Abstract
A total of 173 bacterial strains were isolated from different sources at different regions such as fermented foods, shrimp guts, sea water, mangrove water, and sediments. These bacteria were screened against white spot syndrome virus (WSSV) infection in Palaemon paucidens. Based on mortality, white spot level, and healthiness, three bacterial strains were selected and identified using 16S rRNA gene sequencing. These bacterial strains were Bacillus subtilis KA1, B. licheniformis KA2, and B. subtilis KA3. WSSV challenge test in pilot scale was conducted using Litopenaeus vannamei with B. subtilis KA1 and B. subtilis KA3. The survival ratio of shrimp was 0% for WSSV control after 17th days, 84% for B. subtilis KA1 plus WSSV after 26th days, and 28% for B. subtilis KA3 with WSSV after 26th days. B. subtilis KA1 showed good growth at 18–37 °C in with and without 3% NaCl, and therefore can be applied to aquaculture at low to high temperatures. B. subtilis KA1 produced protease and lipase which can increase digestion to shrimp; exhibited antibacterial activity against Vibrio parahaemolyticus; and significantly increased the survival of WSSV challenged shrimps.
Keywords: White spot syndrome virus, Schrimp, Bacillus subtilis, Probiotics, Vibrio parahaemolyticus
Highlights
-
•
WSSV is a big problem around the world.
-
•
WSSV control protease and lipase producing B. subtilis KA1.
-
•
The 84% survival for B. subtilis KA1 after 26th days and 28% for B. subtilis KA3.
1. Introduction
Diseases are one of the major factors that increase shrimp mortality. Particularly, these diseases are caused by viruses, bacteria, fungi, parasites, and natural factor like algal blooms. Viral infections are the most crucial problem in aquaculture, wherein shrimps are mostly infected in their post-larval stages. In addition, certain viral infections cause mortality and deformations as well as obstruct growth. More than 20 viruses have been reported as pathogenic to shrimp, particularly white spot syndrome virus (WSSV). Considered as a serious pathogen, WSSV is a large DNA virus from Whispovirus and belongs to Nimaviridae family. WSSV is wide spread mostly shrimp producing countries, such as Bangladesh, Cambodia, China, India, Indonesia, and Korea, among others. In shrimp culture, WSSV promotes 80%–100% mortality within 8–10 days. It is present not only in shrimp, but also in all other crustaceans. WSSV is transmitted through cannibalism, death animals and contaminated water; similarly, birds transmit infected shrimps from one place to another [1]. The virus is known to occur in fresh, brackish, and marine water. WSSV multiplies due to environmental stress, temperature, pH, salinity, plankton blooming, molting, and spawning [[2], [3], [4]]. To prevent WSSV infection, several chemicals have been used particularly formaldehyde, malachite green, calcium oxide, and sodium hypochlorite [5]; but these chemicals pose environmental hazards. Beneficial probiotic microbes can otherwise be used against WSSV infection they are a more environment friendly option, and can enhance survival and immunity condition of the shrimp.
Among the bacterial pathogens, Vibrio species cause vibriosis in penaeid shrimp [6]. Vibriosis -is caused by Vibrio parahaemolyticus, a Gram-negative halophilic, non-spore forming, curved rod-shaped bacterium that naturally lives in estuarine and marine environments worldwide [7]. Acute hepatopancreatic necrosis disease (AHPND) is a severe, newly emergent shrimp disease caused by V. parahaemolyticus that has already led to tremendous global losses in the shrimp culture industry [8,9].
Probiotics existing in microbial feed supplements can be beneficial to the host by improving its intestinal microbial balance [10]. With probiotic supplement treat, shrimp losses can be significantly reduced. Shrimp has a non-specific (innate) immune response; the vaccination (even if possible) can only provide short-term protection against pathogens, whereas probiotics provide extensive spectrum against disease. Shrimp culture mainly depends on the feed, and a bacterium producing protease and lipase is added into the culture pond. The enzymes increase the availability of nutrients during digestion, enhance growth rate and support survival. The use of low cost feed is also possible.
In this study, we screened and selected bacterial strains. The strains inhibited WSSV infection to Litopenaeus vannamei and produced protease and lipase that increase feed digestion, and therefore can be applied as probiotics for shrimp aquaculture. The selected strain also exhibited anti-bacterial activity against Vibrio parahaemolyticus, thus causing significant survival of shrimp.
