropagation of Pistacia species26,35,37,54,55,65. The use of computer-based neurofuzzy logic has revealed a linear positive impact of pyridoxine-HCl and nicotinic-acid on promoting some shoot multiplication parameters of pistachio52, but, as far as we know, there is no demonstrative conclusion about the influence of vitamin on the physiological disorders occurred during in vitro culture of other woody species. The ‘IF-THEN’ rules in the present study showed that the thiamin-HCl and glycine impacted differently on three out of the four disorders. Interestingly, the inclusion of High thiamin-HCl (2.70 < Thiamine-HCl < 5.30 mg L−1; Table 3) alleviated strongly the appearance of LN and LC (rules 16–19, 26–29; Table 2). Similarly, the High content of glycine (1.13 < Glycine < 2.00 mg L−1; Table 3) reduced strongly LC and BC (rules 39–40, 51–52; Table 2). POM media has a combination of components which clearly reduced LN and LC including high concentration of thiamin (5.3 mM) compared to 0.1 mM in MS, but very low glycine (0.25 mM) compared to MS, WPM or DKW (2 mM). Then for future POM improvement higher amounts of glycine should be tested in order to reduce BC and LN, especially if UCB1 rootstock is used.
The beneficial effect of sodium, as functional nutrient in plants, on increasing growth and alleviating visual symptoms such as leaf chlorosis and necrosis has been previously documented57,66. However, there is little information related to the response of in vitro plant growth to changes in the sodium concentration of culture media. The use of neurofuzzy logic revealed that sodium has a key role in explaining the variations of two out of the four disorders (STN, and LN), being a single effect for STN parameter or in interaction with thiamine-HCl for LN. Neurofuzzy logic suggests that healthier shoots were obtained when culture medium includes High concentration of sodium. However, treatments with Na+ up to Mid concentration (0.20 < Na+ < 0.8 mM) promotes shoots length of the pistachio cultures, but at higher concentration caused a negative impact on this parameter (Data not shown). This could explain why Na+ at 0.4 mM was selected by a previous model as the optimal for pistachio in vitro multiplication52.
Genotype is a factor impacting three out of the four disorders in the pistachio cultures. In agreement with our previous report51, the cultures of UCB1 showed the lowest and highest frequencies of STN and BC, respectively (rules 11–12, 56–57; Table 2). Also, other studies described that the frequency and type of physiological disorders mediated by unbalanced mineral nutrition varied among genotypes41.
McCown and Sellmer1 have reported the toxic effects of high chloride concentration (30 mM) on different woody species cultured in vitro, when they tried to solve STN problems re-adjusting Ca2+ content of MS medium.
Chloride is an ion highly transported in plants with two principal functions: cell division in leaves and shoots, and maintenance of electrical neutrality since it balances the rapid changes that occur in the level of free cations (e.g. K+, Mg2+ and Na+)57,62. Mid-High chloride concentration (>4.67 mM; Table 3) should be used to reduce STN, LN and LC (leaf ‘bronzing’) disorders (rules 9–10, 20–22 and 37–38; Table 2). On the contrary, Low concentration (<4.67 mM) is recommended to reduce BC abnormality (rules 53, Table 2). The average concentration of chloride in plant culture media is around 3 mM57, however a double amount (6 mM) is used in MS medium. The use of low amounts of chloride in POM, WPM and DKW, 0.47; 1.3 and 2.0 mM, respectively helps to explain the reduction in STN, LN and LC developed in those media, together with the increase in BC. Interesting, although interaction between Cl− and other media components, such as K+ or SO42−, has been pointed out having an effect of growth parameters51, no interaction of chloride with other ions have not been detected here (Table 1).
A range of disorders have also been reported as a result of using insufficient PGRs composition of the culture media12,23–26,35,36,54,67,68. BAP not only has a role in promoting growth parameters of pistachio micropropagation52, but prevents the appearance of some disorders. In agreement with previous literature25,69, the addition of sufficient amount of BAP (1.30 < BAP < 1.50 mg L−1; Table 3) strongly reduces the appearance of STN (rules 6–7; Table 2). However, here BAP was conducted just at two fixed concentrations (1.1 and 1.5 mg L−1) and new experiments should be carried out in order to find the best combination of PGRs for new optimized culture media for pistachio, combining both growth and disorder parameters in the same database.
Wada and coworkers6 asserted the importance of optimizing the nitrogen components content in culture medium to promote multiple elongated shoots and less BC, in diverse pear species. Here, the rules suggest the inclusion of NH4+ at low content (4.12 < NH4+ < 16.42 mM; Table 3) to avoid BC in the pistachio shoots. In fact the WPM media with the lowest NH4+ concentration (5 mM), also produced the lower BC among the control media (MS, POM and DKW). Then, as above mentioned, the final optimization medium for pistachio should include results of several growth parameters along with several physiological disorders to guarantee formulating a robust optimum medium.
