Abstract
In this data article, we present supplementary data related to the research article entitled “Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media” Stan et al., 2019. Data interpretations are included in the related research article Stan et al., 2019. The synthesized starch-coated Fe3O4 nanoparticles (ST-coated Fe3O4 NPs) were analyzed by scanning electron microscopy (SEM) and high resolution transmission electron microscopy (HRTEM) to illustrate the shape and surface coating of nanoparticles. Moreover, the Brunauer-Emmett-Teller (BET) technique was used to evidence starch deposition on magnetite nanoparticles. The obtained nanocomposites were used for adsorption of Optilan Blue (OB) in batch conditions and the optimum agitation speed and point of zero charge (pHpzc) were established. After OB adsorption on ST-coated Fe3O4 NPs, the nanocomposites were analyzed by transmission electron microscopy (TEM), X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR). The stability of starch coated Fe3O4 NPs in the acidic as well as alkaline pH was also evidenced by FTIR spectroscopy. In addition, to test the stability of ST-coated Fe3O4 NPs, leaching experiments were carried out. The experimental data were compared with isotherm and kinetic models in order to determine the most suitable for fitting.
Specifications table
| Subject area | Environmental Engineering |
| More specific subject area | Adsorption |
| Type of data | Table, image, figure |
| How data was acquired | TEM/HRTEM/SEM (STEM Hitachi HD-2700), XRD (Bruker D8), VSM (Bruker ELEXSYS 500), FTIR (JASCO 6100), ICP-OES (Optima 5300DV, Perkin Elmer), Spectrophotometer (UV-VIS, T8) |
| Data format | Analyzed |
| Experimental factors | The effect of four parameters like initial concentration of OB, ST-coated Fe3O4NPs dosage, pH and temperature were examined. After adsorption, the adsorbent was separated with an external magnetic field and the residual dye was determined spectrophotometrically. |
| Experimental features | In the first step Fe3O4NPs were prepared and in the second step the ST-coated Fe3O4NPs were synthesized. The obtained nanocomposites used as adsorbents, were characterized by TEM, XRD, FTIR, BET and VSM techniques. |
| Data source location | Cluj-Napoca, National Institute for Research and Development of Isotopic and Molecular Technologies, Romania |
| Data accessibility | Data are included in this article |
| Related research article | Manuela Stan, Ildiko Lung, Maria-Loredana Soran, Ocsana Opris, Cristian Leostean, Adriana Popa, Florina Copaciu, Mihaela Diana Lazar, Irina Kacso, Teofil-Danut Silipas, Alin Sebastian Porav. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J Taiwan Inst Chem Eng 2019;100:65-73[1]. |
Value of the data
|
1. Data
The presented data are supplementary the research article of J Taiwan Inst Chem Eng 2019; 100:65–73 [1].
The XRD and TEM data for ST-coated Fe3O4 NPs after OB adsorption are summarized in Table 1. The adsorption capacity of the tested adsorbents was compared with other magnetic adsorbents used for dye removal in Table 2. Fig. 1 shows the SEM images of ST-coated Fe3O4 NPs, and the HRTEM images of two nanocomposite samples are illustrated in Fig. 2. The nitrogen adsorption-desorption isotherms and pore radius for Fe3O4 sample before and after starch coating are depicted in Fig. 3.
Table 1.
The average size of crystallites (XRD) and nanoparticles (TEM) of starch-coated Fe3O4 NPs after OB adsorption.
| Magnetite nanoparticles | Starch-coated magnetite nanoparticles after OB dye adsorption |
|
|---|---|---|
| XRD (nm) | TEM (nm) | |
| Fe3O4(av1)a | 14 | 19 |
| Fe3O4(av2) | 13 | 19 |
| Fe3O4(wm) | 14 | 16 |
| Fe3O4 | 16 | 18 |
Abbreviations used: av1 – avocado peel extract, av2 – avocado seed extract, wm – watermelon seed extract, no extract. Additional information can be found in the research article [1].
Table 2.
