1. Introduction
Since the introduction of intraoperative fluoroscopy few decades ago, orthopedic surgeons have been exposed to direct or scatter radiation during many different procedures. The amount of the radiation exposed during the distal locking of the femur and tibia with intramedullary nailing (IMN) is particularly higher.1 Although the use of magnetic nails has lowered the amount of this exposure recently, there is still extreme exposure in percutaneous pinning of the pediatric supracondylar humerus fractures and closed titanium elastic nailing of pediatric fractures, where the surgeon stands closer to the fluoroscope. Particularly in closed reduction of fractures, the radiation beam is directly cast on the hand. The hand exposed to the beam directly (when the hand enters the imaging field) receives 100 times more radiation compared to standing 15 cm away from the fluoroscope.2,3
The International Commission on Radiological Protection (ICRP) determined that the acceptable annual limit of intake for radiation is 20 millisieverts (mSv) for the body, 150 mSv for the eyes and the thyroid and 500 mSv for the hands.4
In this study, we aimed to measure the amount of radiation orthopedists were exposed to during IMN surgeries of lower extremities using the new-generation optically stimulated luminescence (OSL) dosimeters throughout a two-month period and compare our results to both those of the old-generation thermoluminescent dosimeters and the acceptable annual limit of radiation intake set by ICRP. In addition, we aimed to investigate the statistical differences of fluoroscopic measurements of mA (milliampere) and kV (kilovolt) for each case between different IMN surgeries and between the operated and the non-operated sides.
2. Patients and methods
The amount of radiation exposed from the fluoroscope to the surgeon during a total of 40 adult IMN surgeries that took place in our clinic between March and April 2014 (the two-month period) were retrospectively investigated. The same two OSL dosimeters were used in all surgeries. One of the dosimeters was placed on the protective apron, close to the shoulder, and the other underneath it. The same protective apron and neckband was used in all cases. Attention was paid to keep the surgeon's body behind the source tube at all times. The patients were not exposed to additional radiation throughout the study. As the optimal operating time of the dosimeters is two months, the locked IMN surgeries of lower extremities over a two-month period were included in the study.
In all of 40 surgeries, including 13 femoral fractures (subtrochanteric or diaphyseal), 12 tibial diaphysis fractures and 15 intertrochanteric fractures (ITFs), OSL dosimeters were used. One distal locking screw was used in ITFs whereas two screws were inserted in the tibial diaphysis, femoral diaphysis and subtrochanteric femoral fractures. No vertical screw insertion was performed in the femoral diaphysis and subtrochanteric fractures. No fluoroscopy was necessary as the distal locking screws of the short proximal femoral nails (PFNs) used in ITFs were locked using the external guide. However, fluoroscopy was used for check purposes.
Five of the fractures in our group with 13 patients (subtrochanteric or diaphyseal fractures) were subtrochanteric femur fractures and eight were femoral diaphysis fractures. Nine of these patients were male and four were female, with a mean age of 53. According to the AO classification, three of the subtrochanteric fractures were Type A1-1, one was B2-1 and one was A3-1, and four of the femoral diaphysis fractures were Type A2, one was B1, two were B2 and one was C1. The tibial fracture group (12 patients) comprised of seven male and five female patients with a mean age of 34. According to the AO classification, six patients had Type A, four had Type B and two had Type C fractures. The intertrochanteric patient group comprised of nine male and six female patients and had a mean age of 67. According to the AO classification, nine patients had A2-1, three had A2-2, one had A2-3 and two had A3-1 fractures. All patients had closed fractures and traction table was used in femur surgeries. No magnetic nail locking was performed in our cases. All surgeries were performed by the same surgeon. The surgeon was right-handed. The fractures were on the left side in 21 and on the right side in 19 patients.
Ziehm Imaging Vision (Germany) fluoroscopy device was used for intraoperative imaging. The postoperative measurements (in mA, kV and time spent in minutes) of this device was recorded by an assistant blinded to the cases. The fluoroscopy device automatically sets its mA and kV values according to the thickness of the soft tissue.
3. Results
The fluoroscope was used for a total of 16 min and 35 s for the 15 ITFs. The device ran on a mean of 73 kV and 3.3 mA. The fluoroscopy device was used for 15 min in total. For the 13 diaphyseal femur fractures, the device was operated on a mean of 73 kV and 3.1 mA. The total time for fluoroscopy was 36 min and 30 s. For the 12 tibia fractures, the device was operated on a mean of 56 kV and 1.8 mA for 22 min (Table 1). The total time of fluoroscopy in all cases (for all groups) was 75 min and 5 s.
