Abstract
Objectives
Exposure to life stresses can lead to diminution in the capacity of stress response systems to mount a robust response to new challenges, with blunting of dynamic range—the spread between maximal attainable and minimal resting levels. We investigate the association between early-life adversity and the dynamic range of adult diurnal cortisol secretion.
Method
In 35- to 86-year-old adults, cortisol assayed from 16 saliva samples over 4 consecutive days was used to compute diurnal dynamic range and area under the curve (AUC). Economic adversity in childhood was indexed by recalled parental education, family welfare dependence, and perceived financial status; and childhood social adversity by parental separation, death, and abuse.
Results
Adjusted for age, gender, and race/ethnicity, both childhood adversities were strongly associated with smaller adult cortisol diurnal dynamic range, but not with AUC. The association with cortisol dynamic range was explained by adult social and economic variables.
Discussion
Early-life adversity appears to leave a long-term imprint on cortisol secretion dynamics, reducing diurnal dynamic range without increasing total secretion. This points to the importance of examining the adaptation capacity of physiological systems when studying the impact of early-life and chronic stresses on adult health.
Keywords: Childhood adversity, Cortisol evening nadir, Cortisol morning peak, Dynamic range, Stress response
Adversity in childhood has persistent effects on health across the life span, and the underlying biological pathway appears to be dysregulation of the physiological systems of the stress response apparatus (Taylor, Way, & Seeman, 2011). The ability of human physiological systems to respond and adapt to an array of challenges and perceived stressors is critical to maintenance of health and function. In fact, adaptability is a key requisite for the flourishing of nearly every organizational structure in a challenging or changing environment, including for instance, biological species, ecosystems, human communities, and business enterprises (Lasker, 1969).
The body’s adaptation of its physiological milieu in response to demands has been called allostasis (Sterling & Eyer, 1988), and the adaptation capacity of a system is its allostatic reserve. A system’s adaptation capacity depends on its dynamic range—the spread between maximal attainable level when challenged and minimal level at rest. Reduction in dynamic range is seen with usual aging in nearly every physiological and organ system in the human body (Navaratnarajah & Jackson, 2013). Compression of dynamic range is also a price paid by the system for frequent allostasis in the face of repeated or intense challenges.
When adaptation is excessive, because of increased frequency, duration, or severity of challenges, it leads to dysregulation of the system and its stress response. Such dysregulation, the price paid for repeated allostasis, has been called allostatic load, and reduced capacity to adapt is a hallmark of allostatic load (McEwen, 2002; McEwen & Stellar, 1993). In individuals exposed to frequent or sustained stresses, long-term changes in the stress response, in the form of both blunted reactivity and delayed recovery, have been consistently found, particularly in the hypothalamic-pituitary-adrenal (HPA) axis.
HPA Axis
The neuroendocrine HPA axis, in concert with the sympathetic and parasympathetic systems, plays a primary role in the body’s stress response (McEwen, 2002). Dysregulated HPA axis dynamics, in the form of blunted cortisol reactivity to acute stress, has been documented in people with excessive, frequent, or chronic stress (Kudielka, Bellingrath, & Hellhammer, 2006; Lovallo, Farag, Sorocco, Cohoon, & Vincent, 2012), and blunted reactivity, as in burnout and vital exhaustion, has been found to have adverse health consequences (Carroll, Phillips, & Lovallo, 2012; Phillips, Ginty, & Hughes, 2013). We posit that a healthy stress response system is characterized by preserved capacity to mount a robust response (good allostatic reserve), and that the HPA axis’ innate capacity to mount a robust response can be gauged by its diurnal rhythm.
Diurnal Rhythm of Cortisol Secretion
Cortisol secretion in the human body follows a diurnal rhythm, with peak approximately 30 min after waking, steady decrease during the day, and nadir at night. A robust morning peak and a rapid decline after peaking are characteristic of a healthy HPA axis, with the morning rise priming the body for the demands and stresses of the day ahead, and the fast decline making room for surges when stresses are encountered during the day (Fries, Dettenborn, & Kirschbaum, 2009).
Rosmond, Dallman, and Björntorp (1998) showed that the magnitude of diurnal variation in cortisol correlates strongly with dexamethasone suppression of cortisol secretion—a measure of the self-regulatory ability of the HPA axis, and concluded that diminution of diurnal dynamic range is an indicator of HPA axis dysregulation. Consistent with this and with the allostatic load hypothesis, both blunting of the cortisol morning peak and slowing of the daily decline from peak are seen in chronically stressed individuals (Kudielka et al., 2006; Miller, Chen, & Zhou, 2007; Morgan, Cho, Hazlett, Coric, & Morgan, 2002; Pruessner, Hellhammer, & Kirschbaum, 1999).
In a large national study of the cortisol diurnal rhythm, less educated compared with higher educated individuals and nonwhites compared with whites had smaller peaks but higher nadirs, so that their diurnal dynamic range (the dispersion from nadir to peak, a concept borrowed from engineering where it is used in the context of audio amplifiers and speakers, cameras, display devices, and measurement instruments, for example) is compressed, while their total daytime exposure—measured by the area under the curve (AUC) is unaffected (Karlamangla, Friedman, Seeman, Stawksi, & Almeida, 2013). Compression of the diurnal dynamic range of cortisol secretion without change in AUC has been documented in African Americans and Hispanic Americans relative to whites in the United States, and in those from low relative to high socioeconomic status (SES), in multiple other national studies (Cohen et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011). Skinner and colleagues (2011) and Sjogren and colleagues (2006) also document compressed diurnal dynamic range in people who report stressful life events and vital exhaustion, respectively.
Based on these and similar findings from other studies, we postulate that compression of the diurnal dynamic range of cortisol secretion (lower peak and higher nadir) is a manifestation of HPA axis dysregulation resulting from early and chronic life stresses. Accordingly, we hypothesized that early-life adversity would leave its imprint on adult diurnal cortisol rhythm by reducing the diurnal dynamic range.
