Abstract
Purpose
To determine 4-year changes of ocular biometric and dioptric components in Iranian children aged 7–11 years following the first phase.
Methods
468 children were evaluated in the first phase of the study in 2012 and again in 2016–2017. Multi-stage stratified cluster sampling was applied to select the participants. The Topcon autorefractometer and the LENSTAR/BioGraph biometer (WaveLight AG, Erlangen, Germany) were used for cycloplegic refraction and biometry, respectively. All measurements were repeated at 4 years as the baseline assessments.
Results
Of 468 children, 251 (53.6%) were boys. Spherical equivalent (SE) showed a marked myopic shift (P = 0.000) in the second phase which was significantly higher in boys (0.24 vs. 0.18 D) (P < 0.001). Axial length (AL) and anterior chamber depth (ACD) increased by 0.49 ± 0.05 and 0.12 ± 0.02 mm, while lens thickness (LT) and lens power (LP) decreased by 0.08 ± 0.01 mm and 1.59 ± 0.12 D, respectively (P < 0.05). The mean corneal curvature and thickness did not change significantly during 4 years. All biometric component changes were greater in boys. Biometric changes in different age groups showed a decreased LP, increased AL, and increased ACD in most age groups (P < 0.05), while LT and SE did not change significantly in the age groups 9 and 11 years and 8 and 9 years, respectively. Changes in the corneal thickness, diameter, curvature, and refractive astigmatism were not significant in any of the age groups (P > 0.05).
Conclusions
Statistical and clinical changes were seen in AL, ACD, LP, and LT. The changes observed in biometric components (AL, ACD, and LT) had a sinus rhythm.
Keywords: Ocular dioptric component, Children, Biometry, Axial length, Lens power, Keratometry, Age, Sex
Introduction
Coordination among refractive components of the eye [corneal and lens power (LP), anterior chamber depth (ACD), lens thickness (LT), and axial length (AL)] determine the refractive state of the eye. Maintenance of emmetropia in childhood indicates a balance between ocular growth and changes in the focal point of the eye.1 In the early developmental phase, optical defocus is an important factor in regulation of AL changes. Different studies have evaluated the relationship between optical parameters of the eye and its refractive state, and the results have often indicated that AL is a major determinant of the refractive state whose elongation results in myopia.2 However, studies investigating the correlation of biometric/optic parameters have shown the highest correlation between AL and corneal radius of curvature (CR), and these two factors are two important biometric determinants of the refractive state of the eye.3 Some other investigations have shown that the ratio of the AL to CR (AL/CR ratio) has a higher correlation with the refractive state compared to AL alone.4 Although the cornea is the strongest dioptric component of the eye, its role in maintenance of emmetropia is less important than the crystalline lens. The cornea is almost fully developed by the age of 3 years while the lens continues to grow throughout the life through producing new fibers in such a way that during childhood, the lens gets thin axially, and its radius of curvature of the anterior and posterior surfaces increases.5
Cross-sectional studies in school-age children have provided a relative understanding of the algorithm of refractive and biometric changes of the eye. However, prospective studies are more useful since they can provide more accurate information about these changes and control many confounding factors. Evaluation of optical and biometric components changes in school-age children shows that after 4–6 years of age, the refractive state of the eye undergoes different changes in various communities even depending on the place of living (rural or urban).6
Investigation of the developmental course of refractive components in children provides valuable information about natural ocular growth changes. In addition, these studies are necessary in different communities to determine the trend of myopia in order to prepare appropriate plans to assess and follow-up the visual status of children through identifying the risk factors of refractive disorders. In general, studies investigating the prevalence of refractive errors in school-age children, especially those below 15 years, in Iran have shown a markedly higher prevalence of hyperopia compared to East Asian countries.7, 8 Since ethnicity is a determinant of the developmental state and growth of biometric/optical components of the eye, evaluation of the changes of these parameters in Iran school-age children seems useful considering the difference in the prevalence of refractive errors between Iran and other countries.9 Therefore, there may be differences in the growth status of these components in one region compared to other regions that have been overlooked. This study was conducted to evaluate 4-year changes of optical/biometric components in school-age children. A review of the literature showed no similar study in Iran.
Methods
The present study was part of the Dezful Cohort study. The first phase of the study, which had a cross-sectional design, was conducted in 2012. The details of phase 1 have been already published,10 but a brief explanation is presented. The target population of the study was school students of Dezful, a city in the southwest of Iran. In this study, multi-stage stratified cluster sampling was used to select the participants. First, one elementary school, one secondary school, and one high school were randomly selected from boys' and girls’ schools (a total of 6 schools) in Dezful. From each school year in each school (stratum), one class (cluster) was randomly selected, and all of its students were studied. The exclusion criteria were nystagmus, significant corneal opacity, history of wearing contact lens, ocular surgery, seizures, and Arab ethnicity. Ophthalmic and biometric examinations were performed on one day.