Comparison of adsorption capacity/removal efficiency of various magnetic adsorbents for dyes.
| Magnetic Adsorbent | Dye | Isotherm | Adsorption capacity (mg g−1) | Reference |
|---|---|---|---|---|
| Chitosan coated Fe3O4 nanoparticles | Reactive Yellow 145 | Langmuir | 47.62 | [2] |
| Sodium alginate-coated Fe3O4 nanoparticles | Malachite green | Langmuir | 47.84 | [3] |
| PGA-coated Fe3O4 nanoparticles | Methylene blue | Langmuir | 78.67 | [4] |
| L-Serine functionalized Fe3O4 NPs | Rhodamine B | Langmuir | 6.82 | [5] |
| Magnetic Fe3O4@SiO2 starch-graft-poly (acrylic acid) (SPAA) nanocomposite hydrogels | Crystal violet | Langmuir | 80.64 | [6] |
| MNP@St-g-PVS | Methylene blue Malachite green |
Langmuir | 621 567 |
[7] |
| Lignin magnetic nanoparticles (LMNPs) | Methylene blue | Langmuir | 211.42 | [8] |
| Lignin amine magnetic nanoparticles (LAMNPs) | Acid scarlet GR | Langmuir | 176.49 | [8] |
| Fe3O4/Poly (styrene-co-methacrylic acid) | Crystal violet Rhodamine B |
Langmuir | 416.66 69.54 |
[9] |
| Chitosan coated Fe3O4 nanoparticles | Orange I | Langmuir | 180.8 | [10] |
| Starch-coated Fe3O4 NPs | Optilan blue | Langmuir | 86–125 | [1] |
Fig. 1.
SEM images of ST-coated samples: (a) Fe3O4(av1), (b) Fe3O4(av2), (c) Fe3O4(wm) and (d) Fe3O4 samples.
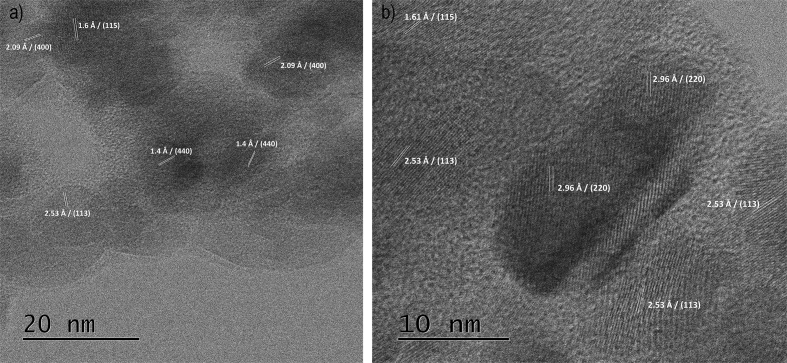
Fig. 2.
HR-TEM images of starch-coated: a) Fe3O4(av2) and b) Fe3O4 samples.
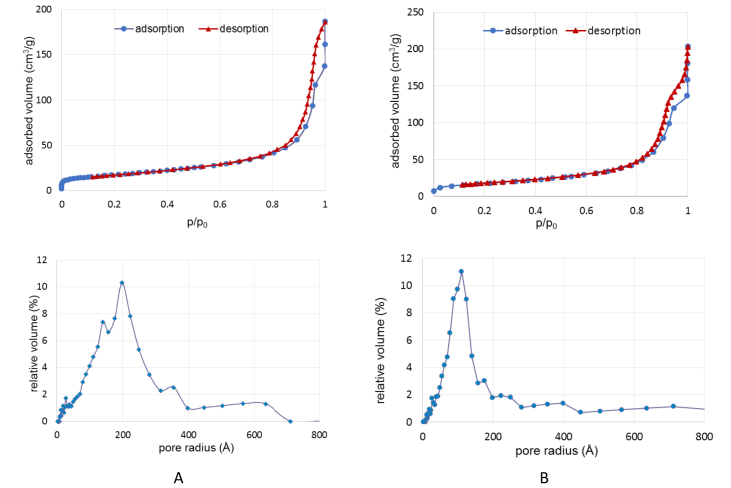
Fig. 3.
Nitrogen adsorption-desorption isotherms and pore radius for Fe3O4 sample. before (A) and after starch coating (B).
TEM, XRD, FTIR and VSM analyses of nanocomposites after OB adsorption are presented in Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8. FTIR analysis was employed to demonstrate the stability of adsorbents in the acidic as well as alkaline media and the spectroscopic evidences are shown in Fig. 9.
Fig. 4.
TEM images after OB adsorption of starch-coated: (a) Fe3O4(av1), (b) Fe3O4(av2), (c) Fe3O4(wm), (d) Fe3O4 samples and the histograms of particle size distribution (e–h).
