Abstract
Background:
HIV-positive persons who use stimulants such as methamphetamine experience greater difficulties in navigating the HIV care continuum. In the era of HIV treatment as prevention (TasP), little is known about the prevalence and correlates of success along the HIV care continuum among people who use stimulants.
Setting:
San Francisco, California USA
Methods:
Cross-sectional study that enrolled 129 HIV-positive men who have sex with men (MSM) from 2013 through 2017 who had biologically confirmed, recent methamphetamine use. Multivariable logistic regressions were built to identify correlates of success across the HIV care continuum.
Results:
Although two-thirds (87/129) of participants had undetectable HIV viral load (<40 copies/mL), only one-in-four (32/129) reported taking at least 90% of their antiretroviral therapy (ART). Those who were homeless in the past year (aOR=0.20; 95% CI=0.06 – 0.65) had 80% lower odds of being undetectable and adherent to ART. Substance use disorder treatment was associated with 77% lower odds of being engaged in HIV care (aOR=0.23; 95% CI=0.06 – 0.84) but also close to 3-fold greater odds of being adherent to ART (aOR=2.91; 95% CI=1.12 – 7.60).
Conclusion:
Despite the fact that many HIV-positive, methamphetamine-using MSM are able to achieve undetectable viral load in this sample, difficulties with ART adherence threaten to undermine the clinical and public health benefits of TasP. Expanded efforts to boost the effectiveness of TasP in this population should focus on meeting the unique needs of homeless individuals, optimizing ART adherence, and facilitating the integration of HIV care with substance use disorder treatment.
Keywords: HIV Care Continuum, Men Who Have Sex with Men, Methamphetamine, Viral Load
1.0. Introduction
HIV-positive persons who use stimulants such as methamphetamine experience greater difficulties with navigating the HIV care continuum that contribute to substantially elevated HIV viral load and faster clinical HIV progression (Adams et al., 2017; Carrico, 2011; Carrico et al., 2011b; Carrico et al., 2014b; Ellis et al., 2003). HIV-positive stimulant users initiate antiretroviral therapy (ART) at lower T-helper (CD4+) cell counts (Carrico et al., 2011a; Kapadia et al., 2005), are less likely to remain engaged in HIV care (Horvath et al., 2013), and are more likely to report difficulties with ART adherence and persistence that contribute to substantially elevated HIV viral load (Carrico et al., 2011b; Ellis et al., 2003). Stimulant users are also more likely to report engaging in HIV transmission risk behavior (Johnson et al., 2008; Morin et al., 2007; Mimiaga et al., 2008), resulting in greater odds of potentially amplified transmission risk where viral load is greater than or equal to 200 copies/mL(Mayer et al., 2014).
In the era of HIV treatment as prevention (TasP), achieving viral suppression (< 200 copies/mL) is crucial to optimize health outcomes and reduce onward HIV transmission rates in high priority populations like stimulant-using MSM (Carrico et al., 2016b). However, relatively little is known about the prevalence and correlates of success along the HIV care continuum among stimulant users in the era of TasP. The goals of the present study were to describe the HIV care continuum in methamphetamine-using men who have sex with men (MSM) and identify correlates of success along the HIV care continuum in this high priority population.
2.0. Methods
HIV-positive, methamphetamine-using MSM residing in the San Francisco Bay Area, were recruited from 2013–2017 for a randomized controlled trial from substance use disorder treatment programs, HIV medical clinics, AIDS service organizations, the community, and referrals from active participants (Carrico et al., 2016a; Carrico et al., in press). At an in-person screening visit, participants completed a signed informed consent. All enrolled participants met the following inclusion criteria: 1) 18 years of age or older; 2) be a man who has sex with men; 3) have documentation of HIV-positive serostatus, and 4) provide a urine or hair sample that was confirmed to be reactive for methamphetamine. Urine toxicology provides evidence of use in the past 3-days and hair toxicology provides evidence of use in the past 1–3 months.
Enrolled participants completed a separate baseline assessment approximately one week later that included a detailed battery of self-report measures, a second urine sample for on-site toxicology testing, and peripheral venous blood sample to measure HIV disease markers (described below). Participants received a $50 pre-loaded debit card for completing each of the study assessments. This study was approved by the Institutional Review Boards for the University of California, San Francisco, the University of Miami, and Northwestern University.