The second phase of the study was conducted 4 years later, from November 2016 to January 2017. In this phase, all students who were 7–11 years old in the first phase were invited to participate in the study. The children were searched from the same schools where the first phase of the study was conducted. If a student had moved to another school, the address of the new school was obtained from the previous school to invite him/her to participate in the second phase of the study.
Five hundred twenty-nine children aged 7–11 years were assessed in the first phase. It should be noted that the results of the first phase were based on the data of part of the study population, and sampling continued after publishing the results. In the second phase, 487 students were invited, 473 of whom participated in the study. This project was approved by the Ethics Committee of Mashhad University of Medical Sciences and was conducted according to tenets of the Declaration of Helsinki.
The participants and their guardians received sufficient information about the study and its objectives. Then informed consent was obtained from the parents, and oral consent was obtained from the students. The inclusion criteria were lack of nystagmus, lack of significant corneal opacity, no history of wearing contact lens, ocular surgery, and seizures.
Examinations were done by the same optometrists who performed examinations in phase one and all measurements were repeated at 4 years as the baseline assessments.
First, the optical biometry was done using the LENSTAR/BioGraph biometer (WaveLight AG, Erlangen, Germany) and AL, ACD, LT in millimeters, and corneal power in diopter (D) were determined. If there were any errors, measurements were repeated 5 min after instilling artificial tears. The validity of the biometric measurements of this device was confirmed in phase one of the study.10 Then non-cycloplegic refraction was measured using Topcon autorefractometer (Topocon KR 8000, Topcon Corporation, Tokyo, Japan). Cycloplegic refraction was evaluated in all students. For this purpose, one drop of cyclopentolate 1% was instilled every 5 min for three times, and autorefraction was repeated 30 min after the last drop. The refraction data were recorded as sphere, negative cylinder, and cylinder axis.
The Bennett's formula was applied to calculate the refractive power of the crystalline lens (LP) using the results of distance cycloplegic refraction, mean corneal power, ACD, LT, and AL.11
The vector analysis method was used to determine the astigmatic difference between the two phases in which the spherocylindrical refraction was transformed to Jackson crossed cylinder format. Using this method, the refractive data (S: sphere, C: cylinder, and α: cylinder axis) were used to calculate M, J0, and J45 as:
| M = S + C/2 J0 = (−C/2) cos (2α) J45 = (−C/2) sin (2α) |
To compute the astigmatic difference in the two phases, the difference between J0 and J45 of the first and second phases’ astigmatisms was calculated separately. To better understand astigmatic changes, the values of power vector were transformed to the conventional spherocylindrical format by applying the following formula:
| C = −2√(J0)2 + (J45) 2 |
All measurements were done under the supervision of an experienced optometrist who did not have access to the data of first phase to prevent any bias and error. All measurements were performed between 9 a.m. and 2 p.m. To avoid mistakes and ambiguity, evaluations started from the right eye.
Data were analyzed with the SPSS.22 (SPSS Inc., Chicago, IL) using paired samples t-test, independent samples t-test, Pearson's correlation coefficient, or if necessary, non-parametric equivalents of the above statistics. P values less than 0.05 were considered significant. Due to the high correlation of the right and left eyes' findings, the data of the right eyes of the participants were analyzed.
Results
After applying the exclusion criteria, the data of 468 out of 473 participants were analyzed, 251 (53.6%) of whom were boys. The mean age of the participants was 13.69 ± 1.59 years.
Table 1 presents the refractive state in two phases of the study according to gender.
Table 1.
Mean and standard deviation (SD) of sphere, cylinder, and spherical equivalent (SE) in two phases of the study in all subjects (n = 468), males (n = 251) and females (n = 217).
| Variables | Phase 1 |
Phase 2 |
P-value | |
|---|---|---|---|---|
| Mean ± SD (95%CI) | Mean ± SD (95%CI) | |||
| Sphere (D) | Male | 0.33 ± 0.92 (0.21–0.45) | 0.12 ± 0.75 (0.02–0.22) | 0.005 |
| Female | 0.15 ± 0.67 (0.05–0.25) | −0.03 ± 1.12 (−0.19 to 0.13) | 0.042 | |
| Total | 0.25 ± 0.82 (0.17–0.33) | 0.06 ± 0.92 (−0.02 to 0.14) | 0.000 | |
| Cylinder (D) | Male | −0.56 ± 0.51 (−0.62 to −0.50) | −0.61 ± 0.59 (−0.69 to −0.53) | 0.310 |
| Female | −0.55 ± 0.49 (−0.61 to −0.49) | −0.54 ± 0.57 (−0.62 to −0.46) | 0.844 | |
| Total | −0.55 ± 0.50 (−0.59 to −0.51) | −0.58 ± 0.58 (−0.64 to −0.52) | 0.396 | |
| SE (D) | Male | 0.05 ± 0.86 (−0.05 to 0.15) | −0.18 ± 0.72 (−0.26 to −0.10) | 0.001 |
| Female | −0.12 ± 0.65 (−0.20 to −0.04) | −0.30 ± 1.26 (0.48 to −0.12) | 0.062 | |
| Total | −0.03 ± 0.78 (−0.11 to 0.05) | −0.23 ± 0.98 (−0.33 to −0.13) | 0.000 | |
SD: Standard deviation; CI: Confidence interval; D: Diopter; SE: Spherical equivalent.