Early-Life Adversity and Adult Cortisol Diurnal Dynamic Range
Childhood is often seen as a sensitive period with potential long-term implications for health (Miller, Chen, & Parker, 2011). Sensitive or critical period models suggest that the health effects of adversity vary by the life course phase in which it is experienced, and that adversity in early life irreparably affects developing biological systems. From a life course epidemiology perspective, early-life adversity increases one’s exposure to and susceptibility to later hardships, creating a “chain of risk” for ill health in adulthood (Ben-Shlomo, Mishra, & Kuh, 2014).
Multiple research studies have demonstrated that early-life adversities, be they economic, emotional, or social, have long-lasting effects on the functioning of the HPA axis, including for example, blunted cortisol reactivity to acute stress in adulthood in those who experienced maltreatment (abuse and/or neglect) in childhood (Carpenter et al., 2007; Goldmann-Mellor, Hamer, & Steptoe, 2012). If early-life adversity does indeed lead to reductions in the HPA axis’ capacity to respond to acute stress, and if the diurnal cortisol dynamic range is a measure of this innate capacity, then early-life adversity should lead to diurnal dynamic range compression.
In fact, lower morning cortisol peak levels are seen in children raised in orphanages (Carlson & Earls, 1997), in sexually abused children (King, Mandansky, King, Fletcher, & Brewer, 2001), and in college students who report parent divorce/separation and/or close family member death in childhood (Meinlschmidt & Helm, 2005), and the diurnal rhythm is flattened in children who experienced early physical or sexual abuse (Cicchetti, Rogosch, Gunnar, & Toth, 2010). Low SES in childhood, however, has been linked to both lower and higher levels of morning cortisol in adulthood, relative to high childhood SES, but the effect appears to be, in most part, mediated by adult SES (Li, Power, Kelly, Kirschbaum, & Hertzman, 2007). Viewed as a whole, while this body of work does demonstrate an important link between severe early adversities and flattening of the diurnal cortisol rhythm in children and young adults, it is not as yet known if this effect persists into adulthood, especially for less severe forms of early-life financial and emotional adversity. This represents a critical gap in our understanding of how early adversity gets under the skin to affect adult health, which this study attempts to fill.
Accordingly, our objective in this study was to examine the associations of both economic and social adversity in early-life with adult cortisol diurnal dynamic range and AUC in a large national sample.
Methods
The data for this study came from the Midlife in the United States Study (MIDUS), a national study initiated in 1995. The first wave surveyed 7,108 English-speaking, noninstitutionalized, 25- to 74-year-old adults, residing in the contiguous 48 states. Of the original 7,108 MIDUS participants, 4,963 (70%) completed a second survey, 9–10 years later. To increase the representation of urban African Americans in the sample, an additional 592 African Americans were recruited from Milwaukee, WI, to participate in the second wave.
A random subsample of 2,022 participants in the second wave also completed a daily telephone diary study about their experiences over eight consecutive days and collected four timed saliva samples per day on four consecutive days, starting from day 2 of the diary study (Almeida, McGonagle, & King, 2009).
Study Sample
Of the 2,022 daily diary study participants, 1,733 provided at least one valid cortisol sample linked with sampling time, for a total of 6,883 days of cortisol data. After excluding data from days when participants awoke before 4 a.m., when the cortisol level in the third daily sample was 10 or more nmol/L higher than the level in the second daily sample (as this likely reflects a time-recording error or cortisol error from consumption of a meal), when participants woke after 11 a.m., and/or when a participant was awake more than 20 hr, days with missing weekend versus workday status, and any cortisol values above 60 nmol/L (to remove measurements affected by medications such as steroids), we were left with a sample of 1,697 participants with 6,334 days of data, and 24,452 cortisol samples. Exactly one of these participants was missing data on race/ethnicity, leaving us with a study sample of size 1,696 (from 1,411 families) for the primary analysis.
Measurements
Early-Life Adversity
Data on adversity in early life were obtained from retrospective self-reports of adverse economic conditions and social relations in childhood, from the first MIDUS survey; the Milwaukee African American subsample provided this information at enrollment in the second wave. For questions about parents, participants were asked to respond about the male and female heads of the household (biological parent, adoptive parent, stepparent, other) who “raised you for most of the time before you turned 17.” Childhood SES was ascertained from household income and parents’ education. Assessment of childhood social adversity was based on reports of family disruption (parental divorce or death) as well as the Conflict Tactics Inventory that captures abuse from family members. Cutpoints were selected to capture any level of adversity in each domain assessed; the resulting adversity score is a count of the number of sources of adversity in childhood. Similar counts of childhood adversities have been used previously in studies of early-life effects on adult health (Danese et al., 2009).
Childhood economic adversity (range, 0–3) was scored as the sum of three 0/1 variables created from responses to survey questions. The first question asked if before age 18, there was ever a period of 6 months or more when the family was on welfare or ADC (Aid to Dependent Children). The second asked, “When you were growing up, was your family (you lived with for the longest time) better off or worse off financially than the average family was at that time?” Possible responses ranged from “a lot better off” to “a lot worse off.” Responses of “somewhat worse off” and “a lot worse off” were coded 1, and the other five (“a lot better off” through “a little worse off”) were coded 0. The third question was about parents’ education and the response was coded 1 if neither parent completed high school or GED and 0 if at least one parent did.
Childhood social adversity (range, 0–3) was also scored as the sum of three 0/1 variables based on responses to survey questions. This adversity score has been referred to as childhood relationship adversity and emotional adversity in some MIDUS publications (Friedman, Karlamangla, Gruenewald, Koretz, & Seeman, 2015). The first two questions asked about experiencing a parent’s death before reaching age 16 and parents’ divorce or separation before age 16. The third variable was based on responses to the Conflict Tactics Inventory (Straus, 1979). Participants reported physical and/or emotional abuse by a parent using a 4-point scale ranging from “never” to “often.” Responses of “sometimes” or “often” were coded 1, and “never” or “rarely” as 0.