Finally, our results demonstrated that some physiological disorders such as SF, hyperhydricity and epinasty were seen infrequently in pistachio and not dependent of the media used. In other species37,41, these disorders have been correlated with deficiency of a wide range of mineral nutrients or insufficient composition of PGRs of culture media, but in the range of our study, those disorders could not be properly modeled.
Conclusions
This study demonstrates that to formulate optimal plant culture medium, the results of both growth parameters and physiological disorders should be considered simultaneously. This fact adds complexity to the design of culture media, increasing the number of treatments to be assayed in proportion to the number of factors and parameters to be taken into account. In this sense computer-based tools such as DOE and AI, have proven to be useful by i) reducing the time and the cost of each experiment (low number of treatments to be tested, only 61 instead of 6,250) but ensuring a well-sampled space design; ii) identifying the key factors affecting each disorder; iii) getting insight about the causes that promote the appearance of physiological disorders in pistachio cultures, and iii) demonstrating that this methodology open a new scenario to design suitable plant tissue culture media. In fact, an AI-designed media for pistachio, POM, was able not only to promote growth parameters but, simultaneously, to reduce physiological disorders, compared with the most used media currently used for pistachio in vitro culture.
Methods
Plant materials and in vitro culture conditions
Shoots of two pistachio rootstocks ‘UCB1’ and P. vera cv. ‘Ghazvini’ were micro-propagated on MS10 and POM52 media supplemented with the vitamin-mixture and PGRs composition described in Table S2. Sucrose (30 g L−1) and agar (5.7 g L−1) were added to each medium. The pH was adjusted to 5.7 prior autoclaving (121 °C, 1 kg cm−2 s−1 for 20 min). The cultures were kept under 16-h photoperiod (white fluorescent tubes; irradiance of 65 µmol m−2 s−1) and day/night temperature of 25/20 ± 2 °C and subcultured into a fresh medium every 30 days.
Pistachio explants of approximately 1 cm in length with 1–2 axillary buds were randomly selected before placing them in glass boxes (180 ml) containing 25 ml of the culture medium. After three successive subcultures (30 days interval) on the same culture media, data on STN, LN, SF, hyperhydricity and epinasty were recorded. Responses for these five physiological disorders were rated as 1 = none, 2 = very low, 3 = moderate, 4 = high. For leaf color disorder (LC) response: 1 = green, 2 = pale green, 3 = pink-edged, 4 = bronze-like or brown was recorded. The BCs were weighed and the results expressed in grams.
Each treatment consisted of two replicates glass boxes (180 ml) sealed with caps, containing five explants each. The experiments were carried out in triplicate.
Design of experiment and data acquisition
The mineral nutrients of MS and POM were subdivided into independent five component factors including (i) KNO3 (ii) NH4NO3, (iii) mesos, (iv) micros, and (v) iron over a range of concentrations expressed in relation to MS medium or POM by attributing 1 × for each as the standard ingredient (Table 4).
Table 4.
Five-factor mineral nutrients used to construct the experimental design space and concentration range expressed as × levels.
| Factors | Mineral nutrients | ×MS | ×POM |
|---|---|---|---|
| 1 | KNO3 | 0.0–1.0× | 0.5–1.5× |
| 2 | NH4NO3 | 0.2–1.1× | 0.5–1.5× |
| 3 (Mesos) |
*Ca(NO3)2.4H2O CaCl2.2H2O KH2PO4 MgSO4.7H2O *K2SO4 *NaH2PO4.H2O |
0.25–3.0× | 0.5–1.5× |
| 4 (Micros) |
MnSO4.4H2O ZnSO4.7H2O CuSO4.5H2O KI CoCl2.6H2O H3BO3 Na2MoO4.2H2O |
0.1–4.0× | 0.5–3.0× |
| 5 (Iron) |
FeSO4.7H2O Na2EDTA.2H2O |
1.0–5.0× | 0.5–3.0× |
*These components have been used only in × POM medium.
For MS experiment, the initial five-factor experimental design was a 23-model-point IV-optimal response surface which was sufficient for modeling a quadratic polynomial51. The designs were augmented to contain five additional points (in total 28 treatments) to detect additional signal (e.g., curvature) possibly not captured in the design as described by Niedz and Evens40 using software Design-Expert®870. However for POM experiment, the initial five-factor design was a 23-model-point IV-optimal augmented in 10 additional points (in total 33 treatments). Another four extra points including well-known basal media: MS, POM, WPM and DKW with their original media composition were used as controls. In total, the database included 65-treatment per rootstock and a total of 130 treatments were assayed (Table 5).