2.1. Measures
2.1.1. Demographics and HIV Disease Markers.
Participants completed a demographic questionnaire assessing age, race/ethnicity, sexual orientation, education, and income. Participants also reported when they were diagnosed with HIV and whether they were homeless at any point in the past year. HIV viral load testing was performed to detect plasma HIV RNA using the Abbott RealTime HIV-1 assay (Abbott Molecular, Inc.; Des Plaines, IL). This assay has a lower limit of detection of 40 copies/mL. CD4+ T-cell count was measured with whole blood using flow cytometry, and assays were performed by Quest Diagnostics.
2.1.2. Engagement in HIV Care.
Participants reported the number of medical appointments they scheduled with their HIV primary care provider and the number of these appointments that they attended in the past six months. Those who scheduled and attended at least one medical appointment with their HIV primary care provider in the past six months were considered engaged in HIV care.
2.1.3. ART Adherence.
Participants reported ART adherence during the past 30 days using the visual analog scale (Giordano et al., 2004). Those who reported being at least 90% adherent were compared to participants who reported taking less than 90% of prescribed ART. ART adherence is essential to remain undetectable, and even among those with an undetectable viral load better ART adherence is associated with lower immune activation and inflammation (Castillo-Mancilla et al., 2016; Castillo-Mancilla et al., 2018). Thus, we also examined the proportion of participants who had an undetectable HIV viral load that also reported being adherent to ART.
2.1.4. Severity of Alcohol and Substance Use.
The Addiction Severity Index was administered to assess the severity of alcohol and other substance use (McLellan et al., 1992). This measure includes the self-reported number of days using multiple illicit substances during the past 30 days, perceived impairment related to substance use, and perceived need for substance use disorder treatment.
2.1.5. Substance Use Disorder Treatment Engagement.
Participants were considered engaged in substance use disorder treatment if they self-reported that they spent at least one night in a residential alcohol or drug treatment facility, received any outpatient substance use disorder treatment, or attended any one-on-one visits with a substance use counselor in the past six months.
2.2. Statistical Analyses.
We began characterizing the HIV care continuum by examining the proportions of participants who were engaged in HIV care, on ART, adherent to ART and had an undetectable viral load (< 40 copies/mL). Bivariate analyses were conducted to examine correlates of each step along the HIV care continuum, and the final multivariable logistic regression models were built by including all independent variables with a p-value < 0.20. The final model examines the associations of any homelessness in the past year and any substance use disorder treatment in the past six months after controlling for age, race/ethnicity, and education. All analyses were conducted in SAS 9.4 (Cary, NC).
3.0. Results
The mean age of the participants was 43.3 (SD = 9.0). Less than half of the participants were White (44%), 29% were Hispanic/Latino, 16% were Black/African American, and 11% were other ethnic minorities or multicultural. Approximately a quarter (26%) graduated from college and two-thirds (67%) had an annual income of less than $16,000. One-third (33%) had been homeless in the past year, and 50% had received any substance use disorder treatment in the past six months. The average length of time since receiving a positive HIV diagnosis was 13.0 (SD=8.5) years, and the median CD4+ T-cell count was 629 (Interquartile Range = 401–784) cells/mm3.
Figure 1 illustrates the proportion of the full study sample achieving success along different steps of the HIV care continuum. The majority of the study sample (85%) had scheduled and kept at least one appointment with an HIV health-care provider in the past six months, and 88% were currently prescribed ART. Although two-thirds (67%) had an undetectable HIV viral load (and 81% were virally suppressed with a viral load of < 200 copies/mL), only 30% self-reported being adherent to ART. One-in-four of those who had an undetectable viral and also reported being adherent to ART.
Figure 1.
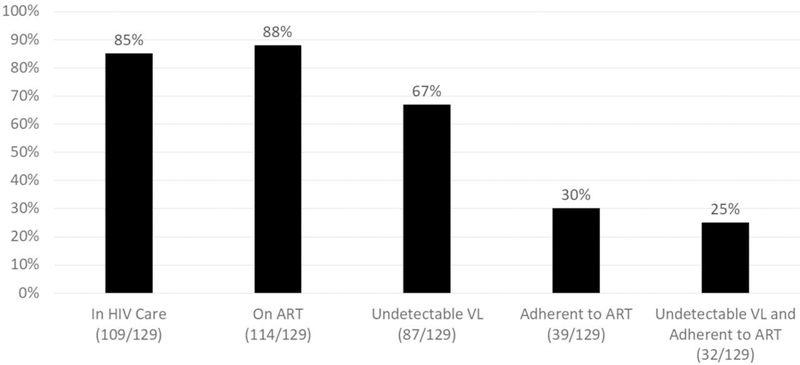
The San Francisco HIV care continuum among methamphetamine-using men who have sex with men (N = 129).