Fig. 5.
XRD patterns for ST-coated Fe3O4 NPs after adsorption of OB.
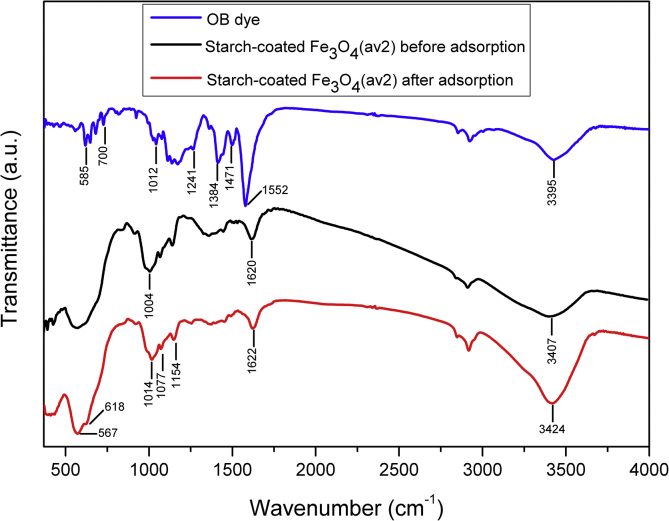
Fig. 6.
FTIR spectra of OB dye and ST-coated Fe3O4 NPs after dye adsorption.
Fig. 7.
FTIR spectra of OB dye and starch-coated Fe3O4(av2) sample. before and after dye adsorption.
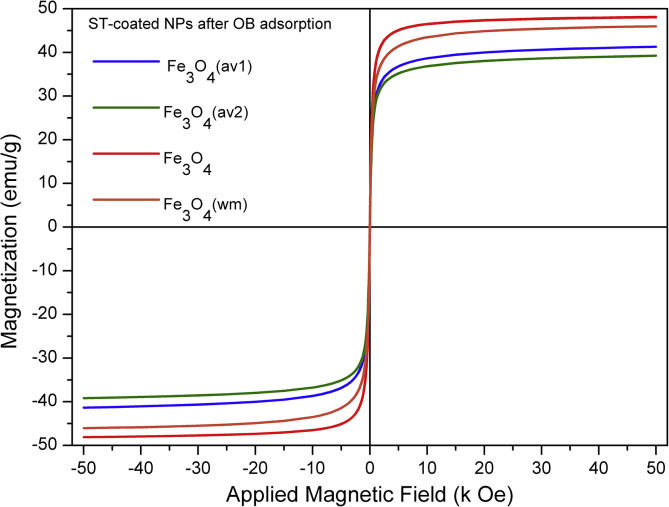
Fig. 8.
The behavior of magnetization vs. the applied magnetic field for ST-coated Fe3O4 NPs after OB dye adsorption.
Fig. 9.
FTIR spectra of starch coated Fe3O4 and Fe3O4(av2) samples (a, b) in the acidic (pH 2) as well as alkaline (pH 10) media.
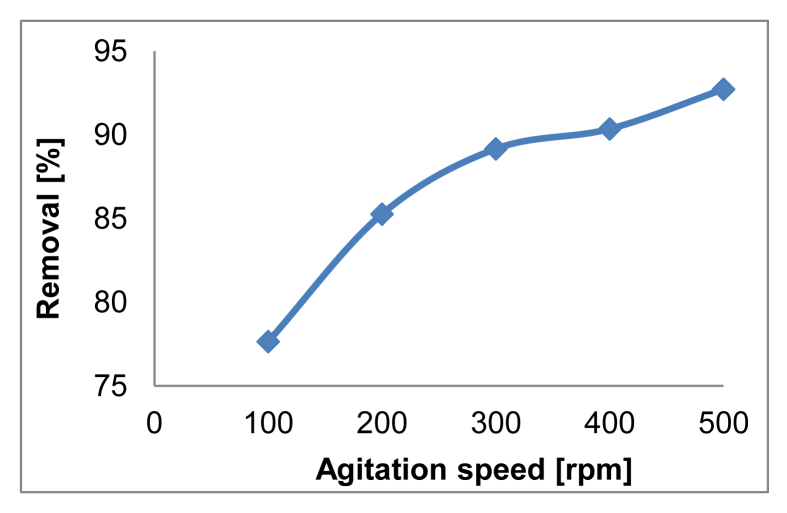
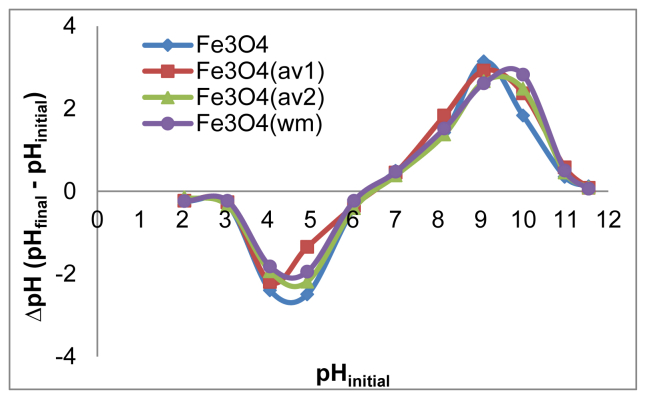
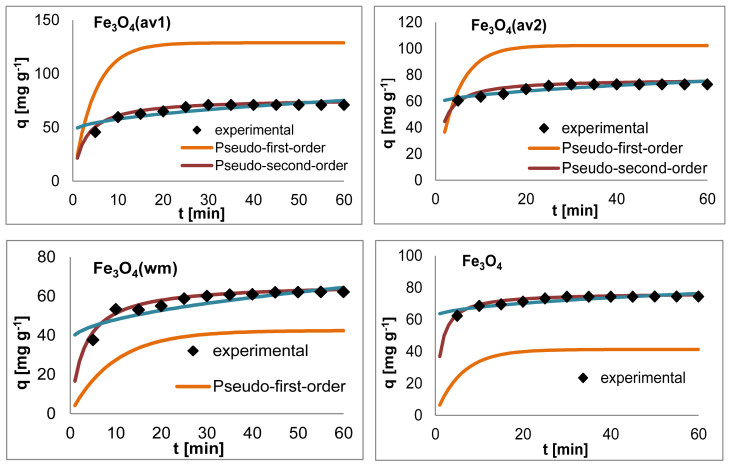
The effect of agitation speed on OB removal is presented in Fig. 10. The pHpzc values for all adsorbents are determined by the position where the resulting curves cut through the pHinitial axis as observed in Fig. 11. Data from leaching experiments, carried out in order to establish complete magnetic separation and the total dissolved iron concentrations by ICP-OES analysis, are shown in Fig. 12. As illustrated in Fig. 13 and Fig. 14, adsorption isotherms and kinetics were modeled and fitted with experimental data in order to determine the interactions that occur between the adsorbent and adsorbate species, the adsorption rate by the adsorbent, and the adsorption mechanism of the solute onto an adsorbent.
Fig. 10.
The effect of agitation speed on OB removal.
Fig. 11.
Point zero charge of ST-coated Fe3O4 NPs.
Fig. 12.
The total dissolved iron concentrations.
Fig. 13.
