Abstract
Purpose
To present a rare anaplastic form of retinal pleomorphic xanthoastrocytoma (PXA) unassociated with phakomatosis.
Methods
A 9-year-old girl, presented with a rapidly growing unilateral intraocular white mass unresponsive to intra-arterial chemotherapy, underwent enucleation with the clinical suspicion of retinoblastoma versus malignant astrocytoma.
Results
Histopathology revealed pleomorphic cells with rosenthal fibers, mitosis, and necrosis. Immunohistochemistry confirmed the diagnosis of anaplastic pleomorphic xanthoastrocytoma (aPXA). The patient had no signs of phakomatosis.
Conclusion
Retinal PXA may occur in patients without phakomatosis and rarely progress toward malignant transformation.
Keywords: Anaplasia, Pleomorphic xanthoastrocytoma, Retinal tumor
Introduction
Pleomorphic xanthoastrocytoma (PXA), the grade II of central nervous system tumors,1 has a favorable prognosis in children and young adults. It represents less than 1% of all brain astrocytic tumors,2 and retina is considered a rare site of its involvement.3 PXA has 75–80% five-year survival rate, and 30% chance of recurrence after resection.4 However, malignant transformation may occur and anaplastic pleomorphic xanthoastrocytoma (aPXA), the grade III of central nervous system tumors, is a new name for the previous entity “pleomorphic xanthoastrocytoma with anaplastic features”.1
Astrocytic tumors occur in 57% of patients with tuberous sclerosis (TSC) and 14% of neurofibromatosis cases while the remaining 29% are attributed to solitary tumors.5
Here, we add a case of retinal aPXA unassociated with phakomatosis to the previous 12 reported cases.6
Case report
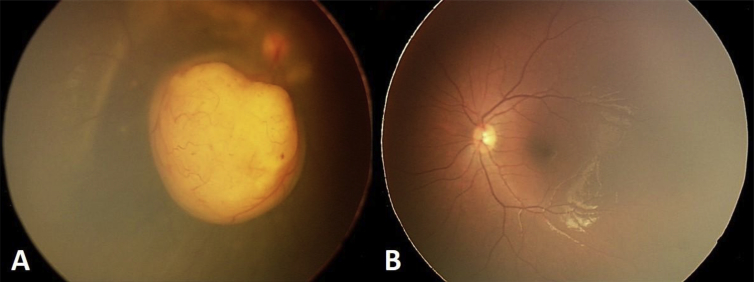
Ophthalmic examination of a 9-year-old girl, with a 4-month history of decreased vision in her right eye, revealed an amelanotic retinal mass with glial tissue strands over the optic disc, areas of vitreous seeding, and serous retinal detachment (Fig. 1). Her vision was 2 m finger counting with 2 + relative afferent pupillary defect. Her intraocular pressure was 16 mmHg.
Fig. 1.
A–B: The fundus photograph of both eyes shows a unilateral well defined amelanotic mass with serous retinal detachment and foci of vitreous seeding in the left eye.
The contralateral eye examinations were unremarkable. Considering the diagnosis of group D retinoblastoma versus activated astrocytoma, four sessions of intra-arterial chemotherapy with Melphalan (5 mg) and Topotecan (1 mg) was performed. However, the rapid tumor growth was unresponsive to the above globe preserving therapy, and progression to neovascular glaucoma occurred within three weeks necessitating enucleation with the clinical suspicion of retinoblastoma versus malignant astrocytoma.
Histopathologic evaluation revealed spindle cells with elongated fibrillary processes and round cells with vesicular cytoplasm, bizarre nuclei, multinucleated giant cells and rosenthal fibers as well as necrosis, and the presence of less than 5 mitoses in 10 high power fields (HPF) with calcospherites and a cystic space inside the tumor (Fig. 2, Fig. 3).
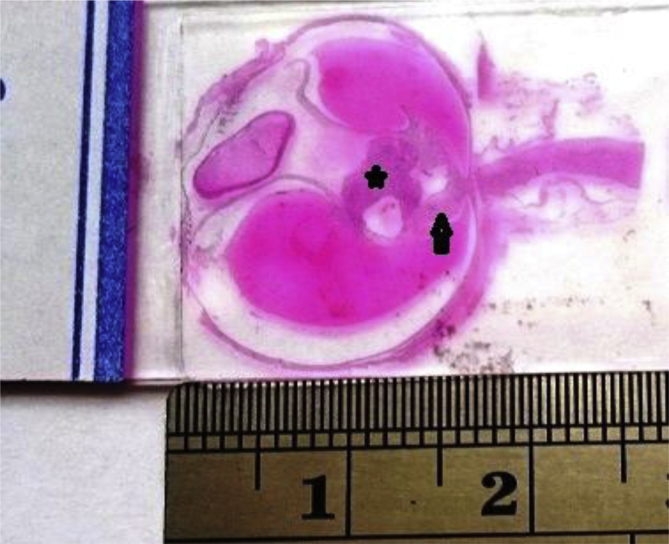
Fig. 2.
