Summary
Severe Cushing syndrome (SCS) is considered an emergency that requires immediate treatment to lower serum cortisol levels. Fluconazole may be considered an alternative treatment in Cushing syndrome when ketoconazole is not tolerated or unavailable. We report a 39-year-old woman with a history of partial pancreaticoduodenectomy due to a periampullary neuroendocrine tumor with locoregional extension. Three years after surgery, she developed liver metastases and was started on 120 mg of lanreotide/month, despite which, liver metastases progressed in the following 6 months. The patient showed extreme fatigue, muscle weakness, delirium, moon face, hirsutism and severe proximal weakness. Laboratory tests showed anemia, hyperglycemia and severe hypokalemia. 24-h urinary free cortisol: 2152 nmol/day (reference range (RR): <276), morning serum cortisol 4883.4 nmol/L (RR: 138–690), ACTH 127.3 pmol/L (RR: 2.2–10). She was diagnosed with ectopic ACTH syndrome (EAS). On admission, she presented with acute upper gastrointestinal tract bleeding and hemodynamic instability. Intravenous fluconazole 400 mg/day was started. After 48 h, her mental state improved and morning cortisol decreased by 25%. The dose was titrated to 600 mg/day which resulted in a 55% decrease in cortisol levels in 1 week, but then had to be decreased to 400 mg/day because transaminase levels increased over 3 times the upper normal level. After 18 days of treatment, hemodynamic stability, lower cortisol levels and better overall clinical status enabled successful bilateral adrenalectomy. This case report shows that intravenous fluconazole effectively decreased cortisol levels in SCS due to EAS.
Learning points:
Severe Cushing syndrome can be effectively treated with fluconazole to achieve a significant improvement of hypercortisolism prior to bilateral adrenalectomy.
Intravenous fluconazole is an alternative treatment when ketoconazole is not tolerated and etomidate is not available.
Fluconazole is well tolerated with mild side effects. Hepatotoxicity is usually mild and resolves after drug discontinuation.
Background
Severe Cushing syndrome (SCS) is considered an emergency that requires immediate diagnosis and treatment to lower serum cortisol levels. SCS has been mostly described in the context of ectopic ACTH syndrome (EAS) (1). The most common tumors associated with EAS are small-cell lung carcinoma, neuroendocrine tumors, pheochromocytoma and medullary thyroid carcinoma (2).
Rapid control of hypercortisolism is mandatory to ensure short-term survival. Emergency reduction of the elevated serum cortisol is rapidly achieved with oral metyrapone and/or ketoconazole. If parenteral therapy is required, intravenous etomidate is rapidly effective. However, etomidate is not available in all countries. Combination therapy with two or more steroidogenesis inhibitors and/or bilateral laparoscopic adrenalectomy (BLA) may be necessary to achieve this goal (1).
Fluconazole may be considered a treatment option in Cushing syndrome when ketoconazole is not tolerated or unavailable. It can be administered either orally or intravenously. There is only a case report about the use of fluconazole in Cushing syndrome in an elderly female patient with EAS (3). Its efficacy and safety in SCS have not been tested.
We report the case of a young woman with SCS due to EAS from a metastatic periampullary neuroendocrine tumor. Given the impossibility of treating the patient with oral ketoconazole or etomidate, intravenous fluconazole was administered to achieve clinical improvement prior to bilateral adrenalectomy.
Case presentation
We report the case of a 39-year-old woman with a history of partial pancreaticoduodenectomy due to a periampullary neuroendocrine tumor with locoregional extension (9/12 metastatic lymph nodes) diagnosed in 2013. Histopathology showed a neuroendocrine tumor, immunohistochemically positive for gastrin, Ki 67 5% (Grade 2). ACTH staining was not performed. Three years later, she developed liver metastases and was treated with a monthly dose of 120 mg of lanreotide. After 6 months, there was progression of the liver metastases and she was started on sunitinib, but it was discontinued at 2 months due to intolerance. At that time, the patient showed extreme fatigue, muscle weakness and delirium. Liver metastases were unchanged by the sunitinib treatment or its discontinuation. Peptide receptor radiotherapy was unavailable at the time.
