Abstract
Purpose
To investigate indocyanine green angiography (ICGA), fluorescein angiography (FA), and enhanced depth imaging optical coherence tomography measured choroidal thickness (EDI-OCT-CT) in the follow-up of inflammatory activity in stromal choroiditis [Vogt-Koyanagi-Harada disease (VKH) and birdshot retinochoroiditis (BRC)] under treatment in order to monitor tapering of therapy or readjustment of therapy in case of subclinical disease recurrence.
Methods
Patients with initial onset disease and/or treatment-naive stromal choroiditis (VKH & BRC) at entry, quiet under therapy, and having had a follow-up of at least four years monitored with dual FA and ICGA and EDI-OCT-CT measurements were analyzed retrospectively. ICGA and FA scores and EDI-OCT-CT values were correlated with therapy, and significant changes of each modality were correlated with disease evolution.
Results
Of the 31 VKH and 29 BRC patients seen from 1995 to 2017 in our center, four patients (2 VKH and 2 BRC patients) fulfilled the inclusion criteria. During tapering, two patients (both VKH) showed no significant ICGA, FA, and EDI-OCT-CT changes (mean follow-up time 5.6 years) and allowed for safe tapering. In the other two (BRC) patients (mean follow-up time 6.25 years), a total of seven significant subclinical changes were demonstrated by ICGA alone after therapy modifications due to side-effects or during attempted tapering of therapy, while FA and EDI-OCT-CT remained unchanged.
Conclusions
ICGA was the most sensitive monitoring modality of stromal choroiditis, able to identify subclinical recurrences following change of therapy and inversely treatment responses after readjusted therapy, events otherwise missed by FA and EDI-OCT. ICGA proved efficient for safe therapy tapering or for timely adjustment of therapy in stromal choroiditis when necessary.
Keywords: Indocyanine green angiography, Stromal choroiditis, Birdshot retinochoroiditis, Vogt-Koyanagi-Harada disease, Treatment, VKH
Introduction
Stromal choroiditis is a subtype of choroiditis where the primary mechanism is the development of inflammatory foci, mostly granulomatous, at the level of the stroma.1, 2, 3 The two most common causes are Vogt-Koyanagi-Harada disease (VKH) and birdshot retinochoroiditis (BRC), both inflammatory processes originating directly from the choroidal stroma, being therefore called primary choroiditis.
More sensitive and precise investigation of the choroid has now been made possible with the availability of indocyanine green angiography (ICGA), often providing information on the presence of subclinical disease.2, 4, 5 Characteristic ICGA findings for VKH6, 7, 8, 9 and BRC10 have been previously reported. ICGA has regularly been strongly advised as a diagnostic and follow-up modality for both these stromal choroiditis entities because of its precision and reactivity to change of disease evolution.4, 6, 10
If left untreated, evolution is detrimental in a majority of VKH and BRC cases. Treatment is largely based on steroidal and non-steroidal immunosuppression, which should be vigorous and prolonged5, 7, 8, 10, 11, 12, 13, 14 and should be started promptly, as has been stressed previously for VKH.15 However, no treatment plan is universally applicable as each case responds differently and the clinician often relies on trial and error to find the appropriate therapy. We have previously shown that ICGA was the best modality to rapidly determine the appropriate treatment in individual cases of stromal choroiditis.2 Once optimal treatment has been found and proper response has been obtained, tapering of therapy, while being attentive to the smallest signs of recurrence represents the next step. In this regard, ICGA has been found to show high sensitivity to detect residual choroidal inflammation during follow-up.11, 12, 16 A similar approach has been suggested in the long-term follow-up of BRC patients.3, 4, 17, 18 Beside precise tapering of therapy in disease under control, such a precise monitoring modality may also be useful when treatment readjustment is needed due to patient intolerance. The aim of the study was to establish whether, on one hand, ICGA can be used in daily practice to accomplish safe tapering of therapy and, on the other hand, whether it is a crucial help to determine treatment adjustment in stromal choroiditis when it has become necessary because of intolerance or side-effects.
Methods
All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki of 1964 and its later amendments. Informed consent was not needed as the study was retrospective and data was anonymized.
Patients with initial-onset disease and/or treatment-naive stromal choroiditis at entry (VKH or BRC) quiet under therapy, and having had long-term (minimum of four years) follow-up with dual fluorescein angiography (FA)/ICGA and enhanced depth imaging optical coherence tomography choroidal thickness measurements (EDI-OCT-CT) seen in our center were included retrospectively in the study. Although management of initial-onset disease is also shown, the present study centred on the follow-up phase when disease was under control. Continuous monitoring was performed in particular during tapering of therapy or when treatment had to be modified due to side-effects, paying attention to subclinical disease evolution, tracking worsening of disease following therapy change or re-improvement when therapy was re-adjusted.
Clinical workup
In addition to the regular ophthalmological workup with slit-lamp examination, fundoscopy, visual acuity (VA), and intraocular pressure (IOP) measurements, patients underwent the complete uveitis workup including laser flare photometry (LFP), computerized visual field (VF) testing, dual FA and ICGA, optical coherence tomography (OCT), and EDI-OCT.