A significant change was seen in the mean sphere and spherical equivalent (SE) between the two phases. This myopic shift was more prominent in boys than girls (0.24 vs. 0.18 D) (P < 0.001).
Table 2 shows the mean and standard deviation (SD) of all biometric parameters, LP, corneal power, and corneal diameter (CD) in all subjects, boys, and girls in the two phases.
Table 2.
Mean and standard deviation (SD) of biometry components, lens power (LP), central corneal thickness (CCT), mean keratometry reading (KR), and corneal diameter (CD) in the two phases of study in all subjects (n = 468), males (n = 251) and females (n = 217).
| Variables | Phase 1 |
Phase 2 |
P-value | |
|---|---|---|---|---|
| Mean ± SD (95%CI) | Mean ± SD (95%CI) | |||
| AL (mm) | Male | 23.01 ± 0.73 (22.91–23.11) | 23.51 ± 0.70 (23.43–23.59) | <0.001 |
| Female | 22.62 ± 0.68 (22.52–22.72) | 23.06 ± 0.72 (22.96–23.16) | <0.001 | |
| Total | 22.83 ± 0.73 (22.77–22.89) | 23.32 ± 0.74 (23.26–23.38) | <0.001 | |
| ACD (mm) | Male | 2.99 ± 0.23 (2.97–3.01) | 3.12 ± 0.26 (3.08–3.16) | <0.001 |
| Female | 2.91 ± 0.28 (2.87–2.95) | 3.01 ± 0.28 (2.97–3.05) | 0.002 | |
| Total | 2.96 ± 0.26 (2.94–2.98) | 3.07 ± 0.27 (3.05–3.09) | <0.001 | |
| LT (mm) | Male | 3.63 ± 0.20 (3.61–3.65) | 3.54 ± 0.21 (3.52–3.56) | <0.001 |
| Female | 3.64 ± 0.20 (3.62–3.66) | 3.59 ± 0.22 (3.55–3.63) | 0.013 | |
| Total | 3.64 ± 0.20 (3.62–3.66) | 3.56 ± 0.21 (3.54–3.58) | <0.001 | |
| CCT (μm) | Male | 551.42 ± 33.48 (547.28–555.56) | 548.42 ± 33.66 (544.44–552.4) | 0.317 |
| Female | 546.75 ± 28.24 (542.99–550.51) | 552.89 ± 32.16 (548.36–557.42) | 0.035 | |
| Total | 549.25 ± 31.21 (546.43–552.07) | 550.27 ± 33.08 (547.27–553.27) | 0.627 | |
| CD (mm) | Male | 12.41 ± 0.40 (12.35–12.47) | 12.45 ± 0.41 (12.39–12.51) | 0.269 |
| Female | 12.27 ± 0.40 (12.21–12.33) | 12.28 ± 0.39 (12.22–12.34) | 0.792 | |
| Total | 12.35 ± 0.41 (12.31–12.39) | 12.38 ± 0.41 (12.34–12.42) | 0.263 | |
| Mean KR (D) | Male | 43.15 ± 1.51 (42.95–43.35) | 43.15 ± 1.41 (42.97–43.33) | 1.000 |
| Female | 43.96 ± 1.54 (43.76–44.16) | 43.79 ± 1.48 (43.57–44.01) | 0.241 | |
| Total | 43.52 ± 1.58 (43.38–43.66) | 43.41 ± 1.47 (43.27–43.55) | 0.270 | |
| LP (D) | Male | 24.83 ± 1.85 (24.59–25.07) | 23.16 ± 1.81 (22.94–23.38) | <0.001 |
| Female | 25.61 ± 1.66 (25.39–25.83) | 24.21 ± 1.77 (23.96–24.46) | <0.001 | |
| Total | 25.19 ± 1.81 (25.03–25.35) | 23.60 ± 1.86 (23.42–23.78) | <0.001 | |
SD: Standard deviation; CI: Confidence interval; AL: Axial length; ACD: Anterior chamber depth; LT: Lens thickness; CCT: Central corneal thickness; CD: Corneal diameter; KR: Keratometry reading; LP: Lens power; D: Diopter.
The results showed an increase in AL and ACD and a decrease in LT and LP. The mean 4-year changes of AL, ACD, and LT were 0.49 ± 0.05, 0.12 ± 0.02, and 0.08 ± 0.01 mm, and the mean 4-year keratometry reading (KR) and LP changes were 0.11 ± 0.10 and 1.59 ± 0.12 D in all subjects, respectively. The changes in all biometric parameters were more prominent in boys than girls (P < 0.001).
