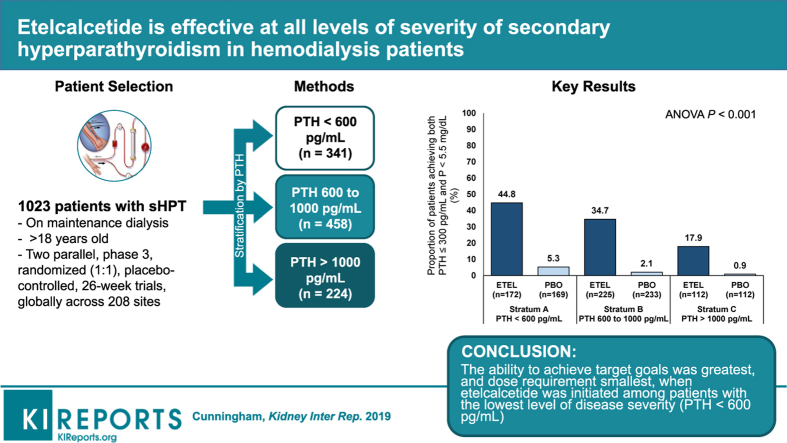
Abstract
Introduction
Calcimimetics improve parameters of secondary hyperparathyroidism (sHPT) but are mostly initiated when patients have severe disease, potentially limiting effectiveness. We evaluated the effects of etelcalcetide on lowering intact parathyroid hormone, calcium, and phosphate at different disease severity levels.
Methods
This analysis examined data from 2 parallel, phase 3, randomized, placebo-controlled, 26-week trials conducted in 1023 adult (≥18 years old) patients with sHPT on maintenance hemodialysis. Etelcalcetide effects by baseline intact parathyroid hormone stratum (<600, 600–1000, and >1000 ng/l) on mean percentage change in intact parathyroid hormone; changes in calcium and phosphate; and achieving serum intact parathyroid hormone ≤300 ng/l, phosphate <1.78 mmol/l, and both combined, were assessed.
Results
Etelcalcetide reduced serum intact parathyroid hormone by a similar percentage across baseline strata. A similar proportion achieved >30% intact parathyroid hormone reduction across strata for the etelcalcetide arms. Parathyroid hormone increased modestly in each placebo-group stratum, most prominently in the lowest stratum. Serum calcium and phosphate concentrations decreased across strata in etelcalcetide-treated patients, with the most pronounced reductions in patients with highest baseline parathyroid hormone. However, the proportion of patients achieving parathyroid hormone, phosphate, and both targets was highest in the lowest baseline parathyroid hormone stratum, where etelcalcetide dose requirements were lowest. Etelcalcetide dose requirement was lowest among patients in the lowest intact parathyroid hormone stratum.
Conclusion
Etelcalcetide effectively lowered serum intact parathyroid hormone, calcium, and phosphate, irrespective of the severity of secondary hyperparathyroidism. The ability to achieve target goals was greatest, and dose requirement smallest, when etelcalcetide was initiated among patients with the lowest level of disease severity.
Keywords: calcium, chronic kidney disease, hemodialysis, mineral metabolism, parathyroid hormone, phosphate
Graphical abstract
Treatment options for sHPT in patients receiving maintenance dialysis include vitamin D receptor activators (VDRAs), calcimimetics, and subtotal parathyroidectomy.1 Current treatment guidelines recommend targeting circulating concentrations of parathyroid hormone (PTH) to approximately 2 to 9 times the normal reference range while avoiding elevated serum concentrations of calcium and phosphate, with an emphasis on serial monitoring and combined control of laboratory values.1, 2 Calcimimetics, calcitriol, and vitamin D analogs are all recommended as potential first-line therapeutic options for patients on dialysis with sHPT.1
Available therapies are often used in combination to take advantage of their additive, at times complementary, or opposing effects. Although VDRAs and calcimimetics synergistically lower PTH, they exhibit opposing effects on serum calcium and phosphate. In clinical practice, VDRAs are routinely prescribed and the oral calcimimetic cinacalcet is commonly added if PTH remains uncontrolled, or when hypercalcemia or hyperphosphatemia limit the ongoing provision or dose escalation of VDRAs. As such, patients can have very high serum concentrations of PTH and/or elevated serum concentrations of calcium and phosphate at the time of calcimimetic initiation.3, 4
The intravenous calcimimetic etelcalcetide (Parsabiv; Amgen Inc., Thousand Oaks, CA), a synthetic peptide calcium-sensing receptor agonist administered 3 times weekly after each hemodialysis session, has been shown to achieve sustained reductions in serum PTH, calcium, and phosphate when tested in 2 placebo-controlled clinical studies in patients receiving maintenance hemodialysis with moderate-to-severe sHPT.5
Here, we report Results of the analyses exploring the effects of etelcalcetide on controlling PTH, calcium, and phosphate based on the underlying severity of sHPT. The role of sHPT severity on dose requirement for etelcalcetide was also evaluated.