Diagnosis and treatment
Diagnostic criteria for VKH were (1) presence of bilateral (granulomatous) uveitis with posterior exudative detachments, (2) ICGA signs of choroiditis characterized by the presence of hypofluorescent dark dots (HDDs) and vasculitis of the choroidal vessels (fuzziness of vessels) in the absence of ocular trauma.1, 12
Treatment of initial onset disease consisted of a combination of steroidal and non-steroidal immunosuppressive therapy from the date of the diagnosis, usually a combination of intravenous (IV) followed by oral corticosteroids as well as cyclosporine (CsA) and mycophenolic acid (Myfortic®), with progressive tapering of corticosteroids over a period of 4–6 months, progressive tapering CsA over a period of 6–9 months, and long-term maintenance of non-steroidal immunosuppressive therapy such as Myfortic®. When persisting or recurring subclinical choroidal inflammation was detected by ICGA, an additional immunomodulator, such as an anti-TNF-α agent, was added.19, 20
Diagnostic criteria for BRC10, 14, 21 included (1) retinal vasculitis in small and large veins in one or both eyes, (2) vitritis in one or both eyes, (3) VF abnormalities in one or both eyes, (4) ICGA lesions in both eyes (required), (5) the presence of HLA-A29 antigen (required), with or without oval, depigmented, birdshot lesions.
Standard therapy consisted of a combination of steroidal and non-steroidal immunosuppressive therapy with progressive tapering of the corticosteroids and long-term maintenance of non-steroidal immunosuppression, usually a combination of CsA and Myfortic®. In case of insufficient response, an additional immunomodulator, often an anti-TNF-α agent, was given.22, 23, 24
Angiography
FA and ICGA were performed simultaneously using a standard protocol, as described previously.11 A Heidelberg Retina Angiograph HRA-2 (Heidelberg Engineering Inc., Heidelberg, Germany) was used for image acquisition. To exclude autofluorescence, signs of any pre-injection fluorescence were sought using the highest flash intensity used for ICGA. At the same time, red-free posterior pole frames were taken. After a bolus injection of 4 mg of Indocyanine Green (ICG) (Cardiogreen; Peaselt, Lorei, Germany), diluted in 5 ml of an Aqua solution, ICGA frames of the posterior pole were taken for approximately 2–3 minutes (early phase angiogram). The ICG fluorescence pattern was then analyzed in detail at 12 ± 3 minutes after ICG injection (intermediate phase of ICGA) by collecting frames of the posterior pole and a minimum of 8 frames of the entire 360° periphery. At the end of the intermediate phase of ICGA, FA was performed. Early FA frames of the posterior pole were captured for up to 2 minutes. Between 4 and 7 minutes, 360° panoramic imaging of the periphery was performed (with a minimum eight frames), followed by the collection of late fluorescein posterior pole frames at 10 minutes. The late ICGA fluorescence pattern was analyzed at 24 ± 4 minutes after ICG injection (late phase ICGA) in the same fashion as for the intermediate phase.
Indocyanine green angiography/Fluorescein angiography scoring
ICGA and FA were graded using a well-established highly reproducible scoring system6, 17, 25, 26 ranging from 0 to 40 for FA and from 0 to 20 for ICGA, which was doubled as ICGA items were half of FA items to reach a comparable score of 40. Each patient had both eyes graded, by two different ophthalmologists, blinded to the clinical data, and the average was calculated and used for calculation. Optic disc fluorescence and leakage, macular edema, retinal vascular or capillary staining and leakage, retinal non-perfusion, neovascularization, pinpoint leaks, staining or pooling were all taken into account in scoring FA. Early stromal vessel hyperfluorescence, choroidal vasculitis, HDDs and optic disk hyperfluorescence were graded for ICGA.
Optical coherence tomography
OCT and EDI-OCT were performed using a Heidelberg Spectralis OCT (Heidelberg Engineering Inc., Heidelberg, Germany) under mydriasis. Choroidal thickness was measured with EDI scans under the macula and, respectively, 1500 μm temporally (T), nasally (N), superiorly (S), and inferiorly. (I).27 Choroidal thickness measurements were performed perpendicular to the retinal pigment epithelium (RPE), going from the posterior RPE edge to the choroid–scleral junction. The mean thickness was established by averaging measurements under the fovea and at 1500 μm T, N, S, and I, averaged for both eyes. In other words, values for the horizontal (3) and vertical (3) scans were obtained for both eyes (a total of 12 measurements) and were averaged at each visit.
Fluorescein angiography (FA) and enhanced depth imaging optical coherence tomography measured choroidal thickness (EDI-OCT-CT).
Evaluation of the sensitivity/reactivity of fluorescein angiography, indocyanine green angiography, and enhanced depth imaging optical coherence tomography measured choroidal thickness
ICGA, FA, and EDI-OCT-CT were analyzed, comparing their ability to account for disease evolution including both improvement and worsening (disease recurrence) following treatment changes. Ideally, any fluctuation in disease pattern following a change of therapy should be captured by these techniques, within a timeframe practicable for close patient monitoring. Significant changes compared to the previous follow-up measurements were defined as a variation of 5 (out of 40) score points for ICGA and FA. This was an arbitrary cut-off value that represents a variation of more than 10% between two ICGA scores.
