Abstract
Objective
To evaluate, in vivo, the impact of ongoing chronic migraine (CM) attacks on the endogenous μ-opioid neurotransmission.
Background
CM is associated with cognitive-emotional dysfunction. CM is commonly associated with frequent acute medication use, including opioids.
Methods
We scanned 15 migraine patients during the spontaneous headache attack (ictal phase): 7 individuals with CM and 8 with episodic migraine (EM), as well as 7 healthy controls (HC), using positron emission tomography (PET) with the selective μ-opioid receptor (μOR) radiotracer [11C]carfentanil. Migraineurs were scanned in two paradigms, one with thermal pain threshold challenge applied to the site of the headache, and one without thermal challenge. Multivariable analysis was performed between the μ-opioid receptor availability and the clinical data.
Results
μOR availability, measured with [11C]carfentanil nondisplaceable binding potential (BPND), in the left thalamus (P-value = 0.005) and left caudate (P-value = 0.003) were decreased in CM patients with thermal pain threshold during the ictal phase relative to HC. Lower μOR BPND in the right parahippocampal region (P-value = 0.001) and right amygdala (P-value = 0.002) were seen in CM relative to EM patients. Lower μOR BPND values indicate either a decrease in μOR concentration or an increase in endogenous μ-opioid release in CM patients. In the right amygdala, 71% of the overall variance in μOR BPND levels was explained by the type of migraine (CM vs. EM: partial-R2 = 0.47, P-value<0.001, Cohen's effect size d = 2.6SD), the severity of the attack (pain area and intensity number summation [P.A.I.N.S.]: partial-R2 = 0.16, P-value = 0.031), and the thermal pain threshold (allodynia: partial-R2 = 0.08).
Conclusions
Increased endogenous μ-opioid receptor-mediated neurotransmission is seen in the limbic system of CM patients, especially in right amygdala, which is highly modulated by the attack frequency, pain severity, and sensitivity. This study demonstrates for the first time the negative impact of chronification and exacerbation of headache attacks on the endogenous μ-opioid mechanisms of migraine patients. ClinicalTrials.gov identifier: NCT03004313
Keywords: Migraine, PET, Opioid, Central pain, MRI, Thermal pain threshold
Abbreviations: CM, chronic migraine; EM, episodic migraine; Ictal, patients during the headache; HC, healthy controls; μOR, μ-opioid receptor; PET, positron emission tomography; BPND, non displaceable binding potential; P.A.I.N.S., pain area and intensity number summation; PAG, periaqueductal gray matter; mPFC, medial prefrontal cortex; STPT, sustained thermal pain threshold; V1, trigeminal ophthalmic region; mCi, Milli-Curie, non-SI unit of radioactivity; MBq, mega-Becquerel, SI derived unit of radioactivity; FWHM, full-width at half maximum resolution; FOV, field of view; T1, longitudinal relaxation; K1, first order kinetic rate constant; fND, free fraction of the radiotracer in non-displaceable tissue; MRI, magnetic resonance imaging; Bmax, concentration of available receptors to the radiotracer; KD, affinity constant of the radioligand for the receptor sites; FAST-SPGR, fast spoiled gradient echo; TE, echo time; TR, repetition time; TI, inversion time; Nex, number of excitations; VAS, pain visual analog scale; FC, functional connectivity; MNI, montreal neurological institute space
Graphical abstract
Highlights
-
•
Increased endogenous μ-opioid neurotransmission in limbic system of chronic migraineurs
-
•
Right amygdala opioid dysfunction is 71% explained by attack frequency, severity and sensitivity.
-
•
Amygdala dysfunction links cognitive-emotional brain mechanisms to migraine suffering.
1. Introduction
Migraine is a potentially progressive disorder, and in a given year, around 3% of individuals in the general population will develop chronic migraine (CM – frequent migraines and headaches on at least 15 days per month), while other 6% develop high frequency episodic headaches (10–14 days of headache per month) (Bigal et al., 2008a). According to the American Migraine Prevalence and Prevention Study, 11.1% of episodic migraine (EM) and 34.3% of CM patients routinely use opioids (Bigal et al., 2008b, Bigal et al., 2009). The number of migraine sufferers that use opioids can be as high as 72% in some in-patient clinics (Nijjar et al., 2010).
In addition to excessive symptomatic medication use, thermal pain threshold (allodynia) is also a risk factor for CM (Benatto et al., 2017), which makes the relationship between opioid use and CM complex, since opioids are potent inducers of hyperalgesia/allodynia (De Felice and Porreca., 2009). Accordingly, opioid use, endogenous opioid release, and μ-opioid receptor (μOR) concentrations are critical elements for the understanding of general pathophysiology and worsening of pain in CM. So far, the only direct investigation of endogenous μ-opioid activation in vivo was done with positron emission tomography (PET) in EM patients with allodynia. This study showed a decrease in μOR non-displaceable binding potential (BPND) with [11C]carfentanil (DaSilva et al., 2014a). μOR BPND is a selective measurement of μOR availability in vivo, and its immediate decrease during EM headache attack (ictal phase) and allodynia mostly suggested the existence of a transitory increase in ictal activation of endogenous μ-opioid neurotransmission in the periaqueductal gray matter (PAG) and medial prefrontal cortex (mPFC), rather than a sharp change in the number of μOR (DaSilva et al., 2014b; Nascimento et al., 2014).
It is unknown, though, whether the chronification and severity of migraine attacks would have a significant negative effect on μOR availability in cognitive and emotional systems of the brain. Hence, to evaluate in vivo the dynamic of endogenous μ-opioid neurotransmission during CM attacks, we used PET molecular imaging to thoroughly investigate the impact of more severe forms of migraine and thermal pain threshold on the human endogenous μ-opioid system activity.