Investigation
Physical examination revealed that the patient had a characteristic moon face, hirsutism and severe proximal weakness. Biochemical testing showed anemia, hyperglycemia and severe hypokalemia. 24-h urinary free cortisol: 2152 nmol/day (reference range (RR): <276), morning serum cortisol 4883.4 nmol/L (RR: 138–690), plasmatic ACTH 127.3 pmol/L (RR:e 2.2–10). She was diagnosed with SCS due to EAS and started treatment with ketoconazole.
The patient was referred to our hospital for bilateral adrenalectomy. On admission, she presented with acute upper gastrointestinal bleeding with hemodynamic instability, subendocardial ischemia and atrial fibrillation in the context of anemia and severe hypokalemia. Hemodynamic support was provided in the intensive care unit (ICU). A Forrest Ib gastric ulcer was found and treated endoscopically.
Treatment
The patient was started on intravenous fluconazole 400 mg/day. Although the initiation dose reported in the literature is 200 mg/day, we decided to start with 400 mg given the severity of the Cushing Syndrome. After 48 h of treatment, her mental state improved and morning cortisol decreased 25%. The dose was titrated to 600 mg/day and cortisolemia decreased 55% in 1 week (Fig. 1). Then the dose had to be decreased to 400 mg/day because the liver transaminases increased 3 times over the normal upper limit. The dose of fluconazole reported in the literature is 200–1200 mg/day (1, 2, 3, 4, 5).
Figure 1.
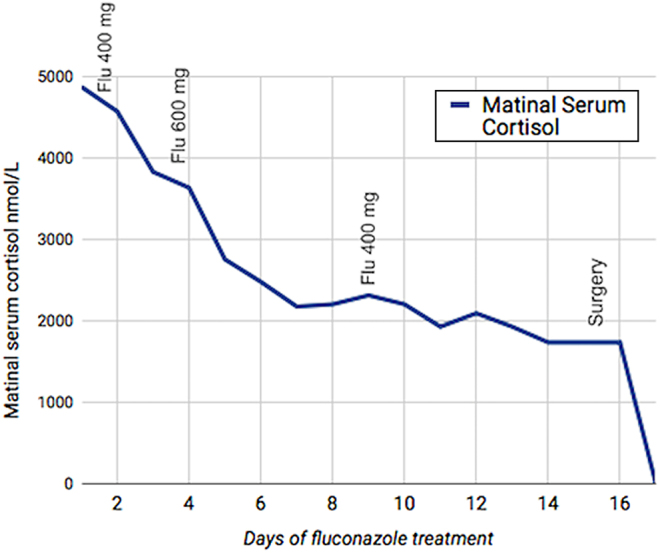
Cortisol response to treatment with fluconazole (Flu). Treatment changes: Day 1: Fluconazole 400 mg, Day 3: Fluconazole 600 mg, Day 9: Fluconazole 400 mg. Surgery was performed at day 15.
Bilirubin and albumin levels, as well as prothrombin time, remained within normal range and the patient did not develop liver failure. Other causes for liver injury were ruled out. Liver enzymes normalized and baseline cortisol decreased by 65%. However, hypokalemia and metabolic alkalosis were severe and only resolved after acetazolamide 250 mg/day was added to spironolactone and amiloride. The patient also developed severe thrombocytopenia, and multiple platelet transfusions were required on a daily basis. Intravenous Gamma globulin was added, but resulted in no consistent platelet increase. A bone marrow biopsy was performed and revealed marked hypocellularity. All viral serology tests were negative. The platelet count improved significantly after the administration of Eltrombopag, a thrombopoietic agent. After 18 days of treatment, hemodynamic stability, lower cortisol levels and better overall clinical status enabled successful bilateral adrenalectomy, which resulted in further improvement of the clinical status of the patient.
The patient was prescribed replacement doses of hydrocortisone and fludrocortisone, and lanreotide 120 LAR. She required prolonged hospitalization in ICU due to sepsis, emphysematous cystitis and disseminated Herpes Zoster infection, which were treated with antibiotics and intravenous acyclovir. She then underwent chemoembolization of her liver metastases which decreased in size after the third cycle.
Outcome and follow-up
Two years after bilateral adrenalectomy, the patient with a periampullary Grade 2 neuroendocrine tumor and liver metastasis, entered a rehabilitation therapy program. She is in good general health and has an active life as a mother.