Methods
This is a secondary analysis of data from 2 parallel, phase 3, randomized, placebo-controlled, 26-week trials conducted in 1023 adult (≥18 years old) patients on maintenance hemodialysis at 208 sites globally from March 12, 2013, to June 12, 2014, and May 12, 2014. The trials were approved by institutional review boards at participating trial sites and were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.5 All patients were on maintenance hemodialysis (stable dialysate calcium concentration ≥2.25 mEq/l) with moderate-to-severe sHPT (intact PTH >400 pg/ml [ng/l]) and an albumin-corrected serum calcium concentration of ≥8.3 mg/dl (2.08 mmol/l). Patients previously prescribed VDRAs, phosphate binders, or calcium supplements must have had no more than a maximum dose change of 50% within the 2 months before screening laboratory assessments and remained on stable doses through randomization. The clinical trial methods, including a complete description of patients, are described in detail by Block et al.5
Patients were excluded if they had received cinacalcet (Sensipar/Mimpara; Amgen Inc.) within 4 weeks of screening, had a parathyroidectomy within 3 months of dosing, were anticipated to undergo a parathyroidectomy or kidney transplant during the treatment period, had a history of cardiovascular disease or cardiac abnormality, had a history of seizure, or were receiving treatment for seizure disorder.
Briefly, eligible patients were randomized 1:1 within 1 day before the first dose of etelcalcetide or placebo. Randomization was stratified by mean screening PTH (A < 600, B 600–1000, and C > 1000 pg/ml), prior cinacalcet use (within 8 weeks before randomization), and region (North America or non–North America). Etelcalcetide was started at 5 mg i.v. per hemodialysis session and titrated every 4 weeks to a maximum of 15 mg in 2.5- or 5.0-mg increments to achieve PTH ≤300 pg/ml. Titration could occur during weeks 5, 9, 13, and 17 based on PTH and serum calcium Results obtained during the prior week. Study drug was temporarily withheld for low PTH (<100 pg/ml × 2), low albumin-corrected calcium (<7.5 mg/dl [1.88 mmol/l]), or symptomatic hypocalcemia, and was restarted at a lower dose. Investigators were blinded to the study PTH, and local-site measurement of PTH was suspended throughout the study. Doses of vitamin D analogs, calcium-based binders/supplements, and dialysate calcium could be changed at the discretion of the investigator; however, such changes would have to be driven by a response to changes in serum calcium concentrations, which were not blinded.
Blood samples were collected predialysis at baseline and post baseline every 2 weeks for PTH. Samples for serum calcium and phosphate were collected weekly through week 27.
Data Analysis
The primary efficacy endpoint for both placebo-controlled studies was the proportion of patients with >30% PTH reduction from baseline during the efficacy-assessment phase (EAP). Secondary endpoints included the proportion of patients with mean PTH ≤300 pg/ml and percent reductions in PTH, corrected serum calcium, and the calcium-phosphate product during the EAP.