Regarding EDI-OCT-CT, previous studies have used 100 μm choroidal thickness variations as a significant cut-off for the subclinical follow-up of diseases such as VKH.28, 29 However, other studies have described chance and physiologic diurnal variations closer to 30 and 60 μm.30, 31 In this study, we have chosen a cut-off of 60 μm. Therefore, any variation between two successive EDI-OCT-CT (ΔOCT) of more than 60 μm was interpreted as a significant change. The main focus of this study was the period of evolution, once stromal choroiditis (ICGA) was under control.
Results
Of the 31 VKH and 29 BRC patients seen from 1995 to 2017 in our center, four patients could be included retrospectively in this study (2 VKH, 2 BRC) as most patients were excluded because of lack of post-initial phase follow-up or non-treatment naive presentation or unavailable data (particularly EDI-OCT) (Table 1). Demographic details are shown in Table 1.
Table 1.
Demographics, diagnoses, duration of follow-up, and number of investigational fluorescein angiography (FA)/Indocyanine green angiography (ICGA)/enhanced depth imaging optical coherence tomography (EDI-OCT) triads included.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Total/Average | |
|---|---|---|---|---|---|
| Age | 45 | 63 | 42 | 38 | |
| Gender | Female | Female | Female | Female | |
| Ethnicity | Caucasian | Caucasian | North African | Caucasian | |
| Diagnosis | BRC | BRC | VKH | VKH | |
| Duration of follow up (years) | 5:1 | 7.4 | 4.0 | 7.2 | Avg: 5.9 |
| Number of triad of ΔFA/ICGA/OCT | 12 | 5 | 4 | 4 | Total: 25 |
| Number of significant ΔOCT | 0 | 0 | 0 | 0 | Total: 0 |
| Number of significant ΔICGA | 5 | 2 | 0 | 0 | Total: 7 |
| (42%) | (40%) | (0%) | (0%) | (28%) | |
| Number of significant ΔFA | 0 | 0 | 0 | 0 | 0 |
BRC: Birdshot retinochoroiditis; VKH: Vogt Koyanagi Harada Disease; Avg: Average; FA: Fluorescein angiography; ICGA: Indocyanine green angiography; OCT: Optical coherence tomography.
The median age of the four female patients was 48.5 years (range, 46–72 years). The average follow-up period was 5.9 years (±1.65). The average number of follow-ups with imaging sessions was 9 (range, 6–15).
A total of 25 successive triads of FA/ICGA/OCT were available. None of these showed a significant change of value after therapy modification for ΔFA (0%), or for ΔEDI-OCT (0%), while 7/25 (28%) follow-ups were identified where ΔICGA was the only investigation detecting subclinical disease evolution. Among those, the absolute average ΔICGA (±5 score points) (or |ΔICGA|) was 7.9 (±2.67) score points.
Details of these 7 significant ΔICGAs are provided in Table 2. Interestingly, despite the fact that among these 7 occurrences (Table 2), the second one was the lowest –yet significant– ΔICGA (ΔICGA = 5), the corresponding imaging studies depicted in Fig. 1 (a to d) undoubtedly demonstrate that such a minimal numerical score variation still represents a substantial visible angiographic evolution.
Table 2.
Detailed findings of the 7 follow-ups identified where only ΔICGAs were significant.
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | Avg. | |
|---|---|---|---|---|---|---|---|---|
| ΔFA | −2.5 | 0 | 2 | −1.5 | −1 | −0.5 | −0.5 | 1.4 |
| ΔICCA | 11 | −5 | 6 | −6.5 | −6 | 11.5 | −9.5 | 7.9 |
| ΔEDI-OCT | −33.7 | −8.4 | −41.4 | 35.3 | −32.2 | 4.3 | 33.9 | 27.0 |
Avg: Average; FA: Fluorescein angiography; ICGA: Indocyanine green angiography; EDI-OCT: enhanced depth imaging optical coherence tomography.
ΔEDI-OCT measures and are in μm.
Fig. 1.
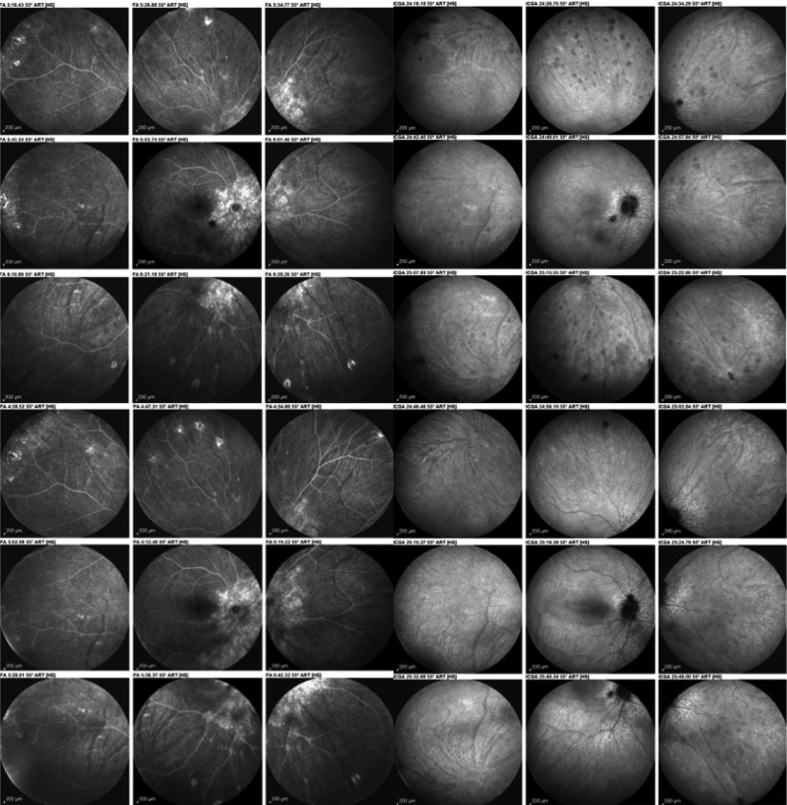
Evolution of fluorescein angiography (FA) and indocyanine green angiography (ICGA) findings in a birdshot retinochoroiditis (BRC) patient after modification of therapy. As shown in Table 2 (2nd column), there is no score change for FA, whereas the score change was 5 for ICGA, which is well illustrated on this figure. FA (2 left sets of 9 frames) before (top left) and after change of therapy (bottom left) have the same aspect. ICGA (2 sets of 9 right frames) shows a substantial decrease of hypofluorescent dark dots (HDDs) (bottom right set of 9 frames). When compared to the situation before therapeutic intervention (top right set of 9 frames).