Adsorption isotherm models fitted to experimental adsorption of OB on ST-coated Fe3O4 NPs at different temperatures (pH 2, mass dosage 0.6 g L−1).
Fig. 14.
Adsorption rate curves (pH 2, dye conc. 50 mg L−1, 308 K and adsorbent dose 0.6 g L−1).
2. Experimental design, materials, and methods
2.1. Materials
Starch-coated Fe3O4 NPs, Optilan Blue MF-GL dye, 0.5 N HCl or 5% NH4OH for pH adjustments.
2.2. Determination of pHpzc of ST-coated Fe3O4 NPs
The pH drift method [11] was used to determine the pH at point of zero charge (pHzpc) of the adsorbents under study. Over 12 mg of adsorbent, 20 mL of 0.01 M NaCl solution was added with the initially pH adjusted in the range of 2–12 by adding 0.5 N HCl or 5% NH4OH. The mixtures were left at room temperature for 48 h, after which the solid material was separated from the solution using an external magnet and the final pH value was measured.
2.3. Adsorption experiments
Optilan Blue adsorption on ST-coated Fe3O4 NPs was performed under batch conditions. The effect of initial dye concentration, pH, temperature and adsorbent dosage on adsorption of OB on ST-coated Fe3O4 NPs were determined. In addition, a study was conducted to determine the optimum agitation speed (100–500 rpm) at which the maximum dye adsorption was accomplished. The solution was adjusted with 0.5 N HCl or 5% NH4OH in order to achieve the desired pH. The adsorbent was separated using an external magnet and the residual dye was measured with a UV–Vis spectrophotometer, recording the absorbance at 629 nm. The obtained experimental data were fitted to different models in order to understand the adsorption behavior of OB on ST-coated Fe3O4 NPs.