2. Methods
2.1. Participants and study design
11 healthy controls (HC), 13 EM, and 8 CM between the ages of 20 and 45 were recruited by advertisement at the University of Michigan and surrounding areas. Enrollment was initiated by phone screening, followed by formal diagnosis as per the International Headache Society Classification (ICHD-3-beta) (Headache Classification. Committe of the International Headache Society, 2004). Exclusion criteria included pregnancy, opioid and hormonal contraceptive use 6 months prior to enrollment, other chronic pain disorders, as well as clinically relevant systemic medical and psychiatric illnesses. A urine drug screening was performed on all subjects to eliminate the possibility of substance abuse, such as cocaine, amphetamine, methamphetamine, marijuana, and opioids.
Ten individuals from the experimental group had to be excluded from the analyses. One CM and 5 EM had missing PET data during migraine attack. 4 HC were not matched for age and gender. The final sample was 7 HC and 15 migraineurs, divided into 8 EM and 7 CM, matched for age and gender. Demographic information is displayed in Tables 1 and 2.
Table 1.
Clinical profile of episodic and chronic migraine participants enrolled in this study.
| Subjects | Gender | Age | Diagnosisa | Chronicity in years | Pain frequencyd | Attack duration (Hours) | Pain intensityb, c | GeoPain craniofacial areae | GeoPain intensityf 1:mild-3:severe | Severityg (P.A.I.N.S.) | STPTcThermal Threshold-°C | Usual abortive medicationh | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic Migraine characteristics | ||||||||||||||
| 1 | Female | 20 | w/ aura | 9 | 16 | 3 | 4 | 56 | 25% | 2 | 112 | 17% | 47.63 | none |
| 2 | Male | 27 | w/o aura | 6 | 30 | 4 | 2 | 10 | 5% | 1 | 10 | 2% | 42.12 | Acetaminophen |
| 3 | Female | 28 | w/ aura | 10 | 16 | 24 | 7 | 5 | 2% | 2 | 10 | 2% | 45.32 | Sumatriptan |
| 4 | Female | 23 | w/o aura | 6 | 17 | 24 | 5 | 8 | 4% | 2 | 16 | 2% | 40.95 | none |
| 5 | Female | 45 | w/o aura | 18 | 30 | 24 | 9 | 32 | 15% | 3 | 96 | 15% | 49.44 | none |
| 6 | Female | 20 | w/o aura | 2 | 15 | 4 | 6 | 83 | 38% | 2 | 166 | 25% | 44.83 | none |
| 7 | Female | 32 | w/o aura | 25 | 16 | 6 | 7 | 53 | 24% | 2 | 106 | 16% | 41.4 | none |
| Episodic Migraine characteristics | ||||||||||||||
| 1 | Male | 21 | w/ aura | 7 | 2 | 12 | 6 | 64 | 29% | 2 | 128 | 19% | 45.2 | Ibuprofen |
| 2 | Female | 26 | w/o aura | 15 | 8 | 12 | 6 | 19 | 9% | 2 | 38 | 6% | 45.91 | Acetaminophen |
| 3 | Female | 21 | w/o aura | 5 | 4 | 12 | 8 | 75 | 34% | 3 | 225 | 34% | 39.53 | none |
| 4 | Female | 38 | w/ aura | 20 | 6 | 72 | 6.2 | 42 | 19% | 2 | 84 | 13% | 36.45 | Acetaminophen |
| 5 | Female | 36 | w/ aura | 20 | 12 | 72 | 8.6 | 30 | 14% | 3 | 90 | 14% | 49.08 | Acetaminophen |
| 6 | Male | 22 | w/ aura | 6 | 8 | 24 | 6.7 | 48 | 22% | 2 | 96 | 15% | 34.33 | Acetaminophen |
| 7 | Male | 26 | w/ aura | 2 | 2 | 5 | 5 | 33 | 15% | 2 | 66 | 10% | 40.68 | none |
| 8 | Male | 26 | w/o aura | 20 | 4 | 6 | 8 | 13 | 6% | 3 | 39 | 6% | 42.77 | Naratriptan |
Based on ICHD-3 beta (However, none of the participants reported visual aura preceding or during the ictal PET scan).
Migraine headache intensity recorded during ictal PET scan.
Measures at ictal PET scan.
Average attack-days per month.
Migraine headache area recorded immediately after ictal PET scan (GeoPain, MoxyTech, Inc. MI).
Migraine headache intensity recorded immediately after ictal PET scan (GeoPain, MoxyTech, Inc. MI).
Pain area and intensity number summation (P.A.I.N.S.) recorded immediately after ictal PET scan (GeoPain, MoxyTech, Inc. MI).
Preventive medication was an exclusion criteria, and abortive medication was not allowed 48 h prior the ictal PET scans.
Table 2.
Clinical profile of healthy controls enrolled in this study.
| Subjects | Gender | Age | STPT thermal threshold-°C |
|---|---|---|---|
| 1 | Male | 24 | 47.43 |
| 2 | Female | 24 | 39.20 |
| 3 | Female | 22 | 49.99 |
| 4 | Female | 41 | 42.06 |
| 5 | Female | 25 | 49.63 |
| 6 | Female | 21 | 43.42 |
| 7 | Female | 33 | 46.31 |
Participants underwent one MRI and one PET on different days. Scanning of patients was performed during the spontaneous headache phase (ictal) of their migraine. CM patients had almost one daily migraine headache attack per month. A pain specialist confirmed the migraine attack prior to the PET session. Patients were asked to abstain from using abortive medications and were only imaged if they had not used these medications within 48 h prior to the scan. They were allowed to take their usual rescue medication only after the imaging.
2.2. Standard protocol approvals, registrations and patient consents
The University of Michigan Institutional Review Board and the Radioactive Drug Research Committee approved the study, and all participants provided written informed consent after the initial screening. ClinicalTrials.gov identifier: NCT03004313.