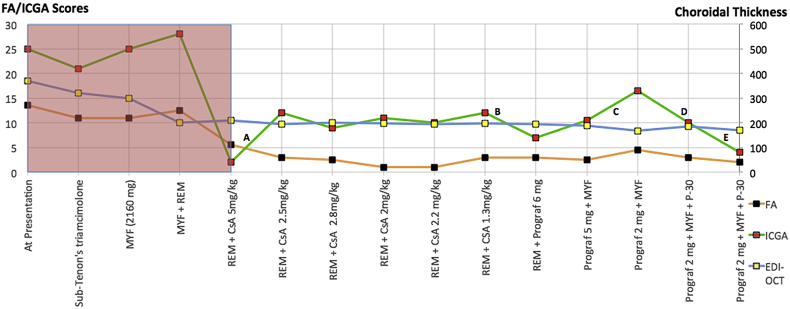
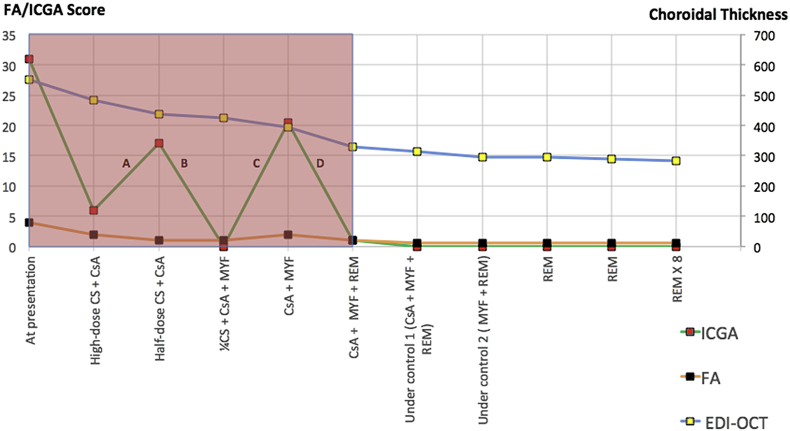
Fine-tuning of therapy in a case of birdshot retinochoroiditis (Fig. 2-patient 1)
Fig. 2.
Fine-tuning of therapy in a birdshot retinochoroiditis (BRC) patient where treatment had to be adjusted because of side-effects of ongoing therapy. MYF: Myfortic®, mycophenolic acid. REM: Remicade®, infliximab, anti-TNF-α agent. CsA: cyclosporine. Prograf® = tacrolimus. P: Prednisone. Pink shadowed area accounts for disease evolution and treatment combinations before disease was under control.
A 45-year-old female patient was diagnosed with BRC and presented with an ICGA score of 25, an FA score of 13.5, and an EDI-OCT-CT of 370 μm. Before disease was under control, several treatments had to be tried, including periocular steroids, mycophenolic acid and the TNF-α blocker infliximab (Remicae®) (grey part of Fig. 2). Because of the failing impact of triple immunosuppressive therapy, it was decided to add CsA (650 mg, 5 mg/kg) which had an effect on the angiographic parameters with a spectacular decrease of the ICGA score from 28 to 2 and a decrease of the FA score from 12.5 to 5.5, while it had no impact on EDI-OCT-CT (A). Obviously cyclosporine was the drug of choice, the immunosuppressant to which this patient was responsive. Once disease was under control, it was further followed by FA, ICGA and EDI-OCT. Unfortunately, the cyclosporine had to be reduced because of renal insufficiency, requiring fine-tuning of dosage or alternate therapy. During this follow-up, a significant ICGA score change occurred in five instances in the absence of change of the other parameters, allowing to detect subclinical choroiditis recurrence leading to readjustment of therapy. It showed that reduced CsA dosage did not control the disease any more, (A) that disease responded to Tacrolimus (Prograf®), (B) that reduced Prograf ® dosage provoked again escape of choroiditis (C), and in two instances, it was shown that the combination of low dose Prograf®, Myfortic® and 30 mg of prednisone was the combination that controlled the disease again. (D and E).
