Abstract
Background
Synovial mesenchymal stem cells (MSCs) are an attractive cell source for cartilage and meniscus regeneration. The optimum cryopreservation medium has not been determined, but dimethylsulfoxide (DMSO) should be excluded, if possible, because of its toxicity. The purposes of our study were to examine the possible benefits of higher concentrations of serum and the effectiveness of 100% serum (without DMSO) for the cryopreservation of synovial MSCs.
Methods
Human synovium was harvested from the knees of four donors with osteoarthritis during total knee arthroplasty. Synovial MSCs (8 × 105 cells) were suspended in 400 μL medium and used as a Time 0 control. The same number of synovial MSCs was also suspended in 400 μL α-MEM medium containing 10% fetal bovine serum (FBS) (5% DMSO, and 1% antibiotic), 95% FBS (and 5% DMSO), or 100% FBS (no DMSO) and cryopreserved at − 80 °C for 7 days. After thawing, the cell suspensions (1.5 μL; 3 × 103 cells) were cultured in 60 cm2 dishes for 14 days for colony formation assays. Additional 62.5 μL samples of cell suspensions (1.25 × 105 cells) were added to tubes and cultured for 21 days for chondrogenesis assays.
Results
Colony numbers were significantly higher in the Time 0 and 95% FBS groups than in the 10% FBS group (n = 24). Colony numbers were much lower in the 100% FBS group than in the other three groups. The cell numbers per dish reflected the colony numbers. Cartilage pellet weights were significantly heavier in the 95% FBS group than in the 10% FBS group, whereas no difference was observed between the Time 0 and the 95% FBS groups (n = 24). No cartilage pellets formed at all in the 100% FBS group.
Conclusion
Synovial MSCs cryopreserved in 95% FBS with 5% DMSO maintained their colony formation and chondrogenic abilities to the same levels as observed in the cells before cryopreservation. Synovial MSCs cryopreserved in 100% FBS lost their colony formation and chondrogenic abilities.
Electronic supplementary material
The online version of this article (10.1186/s12891-019-2700-3) contains supplementary material, which is available to authorized users.
Background
Human synovial mesenchymal stem cells (MSCs) are a promising cell source for regeneration of cartilage and meniscus [1]. In clinical situations, transplantation of synovial MSCs can regenerate cartilage defects in the knee [2]. For this clinical study, we used synovial MSCs just after harvesting. If synovial MSCs could be cryopreserved in a condition that maintained their activity, this would greatly facilitate these transplantations.
The cryopreservation medium utilized thus far for synovial MSC culture used in both basic [3, 4] and translational research [5, 6] has typically been a basic culture medium, such as Dulbecco’s modified Eagle medium (DMEM) or α-minimum essential medium (α-MEM), with added fetal bovine serum (FBS) and dimethyl sulfoxide (DMSO). However, an optimum cryopreservation medium has yet to be identified. We recently reported that synovial MSCs maintained their viability as well as their chondrogenic ability for 48 h when preserved in 100% human serum at 4 °C or 13 °C [7]. This suggests that higher concentrations of serum in the cryopreservation medium improves cell viability.
Our first purpose in the present study was to examine whether a higher concentration of serum in the cryopreservation medium would be beneficial for the preservation of human synovial MSCs. We also focused on DMSO, as it is widely used as a cryoprotective agent; however, it has toxic effects [8–11] and the safety of intraarticular transplantation of DMSO-containing material is not established. If DMSO could be excluded from the cryopreservation medium, then synovial MSCs could be transplanted into the knee joint directly after thawing without requiring any procedure for DMSO removal. Our second purpose was therefore to examine whether 100% serum (without any DMSO) might serve as an effective cryopreservation medium for synovial MSCs.