Table 5.
Five-factor IV-design for MS51 and POM media together with components of original MS, DKW, WPM and POM as controls (65 treatments).
| Treatments | Factor 1 KNO3 | Factor 2 NH4NO3 | Factor 3 Mesos | Factor 4 Micros | Factor 5 Iron | |
|---|---|---|---|---|---|---|
| ×MS medium | #1 | 1.00 | 0.20 | 0.25 | 4.00 | 2.33 |
| #2 | 0.00 | 0.20 | 2.08 | 4.00 | 1.00 | |
| #3 | 1.00 | 1.10 | 2.08 | 0.10 | 1.00 | |
| #4 | 0.00 | 1.10 | 0.25 | 1.40 | 3.67 | |
| #5 | 0.00 | 1.10 | 3.00 | 4.00 | 1.00 | |
| #6 | 0.50 | 0.20 | 3.00 | 0.10 | 5.00 | |
| #7 | 0.00 | 0.20 | 0.25 | 0.10 | 1.00 | |
| #8 | 0.00 | 1.10 | 3.00 | 4.00 | 5.00 | |
| #9 | 0.33 | 0.50 | 0.25 | 0.10 | 5.00 | |
| #10 | 0.00 | 0.50 | 3.00 | 0.10 | 2.33 | |
| #11 | 1.00 | 1.10 | 0.25 | 4.00 | 1.00 | |
| #12 | 0.00 | 0.20 | 1.17 | 2.70 | 5.00 | |
| #13 | 1.00 | 0.65 | 0.25 | 0.10 | 1.00 | |
| #14 | 0.33 | 0.20 | 3.00 | 4.00 | 3.67 | |
| #15 | 1.00 | 0.20 | 1.17 | 0.10 | 3.67 | |
| #16 | 0.67 | 1.10 | 3.00 | 0.10 | 3.67 | |
| #17 | 0.00 | 1.10 | 1.63 | 0.10 | 1.00 | |
| #18 | 1.00 | 0.80 | 3.00 | 4.00 | 2.33 | |
| #19 | 1.00 | 0.80 | 0.25 | 2.70 | 5.00 | |
| #20 | 1.00 | 1.10 | 0.25 | 0.10 | 3.67 | |
| #21 | 0.50 | 0.65 | 1.63 | 2.05 | 3.00 | |
| #22 | 1.00 | 1.10 | 3.00 | 2.70 | 5.00 | |
| #23 | 1.00 | 0.20 | 3.00 | 2.70 | 5.00 | |
| #24 | 0.50 | 0.65 | 1.63 | 2.05 | 2.00 | |
| #25 | 0.67 | 0.20 | 3.00 | 1.40 | 1.00 | |
| #26 | 0.00 | 0.80 | 3.00 | 1.40 | 5.00 | |
| #27 | 0.00 | 0.80 | 0.25 | 4.00 | 2.33 | |
| #28 | 0.33 | 1.10 | 1.17 | 4.00 | 5.00 | |
| ×POM medium | #1 | 1.50 | 1.50 | 1.38 | 2.73 | 0.81 |
| #2 | 1.30 | 0.50 | 1.50 | 0.50 | 3.00 | |
| #3 | 0.50 | 1.50 | 0.50 | 0.50 | 0.50 | |
| #4 | 1.50 | 0.94 | 0.50 | 3.00 | 3.00 | |
| #5 | 0.62 | 1.38 | 0.50 | 0.81 | 2.69 | |
| #6 | 0.50 | 0.50 | 0.93 | 3.00 | 1.48 | |
| #7 | 0.50 | 0.63 | 1.45 | 0.62 | 2.88 | |
| #8 | 1.50 | 1.50 | 1.50 | 0.50 | 0.50 | |
| #9 | 0.86 | 0.50 | 0.50 | 1.72 | 0.50 | |
| #10 | 0.50 | 1.50 | 1.50 | 3.00 | 3.00 | |
| #11 | 0.75 | 0.75 | 1.50 | 2.47 | 0.50 | |
| #12 | 0.50 | 0.50 | 0.50 | 0.50 | 3.00 | |
| #13 | 1.50 | 1.50 | 0.50 | 0.50 | 1.41 | |
| #14 | 0.50 | 0.57 | 1.50 | 3.00 | 3.00 | |
| #15 | 1.50 | 0.50 | 0.97 | 1.84 | 3.00 | |
| #16 | 1.50 | 0.50 | 1.50 | 3.00 | 0.50 | |
| #17 | 0.50 | 1.50 | 0.50 | 3.00 | 3.00 | |
| #18 | 0.50 | 0.50 | 1.50 | 0.50 | 0.50 | |
| #19 | 0.75 | 1.25 | 1.20 | 2.05 | 1.95 | |
| #20 | 1.17 | 0.50 | 0.50 | 3.00 | 1.80 | |
| #21 | 1.38 | 1.47 | 1.38 | 0.70 | 3.00 | |
| #22 | 0.50 | 0.90 | 0.50 | 3.00 | 0.50 | |
| #23 | 0.84 | 0.58 | 0.94 | 1.36 | 2.03 | |
| #24 | 1.50 | 0.50 | 0.50 | 0.50 | 0.50 | |
| #25 | 0.63 | 1.38 | 1.38 | 0.50 | 0.50 | |
| #26 | 1.19 | 1.21 | 0.93 | 1.13 | 0.50 | |
| #27 | 1.50 | 1.50 | 0.50 | 3.00 | 0.50 | |
| #28 | 0.50 | 1.50 | 1.50 | 3.00 | 0.50 | |
| #29 | 1.50 | 1.50 | 1.50 | 3.00 | 3.00 | |
| #30 | 1.50 | 0.85 | 1.50 | 1.58 | 1.76 | |
| #31 | 0.50 | 1.50 | 1.50 | 0.50 | 3.00 | |
| #32 | 0.50 | 0.50 | 0.50 | 3.00 | 3.00 | |
| #33 | 1.50 | 1.50 | 0.50 | 0.50 | 3.00 | |
| Control | MS | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| POM | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| WPM | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| DKW | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Modeling tools