This post hoc analysis evaluated the effects of etelcalcetide by sHPT disease severity (prespecified by baseline PTH stratum, <600, 600–1000, >1000 pg/ml) on absolute PTH reductions and mean percent change and concomitant changes in serum calcium and phosphate following etelcalcetide or placebo administration. In addition, we determined the likelihood of achieving a target PTH ≤300 pg/ml and phosphate <5.5 mg/dl, and the combined endpoint within each PTH stratum. The Cochran-Mantel-Haenszel test was used to compare these laboratory target achievement endpoints between treatment groups in each of the baseline PTH strata. We also report the mean percentage change in PTH, calcium, and phosphate according to the baseline PTH strata. Statistical analyses were performed to compare endpoints across baseline PTH strata in the etelcalcetide group. For comparing the mean laboratory percentage change, we used analysis of variance with pairwise comparison across the 3 strata without adjusting for multiple comparisons. We considered P values < 0.05 as statistically significant and conducted all analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Summary of Placebo-Controlled Studies
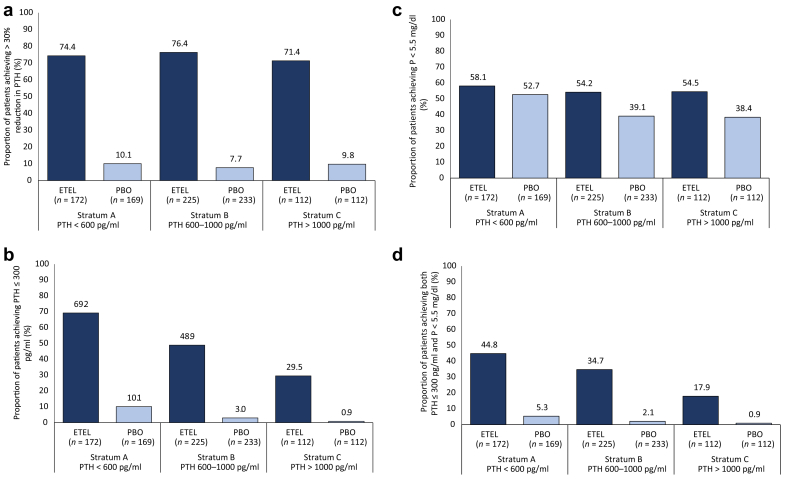
The primary efficacy Results of the 2 placebo-controlled studies were described by Block et al.5 In summary, baseline demographic characteristics were similar across the 2 placebo-controlled studies (N = 1023), with mean (SD) age of 58.2 (14.4) years. Most patients were male (60%); there was a broad distribution by race/ethnicity (66% white, 28% black, 6% other). A history of cinacalcet use was similar between placebo and etelcalcetide treatment arms, 235 of 514 (45.7%) and 240 of 509 (47.2%), respectively. A substantially higher proportion of patients randomized to etelcalcetide achieved the primary efficacy endpoint relative to placebo (74.7% vs. 8.9%; P < 0.001). A substantially higher proportion of patients in the etelcalcetide group also achieved intact PTH ≤300 pg/ml compared with placebo (52% vs. 5%; P < 0.001).
Baseline Laboratory Values by Baseline PTH Strata
The baseline laboratory values for each PTH stratum are shown in Table 1. Among patients with available PTH values at baseline (N = 1023), the patient numbers in each stratum (<600, 600–1000, >1000 pg/ml) were similar between treatment arms. Patients with the highest baseline PTH also had the highest serum concentrations of calcium and phosphate.
Table 1.
Baseline laboratory values by baseline PTH strata
| Mean (SD) | PTH <600 pg/ml |
PTH 600–1000 pg/ml |
PTH >1000 pg/ml |
|||
|---|---|---|---|---|---|---|
| Etelcalcetide (n = 172) | Placebo (n = 169) | Etelcalcetide (n = 225) | Placebo (n = 233) | Etelcalcetide (n = 112) | Placebo (n = 112) | |
| PTH, pg/ml | n = 172 | n = 169 | n = 225 | n = 233 | n = 112 | n = 112 |
| 509.8 (71.0) | 501.9 (66.6) | 780.6 (120.6) | 779.7 (129.2) | 1497.6 (679.1) | 1456.8 (666.8) | |
| Ca, mmol/l | n = 172 | n = 169 | n = 225 | n = 233 | n = 112 | n = 112 |
| 2.39 (0.16) | 2.39 (0.13) | 2.41 (0.17) | 2.42 (0.17) | 2.44 (0.16) | 2.44 (0.18) | |
| P, mmol/l | n = 168 | n = 168 | n = 223 | n = 232 | n = 110 | n = 107 |
| 1.78 (0.436) | 1.73 (0.462) | 1.92 (0.533) | 1.92 (0.472) | 2.01 (0.559) | 2.02 (0.526) | |
| Patients on vitamin D, % | 76 | 70 | 67 | 72 | 61 | 53 |
| Vitamin-D dose, μg/wk | n = 129 | n = 116 | n = 147 | n = 164 | n = 67 | n = 58 |
| 15.35 (13.00) | 15.65 (17.46) | 16.18 (13.63) | 15.23 (12.89) | 17.63 (15.35) | 14.84 (11.50) | |
Vitamin-D dose is presented as intravenous paricalcitol equivalents.
Ca, albumin-corrected serum calcium concentration; P, phosphate; PTH, parathyroid hormone.
Patients in the highest PTH stratum were least likely to be on VDRAs at baseline in both treatment arms. Mean VDRA doses at baseline were generally similar, although etelcalcetide-treated patients had modestly higher mean baseline doses than placebo-treated patients in the 600 to 1000 and >1000 pg/ml strata.