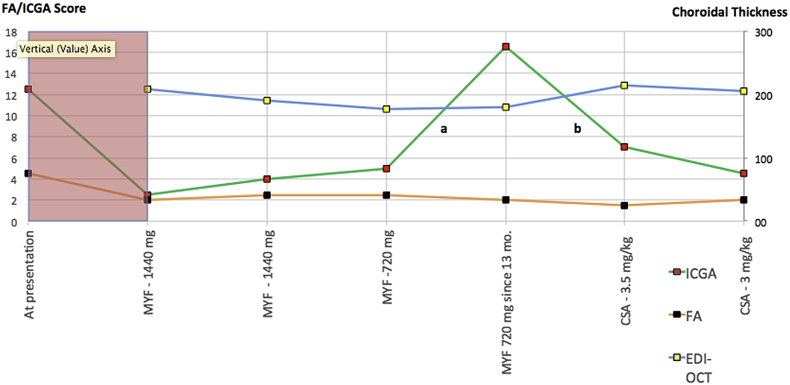
Indocyanine green angiography detected recurrence during tapering in a birdshot retinochoroiditis patient (Fig. 3-patient 2)
Fig. 3.
Recurrence detected by indocyanine green angiography (ICGA) during tapering of therapy in a birdshot retinochoroiditis (BRC) patient. MYF: Myfortic® = mycophenolic acid. CsA: Cyclosporine. Pink shadowed area accounts for disease evolution and treatment combinations before disease was under control.
BRC was diagnosed in a 63-year-old female patient with an ICGA score of 12.5/40 and an FA score of 4.5/40. No EDI-OCT-CT measurement was available. The disease had been left untreated for ±4 years elsewhere. A treatment of mycophenolic acid (1440 mg per day) was introduced with decrease of ICGA scores to between 2 and 4, FA scores between 2 and 2.5, and EDI-OCT-CT between 208 and 190 μm during a 3-year period. Afterwards, the patient asked to try to taper and discontinue Myfortic® therapy. Thirteen months after Myfortic® had been reduced from 1440 mg to 720 mg daily, there was a significant increase of the ICGA score from 5 to 16.5 (a – Fig. 4A) with no change of FA score (point score = 2) (b – Fig. 4B) nor of CT (176 μm). CsA was introduced leading to a decrease of ICGA score from 16.5 to 7 score points (Fig. 4C), 6 months later and stabilization to 4.5 score points at last follow-up with absolutely no significant change of FA score (score = 2) nor EDI-OCT-CT (179 μm). The follow-up of this patient, ICGA allowed to identify subclinical recurrence after reduction of Myfortic® and prompt response to newly introduced CsA. These two events were absolutely not detected by FA nor by EDI-OCT-CT, showing the exquisite sensitivity and reactivity of ICGA in stromal choroiditis.
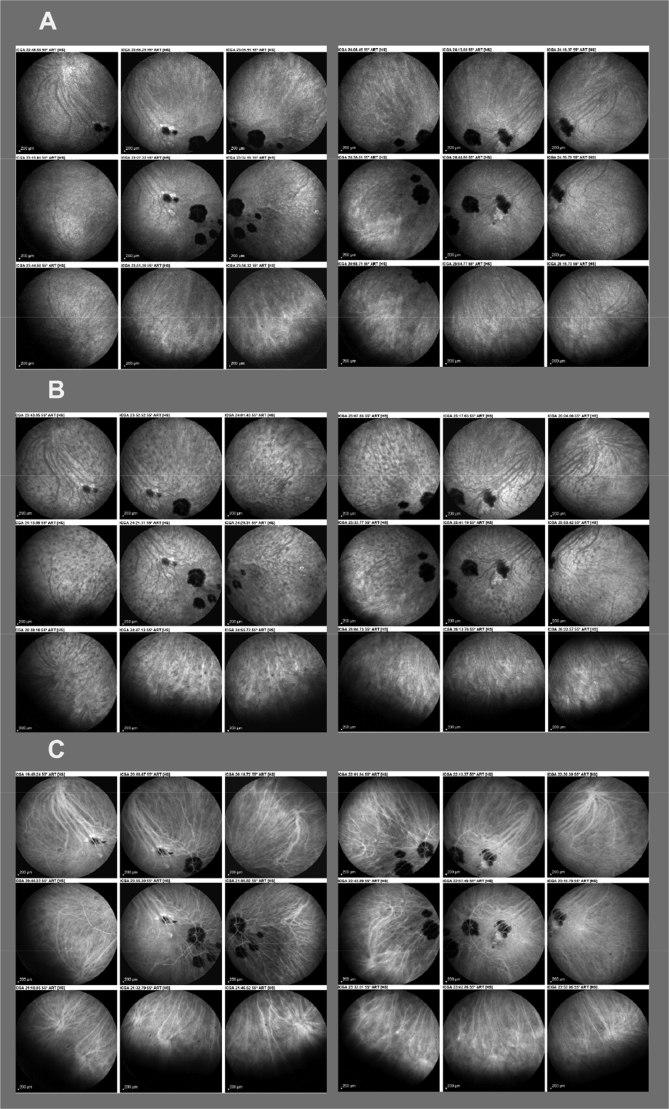
Fig. 4.