2.3. Response to cutaneous thermal stimulus (allodynia)
We applied a sustained thermal pain threshold (STPT) challenge, developed in-house (Nascimento et al., 2014), with adjusted intensity to the trigeminal ophthalmic region (V1) of the participants during the late phase of the PET scan (ictally therefore). A light forehead system was used to hold a 16 mm2 thermal probe (Pathway Model- MEDOC, Ramat Yishai, Israel), which was placed in contact with the forehead area (V1), ipsilateral to the headache. We applied the challenge for 20 min, which was appropriate to estimate μOR availability during a steady-state situation from 40 to 60 min post-radiotracer administration, and to collect enough data points for quantification. The temperature increased 1 °C/s every 10 s starting from a 32 °C baseline to a 50 °C maximum borderline. In this STPT model, the intensity of the temperature was controlled by tapping a mouse used for participants' feedback at every first perception of pain to instantly adjust the temperature back to baseline level.
2.4. Neuroimaging
We used PET for molecular neuroimaging in vivo to evaluate the endogenous μ-opioid response to the pain severity and the frequency of headache attacks in migraine at baseline (rest) and during ictal thermal pain threshold experience. Ninety minutes of PET scan per participant was sufficient to perform an early phase acquisition as a baseline (5–40 min) during headache (at rest), followed by the STPT challenge described above for 20 min in late phase (45–90 min) (thermal pain threshold response). Both phase acquisitions were performed in the same PET ictal session. Participant received [11C]carfentanil, a selective μ-opioid receptor radiotracer, intravenously (Jewett, 2001; Titeler et al., 1989). The total dose injected of [11C]carfentanil was 15 mCi (555 MBq), with a maximum mass of 0.03 μg per kilogram of body weight. Fifty-percent of this dose was administered as a bolus, followed by a continuous and constant infusion rate of the remainder to quickly achieve the steady-state tracer levels (Koeppe et al., 1997). We used a 3D mode Siemens HR+ scanner (Knoxville, TN), with septa retracted, to acquire PET images (full-width at half maximum resolution (FWHM) ~5.5 mm in-plane and 5.0 mm in z-axis). Interactive algorithms were used to reconstruct PET images into a 128 × 128 pixel-matrix in a 24 × 24 cm field of view (FOV), with attenuation and scatter corrections. A motion artifact correction was performed by a linear co-registration through 21 frames of dynamic PET images. We then transformed PET images, on a voxel-by-voxel basis, into two sets of parametric maps: (1) K1 ratio, a tracer transport measure usually used for PET-MRI image co-registration and normalization, (2) BPND (non-displaceable binding potential), a receptor related-measure estimated using a Logan plot with the occipital cortex, a μOR-free reference region, as input function (Logan et al., 1996). BPND is fND × Bmax/KD, where fND is the free fraction of the radiotracer in non-displaceable tissue, and assumed to be constant across the brain, Bmax is the concentration of available receptors to the radiotracer, and KD is the affinity constant of the radioligand for the receptor sites. Acute changes in BPND provide a quantitative index of endogenous opioid release interacting with μORs.
We used a 3 T MRI scanner (GE, Milwaukee, WI, USA) to acquire axial T1-weighted anatomical images using FAST-SPGR pulse sequence with the following parameters: TE/TR/TI = 3.4/10.5/200 ms, flip angle = 25o, FOV = 240 × 240 mm, 256 × 256 × 144 acquisition matrix, 1.5 mm slice thickness, NEX = 1.
PET and MRI images were aligned together before being warped to MNI-ICBM stereotaxic atlas space using a statistical parametric mapping (SPM8-v6313) package in Matlab (MathWorks, R2015b). Therefore, T1 images were co-registered with K1 images using a mutual information algorithm (Meyer et al., 1997). The transformation matrix was then applied to BPND images. A non-linear warping algorithm DARTEL was then performed to standardize the anatomical MR images to MNI space. The transformation matrix was then applied to both BPND and K1 ratio images (Zubieta et al., 2001; Zubieta et al., 2002). We smoothed the normalized and resampled BPND images (2 mm voxels) with a 3-3-2 mm Gaussian Kernel to overcome the inter-subject variability of signal and improve the signal-to-noise ratio. Two patients' images were flipped to have the thermal painful challenge (induced on the right or the left trigeminal ophthalmic side) on the same side of other images for all participants. Image data were therefore presented as right-sided headaches for analysis, as the majority of patients have headaches on the right side.
2.5. Statistical analysis
Two-sample t-test, on a voxel-by-voxel basis (DaSilva et al., 2017), was performed using SPM8 to examine differences in BPND between the following groups: CM and HC, and CM and EM. Voxels with P value of <0.01 were considered to indicate a statistically significant difference as a first stage of group analysis in this study. We also performed a posteriori regions of interest analysis (ROIs) as a second stage of group analysis based on the amygdala, parahippocampal region, caudate, and thalamus, which are related to pain and opioidergic function, as identified in Zubieta et al. studies (Schrepf et al., 2016; Nuechterlein et al., 2016; Harris et al., 2007; Domino et al., 2015; Ray et al., 2011). ROIs that were significant after cluster-level FWE correction (p < 0.05) were extracted for further analysis using MarsBaR (Brett et al., n.d.). A multivariable linear regression was implemented to estimate the association of μOR BPND outcome with clinical data (pain area and intensity, pain visual analog scale (VAS), challenge level, and migraine type (adjusting for age and gender of subject as needed). The multivariable linear regression was performed using Statistical Analysis Software (SAS 9.4). The most parsimonious model was selected removing covariates with P-value >0.1.
Power Analysis: The study had 80%Power to detect an effect size of 1.57SD or larger for group comparisons of CM (n = 7) vs EM (n = 8), and an overall R-Square of 47% or larger for multivariable regression with 3 predictors.