2. Materials and methods
2.1. Isolation of bacteria and culture
Various bacteria were isolated from different sources such as fermented food products (soybean paste and kimchi), shrimp guts, squid guts, mangrove water and sediments, and sea water using tryptic soy agar (TSA) containing 3% NaCl, pH 7.2 incubated at 37 °C for 2 days. A total of 173 morphologically different colonies were isolated. The isolated bacterium was grown in TSB containing 3% NaCl, 200 rpm, at 37 °C for 24 h; these cultures were used for inoculums (cell density 2 × 108) in WSSV challenge experiments.
2.2. Rearing of shrimp
Fresh water shrimp Palaemon paucidens and marine water shrimp L. vannamei were locally purchased and reared for screening and challenge tests. The experimental shrimps were tested for WSSV infections using 2- step PCR test [11], and the WSSV uninfected shrimp were used for the experiments. For the first screening, a glass tank (30 × 21.0 × 26.5 cm) contained 20 numbers of P. paucidens (0.2 g each) in 11 L underground water. The water was aerated for 2 days before the shrimps were added into the water and the shrimps adapted for 2–3 days and were maintained at 18 °C. For the second screening, 100 L culture tank containing 50 L sea-water and 30 numbers of P. paucidens (2 g each) was used. The shrimps adapted for 3 days and the animals were provided with proper aeration. Initially feeding was given with 7.5% of the body weight; then, subsequent feeding was adjusted to 5% of the body weight/day according to the leftover (unutilized feed). The feed was given thrice a day 40% at dawn (6.00 a.m.), 20% at dusk (2.00 p.m.), and 20% at night (10.00 p.m.). Throughout the experiment, the temperature of the tanks was maintained at 25 ± 1 °C using air conditioner. The bottom water in the tank along with excess feed and fecal was siphoned using 2 cm diameter sterilized plastic hose to enhance the survival of the shrimps.
2.3. Preparation of WSSV
One gram of WSSV-infected shrimp was homogenized in 9 mL of TNE buffer (Tris 50 mM, NaCl 100 mM and EDTA 1 mM, pH 7.4), the homogenized was centrifuged at 4000 rpm for 10 min and the supernatant was filtered with 0.25 μM syringe filter and the clear filtrate was stored at −70 °C for further study.
2.4. Screening of bacterial isolates against WSSV using P. paucidens
The 2 mL of bacterial culture broth was added into a glass culture tank containing 20 numbers of P. paucidens (0.2 g each), and 0.5 mL of WSSV extract was added into the culture tank. Based on screening, three bacteria (B. subtilis KA1, B. licheniformis KA2 and B. subtilis KA3) were selected and reconfirmed the WSSV effect in the culture tank containing 30 numbers of P. paucidens (2 g/shrimp). Experimental conditions were the following: tank 1 (-WSSV), tank 2 (+WSSV), tank 3 (B. subtilis KA1+ WSSV), tank 4 (B. licheniformis KA2 + WSSV) and tank 5 (B. subtilis KA3 + WSSV). The behavior of the shrimps was observed everyday as well as their survival ratio, level of white spot formation, overall health status, and the hardness of outer shell.
2.5. Evaluation of inhibition activity of selected isolates against WSSV in pilot scale using L. vannamei
Based on small-scale screening, 2 strains were tested for inhibition of WSSV infection in 50 L marine water with L. vannamei. The water was aerated for two days and 25 numbers of L. vannamei (6 g each) were reared in the 4 tanks (tank 1, L. vannamei control; tank 2, WSSV control; tank 3, B. subtilis KA1 + WSSV; and tank 4, B. subtilis KA3 + WSSV). The L. vannamei was adapted for 2–3 days; after that, 0.1% (cell density 2 × 108) of cultured bacterial isolate was inoculated into the culture tank. After 2 days, 0.01% of WSSV (104 copies) was directly added into the culture tank. During shrimp culture, shrimp mortality was monitored twice daily, and the dead shrimps were examined by PCR to confirm WSSV infection. The level of white spot formation, the overall health status, and hardness of outer shell were also observed.