Methods
Synovial MSCs
This study was approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University, and informed consent was obtained from all study subjects. Human synovium was harvested from the knees of four female donors with osteoarthritis (OA) during total knee arthroplasty operations. The average age and standard deviation of the patients was 71 ± 6 years. The synovium was minced and digested in a solution of 3 mg/mL collagenase (Sigma-Aldrich Japan, Tokyo, Japan) at 37 °C for 3 h and the digested cells were filtered through a 70 μm cell strainer (Greiner Bio-One GmbH, Frickenhausen, Germany). The obtained nucleated cells were suspended in α-MEM (Thermo Fisher Scientific, Rockford IL, USA) supplemented with 1% antibiotic-antimycotic (Thermo Fisher Scientific) and 10% FBS (Thermo Fisher Scientific) and cultured in a cell culture incubator (Astec Co. Ltd., Fukuoka, Japan) in 5% CO2 at 37 °C for 14 days. When the MSCs reached 70–80% confluence, they were replated continuously. Synovial MSCs of passage 2 were used in this study.
Flow cytometry
For cultured synovial MSCs from four donors, surface markers were examined using a FACS Verse instrument (Becton, Dickinson and Company; BD, NJ, USA). The cells before cryopreservation were suspended in FACS buffer using Hank’s balanced salt solution (HBSS) at a density of 5 × 105 cells/mL and stained for 30 min with the antibodies CD44 (PE-Cy7), CD45 (APC-H7), CD73 (V450), CD90 (PE), CD105 (APC) (all from BD), and Ghost Dye Violet 510 for dead cells (Tonbo Biosciences, CA, USA). FlowJo software (Tree Star Software, CA, USA) was used for the analysis.
Differentiation assays
For chondrogenesis, 1.25 × 105 synovial MSCs were suspended in 0.5 mL chondrogenic induction medium consisting of DMEM (Thermo Fisher Scientific) supplemented with 10 ng/mL transforming growth factor-β3 (TGF-β3, Miltenyi Biotec, Bergisch Gladbach, Germany), 500 ng/mL bone morphogenetic protein 2 (BMP-2, Medtronic, Minneapolis, MN, USA), 40 μg/mL proline, 100 nM dexamethasone, 100 μg/mL pyruvate, 50 ng/mL ascorbate-2-phosphate, and 50 mg/mL ITS Premix (Becton Dickinson, San Jose, CA, USA). The cells were pelleted by centrifugation at 450×g for 10 min and then cultured for 21 days. After 21 days, the pellets were sectioned and stained with toluidine blue (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan).
For adipogenesis, 100 synovial MSCs were plated in a 60 cm2 dish and cultured for 14 days in culture medium to make cell colonies. The adherent cells were further cultured in adipogenic induction medium consisting of α-MEM supplemented with 100 nM dexamethasone, 0.5 mM isobutyl-methylxanthine (Sigma-Aldrich), and 50 mM indomethacin (Fujifilm, Wako Pure Chemical Corporation) for an additional 21 days. Adipocytes were stained with oil red O (Muto Pure Chemicals, Tokyo, Japan).
For calcification, 100 synovial MSCs were plated in a 60 cm2 dish and cultured for 14 days in culture medium to make cell colonies. The adherent cells were further cultured in calcification induction medium consisting of α-MEM supplemented with 50 μg/mL ascorbic acid 2-phosphate (Fujifilm Wako Pure Chemical Corporation), 10 nM dexamethasone (Fujifilm Wako Pure Chemical Corporation), and 10 mM β-glycerophosphate (Sigma-Aldrich). After 21 days, calcification was assessed by alizarin red staining (Merck Millipore, Billerica, MA, USA).