2.6. Mobile application for brain mapping
Participants were solicited to map their migraine pain area and headache intensity numerically in order to obtain an objective sensory-discriminative information of the attacks; this was analyzed by using GeoPain (MoxyTech Inc., MI) (DaSilva et al., 2014b), an interactive mobile application developed in-house, during each step of the protocol. As a result, Pain Area and Intensity Number Summation (P.A.I.N.S.) was also obtained for each participant. Different from our previous studies (DaSilva et al., 2014b), we collected P.A.I.N.S data immediately after the thermal PET challenge and sessions in order to be close in time to the thermal pain threshold task, which was the focus of this study. Only in one migraine patient was P.A.I.N.S. data obtained before PET due to time constraint.
3. Results
3.1. Clinical measures
In total, 15 migraine patients were scanned during the spontaneous ictal phase. For CM (1 M/6F), mean age was 28 ± 8.74 years old. They had CM for an average of 10.85 ± 7.96 years, and mean frequency of 20 ± 6.85 days of headache attacks per month. EM sufferers' (4 M/4F) mean age was 27 ± 6.6 years old, and they had been suffering from headaches for 11.87 ± 7.66 years, with a mean of 5.75 ± 3.45 headache attacks per month. Mean age of controls (1 M/6F) was 27.14 ± 10.11 years old. There were no significant age differences among the three groups (P-value = 0.67). No headache attacks were reported within three days of PET scans for EM. Additional clinical information is displayed in Table 1 and Table 2.
At the end of PET scans, mean pain intensity was 5.71 ± 2.29 (CM) and 6.81 ± 1.25 (EM) (visual analog scale (VAS) [1-10]) (Table 1). On a four point scale, values were 2 ± 0.57 (CM) and 2.37 ± 0.51 (EM) (GeoPain [1:mild−3:severe]) (Fig. 1A). The average pain head and facial extension areas for CM was 35.29 ± 29.81 square units for the headache attacks, equivalent to 16% out of 220 square units; resulting in a headache intensity a P.A.I.N.S average of 11%. The average pain head and facial extension areas for EM was 40.5 ± 21.33, equivalent to 18% out of 220 square units; resulting in a headache intensity P.A.I.N.S average of 15% (Table 1). On average, the ictal PET scans for EM occurred 7 h and 36 min (SD 4 h) after onset of the migraine.
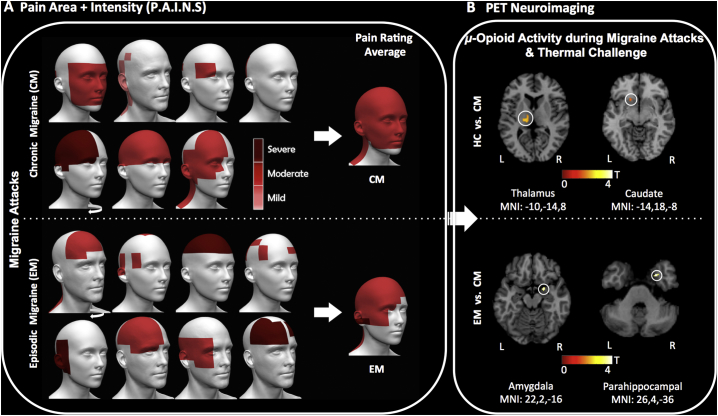
Fig. 1.
Migraine headache severity and endogenous μ-opioid ictal activation during PET. (A) Schematic of individual recorded migraine headaches and facial P.A.I.N.S from the 7 CM and 8 EM patients after the ictal PET session, using the GeoPain mobile application. Sequence of participants follows Table-1, from top left to lower right. The curved white arrows indicate that the head of this patient was flipped to show the most intense headache on the left side. The 3D head images at the center represent the average rating of P.A.I.N.S. Headache color scale: Mild (1 – light red), Moderate (2 – red), and Severe (3 – dark red). (B) PET images with the endogenous μ-Opioid activation during migraine headache attack and trigeminal thermal pain threshold in the left thalamic (MNI_[-10,-14,8]) and left caudate (MNI_[-14,18,-8]) for 7 CM vs. 7 HC (P-values = 0.005 and 0.003, respectively) (top), as well as in the right amygdala (MNI_[22,2,-16]) and the right parahippocampal gyrus (MNI_[26,4,-36]) for 7 CM vs. 8 EM during attack (P-values = 0.002 and 0.001, respectively) (bottom). Both indicate a higher release of endogenous μ-opioid during CM attack and thermal pain threshold. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. μ-opioid receptor BPND during ictal phase
A significant difference in μOR BPND was observed between HC and CM participants during headache attacks and the thermal cutaneous pain threshold challenge (STPT) (late phase scan period). Specifically, reduced regional μOR BPND was noticed in the left thalamus (MNI_[-10,-14,8], P-value = 0.005) and left caudate (MNI_[-14,18,-8], P-value = 0.003) in the CM patients compared to HC (Fig. 1B-top).
When compared to EM patients, a significant decrease in μOR BPND was also noticed in CM patients, but only during late phase analyses (headache attacks + thermal cutaneous pain threshold), indicating enhancements in CM vs EM in endogenous μ-opioid neurotransmission in the limbic system within the right amygdala (MNI_[22,2,-16], P-value = 0.002) and the right parahippocampal voxels in the proximity of the entorhinal cortex (MNI_[22,4,-28], P-value = 0.007 and MNI_[26,4,-36], P-value = 0.001]) (Fig. 1B-bottom). Cluster-wise correction based on the significant ROIs is shown in Table 3.
Table 3.