2.6. Measurement of body weight and survival
| Survival (%) = (Final shrimp number / initial shrimp number) × 100 |
2.7. Detection of protease and lipase activity
The bacterial isolates were screened for protease and lipase activity. For protease, the agar plate contained peptone- 5, beef extract- 3, gelatin- 4, sodium chloride- 30, agar- 20 (g/L), pH 7.2. The bacteria inoculated plates were incubated overnight at 25 and 37 °C for 48 h. After incubation, the plates were stained with HgCl2 and HCl solution (the 100 mL solution containing 15 g HgCl2 and 20 mL HCl), and the protease producing strains were selected based on zone of clearance. The Rhodamine B mediums were used for lipase activity, the medium containing (%) beef extract- 0.5, peptone- 0.5, (NH4)2SO4- 0.5, MgSO4- 0.05, K2HPO4- 0.4, CaCl2- 0.02, NaNO3- 0.2, olive oil emulsion- 12, Rhodamine B (0.1 mg/mL)- 2, agar- 1.8, pH 6.5 and the plates were incubated at 25 and 37 °C for 48 h. The fluorescent hydrolyzed rings of orange color emitted by Rhodamine B around the colonies were observed under UV light.
2.8. Antimicrobial activity of the selected isolates against Vibrio parahaemolyticus
The antimicrobial activity of the selected isolates was assayed using agar diffusion test. Bacterial isolates were grown in TSB containing 3% NaCl at 37 °C in a rotary shaking incubator at 200 rpm for 24 h. V. parahaemolyticus was grown in nutrient broth containing 3% NaCl, 200 rpm at 37 °C for 24 h. The cells of V. parahaemolyticus (cell density 2 × 107) were spread on the agar plates. An 8 mm paper disc was put on top of the agar; then 50 μL culture supernatant of bacterial isolates (cell density 2 × 108) was dropped on the paper disk and incubated at 30 °C for 48 h. The diameter of inhibition zones around the colony was measured.
2.9. Identification of selected isolates
Three selected bacteria were identified by 16S rRNA gene sequencing using 27 F and 1492 R primer. Additionally the carbohydrate utilization of B. subtilis KA1 was examined using API 50 CHI kit (Biomerieux, Lyan, France) according to the manufacturer's instructions and the results were also used for identification.
3. Results and discussion
3.1. Screening of bacteria efficiently inhibiting WSSV
The isolates were tested against WSSV infection with P. paucidens. Based on the survival ratio, white spot, healthiness, and hardness of skeleton, three bacterial isolates were selected. Survival ratio changed with time, and shrimp health conditions are shown in Table 1A. In shrimp control (without WSSV infection), the survival was 60% after 15 days. In WSSV control (without bacterial inoculums), all shrimp died (0% survival) after 15 days. In the tests of bacterial isolates with WSSV infection, the shrimp survival ratios were 26.7–46.7% based on the strains. The three isolates were identified by 16S rRNA gene sequencing, as B. subtilis KA1 (1491 bp), B. licheniformis KA2 (1515 bp), and B. subtilisKA3 (1492 bp). The B. subtilis KA1 was also subjected to a series of biochemical tests. With 97.1% identity, results confirmed the isolate was B. subtilis.
Table 1.
A: Survival and health condition of WSSV- infected fresh water shrimp P. paucidens, B: survival and health condition of WSSV- infected marine water shrimp L. vannamei.