A commercial neurofuzzy logic software, FormRules® 4.03 (Intelligensys, Ltd, UK), was used to model the database generated including 65 treatments per rootstock. The original media macro- and micro-nutrients expressed as salts were converted to their corresponding ions, to avoid ion confounding problems described by Niedz and Evens40,71, and merged all in unique database just before modeling. Then, twenty five variables: eighteen calculated ion concentrations (NH4+, NO3−, K+, Ca2+, Mg2+, PO42−, SO42−, Cl−, Fe2+, BO3−, Mn2+, Zn2+, Cu2+, MoO42−, Na+, Co2+, I−, EDTA−), two genotypes (‘Ghazvini’ and ‘UCB1’ rootstocks); four organic compounds: three vitamins (thiamine-HCl, nicotinic-acid and pyridoxine-HCl); glycine and two PGRs (IBA and BA) were included as inputs, and seven physiological disorder responses (STN, LN, LC, BC, SF, hyperhydricity, and epinasty) were selected as outputs (Table S1). The results for STN and BC obtained in MS media correspond to those described elsewhere51 and were included in the dataset (Tables S1 & S2) as controls to compare the effect of the MS versus POM on the physiological disorders.
Modeling was conducted according to our previous methodology52 using the training parameters presented in Table 6. Among statistical fitness criteria available in the software, Structural Risk Minimization (SRM) was selected as it is able to find the best model with the minimum generalization error72.
Table 6.
The training parameters setting with neurofuzzy logic.
| Critical factors for neurofuzzy logic model |
|---|
| Minimization parameters |
| Ridge regression factor:1e−6 |
| Model selection criteria |
| Structural risk minimization (SRM) |
| C1 = 0.8–0.916; C2 = 4.8 |
| Number of set densities: 2 |
| Set densities: 2, 3 |
| Adapt nodes: True |
| Max.Inputs per SubModel: 4 |
| Max. nodes per input: 15 |
Adaptive-spline-modeling-of-data (ASMOD) used by FormRules® enables the models to be split into submodels. The fuzzification processes allows the input values to be express by a word (Low, Medium or High) together with a membership degree between 0 and 148. Figure S2 is presented to facilitate the understanding of the linguistic expressions of the variables (Low, Medium and High) obtained by the neurofuzzy logic model46.
Independent predictive models were obtained for each physiological disorder, the quality of which was evaluated using the coefficient of determination of the training set (Train Set R2) expressed in percentage (for model predictability) and the analysis of variance (ANOVA) parameters (for model accuracy).
Train Set R2 values are calculated by following equation48.
where yi is the experimental point in the data set, yi′ is the predicted point calculated by the model and yi″ is the mean of the dependent variable. Train set R2 values between 70 and 99.9% are indicative of acceptable predictabilities, although if R2 is higher than 99.9%, the model can be overfitted and the model should be readjusted as described in Colbourn and Rowe73. To assess model accuracy, the software uses ANOVA to evaluate statistical differences between predicted and experimental data52,73.