On-Treatment Laboratory Results by PTH Strata
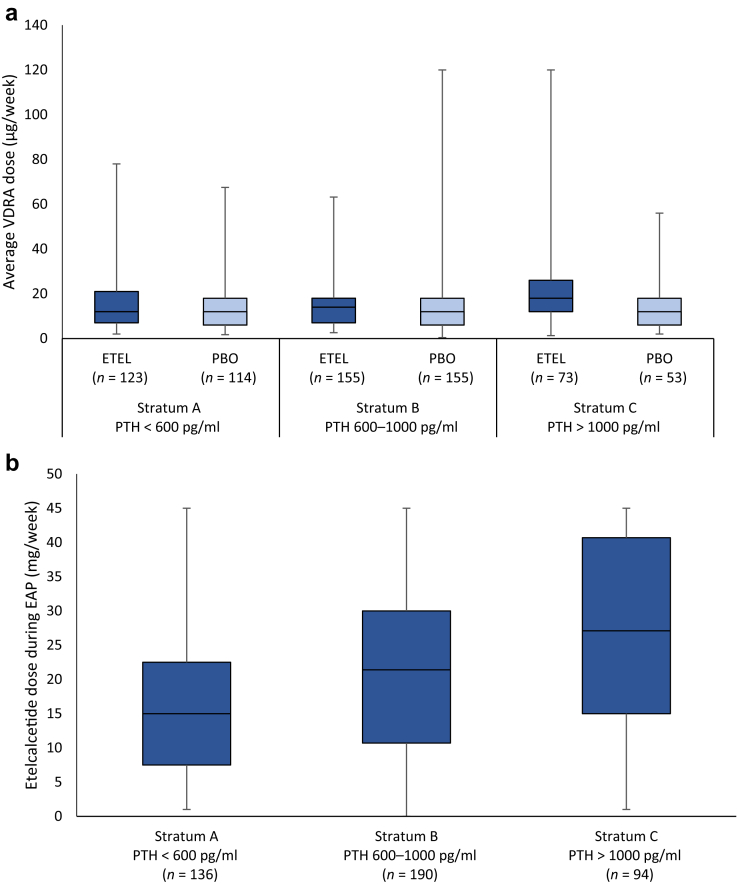
The mean percent changes in laboratory values within each PTH stratum are shown in Table 2. Among patients randomized to etelcalcetide, similar percent reductions in PTH were observed across the 3 baseline PTH strata. Among patients randomized to placebo, PTH concentrations increased, with the largest percent increase observed among patients in the lowest baseline PTH stratum. Etelcalcetide reduced serum calcium and phosphate concentrations in all baseline PTH strata, with the most pronounced changes observed among patients with the highest baseline PTH (P < 0.001 for corrected calcium and P = 0.042 for phosphate). Etelcalcetide-treated patients in the lowest PTH stratum were more likely to achieve discrete targets (PTH ≤300 pg/ml, P < 0.001; and the combination of PTH ≤300 pg/ml + phosphate <1.78 mmol/l, P <0.001) compared with other PTH strata in the etelcalcetide group. Finally, mean doses of etelcalcetide used during the EAP (weeks 20–27) were directly related to the severity of sHPT at baseline.
Table 2.
Percentage change from baseline in mean laboratory values during the EAP by baseline PTH
| Stratum A (PTH <600 pg/ml) |
Stratum B (PTH 600–1000 pg/ml) |
Stratum C (PTH >1000 pg/ml) |
Overall effect of PTH stratum P value |
Multiple comparisons (PTH stratum)a |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Etelcalcetide (n = 172) | Placebo (n = 169) | Etelcalcetide (n = 225) | Placebo (n = 233) | Etelcalcetide (n = 112) | Placebo (n = 112) | A vs. B P value | A vs. C P value | B vs. C P value | ||
| PTH | n = 153 | n = 157 | n = 201 | n = 204 | n = 102 | n = 95 | ||||
| −54.2 (2.3) | 17.1 (3.8) | −58.2 (1.9) | 14.0 (2.6) | −55.5 (3.2) | 5.9 (3.3) | 0.424 | 0.203 | 0.733 | 0.444 | |
| Ca | n = 153 | n = 157 | n = 201 | n = 204 | n = 102 | n = 95 | ||||
| −5.1 (0.6) | 1.4 (0.4) | −7.5 (0.6) | 0.7 (0.3) | −8.9 (0.9) | 0.4 (0.4) | < 0.001 | 0.005 | <0.001 | 0.150 | |
| P | n = 150 | n = 156 | n = 200 | n = 202 | n = 100 | n = 90 | ||||
| −5.4 (1.9) | 3.1 (1.9) | −8.2 (2.4) | −3.6 (1.4) | −14.6 (2.2) | −4.6 (2.1) | 0.042 | 0.366 | 0.013 | 0.066 | |
Ca, albumin-corrected serum calcium concentration; EAP, efficacy-assessment phase; P, phosphate; PTH, parathyroid hormone.
Data are mean (SE) percentage changes from baseline during the EAP. Analysis of variance model was applied to generate P values for the overall effect of PTH strata and for multiple comparisons among PTH strata.
Multiple comparisons were conducted only for patients in the etelcalcetide group.