Childhood Total Adversity (Range, 0–6) The two childhood adversity scores above were summed to capture total early-life adversity. The component measures that were used to create these early-life adversity scores have been individually linked to adult cortisol levels in previous studies (Carpenter et al., 2007; Goldmann-Mellor et al., 2012; Li et al., 2007; Meinlschmidt & Helm, 2005). Combining the component adversity measures into a single cumulative adversity metric has many advantages—it allows for the natural covariation of the component adversities in society, it can uncover a dose response (more adversities leading to worse outcomes), and although the components may not really be interchangeable with respect to their impact on outcomes, their individual effects pale in comparison to the effect of a cumulative metric (Dawes, 1979; Evans, 2003). It should be noted that since each of the six potential sources of adversity were scored 0 for no adversity and 1 for any level of adversity (some or more), this total adversity score is a count of the number of sources of adversity in childhood, not a measure of the severity of adversities faced. This adversity score has been linked to allostatic load constructed from dysregulated resting levels of physiological systems (Friedman et al., 2015), but not to system dynamics and allostatic reserve.
For purposes of sensitivity analysis, we created an alternate version of the total adversity score (range, 0–9) that gives more points for severe adversity, by giving an additional point for being “a lot worse off” financially than the average family, two points for neither parent completing and one point for one parent not completing high school or GED, two points for parent death and one point for parent divorce, and an extra point for being abused by a parent “often.”
Adult Cortisol Diurnal Dynamic Range and AUC
On days 2 through 5 of the 8-day diary study, participants provided saliva samples (and sample collection times) in numbered, color-coded salivettes (Sarstedt, Nümbrecht, Germany), four times a day: Immediately upon waking, 30 min after waking, before lunch, and before bed. Participants also provided, during nightly telephone interviews, the previous night’s bedtime, morning waking time, and if employed, whether it was a workday or off day. Cortisol levels were measured using a luminescence immunoassay (IBL, Hamburg, Germany), and natural-log transformed (after adding 1 nmol/L).
Mean log-cortisol over consecutive 15-min intervals, indexed to individual- and day-specific waking time, in four strata (waking time before vs after median, bedtime before vs after median) and in the complete study sample, all consistently showed a morning peak 30 min after waking, steep decline after the peak for 4 hr, followed by a more gradual decline for 10.5 hr, and plateau or upturn later. Using mixed effects regression, we therefore, modeled the day-specific log-cortisol growth curve as a linear spline with three fixed knots at 0.5, 4.5, and 15 hr after waking. All five growth curve parameters (intercept and four slopes) were modeled to vary with the following covariates: average wake-day length (individual-level, averaged over all 8 days), workday vs off day status (day-level), waking time earlier than sample median (day-level), and previous night’s sleep time in three categories: <6 hr, 6–8 hr, and >8 hr (day-level). To account for correlation between members of the same family (twin pairs and siblings), we included a random intercept at the family level. To account for correlations between repeated measurements in the same individual, we included random effects at the individual level for all five growth curve parameters. In addition, to capture correlations between repeated measurements in the same day, we included a random intercept and a random initial decline slope at the day level.
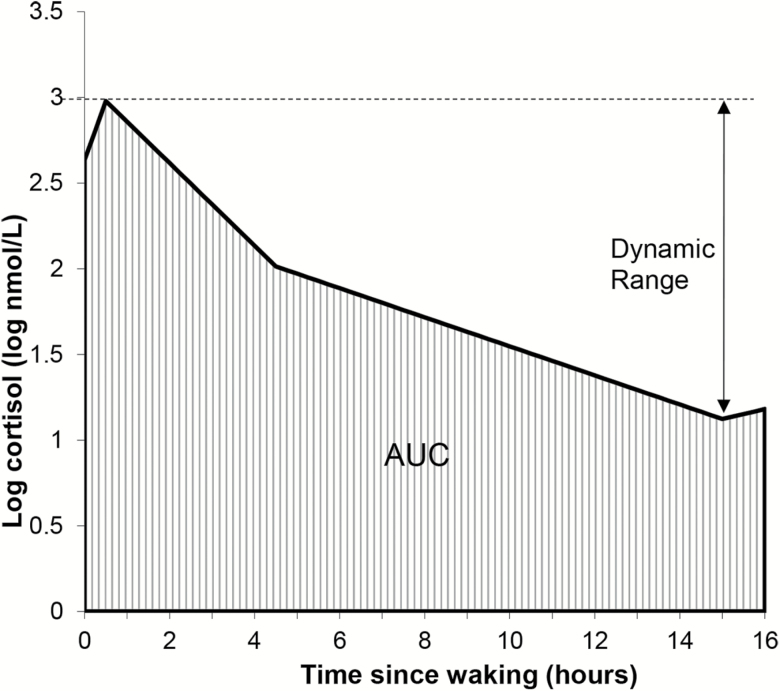
The model estimates of mean intercept and slopes (fixed effects) were combined with corresponding random effects at the family level and individual level, to get individual-specific estimates for the five growth curve parameters to characterize the individual’s intrinsic diurnal rhythm. These were then combined, using standard methods for piecewise-linear curves, to create individual-specific estimates of the log-cortisol morning peak, evening nadir, and AUC—the integrated area under the log-cortisol curve over the first 16 hr after waking; the latter was computed using the trapezoidal formula. The participant’s intrinsic diurnal cortisol dynamic range was calculated as log-cortisol peak minus log-cortisol nadir (Figure 1), which translates to log of the cortisol diurnal peak-to-nadir ratio.
Figure 1.
Diurnal dynamic range and area under the curve (AUC) of daytime cortisol (log-transformed).
Covariates
Demographic and socioeconomic characteristics, including age (in years), race/ethnicity, gender, highest level of educational attainment, and annual household income, were self-reported in the MIDUS survey. We collapsed race into two groups: non-Hispanic white versus all others. The latter group was predominantly African American (160 of 237, or 67.5%), but also included Hispanic Americans, Native Americans, and 55 individuals of mixed race/ethnicity. Education level was collapsed into a three-category variable: high school or GED or less, some college education (<4 years) but did not get a Bachelor’s degree, Bachelor’s degree or more. Annual household income (sum of self-reported earnings, pension, social security, and government assistance for all household members) was converted to family-size-adjusted income-to-poverty ratio (FIPR)—the ratio of household income to the U.S. Census Bureau’s poverty threshold specific to the participant’s age, size of household, and year of data collection. A ratio less than 1 indicates that the family is living under the poverty threshold. Social strain in adulthood (range, 0–3) was calculated as the mean rating over 14 responses about three potential sources of strain (spouse/partner, other family members, friends) to questions regarding how often (not at all: 0, a little: 1, some: 2, a lot: 3) the source “makes too many demands on you,” “criticizes you,” “lets you down when you are counting on him/her,” and “gets on your nerves.” For spouse/partner, ratings on how often he/she “argues with you” and “makes you feel tense” were also included in the average.