Indocyanine green angiography (ICGA) detection of disease recurrence during therapy tapering. A. Disease controlled under Myfortic® (1440 daily). Scars are visible as therapy had been started ∼4 years after disease onset but no active hypofluorescent dark dots (HDDs) are present. B. Thirteen months after reduction of Myfortic dosage to 720 mg, massive reappearance of HDDs, that responded to the introduction to cyclosporine (CsA) with disappearance of HDDs (C).
Two examples of safe tapering of therapy in Vogt-Koyanagi-Harada disease under indocyanine green angiography monitoring
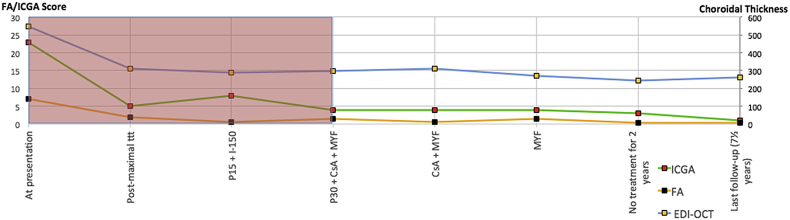
Example 1 (Fig. 5-patient 3)
Fig. 5.
Safe tapering of therapy in a Vogt-Koyanagi-Harada disease (VKH) patient. CS: Corticosteroids, prednisone, ¼CS: Quarter dose of prednisone, CsA: Cyclosporine. MYF: Myfortic® 0 mycophenolic acid. REM: Remicade® REM = infliximab, anti-TNF-α agent. REM X 8 = Remicade every 8 weeks (5 mg/kg). Pink shadowed area shows that several treatment combinations were necessary until disease was under control. MYF: Myfortic, REM: Remicade, CsA: Ciclosporine, P: Prednisone, ttt: Treatment.
VKH was diagnosed in a 42-year-old female patient with an ICGA score of 31/40, an FA score of 4/40, and an EDI-OCT choroidal thickness (CT) of 550 μm at presentation. The grey area of the graphic shows the phase before disease was under control. Three weeks later, under high dose corticosteroid therapy (IV, 500 mg methyl-prednisolone for 3 days followed by 60 mg oral prednisone) + CsA (250 mg), ICGA score decreased to 6, FA score remained low at 2, and EDI-OCT-CT decreased to 482 μm. Three more weeks later, under 35 mg prednisone and the same dose of CsA, the ICGA score re-increased to 17 while the other two parameters did not change significantly (FA score = 1, CT = 438 μm), indicating that the patient probably did not respond to CsA (A). Before disease was under control, several treatments had to be tried, such as mycophenolic acid (1440) that was ineffective when used alone with an increase of the ICGA score to 20.5 without significant change of the other 2 parameters (FA score = 2, CT = 395 μm) (C). After the introduction of infliximab (Remicade®), ICGA score decreased to 1, FA remained at 1 while EDI-OCT-CT also showed a positive evolution progressively decreasing from 395 to 328 μm (D). During the follow-up phase when disease was under control, tapering and discontinuation of both CsA and Myfortic® was achieved without any increase of the ICGA score, not showing any occult choroiditis recurrence. After determining the efficient therapy to which this patient was responsive, ICGA allowed safe tapering.
Example 2 (Fig. 6-patient 4)
Fig. 6.
Safe tapering of therapy in a Vogt-Koyanagi-Harada disease (VKH) patient. Maxinal treatment = methylprednisolone IV (1000 mg per day for 3 days) followed by oral prednisone (1 mg/kg) and azathioprine (2.5 mg/kg). P: Prednisone followed by the dosage in mg. I: Imurek®, azathioprine, followed by the dosage in mg. MYF: Myfortic® = mycophenolic acid, CsA: Cyclosporine. Pink shadowed area accounts for disease evolution and treatment combinations before disease was under control.
VKH was diagnosed in this 38-year-old female patient with an elevated ICGA score of 23/40, a slightly elevated FA score of 7/40, and an EDI-OCT-CT of 500 μm at presentation. Four weeks later, under high dose corticosteroid therapy (IV, 500 mg methyl-prednisolone for 3 days followed by 60 mg oral prednisone) + azathioprine (Imurek®, 150 mg, 2.5 mg/kg), ICGA score decreased to 5, FA score decreased to 2, and CT decreased to 298 μm. Four months later under 15 mg of prednisone and 150 mg of Imurek® (I), there was a slight non-significant increase of the ICGA score to 8 (+3) with no change for FA score nor for CT.
Because of this slight subclinical resurgence of choroiditis and because of intolerance to Imurek®, treatment was changed to prednisone (30 mg) + mycophenolic acid (1440 mg daily) + CsA (250 mg, 4 mg/kg) with a decrease of the ICGA score to 4. The phase of determining the therapy until disease was under control is shown in the grey part of the graph.
With disease under control, CsA and Myfortic® were tapered over a period of 18 months with an ICGA score stable and low of 3. After 4½ years of follow-up without treatment, no recurrence was noted in a patient that can be considered cured.