In addition, the stability of adsorbents in acidic (pH 2) and alkaline (pH 10) media was investigated.
2.4. Characterization and analysis
The surface morphology of nanocomposites was examined from SEM and HRTEM images. BET measurements were also conducted, and evidenced the deposition of starch on the surfaces of magnetite nanoparticles.
The stability of adsorbents was investigated in the acidic pH (2) as well as alkaline pH (10) by FTIR technique in order to underline the possible spectroscopic evidences.
After OB adsorption on ST-coated Fe3O4 NPs, the dried samples were characterized by different techniques such as FTIR, XRD, TEM, and VSM.
2.5. Batch leaching test
For each leaching test, 10 mg of ST-coated Fe3O4 NPs were mixed with 10 mL of water at pH 2 (HCl 0.1 M) in a 20 mL conical flask. The mixture was stirred with 500 rpm for 2 hours at different temperature (25 °C, 35 °C and 45 °C). The nanoparticles were separated using an external magnet and the leachate samples were analyzed by a dual viewing inductively coupled plasma optical emission spectrometer (ICP-OES). The experiment was performed in triplicate.
Acknowledgments
Financial support from the Romanian Ministry of Research and Innovation, Core Programme, Project PN18-03 02 03 is gratefully acknowledged. The authors are grateful to Dr. Marin Senila (INCDO-INOE 2000, Cluj-Napoca) for the ICP-OES measurements.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Stan M., Lung I., Soran M.L., Opris O., Leostean C., Popa A., Copaciu F., Lazar M.D., Kacso I., Silipas T.D., Porav A.S. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019;100:65–73. doi: 10.1016/j.dib.2019.104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalkan N.A., Aksoy S., Aksoy E.A., Hasirci N. Adsorption of reactive Yellow 145 onto chitosan coated magnetite nanoparticles. J. Appl. Polym. Sci. 2012;124:576–584. [Google Scholar]
- 3.Mohammadi A., Daemi H., Barikani M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014;69:447–455. doi: 10.1016/j.ijbiomac.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Inbaraj B.S., Chen B.H. Dye adsorption characteristics of magnetite nanoparticles coatedwith a biopolymer poly(c-glutamic acid) Bioresour. Technol. 2011;102:8868–8876. doi: 10.1016/j.biortech.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 5.Belachew N., Rama Devi D., Basavaiah K. Green synthesis and characterization of L-Serine capped magnetite nanoparticles for removal of Rhodamine B from contaminated water. J. Exp. Nanosci. 2017;12:114–128. [Google Scholar]
- 6.Pourjavadi A., Hosseini S.H., Seidi F., Soleyman R. Magnetic removal of crystal violet from aqueous solutions using polysaccharide-based magnetic nanocomposite hydrogels. Polym. Int. 2013;62:1038–1044. [Google Scholar]
- 7.Pourjavadi A., Abedin-Moghanaki A., Tavakoli A. Efficient removal of cationic dyes using a new magnetic nanocomposite based on starch-g-poly(vinylalcohol) and functionalized with sulfate groups. RSC Adv. 2016;6:38042–38051. [Google Scholar]
- 8.Li X., He Y., Sui H., He L. One-step fabrication of dual responsive lignin coated Fe3O4 nanoparticles for efficient removal of cationic and anionic dyes. Nanomaterials. 2018;8:162. doi: 10.3390/nano8030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayasi M., Karimi M. Synthesis of poly(styrene-co-methacrylic acid)-coated magnetite nanoparticles as effective adsorbents for the removal of crystal violet and Rhodamine B: a comparative study. Polym. Bull. 2017;74:1995–2016. [Google Scholar]
- 10.Du Y., Pei M., He Y., Yu F., Guo W., Wang L. Preparation, characterization and application of magnetic Fe3O4-CS for the adsorption of orange I from aqueous solutions. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108647. e108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustafa G., Tahir H., Sultan M., Akhtar N. Synthesis and characterization of cupric oxide (CuO) nanoparticles and their application for the removal of dyes. Afr. J. Biotechnol. 2013;12:6650–6660. [Google Scholar]