Statistical Analyses
We used mixed effects, linear regression to examine the associations of the three early-life adversity scores with cortisol diurnal dynamic range and AUC, adjusted for age, gender, and race/ethnicity. To account for correlations between siblings from the same family, we used a random intercept at the family level. Separate models were fit for each adversity score (as primary predictor, continuous variable) and each of the two cortisol diurnal characteristics (as dependent variable). Primary models did not include controls for adult SES and social strain, because adult economic and social characteristics may mediate some of the effects of early-life adversity on adult diurnal cortisol rhythms. To capture the direct effects of early-life adversity, a second set of models included education level (categorical), adult FIPR (continuous), and adult social strain as covariates.
Results
In the study sample of 1,696 participants, 41% were between 50 and 64 years old, 43% male, and 86% were non-Hispanic white. Nearly 40% of the samples were college grduates, but 30% had high school or less education (Table 1). Median scores for childhood economic adversity and social adversity were both zero; thus, a majority of the sample reported no economic adversity in childhood and a different majority likewise reported no childhood social adversity. However, a majority of the sample (62%) reported at least one of the six childhood adversities; 38% had one, 15% had two, 5.7% had three, 2.7% had four, and <0.4% of the sample had five adversities. The interquartile range for all three adversity scores was [0,1]; the highest scores were 3 for economic, 2 for social, and 5 for total adversity. Economic and social adversity scores were only minimally correlated with each other (Spearman correlation = .16, p < .0001).
Table 1.
Study Sample Descriptives; Study of Midlife in the United States
| Age (years) | 56.5 (12.1) |
| Men | 734 (43.3%) |
| Non-Hispanic white | 1,459 (86.0%) |
| Education, adult: high school or less | 509 (30.3%) |
| Some college but no Bachelor’s degree | 502 (29.9%) |
| Family-size-adjusted income-poverty ratio as adult | 5.2 (4.2) |
| Social strain, adult (range 0–3) | 0.98 (0.45) |
| Family on welfare (>6 months) as child | 119 (7.0%) |
| Financially worse off than average family | 176 (10.6%) |
| Neither parent completed high school | 441 (26.7%) |
| Parent death in childhood | 113 (6.7%) |
| Parents divorced/separated in childhood | 201 (11.9%) |
| Abuse by parent, sometimes or often | 613 (36.1%) |
| Childhood economic adversity score (range 0–3) | 0.43 (0.68) |
| Childhood social adversity score (range 0–3) | 0.55 (0.65) |
| Childhood total adversity score (range 0–6) | 0.98 (1.03) |
| Cortisol waking value (log nmol/L) | 2.64 (0.35) |
| Cortisol morning peak (log nmol/L) | 2.98 (0.36) |
| Cortisol evening nadir (log nmol/L) | 1.13 (0.46) |
| Cortisol diurnal dynamic range (log nmol/L) | 1.85 (0.43) |
| Cortisol diurnal area under the curve (log(nmol/L)-hr) | 29.1 (5.8) |
Note: Sample size 1,696; Mean (standard deviation) for continuous; number (%) for categories.
Mean diurnal cortisol dynamic range in the sample was 1.85 log-nmol/L, which translates to peak-to-nadir ratio of 6.4; cortisol diurnal dynamic range and AUC were modestly negatively correlated with each other (Pearson correlation r = −.30, p < .0001). As expected, dynamic range was negatively correlated with both early and later decline slopes (r = −.69 and −.91, respectively, p < .0001 for both), so that more positive slopes (or flatter declines) were associated with smaller dynamic range. Dynamic range was only weakly correlated with the morning awakening slope (r = −.08, p = .001). On the other hand, AUC was positively correlated modestly with all slopes, so that both faster morning increase and slower declines from the peak meant higher AUC. As expected, AUC was strongly correlated with both peak and nadir (r = .79 and .90, respectively, p < .0001 for both), but diurnal dynamic range was only modestly correlated with cortisol peak positively (r = .32, p < .0001) and nadir negatively (r = −.67, p < .0001).
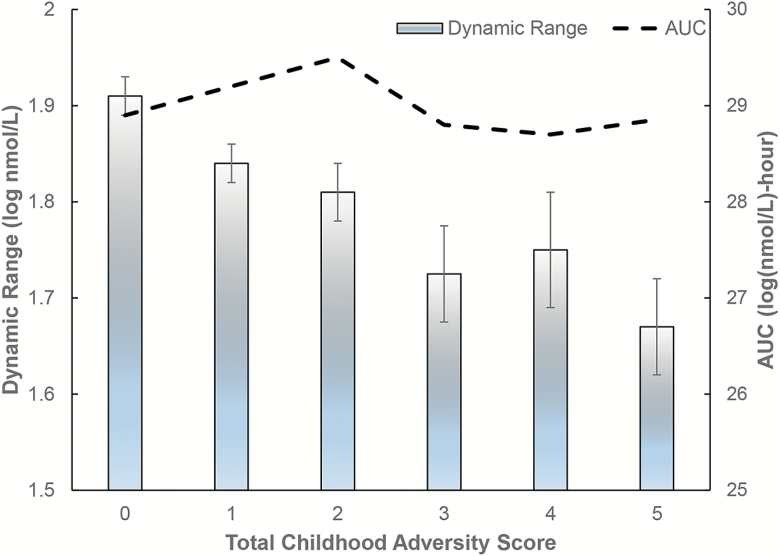
Before adjusting for covariates, mean diurnal dynamic range trended downward with increasing total childhood adversity, but AUC did not show a discernible relationship (Figure 2). Adjusted for age, gender, and race/ethnicity, both economic and social adversity in childhood were strongly associated with reduced diurnal dynamic range in adulthood, and the effect size per additional component of adversity was similar for the two types of adversity, −.039 (p = .01) versus −.034 (p = .03) log-nmol/L (Table 2). For a person who scores the maximum of 3 on the childhood economic adversity scale, this translates to 11% reduction in diurnal cortisol peak-to-nadir ratio. Consistent with the low level of correlation between economic and social adversity in childhood, the effect size per additional component of the total adversity score was similar to the individual effect sizes for the two types of childhood adversity (−.030 log nmol/L, or 3% reduction in diurnal peak-to-nadir ratio, p = .003). This implies a cumulative dose response—the greater the number of component adversities reported across economic and social domains, the greater the compression of dynamic range.