The mean central corneal thickness (CCT), KR, and CD did not change significantly in all subjects, boys, and girls, except for CCT in girls. AL increased by a mean of 0.49 mm in all subjects and by a mean of 0.50 and 0.44 mm in boys and girls, respectively (P < 0.001).
Comparison of the mean changes in biometric/dioptric components (AL, ACD, Mean KR, LT, LP, SE) between the two sexes indicated statistically significant difference (P < 0.001) in all parameters using the independent-samples T test except ACD between the two groups (P = 0.391).
Table 3 shows the mean and SD of biometric and dioptric components and refractive changes in the two phases in different age groups based on age in the first phase.
Table 3.
Mean and standard deviation (SD) of biometric and dioptric components and refractive changes in the two phases separately in different age groups.
| Variables | Age groups (years) based on the age in the first phase (n) Mean ± SD (95%CI) |
|||||
|---|---|---|---|---|---|---|
| 7 (n = 171) | 8 (n = 72) | 9 (n = 55) | 10 (n = 69) | 11 (n = 101) | ||
| AL (mm) | Phase 1 | 22.79 ± 0.60 (22.69–22.89) | 22.73 ± 0.66 (22.57–22.89) | 22.90 ± 0.80 (22.68–23.12) | 22.75 ± 0.97 (22.51–22.99) | 22.99 ± 0.74 (22.85–23.13) |
| Phase 2 | 23.27 ± 0.68 (23.17–23.37) | 23.22 ± 0.59 (23.08–23.36) | 23.16 ± 0.74 (22.96–23.36) | 23.45 ± 0.85 923.25 to 23.65) | 23.48 ± 0.81 (23.32–23.64) | |
| P-value | <0.001 | <0.001 | 0.081 | <0.001 | <0.001 | |
| ACD (mm) | Phase 1 | 2.94 ± 0.22 (2.90–2.98) | 2.92 ± 0.24 (2.86–2.98) | 3.01 ± 0.37 (2.91–3.11) | 2.92 ± 0.26 (2.86–2.98) | 3.00 ± 0.24 (2.96–3.04) |
| Phase 2 | 3.08 ± 0.27 (3.04–3.12) | 3.11 ± 0.26 (3.05–3.17) | 3.02 ± 0.31 (2.94–3.10) | 3.07 ± 0.26 (3.01–3.13) | 3.08 ± 0.26 (3.02–3.14) | |
| P-value | <0.001 | <0.001 | 0.878 | 0.000 | 0.024 | |
| LT (mm) | Phase 1 | 3.66 ± 0.21 (3.62–3.70) | 3.65 ± 0.22 (3.59–3.71) | 3.58 ± 0.16 (3.54–3.62) | 3.67 ± 0.16 (3.63–3.71) | 3.60 ± 0.21 (3.56–3.64) |
| Phase 2 | 3.56 ± 0.23 (3.52–3.6) | 3.52 ± 0.20 (3.48–3.56) | 3.57 ± 0.18 (3.53–3.61) | 3.54 ± 0.24 (3.48–3.60) | 3.60 ± 0.19 (3.56–3.64) | |
| P-value | <0.001 | 0.000 | 0.758 | 0.000 | 1.00 | |
| CCT (μm) | Phase 1 | 547.02 ± 34.96 (541.79–552.25) | 556.21 ± 30.44 (549.17–563.25) | 552.42 ± 27.20 (545.23–559.61) | 547.80 ± 33 (540.02–555.58) | 547.35 ± 24.90 (542.49–552.21) |
| Phase 2 | 552.90 ± 34.32 (547.76–558.04) | 550.22 ± 33.04 (542.60–557.84) | 542.95 ± 33.03 (534.23–551.67) | 553.73 ± 34.53 (545.58–561.88) | 547.48 ± 29.63 (541.7–553.26) | |
| P-value | 0.117 | 0.259 | 0.103 | 0.304 | 0.973 | |
| CD (mm) | Phase 1 | 12.41 ± 0.39 (12.35–12.47) | 12.26 ± 0.42 (12.16–12.36) | 12.24 ± 0.38 (12.12–12.36) | 12.34 ± 0.50 (12.20–12.48) | 12.37 ± 0.36 (12.29–12.45) |
| Phase 2 | 12.41 ± 0.40 (12.35–12.47) | 12.34 ± 0.45 (12.24–12.44) | 12.30 ± 0.35 (12.20–12.40) | 12.39 ± 0.39 (12.29–12.49) | 12.40 ± 0.45 (12.30–12.50) | |
| P-value | 1.00 | 0.272 | 0.391 | 0.513 | 0.601 | |