P-value, Z-value and MNI coordinates of the regions of interest shown in Fig. 1b correspond to the difference in BPND between the late phase of EM and CM (EM > CM), and HC versus CM (HC > CM) scans. *Regions where FWE cluster significance is <0.05.
| Regions of interest | P-value | Z-value | MNI : x y z (mm) |
|---|---|---|---|
| L-Caudate | *0.036 | 2.79 | -14 18 -8 |
| L-Thalamus | 0.005 | 2.59 | -10 -14 8 |
| R-Amygdala | *0.01 | 2.82 | 22 2 -16 |
| R-Parahippocampal | 0.007 | 2.45 | 22 4 -28 |
| R-Parahippocampal | *0.009 | 3.16 | 26 4 -36 |
In multivariate analyses, acute activation of the endogenous μ-opioid neurotransmission interacting with μOR was highly correlated with type of the migraine (CM vs. EM), sensory-discriminative pain severity of the ongoing migraine attacks (P.A.I.N.S.: area vs. intensity), and allodynia (ictal thermal pain threshold) (overall R2 = 0.71) (Fig. 2A). Age and gender effects were not significant and were not included in the final model.
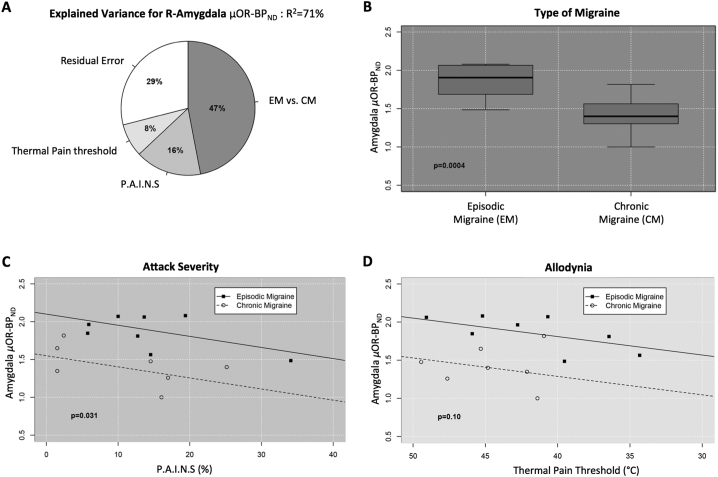
Fig. 2.
Altered μOR of the right amygdala regarding type of migraine, attack severity, and thermal pain threshold. (A) Pie graph illustrating the relative percentages of variance (R2) explained by μOR BPND level in the right amygdala (MNI_[22,2,-16]), during the attack, in response to migraine type, P.A.I.N.S, and cutaneous thermal pain threshold (overall R2 = 71%). Amygdala μOR BPND is mostly affected by the type of migraine (partial-R2 = 47%) compared to P.A.I.N.S (partial-R2 = 16%) and thermal pain threshold sensitivity (partial-R2 = 8%). P.A.I.N.S and thermal pain threshold sensitivity were set at their mean value (85.47 and 43.04 respectively). (B–D) Scatterplot analysis of amygdala μOR BPND in response to each of the three variables shown in (A), adjusted for the other two. (B) Type of migraine: significant lower amygdala μOR BPND for CM vs. EM (P-value<0.001). (C) Attack severity: adjusted linear regression model indicating a significant negative linear correlation between P.A.I.N.S. and amygdala μOR BPND levels (P-value = 0.031). (D) Thermal pain threshold: adjusted linear regression model showing a marginal positive linear correlation between amygdala μOR BPND and cutaneous STPT (P-value = 0.1).
We noticed that the level of endogenous μ-opioid activation during attacks was highly affected by CM vs. EM (standardized beta: β =-0.87, 95%CI = (-1.25,-0.48), P-value<0.001, Cohen's effect size d = 2.6SD), after controlling for P.A.I.N.S. and thermal cutaneous pain threshold) (Fig. 2B). This evidence indicates a higher release of endogenous μ-opioid or loss of μ-opioid receptors available during migraine attacks for CM patients. Controlling for type of migraine and thermal cutaneous thermal pain threshold, which suggests allodynia, there was a negative correlation between μOR BPND and P.A.I.N.S. (β =-0.4, 95%CI = (-0.77,-0.04), P-value = 0.031) (Fig. 2C). This indicates an increase in endogenous μ-opioid interacting with μORs during ongoing pain in intensity and extension combined [P.A.I.N.S]. However, no correlations were found with μOR BPND based on the attack pain intensity or area separately, its chronicity, or the traditional VAS score. As mentioned above, there was a correlation with μOR BPND in the right amygdala for the type of migraine (CM vs. EM), but the correlation disappears when taking in consideration the frequency of the attacks per month alone. Finally, there was a marginally significant relationship between thermal cutaneous thermal pain threshold challenge and μOR BPND in the right amygdala (β = 0.3, 95%CI = (-0.07,0.68), P-value = 0.1) (Fig. 2D). The lower the cutaneous STPT, the more release of endogenous μ-opioid in the right amygdala.
4. Discussion
Our findings show, for the first time in humans, that in CM patients during headache attack under cutaneous thermal pain challenge, there is significantly lower μOR BPND in the thalamus and caudate compared to HC. Additionally, when compared to EM patients during spontaneous headache attacks with increased thermal pain sensitivity (allodynia), CM patients showed that this ictal μOR dysfunction extended to their limbic system, including the parahippocampal region and amygdala, which are highly correlated (71%). Notably, in the right amygdala, such lower levels of μOR BPND greatly correlated with the worsening of the headache attacks and, to some extent, the decrease in the ictal thermal pain threshold. As we noticed more significant changes in the limbic μOR BPND in migraineurs only during the ictal thermal pain challenge, but not at ictal at-rest in the same PET session, the limbic dysfunction in CM implies an acute shift in receptor occupancy by endogenous opioids rather than a temporary change in the number of μORs. Hence, our data indicates that there is a significant increase in endogenous μ-opioid receptor-mediated neurotransmission in the human brain areas associated with negative emotional response during persistence and worsening of the ongoing attack and pain sensitivity (thermal allodynia) in CM (Fig. 2).