In the final multivariate logistic regression models, homelessness in the past year and having received any substance use disorder treatment in the past six months were associated with several steps of the HIV care continuum (Table 1). Those who reported being homeless in the past year had lower odds of an undetectable viral load (adjusted odds ratio [aOR] = 0.35, 95% CI = 0.13 – 0.93; p = 0.035), lower odds of reporting that they were adherent to ART (aOR = 0.22; 95% CI = 0.07 – 0.70; p = 0.010), and lower odds of a undetectable viral load and reporting they were adherent to ART (aOR = 0.20; 95% CI = 0.06 – 0.65; p = 0.008). Any substance use disorder treatment in the past six months was associated with lower odds of being engaged in HIV care (aOR = 0.23; 95% CI = 0.06 – 0.84; p = 0.026) but also almost 3-fold greater odds of being classified as adherent to ART (aOR = 2.91; 95% CI = 1.12 – 7.60; p = 0.029). There were no significant associations of alcohol or substance use severity with the HIV care continuum.
Table 1.
Correlates of success along the San Francisco HIV care continuum among methamphetamine-using men who have sex with men (N = 129)
| In HIV Care | On ART | Undetectable VL | Adherent to ART | Undetectable VL and Adherent | |
|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
|
Age 20–29 30–39 40–49 50+ |
0.74 (0.10, 5.33) 0.71 (0.17, 2.97) - 1.67 (0.35, 8.02) |
0.16 (0.03, 1.07) 0.98 (0.19, 5.01) - 5.94 (0.56, 63.27) |
0.44 (0.10, 2.06) 0.88 (0.25, 3.03) - 0.86 (0.26, 2.89) |
0.48 (0.10, 2.38) 0.71 (0.21, 2.43) - 0.87 (0.27, 2.84) |
0.62 (0.12, 2.99) 0.70 (0.20, 2.45) - 1.16 (0.36, 3.76) |
|
Race/Ethnicity Black/African American White Hispanic/Latino Other Ethnic Minority |
1.02 (0.20, 5.18) - 0.78 (0.19, 3.29) 1.82 (0.18, 18.78) |
0.32 (0.05, 1.98) - 1.49 (0.27, 8.16) 0.75 (0.11, 5.17) |
0.22 (0.06, 0.79) - 0.45 (0.15, 1.37) 0.51 (0.12, 2.27) |
0.87 (0.23. 3.39) - 0.74 (0.25, 2.26) 1.00 (0.22, 4.57) |
0.84 (0.21, 3.39) - 0.85 (0.28, 2.58) 0.94 (0.20, 4.42) |
|
Education <High school High school Graduate Some college College graduate Post-grad |
0.67 (0.09, 4.99) 0.89 (0.22, 3.57) - 5.56 (0.60, 51.37) 1.04 (0.10, 10.85) |
1.12 (0.09, 13.49) 3.79 (0.40, 36.33) - 4.34 (0.44, 42.99) 0.38 (0.06, 2.60) |
2.24 (0.34, 14.63) 1.23 (0.34, 4.43) - 1.10 (0.32, 3.86) 0.21 (0.04, 1.02) |
0.17 (0.01, 3.56) 0.74 (0.22, 2.66) - 1.91 (0.61, 5.98) 0.73 (0.13, 4.02) |
0.24 (0.01, 5.56) 1.07 (0.31, 3.69) - 2.17 (0.71, 6.67) 0.38 (0.06, 2.60) |
|
Homelessness (past year) No Yes |
- 0.35 (0.13, 0.93) |
- 0.22 (0.07, 0.70) |
- 0.20 (0.06, 0.65) |
||
|
Substance Use Disorder Treatment (past 6 months) No Yes |
0.23 (0.06, 0.84) - |
2.91 (1.12, 7.60) - |
VL = Viral Load
4.0. Discussion
In this study of HIV-positive, methamphetamine-using MSM, we found that recent homelessness was significantly associated with lower odds of achieving an undetectable viral load and being classified as adherent to ART. Among HIV-positive MSM in San Francisco, an estimated 96% are aware of their status (CDC, 2016), 93% are on ART (CDC, 2016), and 63–69% of those on ART are at least 90% adherent (San Francisco Department of Public Health, 2017). This underscores the findings that gaps in the care continuum that persist among HIV-positive, methamphetamine-using MSM. Although recent engagement in substance use disorder treatment was associated with lower odds of engagement in HIV care, it was also associated with almost 3-fold greater odds of being classified as adherent to ART. These results indicate that HIV-positive, methamphetamine-using MSM are a high priority population for programs aimed at reducing homelessness and providing substance use disorder treatment, both of which could optimize the clinical and public health benefits of TasP.