Table 1.
Anatomic region of femur fracture, postoperative fluoroscopy measurements, total time and side of surgery.
| Diaphyseal femur fracture | Kilovolts (kV) | Milliamperes (mA) | Minutes (min) | Side |
|---|---|---|---|---|
| Case 1 | 71 | 3.6 | 3 | Left |
| Case 2 | 62 | 2.1 | 2.1 | Left |
| Case 3 | 85 | 2.7 | 3.1 | Right |
| Case 4 | 73 | 2.8 | 2.3 | Left |
| Case 5 | 68 | 3.2 | 1.3 | Right |
| Case 6 | 70 | 3.1 | 3.2 | Right |
| Case 7 | 89 | 3.2 | 3.2 | Right |
| Case8 | 60 | 2.6 | 4.09 | Right |
| Case 9 | 72 | 3.6 | 3.01 | Right |
| Case 10 | 97 | 3.5 | 3.52 | Left |
| Case 11 | 63 | 3.3 | 2.2 | Right |
| Case 12 | 72 | 3.6 | 3.3 | Right |
| Case 13 | 56 | 2.5 | 2.2 | Left |
| Intertrochanteric femur fracture | ||||
| Case 14 | 81 | 4 | 1.03 | Left |
| Case 15 | 70 | 3 | 1 | Left |
| Case 16 | 80 | 3 | 0.50 | Left |
| Case 17 | 71 | 3.6 | 1.47 | Left |
| Case 18 | 80 | 2.2 | 1.23 | Right |
| Case 19 | 71 | 3.5 | 1.23 | Left |
| Case 20 | 69 | 3.5 | 1.31 | Right |
| Case 21 | 62 | 3.1 | 0.54 | Left |
| Case 22 | 63 | 3.2 | 0.5 | Left |
| Case 23 | 100 | 4.2 | 1.2 | Left |
| Case 24 | 89 | 3.2 | 1.2 | Left |
| Case 25 | 78 | 3.9 | 0.36 | Left |
| Case 26 | 60 | 3 | 1.4 | Right |
| Case 27 | 62 | 3 | 1.08 | Right |
| Case 28 | 70 | 3.5 | 1 | Right |
| Diaphyseal tibia fracture | ||||
| Case 29 | 51 | 1.8 | 1.3 | Right |
| Case 30 | 70 | 1.5 | 1.5 | Left |
| Case 31 | 55 | 0.7 | 1.5 | Right |
| Case 32 | 47 | 1.2 | 2.224 | Left |
| Case 33 | 57 | 2.5 | 2 | Right |
| Case 34 | 63 | 1.1 | 0.5 | Right |
| Case 35 | 62 | 3.1 | 1.09 | Left |
| Case 36 | 58 | 2 | 2 | Left |
| Case 37 | 50 | 1.6 | 1.58 | Left |
| Case 38 | 53 | 2.1 | 2.51 | Left |
| Case 39 | 52 | 2 | 2 | Right |
| Case 40 | 54 | 2.3 | 2.31 | Right |
The OSL dosimeter results of the two-month period were; according to the dosimeter on the lead apron, the dose of radiation was 1.61 mSv on the eye, 1.59 mSv on the skin and 1.53 mSv on the body. No significant dose of radiation was detected on the dosimeter underneath the lead apron (below 0.09 mSv).
3.1. Statistical methods and results
Mean, standard deviation, median, minimum, maximum, frequency and percentage values were used in descriptive statistical analysis of the data. Distribution of the data was evaluated with the Kolmogorov-Smirnov test. The ANOVA (Tukey's test) and independent samples t-tests were used in the analysis of quantitative data. Correlation analysis was performed with the Spearman's correlation analysis. The SPSS v.22.0 software was used in all analyses.
The amount of the radiation exposed in the femoral diaphysis fracture and ITF surgeries was significantly higher than that exposed in the tibial diaphysis fracture surgeries (p < 0.05). The difference in the amount of the radiation exposed in femoral diaphysis fracture and ITF fracture surgeries was not significant (p = 0.41610.05) (Table 2).
Table 2.