Data related to achieving study laboratory goals and dose of etelcalcetide across baseline PTH strata are shown in Figure 1. Mean doses of etelcalcetide and VDRA used during the EAP (weeks 20–27) are shown in Figure 2. Etelcalcetide dose was directly related to the severity of sHPT at baseline. Achieving study laboratory goals was not associated with prior cinacalcet use (Supplementary Table S1).
Figure 2.
Weekly vitamin D receptor activators (VDRA) and etelcalcetide (ETEL) dose during the efficacy-assessment phase (EAP) by baseline intact parathyroid hormone (PTH) strata. Data presented as median, 25th percentile and 75th percentile (box) and minimum maximum dose (whiskers). VDRA dose is presented as i.v. paricalcitol equivalents. PBO, placebo.
Figure 1.
Efficacy of etelcalcetide (ETEL) at attaining laboratory targets for (a) intact parathyroid hormone (PTH) >30% reduction, (b) PTH ≤ 300 pg/ml, (c) P < 5.5 mg/dl, and (d) both PTH ≤ 300 pg/ml and P < 5.5 mg/dl, during the efficacy-assessment phase (EAP). Patients with no PTH and/or P measurement during the EAP were considered as not achieving the endpoint. PBO, placebo; P, phosphate.
Conclusion
Etelcalcetide is efficacious at lowering PTH irrespective of the baseline severity of sHPT. Moreover, relative to placebo (along with standard of care for sHPT, including phosphate binders and VDRAs), etelcalcetide reduces serum calcium and phosphate and yields improved overall control of mineral metabolism. As expected, the dose required to achieve these Results depends on the underlying severity of sHPT pretreatment. These findings are consistent with data derived from clinical studies with the oral calcimimetic cinacalcet6 and support the potential clinical strategy of initiating therapy with etelcalcetide earlier in the course of sHPT disease progression, before significant downregulation of parathyroid vitamin D receptor, and calcium-sensing receptor limits responsiveness to VDRAs, calcium salts (or increased calcium concentrations in dialysate), and/or calcimimetic therapies.7
Baseline serum phosphate concentrations were highest in patients with the highest baseline PTH. The direct correlation of PTH and phosphate has been consistently observed in other studies8 and suggests a role of PTH-mediated bone resorption as a contributor to the development of hyperphosphatemia (endogenous hyperphosphatemia) in patients with little or no residual kidney function, as well as the stimulatory effect of phosphate on the parathyroid gland.9, 10, 11 Dietary phosphate restriction will not correct endogenous hyperphosphatemia. It can be hypothesized that patients with more severe sHPT might have more prevalent use of vitamin D, which could also increase calcium and phosphate concentrations. Counterintuitively, in our analysis, patients in the highest PTH stratum had the lowest overall percentage use of VDRA at baseline, suggesting that high serum calcium and/or phosphate concentrations limited the use of VDRAs in this subset. Although we did not control dietary phosphate intake or the use of phosphate binders by protocol, this finding suggests that suboptimally controlled sHPT contributes to poor control of hyperphosphatemia observed in patients receiving maintenance dialysis and may be responsible (indirectly) for some of the excess mortality and morbidity attributed to hyperphosphatemia itself.12
Etelcalcetide treatment resulted in a decrease in serum phosphate in all PTH strata, but the decrease was most pronounced in patients with the highest PTH concentrations at baseline, despite the fact that etelcalcetide-treated patients with the most severe sHPT were also on the highest dose of vitamin D at the end of the trial, suggesting that reductions in serum calcium and phosphate induced by etelcalcetide may have facilitated VDRA use, which would tend to increase serum phosphate via intestinal absorption of dietary phosphate. These data are consistent with previously published cinacalcet studies and suggest that calcimimetic-induced reductions in PTH may help mitigate hyperphosphatemia in patients on dialysis by decreasing phosphate efflux from the bone.11, 13, 14 Patients initiated on etelcalcetide in the lowest PTH stratum were most likely to achieve combined PTH and phosphate targets, once again highlighting the potential benefits of initiating treatment at earlier stages of the disease process. This approach is also consistent with current treatment guidelines recommending therapeutic decisions based on combined achievement of laboratory targets.1
Serum calcium concentrations were highest among patients with the highest PTH at baseline. Furthermore, etelcalcetide-induced reductions in calcium were more pronounced in patients who had the most severe sHPT at baseline. The higher levels of VDRA dosing at the end of the trial in patients with the highest baseline PTH was most likely a response to a more pronounced reduction in serum calcium in patients with more severe sHPT, because investigators were blinded to PTH during the trial. Our observation that baseline sHPT severity predicts the magnitude of calcium reduction after initiation of etelcalcetide is consistent with other studies that have evaluated calcium reduction after initiation of cinacalcet or after parathyroidectomy.15, 16 An observational study of cinacalcet-induced calcium reductions by Brunelli et al.17 showed that patients who had the most prominent reduction in serum calcium were those with the highest baseline concentrations of PTH and alkaline phosphatase. Higher baseline PTH concentrations also have been associated with a higher risk of sustained hypocalcemia and higher needs for calcium supplementation after parathyroidectomy.15, 16, 18, 19, 20 Collectively, these data suggest that patients with more severe sHPT should be monitored most closely for calcimimetic-induced hypocalcemia.