Supplementary information
Acknowledgements
We thank the personnel of Biotechnology Department at Imam Khomeini International University (IKIU) of Iran, for their assistance to carry out a part of the experimental work of this study. Special thank also goes to Xunta de Galicia for financial support (Competitive Reference Groups, ED431C2017/09-FEDER and Programa REDES ED431D-2017/18 respectively).
Author Contributions
E.N.A.: Performed the experiments. G.A.G.: contributed with reagents/materials. E.N.A., G.A.G. and P.P.G.: Conceived and designed the experiments. E.N.A., M.L. and P.P.G.: Contributed DOE/modeling/analysis tools. All authors contributed to writing of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46155-2.
References
- 1.McCown, B. H. & Sellmer, J. C. General media and vessels suitable for woody plant culture. in Cell and Tissue Culture in Forestry (eds Bonga, J. M. & Durzan, D. J.) 4–16 (1987).
- 2.Bairu MW, Jain N, Stirk WA, Doležal K, Van Staden J. Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. South African J. Bot. 2009;75:122–127. doi: 10.1016/j.sajb.2008.08.006. [DOI] [Google Scholar]
- 3.Bosela MJ, Michler CH. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. In Vitro Cell Dev-Pl. 2008;44:316–329. doi: 10.1007/s11627-008-9114-5. [DOI] [Google Scholar]
- 4.Jain N, Bairu MW, Stirk WA, Van Staden J. The effect of medium, carbon source and explant on regeneration and control of shoot-tip necrosis in Harpagophytum procumbens. South African J. Bot. 2009;75:117–121. doi: 10.1016/j.sajb.2008.08.005. [DOI] [Google Scholar]
- 5.Grzegorczyk-Karolak I, Rytczak P, Bielecki S, Wysokińska H. The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tiss. Organ Cult. 2017;128:479–486. doi: 10.1007/s11240-016-1126-y. [DOI] [Google Scholar]
- 6.Wada S, Niedz RP, Reed BM. Determining nitrate and ammonium requirements for optimal in vitro response of diverse pear species. In Vitro Cell Dev-Pl. 2015;51:19–27. doi: 10.1007/s11627-015-9662-4. [DOI] [Google Scholar]
- 7.Wada S, Niedz RP, DeNoma J, Reed BM. Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cell Dev-Pl. 2013;49:356–365. doi: 10.1007/s11627-013-9508-x. [DOI] [Google Scholar]
- 8.Poothong S, Reed BM. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci. Hortic-Amsterdam. 2014;165:132–141. doi: 10.1016/j.scienta.2013.10.040. [DOI] [Google Scholar]
- 9.Reed BM, Wada S, DeNoma J, Niedz RP. Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev-Pl. 2013;49:343–355. doi: 10.1007/s11627-013-9504-1. [DOI] [Google Scholar]
- 10.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 11.Kovalchuk IY, et al. Modeling some mineral nutrient requirements for micropropagated wild apricot shoot cultures. Plant Cell Tiss. Organ Cult. 2017;129:325–335. doi: 10.1007/s11240-017-1180-0. [DOI] [Google Scholar]
- 12.Barghchi M, Alderson PG. In vitro propagation of Pistacia vera L. from seedling tissues. J. Hortic. Sci. 1983;58:435–445. doi: 10.1080/00221589.1983.11515140. [DOI] [Google Scholar]
- 13.Kintzios S, Stavropoulou E, Skamneli S. Accumulation of selected macronutrients and carbohydrates in melon tissue cultures: Association with pathways of in vitro dedifferentiation and differentiation (organogenesis, somatic embryogenesis) Plant Sci. 2004;167:655–664. doi: 10.1016/j.plantsci.2004.05.021. [DOI] [Google Scholar]
- 14.Kevers C, Gaspar T. Soluble, membrane and wall peroxidases, phenylalanine ammonia-lyase, and Lignin changes in relation to vitrification of Carnation tissues cultured in vitro. J. Plant Physiol. 1985;118:41–48. doi: 10.1016/S0176-1617(85)80163-2. [DOI] [Google Scholar]
- 15.Ziv Meira. Quality of micropropagated plants—Vitrification. In Vitro Cellular & Developmental Biology - Plant. 1991;27(2):64–69. doi: 10.1007/BF02632130. [DOI] [Google Scholar]
- 16.Paques M. Vitrification and micropropagation: causes, remedies and prospects. Acta Hortic. 1991;289:283–290. doi: 10.17660/ActaHortic.1991.289.76. [DOI] [Google Scholar]