| A | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Shrimp survival ratio (%) in days |
Shrimp health conditions |
|||||||||||||||||
| 3 | 6 | 9 | 12 | 15 | White spota | Overall health statusb | Hardness of outer skinc | ||||||||||||
| Control | -WSSV | 100 | 86.7 | 66.7 | 60.0 | 60.0 | – | ± | ++ | ||||||||||
| + WSSV | 100 | 73.3 | 53.3 | 20.0 | 0.0 | ++ | ± | ± | |||||||||||
| Isolate +WSSV | B. subtilisKA1 | 100 | 86.7 | 66.7 | 56.7 | 46.7 | ± | ++ | ++ | ||||||||||
| B. licheniformis KA2 | 100 | 76.7 | 60.7 | 45.0 | 26.7 | + | + | ++ | |||||||||||
|
B. subtilis KA3 |
100 |
83.3 |
53.3 |
36.7 |
30.0 |
+ |
++ |
+ |
|||||||||||
| B | |||||||||||||||||||
| Conditions | Shrimp survival ratio (%) in days | Shrimp health condition | |||||||||||||||||
| 6 |
16 |
20 |
21 |
24 |
26 |
White spota |
Overall health statusb |
Hardness of outer skinc |
|||||||||||
| Control | -WSSV | 100 | 96 | 8 | 8 | 8 | 8 | – | + | ++ | |||||||||
| + WSSV | 96 | 96 | 0 | 0 | 0 | 0 | ++ | ± | ± | ||||||||||
| Isolate +WSSV | B. subtilis KA1 | 100 | 96 | 84 | 84 | 84 | 84 | ± | ++ | ++ | |||||||||
| B. subtilis KA3 | 100 | 84 | 60 | 44 | 28 | 28 | + | + | + | ||||||||||
Note: a ++ many white spots, + medium, ± a few, – none. b ++ healthier and more actively moving, + healthy and actively moving, ± less healthy and less moving. c ++ hard, + medium, ± soft.
3.2. Evaluation of selected bacterial isolates against WSSV in pilot scale using L. vannamei
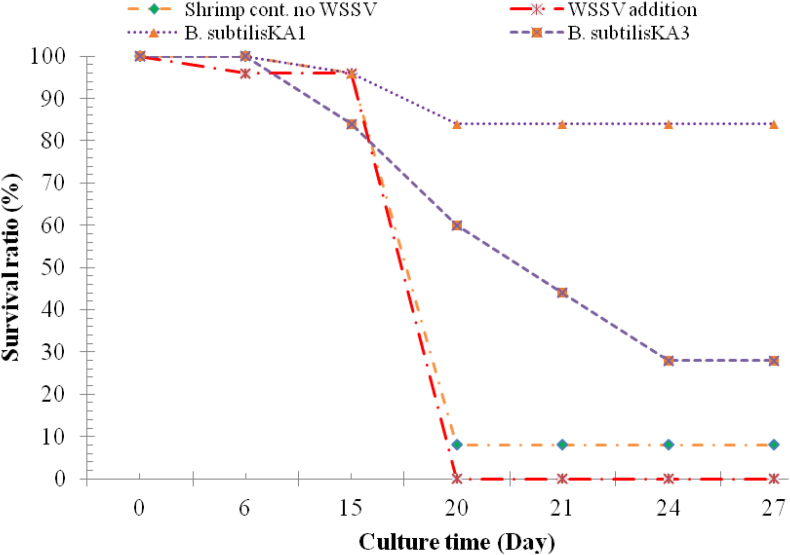
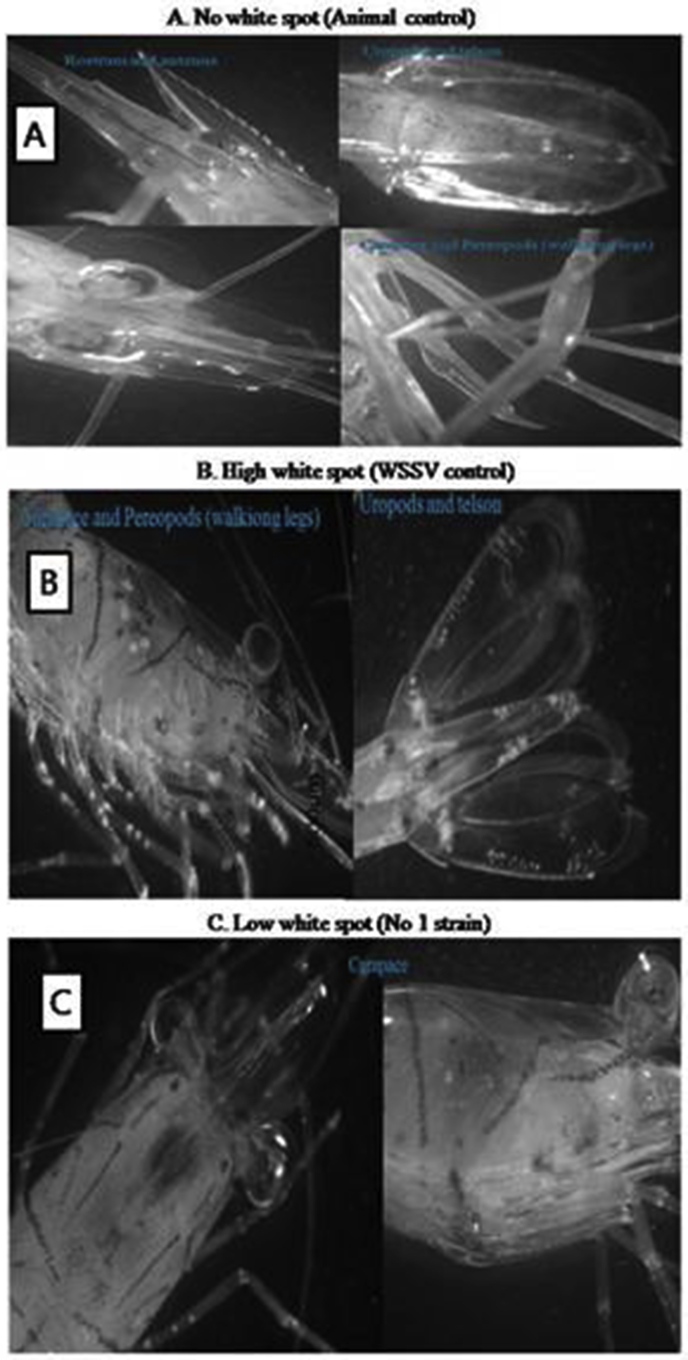
Among the 3 selected isolates, 2 were selected based on survival ratio and shrimp health conditions. The selected B. subtilis KA1 and B. subtilis KA3 were tested further in marine water pilot scale experiment using L. vannamei. Results are shown in Table 1B and Fig. 1. The selection of bacteria improved not only the survival ratio, but also healthiness and hardness as well as reduced WSSV (Fig. 2). The white calcified spots appearing on the exoskeleton were easily identified, and these white spots are diagnostic of white spot disease [12].
Fig. 1.
Survival ratio of WSSV infected L. vannamei.
Fig. 2.