| Mean KR (D) | Phase 1 | 43.40 ± 1.48 (43.18–43.62) | 43.89 ± 1.59 (43.52–44.26) | 43.64 ± 1.45 (43.25–44.03) | 43.46 ± 1.95 (43.01–43.91) | 43.45 ± 1.49 (43.16–43.74) |
| Phase 2 | 43.38 ± 1.41 (43.16–43.60) | 43.60 ± 1.32 (43.29–43.91) | 43.64 ± 1.37 (43.27–44.01) | 43.17 ± 1.67 (42.78–43.56) | 43.39 ± 1.59 (43.08–43.70) | |
| P-value | 0.898 | 0.235 | 1.00 | 0.349 | 0.782 | |
| LP (D) | Phase 1 | 25.44 ± 1.66 (25.19–25.69) | 25.35 ± 1.74 (24.94–25.76) | 24.86 ± 1.89 (24.35–25.37) | 25.24 ± 1.98 (24.77–25.71) | 24.81 ± 1.87 (24.44–25.18) |
| Phase 2 | 23.8 ± 1.74 (23.55–24.05) | 23.76 ± 1.86 (23.33–24.19) | 23.61 ± 2.07 (23.06–24.16) | 23.32 ± 2.00 (22.85–23.79) | 23.33 ± 1.84 (22.98–23.68) | |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Sphere (D) | Phase 1 | 0.28 ± 0.66 (0.18–0.38) | 0.02 ± 0.52 (−0.10 to 0.14) | 0.24 ± 0.98 (−0.01 to 0.49) | 0.58 ± 1.05 (0.33–0.83) | 0.12 ± 0.90 (−0.06 to 0.30) |
| Phase 2 | 0.09 ± 0.84 (−0.03 to 0.21) | 0.05 ± 0.76 (−0.13 to 0.23) | 0.22 ± 0.90 (−0.02 to 0.46) | 0.15 ± 1.11 (−0.10 to 0.40) | −0.14 ± 1.01 (−0.34 to 0.06) | |
| P-value | 0.020 | 0.782 | 0.911 | 0.020 | 0.054 | |
| Cylinder (D) | Phase 1 | −0.56 ± 0.5 (−0.64 to −0.48) | −0.44 ± 0.31 (−0.52 to −0.36) | −0.64 ± 0.67 (−0.82 to −0.46) | −0.57 ± 0.56 (−0.71 to −0.43) | −0.57 ± 0.46 (−0.67 to −0.47) |
| Phase 2 | −0.53 ± 0.46 (−0.59 to −0.47) | −0.59 ± 0.61 (−0.73 to −0.45) | −0.57 ± 0.59 (−0.73 to −0.41) | −0.63 ± 0.72 (−0.81 to −0.45) | −0.66 ± 0.64 (−0.78 to −0.54) | |
| P-value | 0.564 | 0.064 | 0.562 | 0.585 | 0.252 | |
| SE (D) | Phase 1 | 0.01 ± 0.66 (−0.09 to 0.11) | −0.20 ± 0.53 (−0.32 to −0.08) | −0.08 ± 0.94 (−0.33 to 0.17) | 0.30 ± 0.87 (0.10–0.50) | −0.17 ± 0.88 (−0.35 to 0.01) |
| Phase 2 | −0.18 ± 0.91 (−0.32 to −0.04) | −0.24 ± 0.75 (−0.42 to −0.06) | −0.06 ± 0.93 (−0.31 to 0.19) | −0.16 ± 1.04 (−0.41 to 0.09) | −0.47 ± 1.18 (−0.71 to −0.23) | |
| P-value | 0.027 | 0.712 | 0.910 | 0.005 | 0.041 | |
SD: Standard deviation; CI: Confidence interval; AL: Axial length; ACD: Anterior chamber depth; LT: Lens thickness; CCT: Central corneal thickness; CD: Corneal diameter; KR: Keratometry reading; LP: Lens power; SE: Spherical Equivalent; D: Diopter.
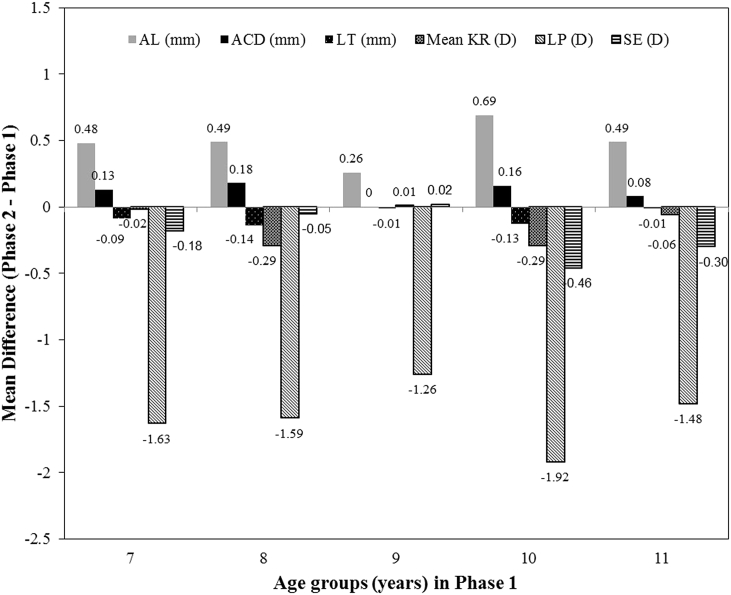
Comparison of biometric and dioptric parameters and refractive changes between the two phases indicates different trends in different age groups. The LP decreased significantly in all age groups (P < 0.05) while AL and ACD increased significantly in all age groups except 9 years. Comparison of LT, sphere, and SE between the two phases in different age groups showed significant changes in the LT in all age groups except 9 and 11 years, in sphere in all age groups except 8, 9, and 11 years, and in SE in all age groups except 8 and 9 years. The changes in CCT, CD, mean KR, and cylinder were not significant in different age groups (P > 0.05). Fig. 1 shows biometric and dioptric changes in different age groups.