Our findings who were homeless in the past year had lower odds of having an undetectable viral load and being adherent to ART build upon prior research where homelessness has emerged as a major predictor of lower ART adherence and HIV disease progression markers (Aidala et al., 2016; Kidder et al., 2007; Moss et al., 2004), even after adjusting for stimulant use (Carrico et al., 2011a). The association of recent substance use disorder treatment with lower odds of being engaged in HIV care may reflect the need to integrate HIV care services better when patients are pursuing substance use disorder treatment. At the same time, substance use disorder treatment was also associated with markedly greater odds of being adherent to ART. This may reflect the success of substance use disorder treatment services with reducing substance use and mitigating co-occurring psychosocial health symptoms that contribute to ART non-adherence. Taken together, results highlight the clear need to develop and test interventions to promote engagement in HIV care and provide positive reinforcement of ART adherence that is integrated into substance use disorder treatment programs for methamphetamine-using MSM.
Our results highlight the need for comprehensive and sustainable structural interventions to address housing instability, which might help optimize the benefits of TasP for methamphetamine-using MSM while promoting engagement with HIV care. Programs addressing homelessness should actively seek collaborations and partnerships with community-based organizations providing services to methamphetamine-using MSM. San Francisco is a well-resourced city where people living with HIV have a great degree of access to substance use disorder treatment and housing services. In 2017, San Francisco’s Department of Homelessness and Supportive Housing released their standard practices for matching people who experience homelessness with available community resources (San Francisco Department of Homelessness and Supportive Housing, 2017). This document also explicitly states that those with a history of or active substance use will not be screened out or deprioritized. Further research is needed to test how multi-level intervention approaches that include housing, case management, HIV care, and accessible substance use disorder treatment could boost the effectiveness of TasP in methamphetamine-using MSM.
This study has some limitations. First, the small sample size and cross-sectional study design restrict the ability to draw conclusions about the causality of study findings. Second, the entire sample was drawn from San Francisco, a well-resourced setting with comprehensive substance use disorder treatment for methamphetamine-using MSM (Carrico et al., 2014a). Although it is unclear whether high prevalence of undetectable viral load would be observed in other settings, this work provides tangible evidence that methamphetamine-using MSM can derive substantial benefits from HIV treatment.
Despite these limitations, this study is among the first to document the HIV care continuum in MSM with biologically confirmed, recent methamphetamine use. Findings indicate that although many HIV-positive, methamphetamine-using MSM are able to achieve undetectable HIV viral load in San Francisco, difficulties with ART adherence threaten to undermine the clinical and public health benefits of TasP in this population. Expanded efforts to address structural factors such as housing as well as facilitate better linkages between substance use disorder treatment and HIV care could optimize the HIV care continuum in this high priority population.
Highlights.
Describes the HIV care continuum in methamphetamine-using sexual minority men
Housing and substance use disorder treatment could optimize the HIV care continuum
Underscores the need to integrate HIV care and substance use disorder treatment
Acknowledgements
We are also thankful for the support of Dr. Teri Leigler who oversaw HIV viral load assays for this project. We are also appreciative of the support of Dr. James Dilley and Ms. Lori Thoemmes who provided field site space for the study team at the Alliance Health Project. Finally, we are grateful to the study participants for choosing to join this project.
Role of Funding Source
This project was supported by the National Institute on Drug Abuse (R01-DA033854; Carrico, Woods, and Moskowitz, PIs). Additional support for this project was provided by the University of California, San Francisco Center for AIDS Research’s Virology Core (P30-AI027763; Volberding, PI). This project was investigator initiated without directives from the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflict declared.