WSSV infection: Animal control (A), WSSV control (B), and low infection with Bacillus subtilis KA1 (C).
In WSSV control, the mortality started on the 6th day, and cumulative mortality (0% survival) reached the 20th day. In the B. subtilis KA1 + WSSV, mortality was started on the 16th day and 84% survival was evident on the 26th day. In B. subtilisKA3 + WSSV, the mortality started on 16th days and 28% survival on 26th day of the experiment (Fig. 1). Hardness of skeleton was the same in control (-WSSV) and B. subtilis KA1 (+WSSV), whereas others were less hard. Healthiness was the best in B. subtilis KA1 (+WSSV), but less in others (Table 2B). At the start of the experiment, L. vannamei weighed 6.2 g/shrimp and it increase to after 15th days 7.5 g and 7.26 g in B. subtilis KA1 (+WSSV) and B. subtilis KA3 (+WSSV), respectively, and at 60th day increased 17 and 15 g, respectively. Rengpipat et al. [13] similarly reported that Bacillus S11 produced better yield, controlled the diseases, and improved the immunity in Penaeus monodon.
Table 2.
Cell growth at various temperatures in 3% NaCl, and protease and lipase activity.
| Conditions |
Cell growth at different temperature (°C) |
Diameter of clear zone around colony (cm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | Cell grown without NaCl |
Cell growth in 3% NaCl |
||||||||
| Protease |
Lipase |
|||||||||
| 18 | 21 | 25 | 37 | 25 | 30 | 25 | 37 | 25 | 37 | |
| B. subtilis KA1 | + | ++ | ++ | ++ | ++ | ++ | 6 | 11 | 3 | 8 |
| B. licheniformis KA2 | ± | ++ | ++ | ++ | ++ | ++ | 5 | 6 | <1 | 7 |
| B. subtilis KA3 | ± | ++ | ++ | ++ | ++ | ++ | 4 | 5 | <1 | 5 |
3.3. Cell growth and the activities of protease and lipase of the selected isolates
The three selected isolates grew well at 21–37 °C. At 18 °C, B. subtilis KA1 showed good growth, whereas B. licheniformis KA2 and B. subtilis KA3 grew poorly (Table 2). Since shrimp culture in Korea starts on May when water temperature is still low at 18 °C, the use of B. subtilis KA1 have more advantage than the two other strains. All the three isolates grew well in 3% NaCl and therefore can be used in marine water aquaculture.