- 17.Hazarika BN. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic-Amsterdam. 2006;108:105–120. doi: 10.1016/j.scienta.2006.01.038. [DOI] [Google Scholar]
- 18.Tabart J, Franck T, Kevers C, Dommes J. Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots. Plant Cell Tiss. Organ Cult. 2015;120:11–18. doi: 10.1007/s11240-014-0568-3. [DOI] [Google Scholar]
- 19.Vinoth A, Ravindhran R. Reduced hyperhydricity in watermelon shoot cultures using silver ions. In Vitro Cell Dev-Pl. 2015;51:258–264. doi: 10.1007/s11627-015-9698-5. [DOI] [Google Scholar]
- 20.Ivanova M, Van Staden J. Nitrogen source, concentration, and NH4+: NO3− ratio influence shoot regeneration and hyperhydricity in tissue cultured Aloe polyphylla. Plant Cell Tiss. Organ Cult. 2009;99:167–174. doi: 10.1007/s11240-009-9589-8. [DOI] [Google Scholar]
- 21.Casanova E, Moysset L, Trillas MI. Effects of agar concentration and vessel closure on the organogenesis and hyperhydricity of adventitious carnation shoots. Biol. Plantarum. 2008;52:1–8. doi: 10.1007/s10535-008-0001-z. [DOI] [Google Scholar]
- 22.Lai CC, Lin HM, Nalawade SM, Fang W, Tsay HS. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. J. Plant Physiol. 2005;162:355–361. doi: 10.1016/j.jplph.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 23.González A., Frutos D. Plant Aging. Boston, MA: Springer US; 1990. In Vitro Culture of Pistacia Vera L. Embryos and Aged Trees Explants; pp. 335–338. [Google Scholar]
- 24.Dolcet-Sanjuan R, Claveria E. Improved shoot-tip micropropagation of Pistacia vera L. and the beneficial effects of methyl jasmonate. J. Am. Soc. Hortic. Sci. 1995;120:938–942. doi: 10.21273/JASHS.120.6.938. [DOI] [Google Scholar]
- 25.Onay, A. In vitro organogenesis and embryogenesis of pistachio, Pistacia vera L. (University of Edinburgh, 1996).
- 26.Akdemir H, et al. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tiss. Organ Cult. 2014;117:65–76. doi: 10.1007/s11240-013-0421-0. [DOI] [Google Scholar]
- 27.Iliev I, Kitin P. Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regul. 2011;63:115–129. doi: 10.1007/s10725-010-9540-3. [DOI] [Google Scholar]
- 28.Jackson MB, et al. Ventilation in plant tissue cultures and effects of poor aeration on ethylene and carbon dioxide accumulation, oxygen depletion and explant development. Ann. Bot-London. 1991;67:229–237. doi: 10.1093/oxfordjournals.aob.a088127. [DOI] [Google Scholar]
- 29.Kevers C, Boyer N, Courduroux JC, Gaspar T. The influence of ethylene on proliferation and growth of rose shoot cultures. Plant Cell Tiss. Organ Cult. 1992;28:175–181. doi: 10.1007/BF00055514. [DOI] [Google Scholar]
- 30.Navarro Cuauhtémoc, Teisson Claude, Côte François, Ganry Jacky. Physiology, Growth and Development of Plants in Culture. Dordrecht: Springer Netherlands; 1994. Effect of light intensity and aeration during in vitro growth (stage III of micropropagation) of banana plants (Musa AAA cv. Petite Naine) pp. 215–219. [Google Scholar]
- 31.Driver JA, Kuniyuki AH. In vitro propagation of Paradox walnut rootstock. HortScience. 1984;19:507–509. [Google Scholar]
- 32.Lloyd G, McCown B. Commericially-feasible micropropagation of montain lauerl, Kalmia latifolia, by use of shoot-tip culture. Proceeding Int. Plant Propagators Soc. 1980;30:421–427. [Google Scholar]
- 33.Barghchi M., Alderson P. G. Trees II. Berlin, Heidelberg: Springer Berlin Heidelberg; 1989. Pistachio (Pistacia vera L.) pp. 68–98. [Google Scholar]
- 34.Abousalim A, Mantell SH. A practical method for alleviating shoot-tip necrosis symtoms in in vitro shoot cultures of Pistacia vera cv. Mateur. J. Hortic. Sci. 1994;69:357–365. doi: 10.1080/14620316.1994.11516465. [DOI] [Google Scholar]
- 35.Onay A. Micropropagation of Pistachio from mature trees. Plant Cell Tiss. Org. 2000;60:159–163. doi: 10.1023/A:1006423914951. [DOI] [Google Scholar]
- 36.Barghchi M, Alderson PG. In vitro propagation of Pistacia species. Acta Hortic. 1983;131:49–60. doi: 10.17660/ActaHortic.1983.131.4. [DOI] [Google Scholar]