Discussion
There is only one report of the use of fluconazole in EAS with SCS. An 80-year-old woman with CS of unknown etiology was started on fluconazole 200 mg daily, then gradually titrated up to 1200 mg. After 1 week, fluconazole doses were reduced to 400 mg. Cortisol levels dropped by about 83% and did not increase despite dose reduction. The patient had severe metabolic alkalosis with refractory hypokalemia that responded to the administration of acetazolamide. She then developed pancytopenia caused by myelodysplasia and died 1 month after diagnosis (3). There are two other case reports of the use of fluconazole in the treatment of CS, one in Cushing’s disease and the other in adrenocortical carcinoma, albeit neither were SCS (4, 5).
Ketoconazole and fluconazole have been shown in vitro to inhibit 11B-hydroxylase and 17-hydroxylase activity in human adrenocortical cells in a dose-dependent manner. In that experiment, fluconazole was at least 40% less potent than ketoconazole in the inhibition of cortisol and 11 deoxycortisol production (6).
Ketoconazole has been used worldwide for more than 30 years to lower circulating cortisol levels and is an effective drug with mild side effects. Hepatotoxicity is usually mild and resolves with drug discontinuation (7). Fluconazole is another azole compound and an accepted alternative to ketoconazole in the treatment of fungal infections. There are few reports about the safety of fluconazole treatment in CS, but in a secondary analysis of a multicenter, dose-escalation trial of invasive mycoses, 27% of 85 patients receiving high-dose prolonged treatment with fluconazole experienced symptoms and 42% showed laboratory abnormalities. Some of the most common: headache (3%), hair loss (3%) anorexia (3%), and eosinophilia (12%) may indicate adrenal insufficiency induced by fluconazole although adrenal function was not evaluated in this study. Nevertheless, these symptoms were not progressive and the authors concluded that fluconazole could be safely used and was well tolerated (8).
This case report shows that intravenous fluconazole effectively decreased cortisol levels in a patient with SCS due to EAS. After the improvement of her medical condition, successful bilateral adrenalectomy could be performed.
Patient’s perspective
Her main concerns, while she was with Cushing syndrome, were depression and the physical and psychological impairment to comply with her duties as a mother. Now she is a happy mother who takes care of her home and children.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent
Written informed consent has been obtained from the patient, and a signed copy of the consent form is provided.
Author contribution statement
All three authors T Canteros, V De Miguel and P Fainstein-Day were involved in patient care and contributed to the preparation of this manuscript.
References
- 1.Alexandraki KI, Grossman AB. Therapeutic strategies for the treatment of severe Cushing’s syndrome. Drugs 2016. 76 . ( 10.1007/s40265-016-0539-6) [DOI] [PubMed] [Google Scholar]
- 2.Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Reviews in Endocrine and Metabolic Disorders 2010. 11 . ( 10.1007/s11154-010-9139-z) [DOI] [PubMed] [Google Scholar]
- 3.Schwetz V, Aberer Felix SC, Pieber T. Fluconazole and acetazolamide in the treatment of ectopic Cushing’s syndrome with severe metabolic alkalosis. Endocrinology, Diabetes and Metabolism Case Reports 2015. 2015 150027 ( 10.1530/EDM-15-0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedl M, Maier C, Zettinig G, Nowotny P, Schima W, Luger A. Long-term control of hypercortisolism with fluconazole: case report and in vitro studies. European Journal of Endocrinology 2006. 154 . ( 10.1530/eje.1.02120) [DOI] [PubMed] [Google Scholar]
- 5.Burns K, Christie D, Gunton J. Fluconazole in the treatment of Cushing’s disease. Endocrinology, Diabetes and Metabolism Case Reports 2016. 2016 150115 ( 10.1530/EDM-15-0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J, van Koetsveld PM, de Herder WW, de Jong FH, Feelders RA. Fluconazole inhibits human adrenocortical steroidogenesis in vitro. Journal of Endocrinology 2012. 215 . ( 10.1530/JOE-12-0310) [DOI] [PubMed] [Google Scholar]
- 7.Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, et al. Ketoconazole in Cushing’s disease: is it worth a try? Journal of Clinical Endocrinology and Metabolism 2014. 99 . ( 10.1210/jc.2013-3628) [DOI] [PubMed] [Google Scholar]
- 8.Stevens DA, Diaz M, Negroni R, Montero Gei F, Castro LG, Sampaio SA, Borelli D, Restrepo A, Franco L, Bran JL, et al. Fluconazole Pan-American study group. Safety evaluation of chronic fluconazole therapy. Chemotherapy 1997. 43 . ( 10.1159/000239592) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a