All the three strains showed protease and lipase activities, which are both higher at 37 °C than at 25 °C. Among the isolates, B. subtilis KA1 produced the highest activities of protease and lipase. Shrimp feed contains 50–60% protease, 10% lipase, and others. These enzymes directly promoted growth and digestion and indirectly reduced (feed conversion ratio) feed taking. In addition, the main functions of the enzymes are increase of digestion (infection time the shrimp not taking feeds) and the reduction of mortality rate. Previous research [14] has reported protease producing Bacillus sp. Mk22 which enhanced shrimp survivals and reduced osmotic stress during V. parahaemolyticus and WSSV infection. B. subtilis produced high amount of secondary metabolites like antibiotics, fine chemicals and enzymes, as well as heterologous proteins, antigens and vaccines [[15], [16], [17]]. Microbial lipases are widely utilized because of their substrate specificity, availability, and catalytic activities [18].
3.4. Anti- vibrio activity of the selected isolates
B. subtilis KA1 showed relatively high activity (4 mm clear zone) against V. parahaemolyticus; whereas B. licheniformis KA2 and B. subtilis KA3 showed low activity (1 mm clear zone). Vibrio species are opportunistic pathogens and predominant in shrimp farming water under normal conditions [19]. Viruses are usually the main pathogens infecting shrimp as they reduce shrimp immunity, whereas superimposed bacterial infections accelerate shrimp mortality [20]. V. parahaemolyticus infection accelerated the proliferation of WSSV in L. vannamei and vice versa, and the combined proliferation of both V. parahaemolyticus and WSSV led to massive death of L. vannamei [21]. In addition to this superimposition, the opportunistic marine pathogen V. parahaemolyticus becomes highly virulent by acquiring a unique AHPND-associated plasmid that expresses a deadly toxin [22]. Probiotics and competitive exclusion agents are considered to enhance gut microflora by preventing the colonization of the gastrointestinal tract by pathogenic bacteria [23,24].
In conclusion, B. subtilis KA1 is able to control both WSSV and V. parahaemolyticus. B. subtilis KA1 also showed good growth at 18–37 °C and in 3% NaCl. Therefore, it can be applied to marine aquaculture at low to high temperatures. B. subtilis KA1 produced higher levels of protease and lipase which can increase feed digestion. Hence, B. subtilis KA1 obtained from this investigation shows strong potential for application as probiotics to shrimp aquaculture.
Conflicts of interest
There is no conflict of interest.
References
- 1.Chou H.Y., Huang C.Y., Lo C.F., Kou G.H. Studies on transmission of white spot syndrome associated baculovirus (WSBV) in Penaeus monodon and P. japonicus via waterborne contact and oral ingestion. Aquaculture. 1998;164:263–276. [Google Scholar]
- 2.Van Thuong K., Van Tuan V., Li W., Sorgeloos P., Bossier P., Nauwynck H. Effects of acute change in salinity and moulting on the infection of white leg shrimp (Penaeus vannamei) with white spot syndrome virus upon immersion challenge. J. Fish Dis. 2016;39(12):1403–1412. doi: 10.1111/jfd.12471. [DOI] [PubMed] [Google Scholar]
- 3.He W., Rahimnejad S., Wang L., Song K., Lu K., Zhang C. Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2017;70:164–173. doi: 10.1016/j.fsi.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Suo Y., Li E., Li T., Jia Y., Qin J.G., Gu Z., Chen L. Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2017;63:87–96. doi: 10.1016/j.fsi.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Nakano H., Hiraoka M., Sameshima M., Kimura T., Momoyama K. Inactivation of penaeid rod-shaped DNA virus (PRDV), the causative agent of Penaied Acute Viremia (PAV), by some chemical and physical treatments. Fish Pathol. 1998;33:65–72. [Google Scholar]