- 37.Marín JA, García E, Lorente P, Andreu P, Arbeloa A. A novel approach for propagation of recalcitrant pistachio cultivars that sidesteps rooting by ex vitro grafting of tissue cultured shoot tips. Plant Cell Tiss. Organ Cult. 2016;124:191–200. doi: 10.1007/s11240-015-0871-7. [DOI] [Google Scholar]
- 38.Parfitt DE, Almehdi AA. Use of high CO2 atmosphere and medium modifications for the successful micropropagation of pistachio. Sci. Hortic-Amsterdam. 1994;56:321–329. doi: 10.1016/0304-4238(94)90050-7. [DOI] [Google Scholar]
- 39.Niedz RP, Evens TJ. Design of experiments (DOE)—history, concepts, and relevance to in vitro culture. In Vitro Cell Dev-Pl. 2016;52:547–562. doi: 10.1007/s11627-016-9786-1. [DOI] [Google Scholar]
- 40.Niedz RP, Evens TJ. Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev-Pl. 2007;43:370–381. doi: 10.1007/s11627-007-9062-5. [DOI] [Google Scholar]
- 41.Reed BM, Wada S, DeNoma J, Niedz RP. Mineral nutrition influences physiological responses of pear in vitro. In Vitro Cell Dev-Pl. 2013;49:699–709. doi: 10.1007/s11627-013-9556-2. [DOI] [Google Scholar]
- 42.Poothong S, Reed BMI. CaCl2, MgSO4, and KH2PO4 improve the growth of micropropagated red raspberries. In Vitro Cell Dev-Pl. 2015;51:648–658. doi: 10.1007/s11627-015-9720-y. [DOI] [Google Scholar]
- 43.Hand C, Maki S, Reed BM. Modeling optimal mineral nutrition for hazelnut micropropagation. Plant Cell Tiss. Organ Cult. 2014;119:411–425. doi: 10.1007/s11240-014-0544-y. [DOI] [Google Scholar]
- 44.Prasad, V. S. S. & Gupta, S. D. Applications and potentials of artificial neural networks in plant tissue culture. In Plant Tissue Culture Engineering 47–67, 10.1007/978-1-4020-3694-1_3 (Springer, 2006).
- 45.Gago J, Landín M, Gallego PP. Artificial neural networks modeling the in vitro rhizogenesis and acclimatization of Vitis vinifera L. J. Plant Physiol. 2010;167:1226–1231. doi: 10.1016/j.jplph.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 46.P. Pedro, Gago Jorge, Landi Mariana. Artificial Neural Networks - Methodological Advances and Biomedical Applications. 2011. Artificial Neural Networks Technology to Model and Predict Plant Biology Process. [Google Scholar]
- 47.Colbourn EA. Neural computing: enable intelligent formulations. Pharm. Tech. Suppl. 2003;10:16–20. [Google Scholar]
- 48.Shao Q, Rowe RC, York P. Comparison of neurofuzzy logic and neural networks in modelling experimental data of an immediate release tablet formulation. Eur. J. Pharm. Sci. 2006;28:394–404. doi: 10.1016/j.ejps.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Nezami-Alanagh E, et al. Design of tissue culture media for efficient Prunus rootstock micropropagation using artificial intelligence models. Plant Cell Tiss. Organ Cult. 2014;117:349–359. doi: 10.1007/s11240-014-0444-1. [DOI] [Google Scholar]
- 50.Ayuso M, Ramil-Rego P, Landin M, Gallego PP, Barreal ME. Computer-assisted recovery of threatened plants: Keys for breaking seed dormancy of Eryngium viviparum. Front. Plant Sci. 2017;8:1–10. doi: 10.3389/fpls.2017.02092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nezami-Alanagh E, Garoosi G-A, Landín M, Gallego PP. Combining DOE with neurofuzzy logic for healthy mineral nutrition of pistachio rootstocks in vitro culture. Front. Plant Sci. 2018;9:1–12. doi: 10.3389/fpls.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nezami-Alanagh E, Garoosi G-A, Maleki S, Landín M, Gallego PP. Predicting optimal in vitro culture medium for Pistacia vera micropropagation using neural networks models. Plant Cell Tiss. Organ Cult. 2017;129:19–33. doi: 10.1007/s11240-016-1152-9. [DOI] [Google Scholar]
- 53.Akdemir, H., Yıldırım, H., Tilkat, E., Onay, A. & Özden Çiftçi, Y. Prevention of shoot tip necrosis responses in in vitro-proliferated mature pistachio plantlets. In In Vitro biology meeting of the SIVB. Seattle P-2003, 10.1007/s11626-012-9504-1 (2012).