Figure 2.
Mean adult diurnal cortisol dynamic range and area under the curve (AUC) at different levels of total childhood adversity score. Error bars indicate the standard error in the mean for dynamic range.
Table 2.
Adjusted Associations of Early-Life Adversity with Cortisol Diurnal Dynamic Range and AUC; Study of Midlife in the United States
| Before controlling for adult SES and social straina |
After controlling for adult SES and social strainb |
|
|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | |
| Dynamic range (log nmol/L) | ||
| Economic adversity (0–3) | −0.039 (−0.069, −0.009)* | −0.024 (−0.054, 0.007) |
| Social adversity (0–3) | −0.034 (−0.065, −0.003)* | −0.020 (−0.052, 0.012) |
| Total adversity (0–6) | −0.030 (−0.049, −0.011)** | −0.018 (−0.039, 0.002)# |
| AUC (log(nmol/L)-hr) | ||
| Economic adversity (0–3) | 0.17 (−0.23, 0.58) | 0.12 (−0.30, 0.53) |
| Social adversity (0–3) | −0.10 (−0.52, 0.32) | −0.11 (−0.54, 0.32) |
| Total adversity (0–6) | 0.03 (−0.23, 0.30) | 0.00 (−0.27, 0.28) |
Note: AUC = area under the log-cortisol curve; CI = confidence interval; SES = socioeconomic status. Associations are from results of mixed effects models with dynamic range and AUC as dependent variables (in separate models), and include a random intercept at the family level. Effect sizes are per additional adversity (on integer scale, range 0–3 or 0–6). Separate models were run for each of the three childhood adversity scores as primary predictor.
aAdjusted for age (continuous, years), gender, race/ethnicity (non-Hispanic white vs rest). Sample size: 1,696 (from 1,411 families).
bAdjusted for age, gender, race/ethnicity, adult family-size-adjusted income-poverty ratio (continuous), education level (high school or GED or less, some college but did not get Bachelor’s degree, versus obtained Bachelor’s degree), and adult social strain. Sample size: 1,606 (from 1,335 families).
# p < .1. *p < .05. **p < .005.
In contrast to the associations with the diurnal dynamic range of cortisol, childhood adversity was not significantly associated with diurnal cortisol AUC—the cumulative total exposure to cortisol over the day—in adulthood (Table 2). In sensitivity analysis, adjusted for the same covariates, the alternate total adversity score (with more points for more severe adversity; score range, 0–9) was significantly associated with diurnal dynamic range (effect size, −0.016 log nmol/L, p = .02) but not with AUC (p = .9).
Associations with cortisol dynamic range were attenuated by a third when controls for adult SES (education and family-size-adjusted income) and adult social strain were added, and the associations were no longer statistically significant (Table 2); for example, the association of total adversity with dynamic range weakened from 3% reduction in peak-to-nadir ratio per additional domain of childhood adversity (−-.030 log nmol/L, p = .003) to 1.8% reduction (−.018 log nmol/L, p = .08). Age, race/ethnicity, and education, also had significant independent associations with diurnal cortisol dynamic range in the expected directions (in the final models with total childhood adversity): Dynamic range decreased with increasing age (p < .0001), was greater in non-Hispanic whites than in the rest (p < .0001), smaller in those who had less education in a graded fashion, and also smaller in those who reported more social conflict in adulthood (Table 3). Gender and family-size-adjusted adult income did not have independent associations with diurnal dynamic range (Table 3).
Table 3.
Adjusted Associations With Cortisol Diurnal Dynamic Range and AUC—Results of Full Models; Study of Midlife in the United States
| Dynamic range (log nmol/L) | AUC (log(nmol/L)-hr) | |
|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | |
| Age (per decade) | −0.048 (−0.066, −0.031)*** | 0.11 (0.09, 0.14)*** |
| Gender: men | −0.023 (−0.064, 0.018) | 1.43 (0.89, 1.98)*** |
| Race/ethnicity: not white | −0.296 (−0.355, −0.237)*** | −0.15 (−0.95, 0.64) |
| Education: high school or less | −0.087 (−0.139, −0.035)** | −0.44 (−1.14, 0.26) |
| some college | −0.052 (−0.102, −0.003)* | −0.34 (−1.01, 0.33) |
| FIPR, adult | 0.003 (−0.002, 0.008) | −0.01 (−0.07, 0.06) |
| Social strain, adult (0–3) | −0.053 (−0.098, −0.007)* | 0.18 (−0.43, 0.79) |
| Total early-life adversity (0–6) | −0.018 (−0.039, 0.002)# | 0.00 (−0.27, 0.28) |
Note: AUC = area under the log-cortisol curve; CI = confidence interval; FIPR = family size adjusted income to poverty ratio. Associations are from results of mixed effects models with dynamic range and AUC as dependent variables (in separate models), and include a random intercept at the family level. Sample size: 1,606 (from 1,335 families). Reference groups: women (for gender), non-Hispanic white (for race/ethnicity), Bachelor’s degree (for education).
# p < .1. *p < .05. **p < .005. ***p < .0001.
In contrast, only age and gender had significant associations with diurnal cortisol AUC, with larger AUC in older individuals and in men, relative to younger individuals and women, respectively. Race/ethnicity, and adult SES and social conflict had no association with diurnal AUC (Table 3).