Amount of the radiation exposed in femoral diaphysis fracture.
| Dependent Variable: miliamper_mA_ | |||
|---|---|---|---|
| Diaphyseal femur fracture | Intertrochanteric femur fracture | Diaphyseal tibia fracture | |
| Diaphyseal femur fracture | 0.4161 | <.0001 | |
| Intertrochanteric femur fracture | 0.4161 | <.0001 | |
| Diaphyseal tibia fracture | <.0001 | <.0001 | |
The amount of the radiation absorbed by the right and the left side showed no significant difference (p = 0.434) (Table 3).
Table 3.
Radiation distribution among anatomic regions.
| Fluoroscopy milliamperes |
P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Min-Max | Median | Mean ± SD | |||||||
| Type of Surgery | |||||||||
| Diaphyseal femur fracture | 2.1 | – | 3.6 | 3.2 | 3.1 | ± | 0.5 |
0.000 |
|
| Intertrochanteric femur fracture | 2.2 | – | 4.2 | 3.2 | 3.3 | ± | 0.5 | ||
| Diaphyseal tibia fracture |
0.7 |
– |
3.1 |
1.9 |
1.8 |
± |
0.7 |
||
| Side | Right | 0.7 | – | 3.6 | 3.0 | 2.7 | ± | 0.8 | 0.434 |
| Left | 1.2 | – | 4.2 | 3.1 | 2.9 | ± | 0.9 | ||
ANOVA (Tukey's test).
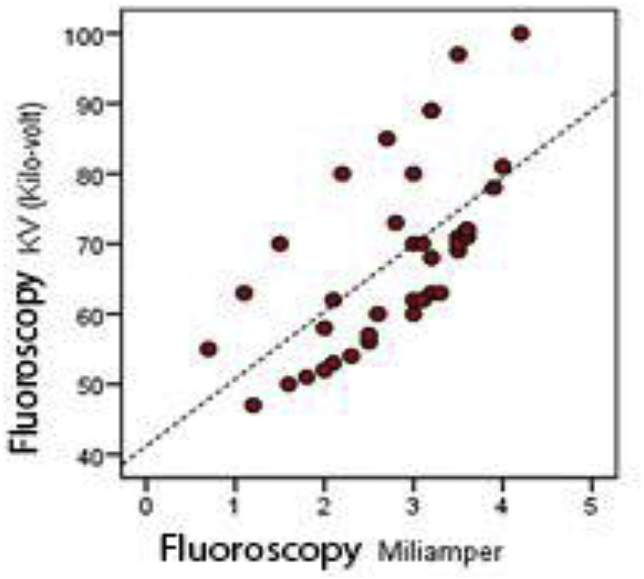
No significant correlation was detected between the amount of the radiation absorbed and duration of the surgery (p > 0.05). However, the amount of the radiation absorbed showed positive correlation with the fluoroscopy kV (p < 0.05) (Table 4, Fig. 1).
Table 4.
Correlation of radiation dose with fluoroscopy kV.
| Duration (min) | Fluoroscopy kV (Kilovolt) | ||
|---|---|---|---|
| Fluoroscopy milliamperes | r | −0.104 | 0.680 |
| p | 0.524 | 0.000 | |
Spearman's correlation.
Positive correlation was found between radiation dose and fluoroscopy kV.
Graphic 1.
Disribution of radiation dose with fluoroscopy kV.
Conclusively, according to the dosimeter on the lead apron, the dose of radiation was 1.61 mSv on the eye, 1.59 mSv on the skin and 1.53 mSv on the body. No significant dose of radiation was detected on the OSL dosimeter underneath the protective lead apron (below 0.09 mSv).