Discussion
The appraisal and evaluation of inflammatory involvement of different eye structures in uveitis has reached a high level of precision in the last decades. This is now also the case for the choroid, which can be precisely investigated thanks to ICGA.1, 3, 4, 6
In this study, we have found that ICGA was the most sensitive modality among the available investigations to detect subtle changes in choroidal inflammatory involvement in patients under treatment. At the onset of treatment significant parallel decrease of values among the 3 investigative modalities could be noted. However, after the post-acute phase and after disease had been under control, neither EDI-OCT nor FA showed any subclinical changes, suggesting that they are of limited use in this type of uveitis. In contrast, in more than one fourth of imaging follow-up triads including ICGA, FA and CT, ICGA showed significant changes, while the other two parameters failed to demonstrate any subclinical disease evolution. The fact that all subclinical changes detected by ICGA occurred in BRC patients indicates that BRC is more resistant to treatment, whereas VKH responds more readily to therapy as the sole origin of the inflammatory process occurs exclusively within the choroid which responds more readily to therapy.24 This explains that no clinical or subclinical recurrences were noted for VKH after adequate therapy had been applied with disease under control, although therapy was discontinued in one patient and reduced to a minimum in the other. On the other hand in BRC, therapy could not be stopped. As has been reported earlier, it is also noteworthy that during initial-onset disease, the retina (FA) is minimally involved in VKH, while there was a substantially higher FA score present in BRC.17
The strength of our study resides in the fact that our patients benefited from multiple imaging follow-up triads, and the long-term follow-up allowing for analysis of trends and fine readjustments of therapy. Indeed, as is the case with rare diseases, the number of patients included is still small and additional data have to be collected to confirm the results of this pilot study.
This study strongly indicates that ICGA should be an essential part of the routine follow-up of stromal choroiditis, and that fine-tuning of medications can and should be safely guided by this monitoring modality. Moreover, the arbitrary cut-off value defining a significant change on ICGA bared highly specific results, speaking in favour of the system, however underestimating its power. Further studies with more flexible definitions of significant ICGA changes (<5 score points variations), should show even stronger results.
As already indicated previously, EDI-OCT does not appear as a reliable modality for precise follow-up of stromal choroiditis and fine-tuning of therapy as it lacks reactivity and does not detect subclinical evolution. The same is true for FA, which is understandable and obvious as the main inflammatory reaction takes place in the choroid. After this pilot study, additional data need to be generated to confirm our findings.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Bouchenaki N.H.C. Stromal choroiditis. In: Pleyer U., Mondino B., editors. Essentials in Ophthalmology: Uveitis and Immunological Disorders. Springer; Berlin Heidelberg, New York: 2004. pp. 234–253. [Google Scholar]
- 2.Balci O., Gasc A., Jeannin B., Herbort C.P., Jr. Enhanced depth imaging is less suited than indocyanine green angiography for close monitoring of primary stromal choroiditis: a pilot report. Int Ophthalmol. 2017;37(3):737–748. doi: 10.1007/s10792-016-0303-7. [DOI] [PubMed] [Google Scholar]
- 3.Balci O., Jeannin B., Herbort C.P., Jr. Contribution of dual fluorescein and indocyanine green angiography to the appraisal of posterior involvement in birdshot retinochoroiditis and Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2018;38(2):527–539. doi: 10.1007/s10792-017-0487-5. [DOI] [PubMed] [Google Scholar]
- 4.Reddy A.K., Gonzalez M.A., Henry C.R., Yeh S., Sobrin L., Albini T.A. Diagnostic sensitivity of indocyanine green angiography for birdshot chorioretinopathy. JAMA Ophthalmol. 2015;133(7):840–843. doi: 10.1001/jamaophthalmol.2015.0822. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi L.A. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151(5):745–751. doi: 10.1016/j.ajo.2011.01.043. e741. [DOI] [PubMed] [Google Scholar]
- 6.Abouammoh M.A., Gupta V., Hemachandran S., Herbort C.P., Abu El-Asrar A.M. Indocyanine green angiographic findings in initial-onset acute Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2016;94(6):573–578. doi: 10.1111/aos.12974. [DOI] [PubMed] [Google Scholar]
- 7.Miyanaga M., Kawaguchi T., Miyata K., Horie S., Mochizuki M., Herbort C.P. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010;54(5):377–382. doi: 10.1007/s10384-010-0853-6. [DOI] [PubMed] [Google Scholar]
- 8.Bouchenaki N., Herbort C.P. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease. Ophthalmology. 2001;108(1):54–64. doi: 10.1016/s0161-6420(00)00428-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang P., Zhong Y., Du L. Development and evaluation of diagnostic criteria for vogt-koyanagi-harada disease. JAMA Ophthalmol. 2018;136(9):1025–1031. doi: 10.1001/jamaophthalmol.2018.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fardeau C., Herbort C.P., Kullmann N., Quentel G., LeHoang P. Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology. 1999;106(10):1928–1934. doi: 10.1016/S0161-6420(99)90403-7. [DOI] [PubMed] [Google Scholar]