Discussion
Consistent with the hypothesis that life stresses lead to compression of the cortisol dynamic range, we find that childhood adversity is indeed associated with smaller diurnal cortisol dynamic range, but is not associated with total cortisol secretion over the day. Thus, HPA axis dysregulation in those exposed to early-life stresses appears to take the form of dynamic range reduction, not increased secretion of cortisol as previously hypothesized.
The finding of a dose response, with greater compression of dynamic range in those with more adversities in childhood, is consistent with a causal relationship between early-life adversity and HPA axis dysregulation. The effect size is substantial; a score of 4 on the total childhood adversity scale (range, 0–6) is associated with 11% reduction in the diurnal peak-to-nadir ratio. The effect of each additional childhood adversity component on diurnal cortisol dynamic range (3% reduction in peak-nadir ratio) is comparable to the effect of 5 years of aging (2.4% reduction). Although a causal link from early-life adversity to adult diurnal cortisol dynamic range cannot be inferred from this study, because the associations seen were explained by adult economic and social variables, these findings, together with those of other studies, do suggest that compression of the diurnal cortisol dynamic range is the hallmark of HPA axis dysregulation in the face of life events and chronic stresses. We posit that such compression of dynamic range implies loss of allostatic reserve, which in extreme cases, leads to allostatic burnout.
Our findings are consistent with the previous finding in a small study of fibromyalgia patients that the diurnal cortisol rhythm is flattened in women who report physical abuse in childhood (Weissbecker, Floyd, Dedert, Salmon, & Sephton, 2006), and confirms recent findings from MIDUS of lower morning levels and flatter diurnal rhythms in adults who report childhood adversity (Zilioli et al., 2016). Work by Gunnar and Vazquez (2001) suggests that the effects of adversity on the diurnal cortisol rhythm start accumulating in childhood.
Our study also confirmed previous findings of smaller diurnal cortisol dynamic range in minority race/ethnicity groups (seen here even after controlling for early-life adversity and adult SES and social strain), and the absence of an association of SES, social strain, or race/ethnicity with total cortisol exposure, or AUC (Cohen et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Karlamangla et al., 2013; Skinner et al., 2011).
While others have documented relationships between early-life adversity and various aspects of the adult diurnal cortisol rhythm, such as morning peak level and slope of afternoon/evening decline, this is the first study to explicitly demonstrate compression of diurnal dynamic range in adults exposed to early-life adversity, and show that the total exposure to cortisol over the daytime (the AUC) is not affected. It brings to the forefront the importance of examining system dynamics above and beyond the current focus in the allostatic load literature on basal levels and integrated exposures such as overnight urinary levels of hormones. It should also be noted that compression of dynamic range without an effect on AUC necessarily implies reduction in peak level and elevation of nadir. The dynamic range construct captures these two effects that are in opposite directions within a single entity.
Health Consequences of Reduced Cortisol Diurnal Dynamic Range
Consistent with the allostatic load hypothesis that a reduction in adaptation capacity (or allostatic reserve) of regulatory physiological systems is a biological mechanism by which chronic life stresses impact health, blunted cortisol reactivity to acute stress has been shown to predict a variety of chronic diseases (de Rooij, 2013). If compression of the diurnal cortisol dynamic range is an indicator of reduction in allostatic reserve, then it should also have health consequences.
Pioneering work by Dallman and colleagues (1994) in rats and Rosmond and colleagues (1998) in humans established the importance of a robust diurnal cortisol dynamic range to the self-regulatory ability of the HPA axis, and its role in preventing the development of abdominal obesity and metabolic abnormalities. As predicted by their work, flatter cortisol trajectories over the waking day have been linked cross-sectionally to atherosclerosis (Toledo-Corral et al., 2013) and to poor cognitive functioning (Fiocco, Wan, Weekes, Pim, & Lupien, 2006; Johar et al., 2015). Experiments by Liston and colleagues (2013) in mouse models provide the neurological underpinning for the latter observations: They show that the diurnal cortisol peak is critical for postsynaptic dendritic spine formation in the brain cortex and promotes learning, and that the cortisol nightly trough is needed to stabilize the newly formed spines and promotes retention. On the other hand, increases in the total daytime exposure to cortisol may not be deleterious to cognitive functioning. This is also suggested by observations that rewarding experiences, such as living in an enriched environment, physical activity, sexual experience, and parenting, which increase cortisol levels, enhance (rather than diminish) adult neurogenesis (Schoenfeld & Gould, 2012).
In older women, Varadhan and collegues (2008) also document cross-sectional association between smaller diurnal dynamic range and frailty, and increased mortality risk in relation to flattened diurnal cortisol rhythm has been documented in British civil servants (Kumari, Shipley, Stafford, & Kivimaki, 2011) and in cancer patients (Sephton et al., 2013). Thus, cortisol diurnal dynamic range compression appears to have great import on health, functioning, and survival, and may represent a major biological pathway by which early-life stresses influence adult health and functioning.
Diurnal Dynamic Range Versus Stress Reactivity
Work by Rosmond and colleagues (1998) indicates that the magnitude of the acute cortisol response to stress (i.e., reactivity), at least as measured using a standardized challenge in a laboratory, is associated with adverse health only in individuals with suppressed diurnal dynamic range, and not in individuals with robust diurnal variation. This might explain the contradictory findings on the health consequences of heightened versus dampened reactivity in the literature, and suggests that diurnal dynamic range might well be a better index of system dysregulation. The diurnal dynamic range toolbox may also be useful in assessing the health of other regulatory physiological systems: Rief and colleagues (2010) have documented flattening of the circadian rhythm of the sympathetic adrenal system in people with chronic stress.