Fig. 1.
Bar chart of axial length (AL), anterior chamber depth (ACD), lens power (LP), lens thickness (LT), mean keratometry reading (KR), and spherical equivalent (SE) changes in different age groups.
According to Fig. 1, the trend of biometric/dioptric parameters changes indicated an increase in AL and ACD and a decrease in LP and LT, causing a myopic shift in all age groups except 9 years.
During 4 years, the age group 10 years experienced the largest changes and the age group 9 years had the smallest disparity in the majority of parameters when compared to the first phase.
Discussion
This study showed a myopic shift of −0.21 D as well as an increase in AL and ACD and a marked decrease in LT and LP in 4-year follow-up of Iranian children aged 7–11 years. Comparison of the mean changes in biometric/dioptric components in the two sexes indicated statistically significant difference in all parameters except ACD between the two groups. These changes were usually more prominent in boys.
Spherical equivalent changes
The mean SE changes during 4 years were more prominent in boys and far smaller than previous reports,12, 13 which could be due to differences in the ethnicity (Iranian vs. Hong Kong Chinese),12 study type, sample size, and use of cycloplegic agents. Consistent with the results of Pointer et al, the present study found no significant cylinder changes in different age groups.13 Comparison of SE between boys and girls in the two phases showed a significant difference, which was contrary to the results of studies reporting lack of gender difference in refractive errors and confirming the results of studies indicating higher myopia in women.2, 9
Corneal characteristics changes (mean keratometry, central corneal thickness, and corneal diameter)
No significant change in the CCT and CD in all subjects and different age groups was consistent with the results of previous studies.10, 14, 15 While, CD showed an increase of about 0.15 mm in boys, which was in good agreement with previous studies.10, 14 Reporting the increase in CCT in Singaporean boys aged 9–10 years compared to girls,15 could be in part due to differences in ethnicity and the use of an automated and noncontact pachymeter mounted on a slit-lamp as measurement technique.
Comparison of the mean corneal power in both phases showed the girls’ corneas were markedly steeper, which was in line with previous studies.9, 10, 14, 16, 17 The lack of significant change in the mean KR in this 4-year longitudinal study was consistent with studies reporting no considerable changes in the corneal curvature approximately after the age of 3 years.9, 10, 16, 17 Contrary to previous studies, marked corneal flattening of 0.33 and 0.44 D was reported in longitudinal evaluation of children aged 6–14 and 6–18 years, respectively.18
Anterior chamber depth changes
Increase in ACD in almost all age groups confirmed the results of a previous studies in Nepali and Taiwan students.18, 19 However, no significant ACD change in a study of Tibetan children 6–16 years could be due to differences in the measurement technique (ultrasound vs. optical biometry), study type (cross-sectional vs. longitudinal), and age range.20 One explanation for ACD changes is a coordinated increase in AL that increases the ACD through physical stretching applied to the lens and changing its thickness. Another reason is that during ocular growth, following the growth of the sclera in the area between the limbus and ciliary muscle attachment zone, the tension of the posterior ciliary muscle applied on the posterior zonular fibers moves the lens backward and deepens the ACD. This situation is especially reported in myopia.
Similar to previous reports, ACD was deeper in boys than girls.9, 10, 14, 16, 17
Calculated crystalline lens power and lens thickness changes
LT in the second phase and LP calculated by the Bennett's formula in both phases were greater in girls than boys, which was contrary to the results of a study that reported no difference in LT between boys and girls and a higher LP in boys.16 The reason for this difference may be the type of this study (cross-sectional). However, Li et al (2016) reported that girls have more powerful lenses.14 Although there is no clear explanation for the difference in refractive/biometric components between boys and girls, the reason for thinner and lower LP in boys may be further increase in the AL, resulting in flattening of lens curvatures and decreased LT through physical stretching.