Freezing and thawing methods
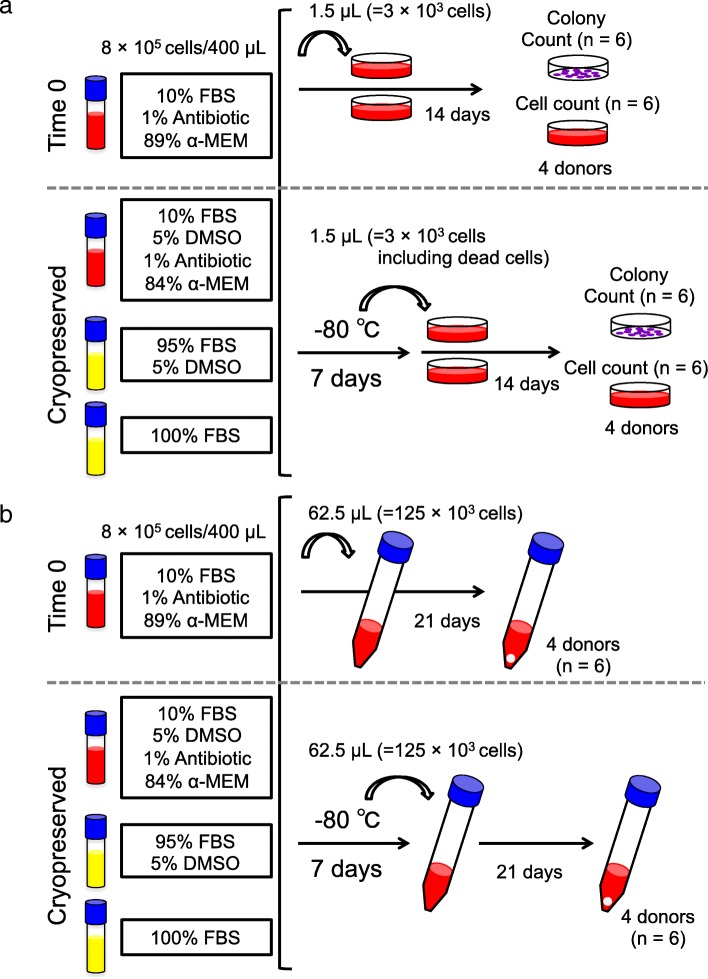
8 × 105 synovial MSCs were allocated into 4 freezing tubes (Sumitomo Bakelite, Tokyo, Japan) and suspended in 400 μL medium (Fig. 1). For the “Time 0” group, the cells were suspended in medium consisting of 10% FBS, 1% Antibiotic, and 89% α-MEM. For the “10% FBS” group, the cells were suspended in medium consisting of 10% FBS, 5% DMSO (CultureSure DMSO; Fujifilm Wako Pure Chemical Corporation), 1% Antibiotic, and 84% α-MEM. For the “95% FBS” group, the cells were suspended in medium consisting of 95% FBS and 5% DMSO. For the “100% FBS” group, the cells were suspended in 100% FBS. The tubes in the “10% FBS” group, the “95% FBS” group, and the “100% FBS” group were put in a bio freezing vessel (Bicell, Japan Freezer, Tokyo, Japan), and kept in a freezer at − 80 °C for 7 days (Fig. 1). Then, the tubes were removed out of the freezing vessel and the frozen cells in the tube were thawed using a frozen cell thawing device (ThawSTAR, Astero Bio, Menlo Park CA, USA).
Fig. 1.
Scheme for the colony formation and chondrogenesis assays. a Colony formation assay. Synovial mesenchymal stem cells (MSCs; 8 × 105 cells) were suspended in 400 μL medium containing 10% fetal bovine serum (FBS) as a time 0 control. Synovial MSCs (8 × 105 cells) suspended in 400 μL medium containing 10% FBS, 95% FBS, or 100% FBS were cryopreserved at − 80 °C for 7 days and thawed. A 1.5 μL volume of cell suspension (containing 3 × 103 cells, including living and dead cells) was added to 60 cm2 dishes for colony formation and cell proliferation assays. b Chondrogenesis assay. A 62.5 μL volume of cell suspension (containing 1.25 × 105 cells, including living cells and dead cells) was added to 15 mm tubes for chondrogenesis assays
Colony formation
A 1.5 μL volume of cell suspension (containing 3 × 103 cells, including living cells and dead cells) was added to twelve 60 cm2 dishes, 10 mL α-MEM containing 10% FBS was added to each dish, and the cells were cultured for 14 days (Fig. 1a). Six dishes were stained with crystal violet to count the total numbers of cell colonies. Only colonies greater than 2 mm in diameter or showing distinct staining were counted. Cells were harvested from the other 6 dishes, and the cell numbers per dish were counted with a hemocytometer. The cell number per colony was determined by counting the cell number per dish for 6 dishes (dishes A, B, C, D, E, and F), and the colony numbers per dish were counted for 6 additional dishes (dishes G, H, I, J, K, and L). The cell number per colony was calculated based on the cell number from dish A divided by the colony number from dish G, and this calculation was repeated for the remaining pairs of dishes (i.e., B and H, C and I, D and J, E and K, and F and L.) The mean and standard deviation were then determined for cell number per colony (4 donors, n = 24) [12].