Conclusion
The findings of this study have to be interpreted in the context of study limitations, which include the observational study design which precludes definitive causal inference, the retrospective recall of early-life circumstances which could be biased by adult health and social circumstances, the small proportion of participants who reported being financially worse off than average (10%), overrepresentation of non-Hispanic whites in the study sample (86%), and use of a nonstandard early-life adversity scale. The study design does not allow control for potential confounding by health behaviors and adult health, which are likely to also be pathway variables in the link between early-life circumstances and adult cortisol regulation. In addition, information on pregnancy and menopause status at the time of the cortisol collection was not available. However, data collected at other times of contact with MIDUS participants (at the time of the survey and/or in-person biomarker collection) indicate that premenopausal women comprised less than 6% of the MIDUS sample. Strengths of the study include the large national sample, multiple days of cortisol collection, the novel focus on dynamic range, and the contrast between effects on dynamic range and total cumulative exposure. In light of these strengths and limitations, we conclude that our findings add to the large body of evidence on the lifelong consequences of early-life adversity, and shed light on a major physiological mechanism by which early-life influences adult health and successful aging. Future work will look at the role of HPA axis dynamic range compression in mediating the impact of early-life adversity on various aspects of late life health, such as osteoporosis, cardiovascular disease, and physical and cognitive decline.
Funding
This work was supported by the U.S. National Institutes on Health under grants P01-AG020166, R01-AG019239, R01-AG033067, R01-AG020166, P30-AG028748, P30-AG017265, M01-RR00865, U24-AG047867, and the UK Economic and Social Research Council and the Biotechnology and Biological Sciences Research Council under award number ES/M00919X/1. The first wave of MIDUS was supported by the John D. and Catherine T. MacArthur Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of Interest
None reported.
Author Contributions
A. S. Karlamangla planned the study, supervised the analysis, and wrote the article. S. S. Merkin performed data analysis and contributed to revising the article. D. M. Almeida planned and supervised the cortisol data collection, and helped to revise the article. E. M. Friedman contributed to the data analysis, modeled the diurnal cortisol rhythms, and helped to revise the article. J. A. Mogle participated in the cortisol data collection and cleaning, helped interpret the data, and contributed to revising the manuscript. T. E. Seeman conceptualized and helped to plan the study and revise the manuscript.
References
- Almeida D. M., McGonagle K., & King H (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55, 219–237. doi: 10.1080/19485560903382338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Mishra G., & Kuh D (2014). Life course epidemiology. In Ahrens W., & Pigeot I. (Eds.), Handbook of epidemiology (2nd ed., pp. 1521–1549). New York, NY: Springer. [Google Scholar]
- Carlson M., & Earls F (1997). Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences, 807, 419–428.doi: 10.1111/j.1749–6632.1997.tb51936.x [DOI] [PubMed] [Google Scholar]
- Carpenter L. L., Carvalho J. P., Tyrka A. R., Wier L. M., Mello A. F., Mello M. F.,…Price L. H (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry, 62, 1080–1087. doi: 10.1016/j.biopsych.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Phillips A. C., & Lovallo W. R (2012). The behavioural and health corollaries of blunted physiological reactions to acute psychological stress: Revising the reactivity hypothesis. In Wright R. A. & Gendolla G. H. E. (Eds.), Motivation perspectives of cardiovascular response (pp. 243–263). Washington, DC: APA Press. [Google Scholar]
- Cicchetti D., Rogosch F. A., Gunnar M. R., & Toth S. L (2010). The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development, 81, 252–269. doi: 10.1111/j.1467-8624.2009.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Schwartz J. E., Epel E., Kirschbaum C., Sidney S., & Seeman T (2006). Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine, 68, 41–50. doi: 10.1097/01.psy.0000195967.51768.ea [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Akana S. F., Levin N., Walker C. D., Bradbury M. J., Suemaru S., & Scribner K. S (1994). Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Annals of the New York Academy of Sciences, 746, 22–31; discussion 31. [DOI] [PubMed] [Google Scholar]
- Danese A., Moffitt T. E., Harrington H., Milne B. J., Polanczyk G., Pariante C. M.,…Caspi A (2009). Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics & Adolescent Medicine, 163, 1135–1143. doi: 10.1001/archpediatrics.2009.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes R. M. (1979). The robust beauty of improper linear models in decision making. American Psychologist, 34, 571. doi: 10.1037/0003-066X.34.7.571 [Google Scholar]
- DeSantis A. S., Adam E. K., Doane L. D., Mineka S., Zinbarg R. E., & Craske M. G (2007). Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. The Journal of Adolescent Health, 41, 3–13. doi: 10.1016/j.jadohealth.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Evans G. W. (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology, 39, 924–933. doi: 10.1037/0012-1649.39.5.924 [DOI] [PubMed] [Google Scholar]
- Fiocco A. J., Wan N., Weekes N., Pim H., & Lupien S. J (2006). Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress (Amsterdam, Netherlands), 9, 143–152. doi: 10.1080/10253890600965674 [DOI] [PubMed] [Google Scholar]
- Friedman E. M., Karlamangla A. S., Gruenewald T. L., Koretz B., & Seeman T. E (2015). Early life adversity and adult biological risk profiles. Psychosomatic Medicine, 77, 176–185. doi: 10.1097/PSY.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Dettenborn L., & Kirschbaum C (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72, 67–73. doi: 10.1016/j.ijpsycho.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Goldmann-Mellor S., Hamer M., & Steptoe A (2012). Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology, 37, 1755–1768. doi: 10.1016/j.psyneuen.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Gunnar M. R., & Vazquez D. M (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. doi: 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Hajat A., Diez-Roux A., Franklin T. G., Seeman T., Shrager S., Ranjit N.,…Kirschbaum C (2010). Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology, 35, 932–943. doi: 10.1016/j.psyneuen.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johar H., Emeny R. T., Bidlingmaier M., Lacruz M. E., Reincke M., Peters A.,…Ladwig K. H (2015). Lower morning to evening cortisol ratio is associated with cognitive impairment in men but not women: An analysis of 733 older subjects of the cross-sectional KORA-Age study. Psychoneuroendocrinology, 51, 296–306. doi: 10.1016/j.psyneuen.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Karlamangla A. S., Friedman E. M., Seeman T. E., Stawksi R. S., & Almeida D. M (2013). Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the national study of daily experiences. Psychoneuroendocrinology, 38, 2585–2597. doi: 10.1016/j.psyneuen.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. A., Mandansky D., King S., Fletcher K. E., & Brewer J (2001). Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences, 55, 71–74. doi: 10.1046/j.1440-1819.2001.00787.x [DOI] [PubMed] [Google Scholar]