Strengths of this study include its large and diverse clinical trial populations; frequent central laboratory measurements of PTH, calcium, and phosphate; and protocol-directed titration of the interventions. In addition to these strengths, our study had limitations aside from those inherent in post hoc analyses. First, we cannot rule out the possibility that differences in phosphate binder use among strata may have influenced our findings following etelcalcetide treatment. However, given the high percentage of subjects with concomitant phosphate binder use (83%), we believe that the effects observed on phosphate are at least in part attributable to etelcalcetide. Second, this study does not address whether severity of sHPT impacts on frequency of adverse events. Given that adverse events occurred in 92% of etelcalcetide-treated and 80% of placebo-treated patients,5 we believe that severity of sHPT at baseline does not dictate frequency of treatment-related adverse events; however, future assessments of adverse events by PTH strata are needed. Finally, although achieving study laboratory goals was not associated with prior cinacalcet use, this analysis did not consider cinacalcet adherence (such data were not collected in this study).
In summary, etelcalcetide effectively lowers serum PTH, calcium, and phosphate across a broad spectrum of sHPT disease severity. However, the ability to achieve target goals is the greatest and the dose requirement the least when treatment is initiated at lower PTH concentrations. Reductions in serum calcium and phosphate were more pronounced in patients with the highest baseline PTH at the time of initiation of etelcalcetide treatment, supporting the concept that reductions in serum calcium and phosphate are mediated by etelcalcetide-induced reductions in PTH, leading to reduced bone turnover and reduced efflux of calcium and phosphate from bone mineral during treatment. Additional experience with etelcalcetide in clinical practice will help to determine whether initiation of etelcalcetide among patients with mild-to-moderate sHPT and normal bone remodeling prevents or ameliorates clinical complications of sHPT. These Results reinforce the view that clinicians should consider the degree of pretreatment sHPT severity when assessing treatment options for the management of chronic kidney disease–mineral and bone disorder in patients on hemodialysis.
Disclosure
JC has received consulting fees from Amgen, Novo Nordisk, OPKO, Vifor, and Kai Pharma. GAB has received research grants from Keryx; consulting fees from Akebia, Amgen, AstraZeneca, Daiichi Sankyo, Keryx, Kirin, Omeros, Ono, and OPKO; and is part of the speakers’ bureau for OPKO. GMC is on the Board of Directors of Satellite Healthcare; owns stock/stock options in Ardelyx, Cricket Health, Durect, DxNow, Eliaz Therapeutics, Outset Medical, Physiowave, and Puracath Medical; has received an institutional grant from Amgen and Janssen; has received consulting fees from AMAG, Gilead, and Sanafit; has DSMB membership for Bayer, Bristol-Myers Squib, and ReCor; and is on the Trial Steering Committee for Akebia and AstraZeneca. KC and JI are employees of Amgen and own Amgen stock. PE has received research grants from Amgen, consulting fees from Vifor FMC, and is part of the speakers’ bureau for Amgen, B. Braun, and Vifor FMC. YS is an employee of Amgen. PUT has received research grants from Amgen, Astellas, GSK, and Hemotech, and consulting fees from Amgen and Vifor FMC. DAB receives research grants from the National Institutes of Health, Renal Research Institute; consulting fees from Amgen, Relypsa/Vifor/Fresenius, Sanofi/Genzyme, Novo Nordisk/Covance and Tricida; and owns stock in Amgen and Tricida. No further disclosures were reported.
Acknowledgments
This study was funded by Amgen Inc., Thousand Oaks, CA (Amgen studies 20120229 [NCT01785849], 20120230 [NCT01788046]). The authors thank Holly Tomlin, MPH, CMPP (Amgen Inc. at time of writing, currently Tomlin Health Sciences Communications) and William W. Stark Jr, PhD (employee and stockholder, Amgen Inc.) and Jonathan Plumb, PhD (Fishawack Communications Inc., funded by Amgen Inc.), for medical writing and journal formatting assistance. All authors critically evaluated and provided their interpretations of the data for earlier drafts of the manuscript and approved the final draft for submission in accordance with ICMJE criteria for authorship.