The decrease in LP in all age groups was in line with studies indicating that this reduction continues slowly after the age of 10 years.5, 18, 21 A marked decrease in the LP in the age group 10 years in the present study is justifiable on the basis of increased near-work activity and AL changes. Therefore, LP changes can not be explained by a simple developmental process independent of changes in AL or refractive errors5; otherwise, they may induce a myopic or even a hyperopic shift. The reduced LP in all age groups did not follow a similar pattern, and no significant thinning was observed in the age group 9 and 11 years. This finding confirmed previous results indicating a decrease in LP and LT by 10 years of age, followed by a gradual increase in LT and further decrease in LP.5 Increased LT reported after age 9.5 years is contrary to the decrease seen in the age group 10 years and lack of significant change in the age group 11 years.21 A study in Taiwanese schoolchildren showed that lens becomes thinner in children 7–13 years, and then its thickness increases after 15 years of age, which is consistent with our findings.19 On the contrary, lack of LT change with ageing in Nepali children aged 6–16 years could be due to ethnic differences.20 Although decreased LP and LT can be explained by physical stretching following equatorial ocular growth in children up to 10 years of age, the decrease in LP in the age group 11 years may be attributed to changes in other components like crystalline lens gradient refractive index.22 It seems that the lens, as a major optical component of the anterior segment, is responsible for maintaining a balance in the refractive state, and its power and thickness decrease to compensate for the increase in AL during ocular growth.
Axial length changes
AL increases rapidly until 3 years of age; after that, it increases by only 1 mm during 10 years.22 However, AL changes until 25–30 years of age are responsible for myopia and decreased prevalence of hyperopia. The mean AL increased by 0.49 mm during 4 years follow-up in this study while these changes were markedly larger in studies conducted in East Asian countries due to the higher prevalence of myopia.12 Comparison of mean biometric/optical parameters between girls and boys showed a significant difference in AL in favor of boys, which was consistent with the results of previous studies.9, 10, 14, 16, 17
Concurrent spherical equivalent and biometric components changes in different age groups
The initial myopic shift in the age group 7 years is probably related to a sudden increase in the frequency of near-work activities due to starting school and a sudden decrease in outdoor activities. In parallel with this dioptric change, a marked increase in AL and ACD and a considerable decrease in LP and LT were also seen. In the age group 8 years, despite rather similar refractive changes as in the age group 7 years, SE changes were not remarkable, which could be due to the higher percentage of myopic patients in this age group compared to the age group 7 years [considering the 95% confidence interval (CI) in both groups]. On the other hand, the mean changes of the corneal power, although non-significant, indicated more flattening of the cornea (0.29 D vs. 0.02 D).
The smallest SE change was seen in the age group 9 years, which can be explained by changes in optical/biometric components. In this group, AL changes were almost half of previous age groups, and the changes in ACD, LT, and corneal power were not significant. Based on AL changes between the two phases, a myopic shift of 1 D was expected that was neutralized with a 1.25 D decrease in LP, causing no significant change in SE.
The largest SE, AL, and LP changes were seen in the age group 10 years, which could be related to an increase in near-work demand at higher school/educational levels and other close-work activities such as mobile or computer use. This finding is in line with the result of the study that shows the greatest change in refraction and AL are in the early years of myopia development and their changes decrease thereafter.23 Moreover, studies showed that the intensity of near-work has a more important role in causing myopia than its duration.24 The changes seen in the age group 11 years were smaller than the age group 10 years, which could be attributed to the age-related decrease in AL changes.
One of the limitations of this study was that we did not evaluate confounding factors such as parents’ refractive status and frequency of near-work activity as factors contributing to refractive and AL changes. In addition, we did not investigate the effect of accommodation on LT measurement due to performing biometry before cycloplegic refraction. Moreover, it was not possible to provide the ocular growth curve of different dioptric components because the data were collected in only two phases. Another concern is regarding the ethnicity of the study population, the southwest of Iran consists of various ethnic groups such as Arab, Fars, and others, but this study only included Dezful students, and Arab students were excluded from the study. Although the Lurs race was in our sample, the subgroup analysis could not provide meaningful results due to their low number.
In summary, considering the definition of a clinically significant change in dioptric components as an at least 0.5 D change in SE and KR, 0.1 mm change in LT, 0.75 D change in crystalline LP, 0.2 mm change in AL, and 0.1 mm change in ACD, the results of this 4-year longitudinal study showed clinically significant changes in AL (0.49 mm increase), ACD (0.11 mm increase), LT (0.08 mm decrease), and LP (1.59 D decrease) in Iranian children aged 7–11 years. Our study showed that increased intensity of near-work activity (hard copies or video display terminals) at higher school/educational levels is associated with significant changes in AL. Therefore, protective measures like the use of positive lenses and atropine drops for close-work activities may help to control refractive and AL changes during this time. The same applies to children at the start of school, which is associated with increased near-distance activities and reduced outdoor activities.
Footnotes
Financial support: This project was supported by Mashhad University of Medical Sciences.