The limbic system is responsible in great part for the processing of our emotional and motivational responses, decision-making, and memory consolidation. Migraine has been linked to memory disturbances and depression, especially with higher frequency of attacks (Calandre et al., 2002; Seng et al., 2017), and is also associated with altered functional connectivity and structural plasticity in key limbic structures, including the parahippocampal area (e.g., enthorhinal cortex), hippocampus, and amygdala (Liu et al., 2017). Neuroimaging studies of low and high frequency migraine patients have reported morphometric changes in the enthorhinal cortex as one the main distinguishers of CM (Schwedt et al., 2015). This is a well-known limbic structure that modulates anxiety-driven hyperalgesia (Veinante et al., 2013), plays an important role in memory formation and consolidation, and is the main interface between the hippocampus and neocortex. Hippocampal volume has also correlated negatively with the headache frequency in chronic migraineurs (Liu et al., 2017). Such neuroplasticity in migraineurs seems to extend to other cortico-limbic regions associated with attack-related thermal pain threshold, and increased functional connectivity in the amygdala with other brain regions has been observed in CM compared to EM patients (Chen et al., 2017).
The amygdala is well-known for its ability to activate arousal systems that affect information processing across the brain, triggering response-behavioral and neurophysiological changes that induce bottom-up attentional control over memory retrieval and sensory processing (LeDoux et al., 2017). In fact, in rodent models with noxious stimuli, opioidergic dysfunction in the amygdala has been strongly connected to an increase in anxiety-like behaviors (Narita et al., 2006a, Narita et al., 2006b), especially when there is persistence of the pain inputs. Not surprisingly, recent studies have demonstrated an enhanced response to negative and aversive stimuli in migraine Wilcox et al., 2016). Furthermore, the involvement of the amygdala in chronic pain and migraine suffering might also be modulated by its lateralization of function (Liu et al., 2017). Nociceptive inputs equally reach both the left and right amygdala, but it is the right amygdala that plays a major role in the processing of nociceptive and emotional components of pain (Carrasquillo and Gereau, 2008), which takes place through different opioid receptor-mediated mechanisms, including μ and κ, as demonstrated in recent migraine and thermal pain threshold animal models (Xie et al., 2017). This is consistent with our current findings in vivo showing that the variance of ictal μ-opioid neurotransmission in the right amygdala of migraineurs during the ongoing attacks is mostly (71%) dictated by the clinical combination of their type of migraine (CM > EM), the severity of the ongoing headache, and thermal pain sensitivity (Fig. 2).
Three main observations can be taken from our data about the clinical impact of CM in the ictal endogenous μ-opioid mechanisms in the right amygdala. First, the CM vs. EM difference used in our study, based on the International Headache Society classification, suggests that ≥15 headache days/month frequency may have some pathophysiological significance regarding opioidergic dysfunction in migraine. Second, the pain intensity or area measurements of the ongoing headache attacks alone didn't show individual correlation with the limbic opioid activation, but only when both were combined to give a more precise picture of the sensory-discriminative aggravation of the attacks. Third, the ictal migraine thermal pain sensitivity (allodynia) contribution to the μ-opioid activation in the right amygdala is highly consistent with the concept of sensitized circuits in CM patients promoting amplification of nociception and increased emotional responses (Chen et al., 2017). When the CM patient is challenged with innocuous stimuli during the persistent attacks, the amygdala seems to contribute to sensitized avoidance-behavior neuromechanisms that help to protect the patient from interaction with the environment (e.g., exposure to heat, sound, light) until the headache subsides (DaSilva et al., 2017).
The sensory process and modulatory neuromechanisms in repeated migraine attacks with thermal pain threshold have also been reported in CM animal and human models. In a rat model of CM induced by repeated meningeal infusion of inflammatory soup, there were notable changes in functional connectivity (FC) between PAG with PFC, thalamus, and other regions involved in nociception (Jia et al., 2017). In humans, similar changes in FC where found in high frequent episodic migraineurs between PAG and PFC (Solstrand and Dahlberg et al., 2018). We previously demonstrated an increase in μ-opioid receptor-mediated neurotransmission in PAG and mPFC during a spontaneous EM attacks and allodynia (DaSilva et al., 2014a, DaSilva et al., 2014b), when compared to the interictal phase. Herein, when ictal CM attacks where compared with HCs during thermal challenge, we noticed that μ-opioid system activity was also increased in the thalamus and caudate, confirming that important brain regions generally involved in sensory processing and modulation of pain are also affected at the circuit level, facilitating ascending cephalic pain inputs with inefficient inhibitory pain modulatory responses to external stimuli (Jia and Yu., 2017; Wunderlich et al., 2011). Simultaneously, this inefficiency of the overflowing opioidergic system in CM could be potentially induced at a certain level by receptor-mediated mechanisms like opioid-induced hyperalgesia associated with central sensitization, including expansion of receptive fields, increased transmitter release, long-term potentiation, N-methyl-d-aspartate mechanisms as well as medication overuse-headaches, which are commonly linked to repeated exposure to exogenous μ-opioids like morphine (Okada-Ogawa et al., 2009), but this remains unclear with endogenous μ-opioids.
Some limitations are worth mentioning. Sample size limits further adjustments, although it is difficult to substantially increase sample in studies using techniques that require prolonged testing and where patients cannot use rescue medication during an ongoing and spontaneous severe migraine attack. Nonetheless, the reported findings are sufficiently large to be detected with the current sample size. The Power Analysis under Statistical Analysis section shows the magnitude of the effect size that the study can detect with sufficient (80%) power. In our data, the difference among the region identified had a large magnitude. We believe such findings will add to the literature, however, as is the case with many other studies, they need to be further investigated.