Role of the Funding Source
The study was designed and conducted by Amgen Inc. (Thousand Oaks, CA). The authors, in collaboration with Amgen Inc., collected and interpreted the data, wrote the report, and made the decision to submit the article for publication.
Data Sharing Statement
There is a plan to share data. This may include de-identified individual patient data for variables necessary to address the specific research question in an approved data-sharing request; also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data sharing requests relating to data in this manuscript will be considered after the publication date and (i) this product and indication (or other new use) have been granted marketing authorization in both the United States and Europe, or (ii) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors reviews requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen’s sole discretion and without further arbitration. On approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at the following: http://www.amgen.com/datasharing
Footnotes
Table S1. Logistic regression model adjusted for stratification factors for achieving laboratory targets.
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
Logistic regression model adjusted for stratification factors for achieving laboratory targets.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59. [DOI] [PMC free article] [PubMed]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO Clinical Practice Guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]; Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO Clinical Practice Guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130. [DOI] [PubMed]
- 3.Bover J., Ureña P., Ruiz-Garcia C. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161–174. doi: 10.2215/CJN.01760215. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bover J, Ureña P, Ruiz-Garcia C, et al. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161-174. [DOI] [PMC free article] [PubMed]
- 4.Frãzao J., Rodriguez M. Secondary hyperparathyroidism disease stabilization following calcimimetic therapy. NDT Plus. 2008;1(suppl 1):i12–i17. doi: 10.1093/ndtplus/sfm039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Frazao J, Rodriguez M. Secondary hyperparathyroidism disease stabilization following calcimimetic therapy. NDT Plus. 2008;1(suppl 1):i12-i17. [DOI] [PMC free article] [PubMed]
- 5.Block G.A., Bushinsky D.A., Cunningham J. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146–155. doi: 10.1001/jama.2016.19456. [DOI] [PubMed] [Google Scholar]; Block GA, Bushinsky DA, Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146-155. [DOI] [PubMed]
- 6.Frazao J.M., Messa P., Mellotte G.J. Cinacalcet reduces plasma intact parathyroid hormone, serum phosphate and calcium levels in patients with secondary hyperparathyroidism irrespective of its severity. Clin Nephrol. 2011;76:233–243. doi: 10.5414/cn106965. [DOI] [PubMed] [Google Scholar]; Frazao JM, Messa P, Mellotte GJ, et al. Cinacalcet reduces plasma intact parathyroid hormone, serum phosphate and calcium levels in patients with secondary hyperparathyroidism irrespective of its severity. Clin Nephrol. 2011;76:233-243. [DOI] [PubMed]
- 7.Sudhaker Rao D., Han Z.H., Phillips E.R. Reduced vitamin D receptor expression in parathyroid adenomas: implications for pathogenesis. Clin Endocrinol (Oxf) 2000;53:373–381. doi: 10.1046/j.1365-2265.2000.01081.x. [DOI] [PubMed] [Google Scholar]; Sudhaker Rao D, Han ZH, Phillips ER, et al. Reduced vitamin D receptor expression in parathyroid adenomas: implications for pathogenesis. Clin Endocrinol (Oxf). 2000;53:373-381. [DOI] [PubMed]
- 8.Block G., Do T.P., Collins A.J. Co-trending of parathyroid hormone and phosphate in patients receiving hemodialysis. Clin Nephrol. 2016;85:142–151. doi: 10.5414/CN108629. [DOI] [PubMed] [Google Scholar]; Block G, Do TP, Collins AJ, et al. Co-trending of parathyroid hormone and phosphate in patients receiving hemodialysis. Clin Nephrol. 2016;85:142-151. [DOI] [PubMed]
- 9.Almaden Y., Hernandez A., Torregrosa V. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 1998;9:1845–1852. doi: 10.1681/ASN.V9101845. [DOI] [PubMed] [Google Scholar]; Almaden Y, Hernandez A, Torregrosa V, et al. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 1998;9:1845-1852. [DOI] [PubMed]
- 10.Li J., Molnar M.Z., Zaritsky J.J. Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant. 2013;28:1516–1525. doi: 10.1093/ndt/gfs598. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li J, Molnar MZ, Zaritsky JJ, et al. Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant. 2013;28:1516-1525. [DOI] [PMC free article] [PubMed]