4. Discussion
Fluoroscopy is often used in orthopedic surgeries as it provides the real-time images of the skeletal system. Originally intended for the imaging of fracture reduction and placement of orthopedic implants, fluoroscopy has found itself new areas of application today with the employment of minimally invasive surgeries.5 However, the widespread use of this technique leaves not only the surgeon but also the anesthetists, nurses, assistant staff and the patient prone to the harmful effects of ionized radiation.6,7 Ionized radiation is emitted in all directions from the tube during fluoroscopy.2
In orthopedic trauma surgeries, the studies on exposure to radiation were conducted using thermoluminescent dosimeters.8, 9, 10, 11, 12, 13 The OSL dosimeter, developed after the year 2000, was used in our study.14, 15, 16, 17, 18 We preferred this type of dosimeter as it is not affected by heat or humidity and it can provide information about the radiation exposed to the eye and the skin by using custom distance calculations.19 Re-readability of the OSL dosimeter makes it a better choice over the thermoluminescent dosimeters in medical procedures. The device enables access to the previously stored radiation exposure data.20 Inside the OSL dosimeter, an A12O3:C crystal layer is sandwiched between two 0.3 mm-thick layers of polyester sensitive to radiation. These dosimeters can measure the gamma, X-ray and beta radiation between the 5 keV and 40 MeV energy range and unlike thermoluminescent dosimeters, they are not affected by heat or humidity. The aluminum oxide (A12O3:C) used in OSL dosimeters is produced by the crystal growth division of Landauer Inc. in the US. The optimal operating time of OSL dosimeters is two months.21 In addition, these dosimeters are used by ICRP to identify the effects of external irradiation on the human body, taking the characteristics of the body into account. The personal dose equivalents of Hp(10) for whole body, Hp(0.07) for the skin and Hp(0.03) for the lens of the eye can be individually measured.
In our study, the fluoroscopy device was run at 1.8 mA and 56 kV for tibia fractures, at 3.3 mA and 73 kV for ITFs and at 3.1 mA and 73 kV for subtrochanteric femur and femoral diaphysis fractures. These values support the hypothesis that the radiation exposure with high mA and kV in femoral fractures is four times higher than that in tibial fractures.3 Lower operating values in mA and kV is suggested for protection from radiation.
According to a study, the hand of an orthopedic surgeon who performs 75 IMN operations with proximal and distal locking each year receives one tenth of the radiation exposure recommended for the hand.22 In our study, after 40 IMN operations, the dosimeter placed on the apron and close to the left shoulder read 1.53 mSv, which may be accepted a low amount considering that the hand receives 30 to 40 times more radiation than the body.3 Without the use of protective gear for the eye and hand, the recommended limit for radiation exposure is exceeded after 300 cases per year, a finding compatible with ours.23
In a study performed using thermoluminescent dosimeter, the amount of radiation exposed to when standing 40 cm away from the fluoroscopy device for 7 min was 0.17 mSv and when the distance was 80 cm, the exposure decreased to 0.02 mSv.18 Our results with the OSL type dosimeter is about 1.5 times more based on minute count and regardless of the distance. Senior surgeons are exposed to lower amounts of radiation during the same surgical interventions in comparison to resident surgeons.19,24
The hand is the most exposed part of our body to radiation. It is a fact that the radiation emission is inversely related to the distance squared. Accordingly, it is known that the hand is exposed 30 to 40 times more radiation than the body.3 Measurements on multiple parts of our body (eyes, fingers, thyroid, wrists, toes … etc.) is costly and not convenient for the surgeon; however, it provides us with better results regarding spatial dose distribution.25 Radiation doses measured with a single dosimeter underneath the apron may not be reliable. The use of two dosimeters; one underneath the lead apron and the other on the left shoulder or over the neckband or on the hand has been recommended.26,27
All radiation doses should be kept at the minimum possible during surgeries. To achieve this, optimization rules, known as ALARA (as low as reasonably achievable), have been introduced.28 The aim with this optimization is not to zeroize the irradiation but to lower the risks to an acceptable level under given conditions. ALARA requires the knowledge and implementation of the rules to receive the minimum dose possible throughout the professional life29 and includes guidelines about the distance to the fluoroscope and the importance of fluoroscopy use time, use of dosimeter, maintaining and using all protective gear, positioning the X-ray tube, etc.30,31
The weakness of the study is the short duration of the study with small number of cases. A multi-center, prospective study with a longer duration and more number of cases could reaffirm the findings of this study.