Low power section of the enucleated globe shows serous retinal detachment, a glial tissue over the optic disc (arrow) and the tumor (asterisks) with internal cystic space.
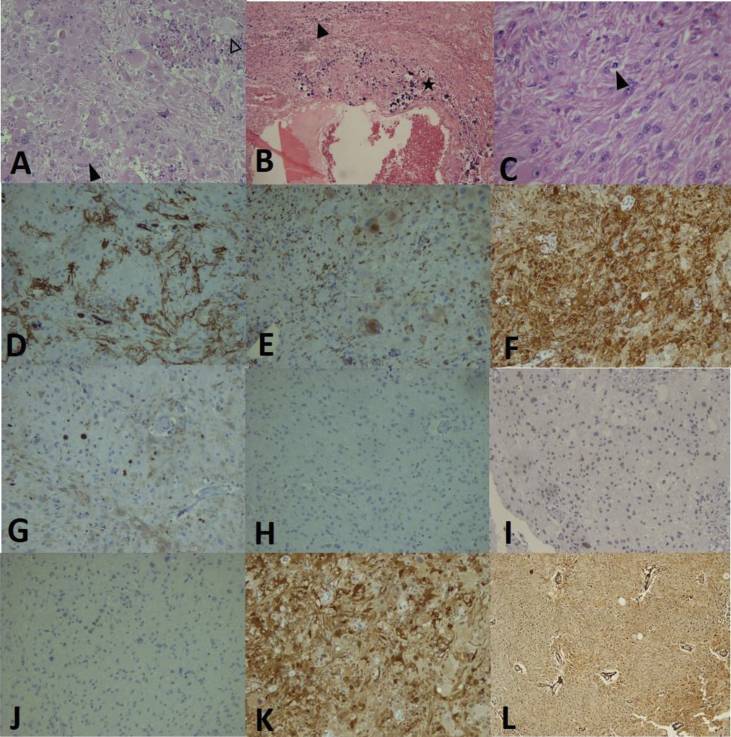
Fig. 3.
Histological and immunohistochemical findings (Olympus microscope BX41 and Olympus camera DP12, Japan): A, Pleomorphic tumor cells and nuclei with haphazardly arrangement in desmoplastic stroma, xanthomatous cells (white arrowhead) and multinucleated giant cells (black arrowhead) (H&E stain 200x), B: Calcospherites (asterisks) and necrotic areas (black arrowhead) are noted (H&E stain 100x), C: Mitosis and multiple eosinophilic granular bodies among the tumor cells (H&E stain 400x), D: Immunoreactivity for CD34 (200x), E: CD68 immunoreactivity in some tumoral cells (200x), F: Glial fibrillary acidic protein (GFAP) immunoreactivity (200x), G: KI67 reactivity in 10% of the tumor cells (200x), H–J: Non-reactivity for neurofilament, synaptophysin and P53 (200x). K: S100 immune reactivity (200x) and L: Positive reticulin stain (100x).
Moreover, immunohistochemistry evaluation showed a diffuse immune reactivity for glial fibrillary acidic protein (GFAP) and S100 together with focal reactivity for CD34 and CD68. No immune reactivity was noted for P53, synaptophysin and neurofilament. Ki67 immunostaining showed 10% reactivity, respectively (Fig. 3).
Considering the above findings, the aPXA was diagnosed.
Brain MRI and physical examinations did not reveal any neurologic or dermatologic sign of phakomatosis.
Discussion
Our case is an extremely rare case of a rapidly growing anaplastic form of PXA in the retina of a 9-year-old girl with no evidence of phakomatosis.
PXA accounts for less than 1% of all brain astrocytic tumors2 and rarely transforms to anaplasia. Retina is an uncommon site for PXA3 and subsequently anaplastic PXA. Two-thirds of PXAs are associated with phakomatosis.5 Takafumi et al.7 have shown the total reported cases of retinal astrocytomas unassociated with TSC, regardless of being PXA or aPXA. However, Shields et al.6 have classified them and added two new cases of retinal aggressive PXA unassociated with TSC to the ten previously reported retinal aPXA cases.