- 11.Bouchenaki N., Herbort C.P. Indocyanine green angiography guided management of vogt-koyanagi-harada disease. J Ophthalmic Vis Res. 2011;6(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 12.Herbort C.P., Mantovani A., Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007;27(2-3):173–182. doi: 10.1007/s10792-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi T., Horie S., Bouchenaki N., Ohno-Matsui K., Mochizuki M., Herbort C.P. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2010;30(1):41–50. doi: 10.1007/s10792-008-9288-1. [DOI] [PubMed] [Google Scholar]
- 14.Papadia M., Herbort C.P. Indocyanine green angiography (ICGA) is essential for the early diagnosis of birdshot chorioretinopathy. Klinische Monatsblatter fur Augenheilkunde. 2012;229(4):348–352. doi: 10.1055/s-0031-1299224. [DOI] [PubMed] [Google Scholar]
- 15.Herbort C.P., Jr., Abu El Asrar A.M., Takeuchi M. Catching the therapeutic window of opportunity in early initial-onset Vogt-Koyanagi-Harada uveitis can cure the disease. Int Ophthalmol. 2018 doi: 10.1007/s10792-018-0949-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.da Silva F.T., Hirata C.E., Sakata V.M. Indocyanine green angiography findings in patients with long-standing Vogt-Koyanagi-Harada disease: a cross-sectional study. BMC Ophthalmol. 2012;12:40. doi: 10.1186/1471-2415-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabro F., Herbort C.P. Need for quantitative measurement methods for posterior uveitis: comparison of dual FA/ICGA angiography, EDI-OCT choroidal thickness and SUN vitreous haze evaluation in stromal choroiditis. Klinische Monatsblatter fur Augenheilkunde. 2018;235(4):424–435. doi: 10.1055/s-0043-124966. [DOI] [PubMed] [Google Scholar]
- 18.Gillmann K., El Ameen A., Massy R., Fabro F., Gasc A., Herbort C.P., Jr. Assessment of measurement methods of posterior inflammation in stromal choroiditis: the value of quantitative outcome measures versus the presently qualitatively based paradigm. Int Ophthalmol. 2018 doi: 10.1007/s10792-018-0979-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Abu El-Asrar A.M., Dosari M., Hemachandran S., Gikandi P.W., Al-Muammar A. Mycophenolate mofetil combined with systemic corticosteroids prevents progression to chronic recurrent inflammation and development of 'sunset glow fundus' in initial-onset acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2017;95(1):85–90. doi: 10.1111/aos.13189. [DOI] [PubMed] [Google Scholar]
- 20.Abu El-Asrar A.M., Hemachandran S., Al-Mezaine H.S., Kangave D., Al-Muammar A.M. The outcomes of mycophenolate mofetil therapy combined with systemic corticosteroids in acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2012;90(8):e603–e608. doi: 10.1111/j.1755-3768.2012.02498.x. [DOI] [PubMed] [Google Scholar]
- 21.Herbort C.P., Jr., Pavesio C., LeHoang P. Why birdshot retinochoroiditis should rather be called 'HLA-A29 uveitis'? Br J Ophthalmol. 2017;101(7):851–855. doi: 10.1136/bjophthalmol-2016-309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knecht P.B., Papadia M., Herbort C.P., Jr. Early and sustained treatment modifies the phenotype of birdshot retinochoroiditis. Int Ophthalmol. 2014;34(3):563–574. doi: 10.1007/s10792-013-9861-0. [DOI] [PubMed] [Google Scholar]
- 23.Papadia M., Herbort C.P., Jr. New concepts in the appraisal and management of birdshot retinochoroiditis, a global perspective. Int Ophthalmol. 2015;35(2):287–301. doi: 10.1007/s10792-015-0046-x. [DOI] [PubMed] [Google Scholar]
- 24.Papadia M., Herbort C.P. Reappraisal of birdshot retinochoroiditis (BRC): a global approach. Graefe's Arch Clin Exp Ophthalmol Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013;251(3):861–869. doi: 10.1007/s00417-012-2201-7. [DOI] [PubMed] [Google Scholar]
- 25.Tugal-Tutkun I., Herbort C.P., Khairallah M., Mantovani A. Interobserver agreement in scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation. Ocul Immunol Inflamm. 2010;18(5):385–389. doi: 10.3109/09273948.2010.489730. [DOI] [PubMed] [Google Scholar]
- 26.Tugal-Tutkun I., Herbort C.P., Khairallah M. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis) Int Ophthalmol. 2010;30(5):539–552. doi: 10.1007/s10792-008-9263-x. [DOI] [PubMed] [Google Scholar]
- 27.Fong A.H., Li K.K., Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina (Philadelphia, Pa) 2011;31(3):502–509. doi: 10.1097/IAE.0b013e3182083beb. [DOI] [PubMed] [Google Scholar]
- 28.Keane P.A., Allie M., Turner S.J. Characterization of birdshot chorioretinopathy using extramacular enhanced depth optical coherence tomography. JAMA Ophthalmol. 2013;131(3):341–350. doi: 10.1001/jamaophthalmol.2013.1724. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama M., Keino H., Okada A.A. Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina (Philadelphia, Pa) 2012;32(10):2061–2069. doi: 10.1097/IAE.0b013e318256205a. [DOI] [PubMed] [Google Scholar]
- 30.Tan C.S., Ouyang Y., Ruiz H., Sadda S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M., Yang X.F., Jiao X. The diurnal variation pattern of choroidal thickness in macular region of young healthy female individuals using spectral domain optical coherence tomography. Int J Ophthalmol. 2016;9(4):561–566. doi: 10.18240/ijo.2016.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]