5. Conclusion
A threshold value for harmless radiation was not defined in the literature20 and currently it is believed that such a value does not exist.32 In addition, severe adverse effects of high doses of radiation is well known; including cancer, cataract, birth defects, etc.33
In our study, we observed that the amount of radiation exposed during IMN surgeries of femur fractures had a higher statistical significance than IMN surgeries of tibia fractures. On the other hand, the side of the fracture did not have any statistically significant effect on the amount of radiation absorbed. We recommend orthopedists to use OSL dosimeters for repetitive measurements. Our results with the OSL type dosimeters were about 1.5 times higher than those of thermoluminescent dosimeters, based on minute count and regardless of the distance. In addition, we would like to reiterate that the radiation exposed during IMN surgeries of femur fractures was higher than IMN surgeries of tibia fractures.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jcot.2018.07.017.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sanders R., Koval K.J., DiPasquale T., Schmelling G., Stenzler S., Ross E. Exposure of the ortopaedic surgeon to radiation. J Bone Joint Surg Am. 1993;75(3):326–330. doi: 10.2106/00004623-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Arnstein P., Richards A.M., Putney R. The risk from radiation exposure during operative X –ray screening in hand surgery. J Hand Surg Br. 1994;19:393–396. doi: 10.1016/0266-7681(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 3.Nair Kesavachandran Chandrasekharan, Frank Haamann, Albert Neinhaus. Radiation exposure of eyes, thyroid gland and hands in orthopaedic staff: a systematic review. Eur J Med Res. 2012;17(1):28. doi: 10.1186/2047-783X-17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International commission on radiological protection: ICRP publication 60: 1990 recommendations of the international commission on radiological protection. Ann ICRP. 1991;2:1–3. [PubMed] [Google Scholar]
- 5.Çeçen G.S., Gülabi D., Pehlivanoğlu G., Bulut G., Bekler H., Asil K. Radiation in the orthopedic operating theatre. Acta Orthop Traumatol Turcica. 2015;49(3):297–301. doi: 10.3944/AOTT.2015.14.0250. [DOI] [PubMed] [Google Scholar]
- 6.Park M.S., Lee K.M., Lee B. Comparison of operator radiation exposure between C-arm and O-arm fluoroscopy for orthopaedic surgery. Radiat Protect Dosim. 2012;148:431–438. doi: 10.1093/rpd/ncr149. [DOI] [PubMed] [Google Scholar]
- 7.Blakely E.A. Biological effects of cosmic radiation: deterministic and stochastic. Health Phys. 2000;79:495–506. doi: 10.1097/00004032-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Han B., Shi Z., Fu Y., Ye Y., Jing J., Li J. Comparison of free-hand fluoroscopic guidance and electromagnetic navigation in distal locking of femoral intramedullary nails. Medicine (Baltim) 2017 Jul;96(29) doi: 10.1097/MD.0000000000007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehlman C.T., DiPasquale T.G. Radiation exposure to the orthopaedic surgical team during fluoroscopy: ‘how far away is far enough?’. J Orthop Trauma. 1997;11:392–398. doi: 10.1097/00005131-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sanders R., Koval K.J., DiPasquale T. Exposure of the orthopaedic surgeon to radiation (see comments) J Bone Joint Surg [Am] 1993;75-A:326–330. doi: 10.2106/00004623-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 11.O'Rourke P.J., Crerand S., Harrington P., Casey M., Quinlan W. Risks of radiation exposure to orthopaedic surgeons. J R Coll Surg Edinb. 1996;41:40–43. [PubMed] [Google Scholar]
- 12.Muller L.P., Suffner J., Wenda K., Mohr W., Rommens P.M. Radiation exposure to the hands and the thyroid of the surgeon during intramedullary nailing. Injury. 1998;29:461–468. doi: 10.1016/s0020-1383(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 13.Alonso J.A., Shaw D.L., Maxwell A., Mcgill G.P. Scattered radiation during fixation of hip fractures is distance alone enough protectıon? The Journal of Bone And Joınt Surgery. august 2001;83-b(6) doi: 10.1302/0301-620x.83b6.11065. [DOI] [PubMed] [Google Scholar]
- 14.Jablon S., Bailar J.C., 3rd The contribution of ionizing radiation to cancer mortality in the United States. Prev Med. 1980;9:219–226. doi: 10.1016/0091-7435(80)90079-1. [DOI] [PubMed] [Google Scholar]