No gender or racial predilection for PXA has been reported in the literature.8 There are many clinical and histopathological similarities between retinal aPXAs with and without TSC, except for enucleation at older ages in aPXA patients without TSC.9 Retinal astrocytic hamartomas associated with TSC generally show a low tendency to grow.10, 11 Our case is similar to Shields’ review on acquired aPXAs6 in which most of their cases had unilateral and solitary tumors, and also that blind painful eye or progressive growth were the most common causes of enucleation. Our case also had a rapidly growing tumor unresponsive to the chemotherapy. A wide age range at the enucleation (i.e., between 1 and 56 years) has been reported for retinal aPXAs without TSC.6 Our case did not show the signs of TSC in systemic evaluation. However, later progression to TSC is unpredictable.9
Histologically, PXA is composed of spindle cells, xanthomatous cells, bizarre mononuclear or multinucleated giant cells, mitotic index (MI) less than five in ten HPF, eosinophilic granular bodies (rosenthal fibers), and calcospherites with numerous reticulin fibers.1, 9, 12, 13 The anaplastic form of PXA (aPXA), which is associated with shorter survival and more recurrence rate,2 is histologically defined as PXA with more than five MI in ten HPF, cellular anaplasia, high Ki67 labeling indices (LI), and decreased reticulin fibers with or without necrosis,2, 8, 12 but still maintains eosinophilic granular bodies, desmoplasia and xanthomatous changes which are characteristics for PXA and differentiate it from glioblastoma.14
It is noteworthy that 1–5% of PXAs and 4–22% of aPXAs are immune-reactive for Ki67 LI12 which is a more reliable index than MI for proliferation potential estimation.12 The 10% immunoreactivity for Ki67 in our case is compatible with the reported Ki67 LI of aPXA in the literature.12 Regarding the Korshunov grading system for PXA,15 our case with the rapid tumor growth, moderately high Ki67 LI, greater nuclear pleomorphism and necrosis than conventional PXA could not be considered a benign PXA. Moreover, previous chemotherapies could affect the mitotic rate.
Both neuroglial and histiocytic features of PXA were detected in our case.3, 12, 16 Neural differentiation is important for PXA to differentiate it from astrocytoma or glioblastoma.12
Only half of PXAs express CD34. This immunoreactivity is more apparent in PXA than in aPXA.12 Only few cells of our case expressed CD34.
PXA and aPXA do not show a significant difference in P53 expression.12 Generally, more than 50% of P53 immunoreactivity indicates the presence of TP53 mutation.17 In our case, immunoreactivity for P53 was negative.
Common clinical differential diagnoses for PXA include retinoblastoma, choroidal melanoma, Coat's disease, capillary hemangioma, and choroidal osteoma. Less dilated tortuous vessels, the presence of subretinal yellow exudation, and entirely amelanotic feature are in favor of astrocytic hamartomas.9, 18
Globe preserving therapies such as brachytherapy, endoresection and photodynamic therapy have vision threatening risks19, 20, 21 and chemotherapy, similar to our case, is generally ineffective for PXA and aPXA.8, 22
As a conclusion, diagnosis of aPXA should be kept in mind in the case of retinal white mass with an aggressive course in young children without phakomatosis.
Acknowledgement
The authors wish to thank Rassoul Akram Hospital Clinical Research Development Center (RCRDC) for its editing assists.