5. Conclusion
The weight of evidence from our current findings is that, overall, migraineurs have increased μ-opioid receptor-mediated neurotransmission in the pain perception and modulation brain regions during the attacks (Fig. 3). However, with clinical chronification and aggravation of the attacks and increase in pain sensitivity (e.g., allodynia), which is largely the case in CM patients, dysfunctional μ-opioid activity escalates to the parahippocampal region as well as to the right amygdala. This flawed opioidergic activity in the limbic system provides the neuromechanistic explanation that likely contributes to the increased cognitive and emotional impact of pain on CM patients, enhanced global sensitivity to the environment evidenced by their thermal pain thresholds, and possibly their higher risk for medication overuse headache, and substance overuse, including opiates.
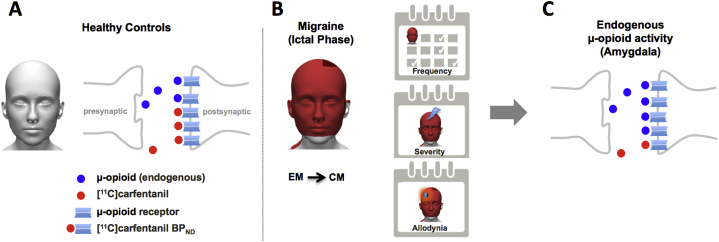
Fig. 3.
Illustration summarizes the finding for endogenous μ-Opioid release. (A) Stable levels of endogenous μ-Opioid for HC. (B) Illustration of a participant suffering severe headache attacks during CM (≥15 attacks/month) and exposed to a cutaneous STPT challenge. (C) Sudden increase in endogenous μ-opioid release in the limbic system (right amygdala) of CM patients during the ongoing headache attacks and allodynia relative to EM.
Statement of authorship
| Name | Location | Role | Contribution |
|---|---|---|---|
| Hassan Jassar, PhD | University of Michigan, Ann Arbor, Michigan | Author | Performed neuroimaging analyses of data; interpreted the results; drafted the manuscript for intellectual content |
| Thiago D. Nascimento, DDS, MS | University of Michigan, Ann Arbor, Michigan | Author | Conducted the data acquisition; interpreted the results; drafted the manuscript for intellectuel content |
| Niko Kaciroti, PhD | University of Michigan, Ann Arbor, Michigan | Author | Performed statistical analysis of results |
| Marcos F. DosSantos, DDS, PhD, | University of Michigan, Ann Arbor, Michigan | Author | Conducted the data acquisition |
| Theodora Danciu, DDS, DMedSc | University of Michigan, Ann Arbor, Michigan | Author | Conducted the data acquisition |
| Robert A. Koeppe, PhD | University of Michigan, Ann Arbor, Michigan | Author | Revised the manuscript for intellectual content |
| Yolanda R. Smith, MD | University of Michigan, Ann Arbor, Michigan | Author | Conducted the data acquisition |
| Marcelo E Bigal, MD, PhD | Ventus Therapeutics, Montreal, Canada | Author | Drafted the manuscript for intellectual content |
| Frank Porreca, PhD | University of Arizona, Tucson, Arizona | Author | Drafted the manuscript for intellectual content |
| Kenneth L. Casey, MD | University of Michigan, Ann Arbor, Michigan | Author | Drafted the manuscript for intellectual content |
| Jon-Kar Zubieta, MD, PhD | Stony Brook University, Stony Brook, New York | Author | Drafted the manuscript for intellectual content |
| Alexandre F. DaSilva, DDS, DMedSc | University of Michigan, Ann Arbor, Michigan | Author | Conceived and designed the research; interpreted the results; drafted the manuscript for intellectual content; contributed to this work in his personal capacity |
Funding
Supported by grants from the National Institute of Health – National Institute of Neurological Disorders and Stroke (NIH-NINDS K23 NS062946, R01 NS094413), USA; the Dana Foundation's Brain and Immuno-Imaging Award, USA; and the Migraine Research Foundation Research Grant Award, USA. This study is not industry-sponsored.
Disclosures
Dr. A. F. DaSilva co-created GeoPain (previously named PainTrek), and also co-founded MoxyTech Inc. that licensed the technology from the University of Michigan. Authors declare that they have no conflict of interest.
Dr. A. F. DaSilva had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Marcelo Bigal is the President and CEO of Ventus Therapeutic but has no conflict of interest with the study regarding financial support. His contribution was strictly scientific.
Acknowledgments
We thank the technologists J. M. Rothley, E.J. McKenna, A.R. Weeden, P. K. and C. Hendriks for the help with PET scan at the PET Center Nuclear Medicine, and the Functional MRI Laboratory personnel, S. Peltier and K. Newnham.
Contributor Information
Hassan Jassar, Email: hjassar@umich.edu.
Niko Kaciroti, Email: nicola@umich.edu.
Alexandre F. DaSilva, Email: adasilva@umich.edu.
References
- Benatto M.T., Florencio L.L., Carvalho G.F. Cutaneous allodynia is more frequent in chronic migraine, and its presence and severity seems to be more associated with the duration of the disease. Arq. Neuropsiquiatr. 2017;75:153–159. doi: 10.1590/0004-282X20170015. [DOI] [PubMed] [Google Scholar]
- Bigal M.E., Ashina S., Burstein R. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–1533. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal M.E., Serrano D., Buse D., Scher A., Stewart W.F., Lipton R.B. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Bigal M.E., Borucho S., Serrano D., Lipton R.B. The acute treatment of episodic and chronic migraine in the USA. Cephalalgia. 2009;29:891–897. doi: 10.1111/j.1468-2982.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton, JL, Valabrague, R, Poline, JB. Region of interest analysis using an SPM toolbox for SPM 99. NeuroImage;16:497.