- 15.Tse V., Lising J., Khadra M. Radiation exposure during fluoroscopy: should we be protecting our thyroids? Aust N Z J Surg. 1999;69:847–848. doi: 10.1046/j.1440-1622.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 16.Faure E. X-rays-induced secretion of cellular factor(s) that enhance(s) HIV-1 promoter transcription in various nonirradiated transfected cell lines. Cell Mol Biol (Noisy-Le-Grand) 1998;44:1275–1292. [PubMed] [Google Scholar]
- 17.Mehlman C.T., DiPasquale T.G. Radiation exposure to the orthopaedic surgical team during fluoroscopy: “how far away is far enough?”. J Orthop Trauma. 1997;11:392–398. doi: 10.1097/00005131-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Barry T.P. Radiation exposure to an orthopedic surgeon. Clin Orthop Relat Res. 1984;182:160–164. [PubMed] [Google Scholar]
- 19.Sinclair W.K. Radiation protection recommendations on dose limits: the role of the NCRP and the ICRP and future developments. Int J Radiat Oncol Biol Phys. 1995;31:387–392. doi: 10.1016/0360-3016(94)00275-p. [DOI] [PubMed] [Google Scholar]
- 20.Pradhan A.S., Lee J.I., Kim J.L. Recent developments of optically stimulated luminescence materials and techniques for radiation dosimetry and clinical applications. J Med Phys. 2008 Jul-Sep;33(3):85–99. doi: 10.4103/0971-6203.42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yukihara E.G., McKeever S.W.S. Optically stimulated luminescence (OSL) dosimetry in medicine. Phys Med Biol. 2008;53:R351–R379. doi: 10.1088/0031-9155/53/20/R01. [DOI] [PubMed] [Google Scholar]
- 22.Blattert T.R., Fill U.A., Kunz E., Panzer W., Weckbach A., Regulla D.F. Skill dependence of radiation exposure for the orthopaedic surgeon during interlocking of nailing of long-bone shaft fractures: a clinical study. Arch Orthop Trauma Surg. 2004;124:659–664. doi: 10.1007/s00402-004-0743-9. [DOI] [PubMed] [Google Scholar]
- 23.Mroz T.E., Yamashita T., Davros W.J., Lieberman I.H. Radiation exposure to the surgeon and the patient during kyphoplasty. J Spinal Disord Tech. 2008;21:96–100. doi: 10.1097/BSD.0b013e31805fe9e1. [DOI] [PubMed] [Google Scholar]
- 24.Uffmann M., Schaefer-Prokop C. Digital radiography: the balance between image quality and required radiation dose. Eur J Radiol. 2009;72(2):202–208. doi: 10.1016/j.ejrad.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 25.Vano E., Gonzales L., Guibelalde E., Fernandez J.M., Ten J.I. Radiation exposure to medical staff in interventional and cardiacradiology. Br J Radiol. 1998;71:954–960. doi: 10.1259/bjr.71.849.10195011. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner K., Harrison R.M. Estimation of effective dose equivalent to staff in diagnostic radiology. Phys Med Biol. 1988;33:83–91. doi: 10.1088/0031-9155/33/1/008. [DOI] [PubMed] [Google Scholar]
- 27.Niklason M.T., Marx M.V., Chan H.P. Interventional radiologysts: occupational radiation doses and risks. Radiology. 1993;187:729–733. doi: 10.1148/radiology.187.3.8497622. [DOI] [PubMed] [Google Scholar]
- 28.Strauss1 Keith J., Kaste Sue C. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—a white paper executive summary. Pediatr Radiol. 2006 Sep;36(Suppl 2):110–112. doi: 10.1007/s00247-006-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan D.J., Patel J.N., Liporace F.A., Yoon R.S. Intraoperative radiation safety in orthopaedics: a review of the ALARA (As low as reasonably achievable) principle. Patient Saf Surg. 2016 Dec 12;10:27. doi: 10.1186/s13037-016-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahler D.M. Navigated long-bone fracture reduction. J Bone Joint Surg Am. 2009;91(Suppl 1):102–107. doi: 10.2106/JBJS.H.01286. [DOI] [PubMed] [Google Scholar]
- 31.Larson B.J., Egbert J., Goble E.M. Radiation exposure during fluoroarthroscopically assisted anterior cruciate reconstruction. Am J Sports Med. 1995;23:462–464. doi: 10.1177/036354659502300416. [DOI] [PubMed] [Google Scholar]
- 32.Madan S., Blakeway C. Radiation exposure to surgeon and patient in intramedullary nailing of the lower limb. Injury. 2002;33:723–727. doi: 10.1016/s0020-1383(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 33.Seibert J.A. Digital radiography: image quality and radiation dose. Health Phys. 2008;95:586–598. doi: 10.1097/01.HP.0000326338.14198.a2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.