- 6.Saulnier D., Haffner P., Goarant C., Levy P., Ansquer D. Experimental infection models for shrimp vibriosis studies: a review. Aquaculture. 2000;191:133–144. [Google Scholar]
- 7.Wu Y., Wen J., Ma Y., Ma X., Chen Y. Epidemiology of food-borne disease outbreaks caused by Vibrio parahaemolyticus, China, 2003-2008. Food Control. 2014;46:197–202. [Google Scholar]
- 8.Lightner D.V., Redman R.M., Pantoja C.R., Noble B.L., Tran L. Early mortality syndrome affects shrimp in Asia. Global Aquaculture Advocate. 2012;15:40. [Google Scholar]
- 9.Nunan L., Lightner D., Pantoja C., Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Org. 2014;111(1):81–86. doi: 10.3354/dao02776. [DOI] [PubMed] [Google Scholar]
- 10.Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 11.Lo C.F., Leu J.H., Ho C.H., Chen C.H., Peng S.E., Chen Y.T., Chou C.M., Yeh P.Y., Huang C.J., Chou H.Y., Wang C.H., Kou G.H. Detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimps using polymerase chain reaction. Dis. Aquat. Org. 1996;25:133–141. [Google Scholar]
- 12.Chou H.Y., Huang C.Y., Wang C.H., Chiang H.C., Lo C.F. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 1995;23:165–173. [Google Scholar]
- 13.Rengpipat S., Phianphak W., Piyatiratitivorakul S., Menasveta P. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture. 1998;167(3–4):301–313. [Google Scholar]
- 14.Sekar Ashokkumar, Packyam Mayavu, Kim Keun. Growth enhancement of shrimp and reduction of shrimp infection by Vibrio parahaemolyticus and white spot syndrome virus with dietary administration of Bacillus sp. Mk22., Microbiol. Biotechnol. Lett. 2016;44(3):261–267. [Google Scholar]
- 15.Westers L., Westers H., Quax W.J. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim. Biophys. Acta. 2004;1694:299–310. doi: 10.1016/j.bbamcr.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 17.Valdez A., Yepiz-Plascencia G., Ricca E., Olmos J. First Litopenaeus vannamei WSSV 100% oral vaccination protection using CotC:Vp26 fusion protein displayed on Bacillus subtilis spores surface. J. Appl. Microbiol. 2014;117:347–357. doi: 10.1111/jam.12550. [DOI] [PubMed] [Google Scholar]
- 18.Kademi A., Fakhreddine L., Baratti J. Purification and characterization of a thermostable esterase from the moderate thermophile Bacillus circulans. Appl. Microbiol. Biotechnol. 2000;54:173–179. doi: 10.1007/s002530000353. [DOI] [PubMed] [Google Scholar]
- 19.Oxley A.P.A., Shipton W., Owens L., McKay D. Bacterial flora from the gut of the wild and cultured banana prawn, Penaeus merguiensis. J. Appl. Microbiol. 2002;93(2):214–223. doi: 10.1046/j.1365-2672.2002.01673.x. [DOI] [PubMed] [Google Scholar]
- 20.Lightner D.V., Redman R.M., Bell T.A. Observations on the geographic distribution, pathogenesis and morphologyof the baculovirus from Penaeus monodon Fabricius. Aquaculture. 1983;32(3–4):209–233. [Google Scholar]
- 21.Zhang X., Song X., Huang J. Impact of Vibrio parahaemolyticus and white spot syndrome virus (WSSV) co-infection on survival of penaeid shrimp Litopenaeus vannamei. Chin. J. Oceanol. Limnol. 2016;34(6):1278–1286. [Google Scholar]
- 22.Lee C.T., Chen I.T., Yang Y.T., Ko T.P., Huang Y.T., Huang J.Y., Huang M.F., Lin S.J., Chen C.Y., Lin S.S., Lightner D.V., Wang H.C., Wang A.H., Wang H.C., Hor L.I., Lo C.F. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. U.S.A. 2015;112(34):10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green D.H., Wakeley P.R., Page A.B., Baccigalupi L., Ricca E., Cutting S.M. Characterization of two Bacillus probiotics. Appl. Environ. Microbiol. 1999;65:4288–4294. doi: 10.1128/aem.65.9.4288-4291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoa N.T., Baccigalupi L., Huxham A., Smertenko A., Van P.H., Ammendola S., Ricca E., Cutting S.M. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 2000;66:5241–5251. doi: 10.1128/aem.66.12.5241-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]