- Calandre E.P., Bembibre J., Arnedo M.L., Becerra D. Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia. 2002;22:291–302. doi: 10.1046/j.1468-2982.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y., RWt Gereau. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol. Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Chen X., Liu M., Dong Z., Ma L., Yu S. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J. Headache Pain. 2017;18:7. doi: 10.1186/s10194-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva A.F., Nascimento T.D., Love T. 3D-neuronavigation in vivo through a patient's brain during a spontaneous migraine headache. J. Vis. Exp. 2014;(88):e50682. doi: 10.3791/50682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva A.F., Nascimento T.D., DosSantos M.F. Association of mu-opioid activation in the prefrontal cortex with spontaneous migraine attacks - brief report I. Ann. Clin. Transl. Neurol. 2014;1:439–444. doi: 10.1002/acn3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva A.F., Nascimento T.D., Jassar H. Dopamine D2/D3 imbalance during migraine attack and allodynia in vivo. Neurology. 2017;88:1634–1641. doi: 10.1212/WNL.0000000000003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Porreca F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia. 2009;29:1277–1284. doi: 10.1111/j.1468-2982.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino E.F., Hirasawa-Fujita M., Ni L., Guthrie S.K., Zubieta J.K. Regional brain [(11)C]carfentanil binding following tobacco smoking. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;59:100–104. doi: 10.1016/j.pnpbp.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Clauw D.J., Scott D.J., McLean S.A., Gracely R.H., Zubieta J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification. Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranialneuralgias, and facial pain. Cephalalgia. 2004:1–160. 2nd edn. [PubMed] [Google Scholar]
- Jewett D.M. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl. Med. Biol. 2001;28:733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- Jia Z., Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin. 2017;14:130–140. doi: 10.1016/j.nicl.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Tang W., Zhao D., Yu S. Disrupted functional connectivity between the periaqueductal gray and other brain regions in a rat model of recurrent headache. Sci. Rep. 2017;7:3960. doi: 10.1038/s41598-017-04060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe R.A., Frey K.A., Kume A., Albin R., Kilbourn M.R., Kuhl D.E. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J. Cereb. Blood Flow Metab. 1997;17:919–931. doi: 10.1097/00004647-199709000-00001. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Brown R. A higher-order theory of emotional consciousness. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E2016–E2025. doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Chou K.H., Lee P.L. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia. 2017;37:1329–1336. doi: 10.1177/0333102416678624. [DOI] [PubMed] [Google Scholar]
- Logan J., Fowler J.S., Volkow N.D., Wang G.J., Ding Y.S., Alexoff D.L. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Meyer C.R., Boes J.L., Kim B. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med. Image Anal. 1997;1:195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- Narita M., Kaneko C., Miyoshi K. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M., Kuzumaki N., Narita M. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J. Neurochem. 2006;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- Nascimento T.D., DosSantos M.F., Lucas S. Mu-opioid activation in the midbrain during migraine allodynia - brief report II. Ann. Clin. Transl. Neurol. 2014;1:445–450. doi: 10.1002/acn3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijjar S.S., Gordon A.S., Clark M.D. Entry demographics and pharmacological treatment of migraine patients referred to a tertiary care pain clinic. Cephalalgia. 2010;30:87–91. doi: 10.1111/j.1468-2982.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein E.B., Ni L., Domino E.F., Zubieta J.K. Nicotine-specific and non-specific effects of cigarette smoking on endogenous opioid mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;69:69–77. doi: 10.1016/j.pnpbp.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Ogawa A., Porreca F., Meng I.D. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J. Neurosci. 2009;29:15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Ruparel K., Newberg A. Human mu opioid receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A., Harper D.E., Harte S.E. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 2016;157:2217–2225. doi: 10.1097/j.pain.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chong C.D., Wu T., Gaw N., Fu Y., Li J. Accurate classification of chronic migraine via brain magnetic resonance imaging. Headache. 2015;55:762–777. doi: 10.1111/head.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng E.K., Buse D.C., Klepper J.E. Psychological factors associated with chronic migraine and severe migraine-related disability: an observational study in a tertiary headache center. Headache. 2017;57:593–604. doi: 10.1111/head.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstrand Dahlberg L., Linnman C.N., Lee D., Burstein R., Becerra L., Borsook D. Responsivity of periaqueductal gray connectivity is related to headache frequency in episodic migraine. Front. Neurol. 2018;9:61. doi: 10.3389/fneur.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeler M., Lyon R.A., Kuhar M.J. Mu opiate receptors are selectively labelled by [3H]carfentanil in human and rat brain. Eur. J. Pharmacol. 1989;167:221–228. doi: 10.1016/0014-2999(89)90582-7. [DOI] [PubMed] [Google Scholar]
- Veinante P., Yalcin I., Barrot M. The amygdala between sensation and affect: a role in pain. J. Mol. Psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox S.L., Veggeberg R., Lemme J. Increased functional activation of limbic brain regions during negative emotional processing in migraine. Front. Hum. Neurosci. 2016;10:366. doi: 10.3389/fnhum.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich A.P., Klug R., Stuber G., Landwehrmeyer B., Weber F., Freund W. Caudate nucleus and insular activation during a pain suppression paradigm comparing thermal and electrical stimulation. Open Neuroimaging J. 2011;5:1–8. doi: 10.2174/1874440001105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.Y., De Felice M., Kopruszinski C.M. Kappa opioid receptor antagonists: a possible new class of therapeutics for migraine prevention. Cephalalgia. 2017;37:780–794. doi: 10.1177/0333102417702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Smith Y.R., Bueller J.A. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zubieta J.K., Smith Y.R., Bueller J.A. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]