- Kudielka B. M., Bellingrath S., & Hellhammer D. H (2006). Cortisol in burnout and vital exhaustion: An overview. Giornale Italiano Di Medicina Del Lavoro Ed Ergonomia, 28, 34–42. [PubMed] [Google Scholar]
- Kumari M., Shipley M., Stafford M., & Kivimaki M (2011). Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. The Journal of Clinical Endocrinology and Metabolism, 96, 1478–1485. doi: 10.1210/jc.2010-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker G. W. (1969). Human biological adaptability. The ecological approach in physical anthropology. Science (New York, N.Y.), 166, 1480–1486.doi: 10.1126/science.166.3912.1480 [DOI] [PubMed] [Google Scholar]
- Li L., Power C., Kelly S., Kirschbaum C., & Hertzman C (2007). Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology, 32, 824–833. doi: 10.1016/j.psyneuen.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Liston C., Cichon J. M., Jeanneteau F., Jia Z., Chao M. V., & Gan W. B (2013). Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature Neuroscience, 16, 698–705. doi: 10.1038/nn.3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W. R., Farag N. H., Sorocco K. H., Cohoon A. J., & Vincent A. S (2012). Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry, 71, 344–349. doi: 10.1016/j.biopsych.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2002). Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging, 23, 921–939. doi: 10.1016/S0197-4580(02)00027-1 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. doi: 10.1001/archinte.1993.00410180039004 [PubMed] [Google Scholar]
- Meinlschmidt G., & Heim C (2005). Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology, 30, 568–576. doi: 10.1016/j.psyneuen.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Miller G. E., Chen E., & Parker K. J (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137, 959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Chen E., & Zhou E. S (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Morgan C. A. III, Cho T., Hazlett G., Coric V., & Morgan J (2002). The impact of burnout on human physiology and on operational performance: A prospective study of soldiers enrolled in the combat diver qualification course. The Yale Journal of Biology and Medicine, 75, 199–205. [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah A., & Jackson S. H (2013). The physiology of ageing. Medicine, 41, 5–8. doi: 10.1016/j.mpmed.2012.10.009 [Google Scholar]
- Phillips A. C., Ginty A. T., & Hughes B. M (2013). The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology, 90, 1–7. doi: 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Pruessner M., Hellhammer D. H., & Kirschbaum C (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine, 61, 197–204. doi: 10.1097/00006842-199903000-00012 [DOI] [PubMed] [Google Scholar]
- Rief W., Mills P. J., Ancoli-Israel S., Ziegler M. G., Pung M. A., & Dimsdale J. E (2010). Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosomatic Medicine, 72, 755–762. doi: 10.1097/PSY.0b013e3181f367e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij S. R. (2013). Blunted cardiovascular and cortisol reactivity to acute psychological stress: A summary of results from the Dutch Famine Birth Cohort Study. International Journal of Psychophysiology, 90, 21–27. doi: 10.1016/j.ijpsycho.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Rosmond R., Dallman M. F., & Björntorp P (1998). Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. The Journal of Clinical Endocrinology and Metabolism, 83, 1853–1859. doi: 10.1210/jcem.83.6.4843 [DOI] [PubMed] [Google Scholar]
- Schoenfeld T. J., & Gould E (2012). Stress, stress hormones, and adult neurogenesis. Experimental Neurology, 233, 12–21. doi: 10.1016/j.expneurol.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton S. E., Lush E., Dedert E. A., Floyd A. R., Rebholz W. N., Dhabhar F. S.,…Salmon P (2013). Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior, and Immunity, 30 (Suppl), S163–S170. doi: 10.1016/j.bbi.2012. 07.019 [DOI] [PubMed] [Google Scholar]
- Sjogren E., Leanderson P., & Kristenson M (2006). Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. International Journal of Behavioral Medicine, 13, 193–200. doi: 10.1207/s15327558ijbm1303_2 [DOI] [PubMed] [Google Scholar]
- Skinner M. L., Shirtcliff E. A., Haggerty K. P., Coe C. L., & Catalano R. F (2011). Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology, 23, 1167–1186. doi: 10.1017/S095457941100054X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P., & Eyer J (1988). Allostasis: A new paradigm to explain arousal pathology. In: Fisher S. & Reason J. (Eds.), Handbook of life stress, cognition, and health (pp. 631–651). New York, NY: John Wiley and Sons. [Google Scholar]
- Straus M. A. (1979). Measuring intrafamily conflict and violence: The conflict tactics (CT) scales. Journal of Marriage and Family, 41, 75–88. doi: 10.2307/351733 [Google Scholar]
- Taylor S. E., Way B. M., & Seeman T. E (2011). Early adversity and adult health outcomes. Development and Psychopathology, 23, 939–954. doi: 10.1017/S0954579411000411 [DOI] [PubMed] [Google Scholar]
- Toledo-Corral C. M., Myers S. J., Li Y., Hodis H. N., Goran M. I., & Weigensberg M. J (2013). Blunted nocturnal cortisol rise is associated with higher carotid artery intima-media thickness (CIMT) in overweight African American and Latino youth. Psychoneuroendocrinology, 38, 1658–1667. doi: 10.1016/j.psyneuen.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhan R., Walston J., Cappola A. R., Carlson M. C., Wand G. S., & Fried L. P (2008). Higher levels and blunted diurnal variation of cortisol in frail older women. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 63, 190–195.doi: 10.1093/gerona/63.2.190 [DOI] [PubMed] [Google Scholar]
- Weissbecker I., Floyd A., Dedert E., Salmon P., & Sephton S (2006). Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology, 31, 312–324. doi: 10.1016/j.psyneuen.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Zilioli S., Slatcher R. B., Chi P., Li X., Zhao J., & Zhao G (2016). Childhood adversity, self-esteem, and diurnal cortisol profiles across the life span. Psychological Science, 27, 1249–1265. doi: 10.1177/0956797616658287 [DOI] [PMC free article] [PubMed] [Google Scholar]