References
- Adams JW, Bryant KJ, Edelman JE, Fiellin DA, Gaither JR, Gordon AJ, Gordon KS, Kraemer KL, Mimiaga MJ, Operario D, Tate JP, van den Berg JJ, Justice AC, Marshall BDL, 2017. Association of Cannabis, Stimulant, and Alcohol use with Mortality Prognosis Among HIV-Infected Men. AIDS Behav 22, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidala AA, Wilson MG, Shubert V, Gogolishvili D, Globerman J, Rueda S, Bozack AK, Caban M, Rourke SB, 2016. Housing status, medical care, and health outcomes among people living with HIV/AIDS: A systematic review 106, e1–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, 2011. Substance use and HIV disease progression in the HAART era: Implications for the primary prevention of HIV. Life Sci 88, 940–947. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED, 2011a. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS 25, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Flentje A, Gruber VA, Woods WJ, Discepola MV, Dilworth SE, Neilands TB, Jain J, Siever MD, 2014a. Community-based harm reduction substance abuse treatment with methamphetamine-using men who have sex with men. J. Urban Health 91, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Gomez W, Jain J, Shoptaw S, Discepola MV, Olem D, Lagana-Jackson J, Andrews R, Neilands TB, Dilworth SE, Evans JL, Woods WJ, Moskowitz JT, 2018. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug Alcohol Depend In Press. 10.1016/j.drugalcdep.2018.07.029 [DOI] [PMC free article] [PubMed]
- Carrico AW, Jain J, Discepola MV, Olem D, Andrews R, Woods WJ, Neilands TB, Shoptaw S, Gomez W, Dilworth SE, Moskowitz JT, 2016a. A community-engaged randomized controlled trial of an integrative intervention with HIV-positive, methamphetamine-using men who have sex with men. BMC Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Riley ED, Johnson MO, Charlebois ED, Neilands TB, Remien RH, Lightfoot MA, Steward WT, Weinhardt LS, Kelly JA, Rotheram-Borus MJ, Morin SF, Chesney MA, 2011b. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of antiretroviral therapy utilization. J. Acquir. Immune Defic. Syndr 56, 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Shoptaw S, Cox C, Stall R, Li X, Ostrow DG, Vlahov D, Plankey MW, 2014b. Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr 67, 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Zepf R, Meanley S, Batchelder A, Stall R, 2016b. When the party is over: A systematic review of behavioral interventions for substance-using men who have sex with men. J. Acquir. Immune Defic. Syndr 73, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ Jr., Gardner EM, Macatangay BJ, Breen EC, Jacobson LP, Anderson PL, Wada NI, 2016. Suboptimal cART adherence is associated with higher levels of inflammation despite HIV suppression. Clin. Infect. Dis 63, 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Mancilla JR, Morrow M, Boum Y, Byakwaga H, Haberer JE, Martin JN, Bangsberg D, Mawhinney S, Musinguzi N, Huang Y, Tracy RP, Burdo TH, Williams K, Muzoora C, Hunt PW, Siedner MJ, 2018. Higher art adherence is associated with lower systemic inflammation in treatment-naive Ugandans who achieve virologic suppression. J. Acquir. Immune Defic. Syndr 77, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I, Group HIVNRC, 2003. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J. Infect. Dis 188, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR, 2004. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin. Trials 5, 74–79. [DOI] [PubMed] [Google Scholar]
- Horvath KJ, Carrico AW, Simoni J, Boyer EW, Amico KR, Petroll AE, 2013. Engagement in HIV medical care and technology use among stimulant-using and nonstimulant-using men who have sex with men. AIDS Res. Treat 2013, 121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco Department of Homelessness and Supportive Housing, 2017. San Francisco coordinated entry standards http://hsh.sfgov.org/wp-content/uploads/2017/04/DRAFT-San-Francisco-Coordinated-Entry-Standards-2.pdf.
- Johnson MO, Carrico AW, Chesney MA, Morin SF, 2008. Internalized heterosexism among HIV-positive, gay-identified men: implications for HIV prevention and care. J. Consult. Clin. Psychol 76, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia F, Cook JA, Cohen MH, Sohler N, Kovacs A, Greenblatt RM, Choudhary I, Vlahov D, 2005. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use. Addiction 100, 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder DP, Wolitski RJ, Campsmith ML, Nakamura GV, 2007. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am. J. Public Health 97, 2238–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KH, Skeer MR, O’Cleirigh C, Goshe BM, Safren SA, 2014. Factors associated with amplified HIV transmission behavior among American men who have sex with men engaged in care: implications for clinical providers. Ann. Behav. Med 47, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat 9, 199–213. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Fair AD, Mayer KH, Koenen K, Gortmaker S, Tetu AM, Hobson J, Safren SA, 2008. Experiences and sexual behaviors of HIV-infected MSM who acquired HIV in the context of crystal methamphetamine use. Aids Educ. Prev 20, 30–41. [DOI] [PubMed] [Google Scholar]
- Morin SF, Myers JJ, Shade SB, Koester K, Maiorana A, Rose CD, 2007. Predicting HIV transmission risk among HIV-infected patients seen in clinical settings. AIDS Behav 11, S6–16. [DOI] [PubMed] [Google Scholar]
- Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, Bangsberg DR, 2004. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: A prospective study. Clin. Infect. Dis 39, 1190–1198. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. HIV infection risk, prevention, and testing behaviors among men who have sex with men—National HIV behavioral surveillance, 20 U.S. Cities, 2014 https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
- San Francisco Department of Public Health, 2017. HIV Epidemiology Annual Report, 2016 https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/Annual-Report-2016-20170831.pdf.