- 11.Frãzao J.M., Braun J., Messa P. Is serum phosphorus control related to parathyroid hormone control in dialysis patients with secondary hyperparathyroidism? BMC Nephrol. 2012;13:76. doi: 10.1186/1471-2369-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]; Frazao JM, Braun J, Messa P, et al. Is serum phosphorus control related to parathyroid hormone control in dialysis patients with secondary hyperparathyroidism? BMC Nephrol. 2012;13:76. [DOI] [PMC free article] [PubMed]
- 12.Block G.A., Klassen P.S., Lazarus J.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]; Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208-2218. [DOI] [PubMed]
- 13.Cooper K., Quarles D., Kubo Y. Relationship between reductions in parathyroid hormone and serum phosphorus during the management of secondary hyperparathyroidism with calcimimetics in hemodialysis patients. Nephron Clin Pract. 2012;121:c124–c130. doi: 10.1159/000345164. [DOI] [PubMed] [Google Scholar]; Cooper K, Quarles D, Kubo Y, et al. Relationship between reductions in parathyroid hormone and serum phosphorus during the management of secondary hyperparathyroidism with calcimimetics in hemodialysis patients. Nephron Clin Pract. 2012;121:c124-c130. [DOI] [PubMed]
- 14.Zitt E., Fouque D., Jacobson S.H. Serum phosphorus reduction in dialysis patients treated with cinacalcet for secondary hyperparathyroidism Results mainly from parathyroid hormone reduction. Clin Kidney J. 2013;6:287–294. doi: 10.1093/ckj/sft026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zitt E, Fouque D, Jacobson SH, et al. Serum phosphorus reduction in dialysis patients treated with cinacalcet for secondary hyperparathyroidism Results mainly from parathyroid hormone reduction. Clin Kidney J. 2013;6:287-294. [DOI] [PMC free article] [PubMed]
- 15.Kravietz A.M., Buicko J.L., Parreco J.P. Thirty-day readmissions following parathyroidectomy: evidence from the National Readmissions Database, 2013–2014. Am J Otolaryngol. 2018;39:82–87. doi: 10.1016/j.amjoto.2018.01.006. [DOI] [PubMed] [Google Scholar]; Kravietz AM, Buicko JL, Parreco JP, et al. Thirty-day readmissions following parathyroidectomy: evidence from the National Readmissions Database, 2013-2014. Am J Otolaryngol. 2018;39:82-87. [DOI] [PubMed]
- 16.Li J.G., Xiao Z.S., Hu X.J. Total parathyroidectomy with forearm auto-transplantation improves the quality of life and reduces the recurrence of secondary hyperparathyroidism in chronic kidney disease patients. Medicine (Baltimore) 2017;96:e9050. doi: 10.1097/MD.0000000000009050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li JG, Xiao ZS, Hu XJ, et al. Total parathyroidectomy with forearm auto-transplantation improves the quality of life and reduces the recurrence of secondary hyperparathyroidism in chronic kidney disease patients. Medicine (Baltimore). 2017;96:e9050. [DOI] [PMC free article] [PubMed]
- 17.Brunelli S.M., Dluzniewski P.J., Cooper K. Management of serum calcium reductions among patients on hemodialysis following cinacalcet initiation. Pharmacoepidemiol Drug Saf. 2015;24:1058–1067. doi: 10.1002/pds.3845. [DOI] [PubMed] [Google Scholar]; Brunelli SM, Dluzniewski PJ, Cooper K, et al. Management of serum calcium reductions among patients on hemodialysis following cinacalcet initiation. Pharmacoepidemiol Drug Saf. 2015;24:1058-1067. [DOI] [PubMed]
- 18.Puccini M., Ceccarelli C., Meniconi O. Near total parathyroidectomy for the treatment of renal hyperparathyroidism. Gland Surg. 2017;6:638–643. doi: 10.21037/gs.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Puccini M, Ceccarelli C, Meniconi O, et al. Near total parathyroidectomy for the treatment of renal hyperparathyroidism. Gland Surg. 2017;6:638-643. [DOI] [PMC free article] [PubMed]
- 19.Yajima A., Ogawa Y., Takahashi H.E. Changes of bone remodeling immediately after parathyroidectomy for secondary hyperparathyroidism. Am J Kidney Dis. 2003;42:729–738. doi: 10.1016/s0272-6386(03)00909-0. [DOI] [PubMed] [Google Scholar]; Yajima A, Ogawa Y, Takahashi HE, et al. Changes of bone remodeling immediately after parathyroidectomy for secondary hyperparathyroidism. Am J Kidney Dis. 2003;42:729-738. [DOI] [PubMed]
- 20.Florescu M.C., Islam K.M., Plumb T.J. Calcium supplementation after parathyroidectomy in dialysis and renal transplant patients. Int J Nephrol Renovasc Dis. 2014;7:183–190. doi: 10.2147/IJNRD.S56995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Florescu MC, Islam KM, Plumb TJ, et al. Calcium supplementation after parathyroidectomy in dialysis and renal transplant patients. Int J Nephrol Renovasc Dis. 2014;7:183-190. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Logistic regression model adjusted for stratification factors for achieving laboratory targets.