Conflicts of interest: The authors declare that they have no conflict of interest.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Flitcroft D.I. Emmetropisation and the aetiology of refractive errors. Eye (Lond) 2014;28(2):169–179. doi: 10.1038/eye.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Liu B., Huang S., Huang W., He M. Relationship between refractive error and ocular biometrics in twin children: the Guangzhou Twin Eye Study. Eye Sci. 2014;29(3):129–133. [PubMed] [Google Scholar]
- 3.Hashemi H., Khabazkhoob M., Emamian M.H. Association between refractive errors and ocular biometry in Iranian adults. J Ophthalmic Vis Res. 2015;10(3):214–220. doi: 10.4103/2008-322X.170340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi H., Khabazkhoob M., Miraftab M. Axial length to corneal radius of curvature ratio and refractive errors. J Ophthalmic Vis Res. 2013;8(3):220–226. [PMC free article] [PubMed] [Google Scholar]
- 5.Iribarren R., Morgan I.G., Chan Y.H., Lin X., Saw S.M. Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol Vis Sci. 2012;53(9):5124–5130. doi: 10.1167/iovs.12-9637. [DOI] [PubMed] [Google Scholar]
- 6.Fan D.S., Cheung E.Y., Lai R.Y., Kwok A.K., Lam D.S. Myopia progression among preschool Chinese children in Hong Kong. Ann Acad Med Singapore. 2004;33(1):39–43. [PubMed] [Google Scholar]
- 7.Yekta A., Fotouhi A., Hashemi H. Prevalence of refractive errors among schoolchildren in Shiraz, Iran. Clin Exp Ophthalmol. 2010;38(3):242–248. doi: 10.1111/j.1442-9071.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 8.Alsaqr A., Abu Sharha A., Fagehi R. The visual status of adolescents in Riyadh, Saudi Arabia: a population study. Clin Ophthalmol. 2018;12:965–972. doi: 10.2147/OPTH.S162319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zadnik K., Manny R.E., Yu J.A. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80(3):226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Hashemi H., Jafarzadehpur E., Ghaderi S. Ocular components during the ages of ocular development. Acta Ophthalmol. 2015;93(1):e74–e81. doi: 10.1111/aos.12498. [DOI] [PubMed] [Google Scholar]
- 11.Bennett A.G. A method of determining the equivalent powers of the eye and its crystalline lens without resort to phakometry. Ophthalmic Physiol Optic. 1988;8(1):53–59. doi: 10.1016/0275-5408(88)90089-0. [DOI] [PubMed] [Google Scholar]
- 12.Lam C.S., Edwards M., Millodot M., Goh W.S. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci. 1999;76(6):370–380. doi: 10.1097/00006324-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Pointer J.S. A 6-year longitudinal optometric study of the refractive trend in school-aged children. Ophthalmic Physiol Optic. 2001;21(5):361–367. [PubMed] [Google Scholar]
- 14.Li S.M., Iribarren R., Kang M.T. Corneal power, anterior segment length and lens power in 14-year-old Chinese children: the anyang childhood eye study. Sci Rep. 2016;6:20243. doi: 10.1038/srep20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong L., Saw S.M., Siak J.K., Gazzard G., Tan D. Corneal thickness determination and correlates in Singaporean schoolchildren. Invest Ophthalmol Vis Sci. 2004;45(11):4004–4009. doi: 10.1167/iovs.04-0121. [DOI] [PubMed] [Google Scholar]
- 16.Twelker J.D., Mitchell G.L., Messer D.H. Children's ocular components and age, gender, and ethnicity. Optom Vis Sci. 2009;86(8):918–935. doi: 10.1097/opx.0b013e3181b2f903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojaimi E., Rose K.A., Morgan I.G. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005;46(8):2748–2754. doi: 10.1167/iovs.04-1324. [DOI] [PubMed] [Google Scholar]
- 18.Garner L.F., Stewart A.W., Owens H., Kinnear R.F., Frith M.J. The Nepal Longitudinal Study: biometric characteristics of developing eyes. Optom Vis Sci. 2006;83(5):274–280. doi: 10.1097/01.opx.0000215251.27409.16. [DOI] [PubMed] [Google Scholar]
- 19.Lin L.L., Shih Y.F., Tsai C.B. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999;76(5):275–281. doi: 10.1097/00006324-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Garner L.F., Yap M.K., Kinnear R.F., Frith M.J. Ocular dimensions and refraction in Tibetan children. Optom Vis Sci. 1995;72(4):266–271. doi: 10.1097/00006324-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Jones L.A., Mitchell G.L., Mutti D.O., Hayes J.R., Moeschberger M.L., Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005;46(7):2317–2327. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- 22.Zadnik K., Mutti D.O., Fusaro R.E., Adams A.J. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci. 1995;36(8):1581–1587. [PubMed] [Google Scholar]
- 23.Xiang F., He M., Morgan I.G. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology. 2012;119(7):1478–1484. doi: 10.1016/j.ophtha.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Ip J.M., Saw S.M., Rose K.A. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49(7):2903–2910. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]