- 54.Tilkat E, Işıkalan Ç, Onay A. In vitro propagation of khinjuk pistachio (Pistacia khinjuk Stocks) through seedling apical shoot tip culture. Propag. Ornam. Plants. 2005;5:1–5. [Google Scholar]
- 55.Barghchi M, Alderson PG. The control of shoot tip necrosis in Pistacia vera L. in vitro. Plant Growth Regul. 1996;20:31–35. doi: 10.1007/BF00024054. [DOI] [Google Scholar]
- 56.Gago J, Pérez-Tornero O, Landín M, Burgos L, Gallego PP. Improving knowledge of plant tissue culture and media formulation by neurofuzzy logic: A practical case of data mining using apricot databases. J. Plant Physiol. 2011;168:1858–1865. doi: 10.1016/j.jplph.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 57.George, E. F., Hall, M. A. & Klerk, G.-J. De. The Components of plant tissue culture media I: Macro- and micro-nutrients. in Plant Propagation by Tissue Culture (eds George, E. F., Hall, M. A. & De Klerk, G.-J.) 1, 65–113 (Springer Netherlands, 2007).
- 58.Taiz, L. & Ziger, E. Mineral nutrition. in Plant physiology 67–75 (Sinauer Associates, 2002).
- 59.Akin M, Eyduran E, Niedz RP, Reed BM. Developing hazelnut tissue culture medium free of ion confounding. Plant Cell Tiss. Organ Cult. 2017;130:483–494. doi: 10.1007/s11240-017-1238-z. [DOI] [Google Scholar]
- 60.Marschner, H. Mineral nutrition of higher plants. (Wiley, 1996).
- 61.Mengel, K. & Kirkby, E. A. Principales of plant nutrition. (Kluwer Academic Publishers, 2001).
- 62.Hopkins, W. G. & Hüner, N. P. A. Plants and inorganic nutrients. in Introduction to plant physiology (ed. Witman, K.) 241–257 (Wiley, 2004).
- 63.Niedz RP, Hyndman SE, Evens TJ, Weathersbee AA. Mineral nutrition and in vitro growth of Gerbera hybrida (Asteraceae) In Vitro Cell Dev-Pl. 2014;50:458–470. doi: 10.1007/s11627-014-9620-6. [DOI] [Google Scholar]
- 64.Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 65.Benmahioul B, Dorion N, Kaid-Harche M, Daguin F. Micropropagation and ex vitro rooting of pistachio (Pistacia vera L.) Plant Cell Tiss. Organ Cult. 2012;108:353–358. doi: 10.1007/s11240-011-0040-6. [DOI] [Google Scholar]
- 66.Subbarao GV, Ito O, Berry WL, Wheeler RM. Sodium-a functional plant nutrient. Crit. Rev. Plant Sci. 2003;22:391–416. doi: 10.1080/07352680390243495. [DOI] [Google Scholar]
- 67.Barghchi M, Alderson PG. In vitro propagation of Pistacia vera L. and the commercial cultivars Ohadi and Kalleghochi. J. Hortic. Sci. 1985;60:423–430. doi: 10.1080/14620316.1985.11515647. [DOI] [Google Scholar]
- 68.Abousalim, A. Micropropagation and micrografting of pistachio (Pistacia vera and P. atlantica). (University of London, 1990).
- 69.Yang Z, Lüdders P. In vitro propagation of pistachio (Pistacia vera L.) Gartenbauwissenschaft. 1993;59:30–34. [Google Scholar]
- 70.Design-Expert. Stat-Ease, Inc., Minneapolis, MN (2010).
- 71.Niedz RP, Evens TJ. A solution to the problem of ion confounding in experimental biology. Nat. Methods. 2006;3:417–427. doi: 10.1038/nmeth0606-417. [DOI] [PubMed] [Google Scholar]
- 72.Kim, D. Structural risk minimization on decision trees using an evolutionary multiobjective optimization. In of European Conf. on Genetic Programming, LNCS3003, 338–348 (2004).
- 73.Colbourn EA, Rowe RC. Novel approaches to neural and evolutionary computing in pharmaceutical formulation: challenges and new possibilities. Future Med. Chem. 2009;1:713–26. doi: 10.4155/fmc.09.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.