Footnotes
Conflict of interest: The authors have no conflict of interest in any concept or product described in this short report.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Louis D.N., Perry A., Reifenberger G. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Patibandla M.R., Nayak M., Purohit A.K., Thotakura A.K., Uppin M., Challa S. Pleomorphic xanthoastrocytoma with anaplastic features: a rare case report and review of literature with reference to current management. Asian J Neurosurg. 2016;11(3):319. doi: 10.4103/1793-5482.144161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zárate J.O., Sampaolesi R. Pleomorphic xanthoastrocytoma of the retina. Am J Surg Pathol. 1999;23(1):79–81. doi: 10.1097/00000478-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ida C.M., Rodriguez F.J., Burger P.C. Pleomorphic xanthoastrocytoma: natural history and long-term follow-up. Brain Pathol. 2015;25(5):575–586. doi: 10.1111/bpa.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulbright T.M., Fulling K.H., Helveston E.M. Astrocytic tumors of the retina. Differentiation of sporadic tumors from phakomatosis-associated tumors. Arch Pathol Lab Med. 1984;108(2):160–163. [PubMed] [Google Scholar]
- 6.Shields C.L., Shields J.A., Eagle R.C., Jr., Cangemi F. Progressive enlargement of acquired retinal astrocytoma in two cases. Ophthalmology. 2004;111(2):363–368. doi: 10.1016/j.ophtha.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Fuchino T., Hayashi T., Suzuma K. Solitary retinal astrocytoma: a case series. Open J Pathol. 2013;3(2):60–64. [Google Scholar]
- 8.Giannini C., Scheithauer B.W., Burger P.C. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85(9):2033–2045. [PubMed] [Google Scholar]
- 9.Shields J.A., Eagle R.C., Jr., Shields C.L., Marr B.P. Aggressive retinal astrocytomas in four patients with tuberous sclerosis complex. Trans Am Ophthalmol Soc. 2004;102:139–147. [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson D.M. Ophthalmic manifestations of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:17–25. doi: 10.1111/j.1749-6632.1991.tb37744.x. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer-Galler I.E., Robertson D.M. Long-term observation of retinal lesions in tuberous sclerosis. Am J Ophthalmol. 1995;119(3):318–324. doi: 10.1016/s0002-9394(14)71174-2. [DOI] [PubMed] [Google Scholar]
- 12.Hirose T., Ishizawa K., Sugiyama K., Kageji T., Ueki K., Kannuki S. Pleomorphic xanthoastrocytoma: a comparative pathological study between conventional and anaplastic types. Histopathology. 2008;52(2):183–193. doi: 10.1111/j.1365-2559.2007.02926.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahramancetin N., Tihan T. Aggressive behavior and anaplasia in pleomorphic xanthoastrocytoma a plea for a revision of the current WHO classification. CNS Oncol. 2013;2(6):523–530. doi: 10.2217/cns.13.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prayson R.A., Morris H.H., 3rd Anaplastic pleomorphic xanthoastrocytoma. Arch Pathol Lab Med. 1998;122(12):1082–1086. [PubMed] [Google Scholar]
- 15.Korshunov A., Golanov A. Pleomorphic xanthoastrocytomas: immunohistochemistry, grading and clinico-pathologic correlations. An analysis of 34 cases from a single institute. J Neuro Oncol. 2001;52(1):63–72. doi: 10.1023/a:1010648006319. [DOI] [PubMed] [Google Scholar]
- 16.Matyja E., Kroh H., Taraszewska A., Nagańska E., Zabek M., Marchel A. Expression of macrophage/histiocytic antigens in pleomorphic xanthoastrocytomas. Folia Neuropathol. 2003;41(2):89–95. [PubMed] [Google Scholar]
- 17.Kelley T.W., Tubbs R.R., Prayson R.A. Molecular diagnostic techniques for the clinical evaluation of gliomas. Diagn Mol Pathol. 2005;14(1):1–8. doi: 10.1097/01.pdm.0000138207.96718.85. [DOI] [PubMed] [Google Scholar]
- 18.Sawyer J.R., Roloson G.J., Chadduck W.M., Boop F.A. Cytogenetic findings in a pleomorphic xanthoastrocytoma. Canc Genet Cytogenet. 1991;55(2):225–230. doi: 10.1016/0165-4608(91)90081-5. [DOI] [PubMed] [Google Scholar]
- 19.Vilaplana D., Castilla M., Poposki V., Alameda F., Shields C.L. Acquired retinal astrocytoma managed with endoresection. Retina. 2006;26(9):1081–1082. doi: 10.1097/01.iae.0000254888.63519.3c. [DOI] [PubMed] [Google Scholar]
- 20.Eskelin S., Tommila P., Palosaari T., Kivelä T. Photodynamic therapy with verteporfin to induce regression of aggressive retinal astrocytomas. Acta Ophthalmol. 2008;86(7):794–799. doi: 10.1111/j.1755-3768.2007.01151.x. [DOI] [PubMed] [Google Scholar]
- 21.Drummond S.R., Kemp E.G. Retinal astrocytoma managed by brachytherapy. Ophthalmology. 2009;116(3):597. doi: 10.1016/j.ophtha.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Thomas C., Golden B. Pleomorphic xanthoastrocytoma: report of two cases and brief review of the literature. Clin Neuropathol. 1993;12(2):97–101. [PubMed] [Google Scholar]