Comparison of chondrogenesis potential
Synovial MSCs (8 × 105 cells) were suspended in 400 μL medium containing 10% FBS, 95% FBS, or 100% FBS, cryopreserved at − 80 °C for 7 days, and then thawed. A 62.5 μL volume of cell suspension (containing 1.25 × 105 cells, including living and dead cells) was added to six 15 mL tubes (Falcon) containing 0.5 mL chondrogenic induction medium. The cells were pelleted by centrifugation at 450 × g for 10 min and cultured for 21 days (Fig. 1b). The cultured cell pellets were photographed and weighed with a semi micro balance (CPA225D, Sartorius, Gottingen, Germany). The pellets were sectioned and stained with toluidine blue and immunostained with collagen type II (Kyowa Pharma Chemical Co., LTD, Toyama, Japan). The mean and standard deviation were then determined for the pellet weight (4 donors, n = 24).
Statistical analysis
All data were statistically evaluated with the Friedman test and Dunn’s multiple comparison test using GraphPad Prism 6 (GraphPad Software, CA, USA). Data were expressed as mean ± standard deviation (SD). Two-tailed p values < .05 were considered statistically significant.
Results
MSC characteristics
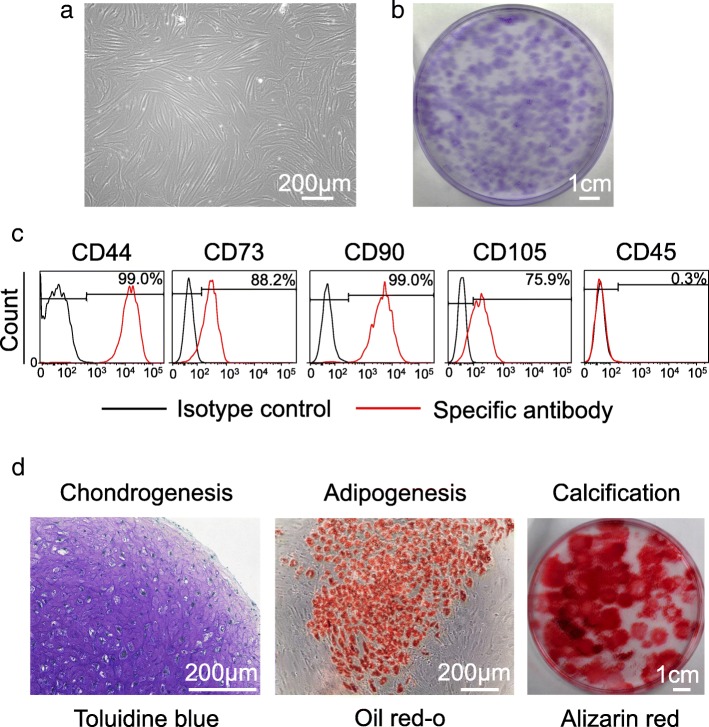
Synovial cells were spindle shaped (Fig. 2a) and formed cell colonies 14 days after the initial plating (Fig. 2b). They stained positive for CD 44, 73, 90, and 105 and negative for CD 45 (Fig. 2c). They showed chondrogenesis, adipogenesis, and calcification potential (Fig. 2d). Overall, they had characteristics of MSCs [13].
Fig. 2.
Characteristics of synovial mesenchymal stem cells (MSCs) as MSCs. a Cell morphology. b Colony morphology. c Representative histograms for surface markers (d) Multidifferentiation
Colony formation
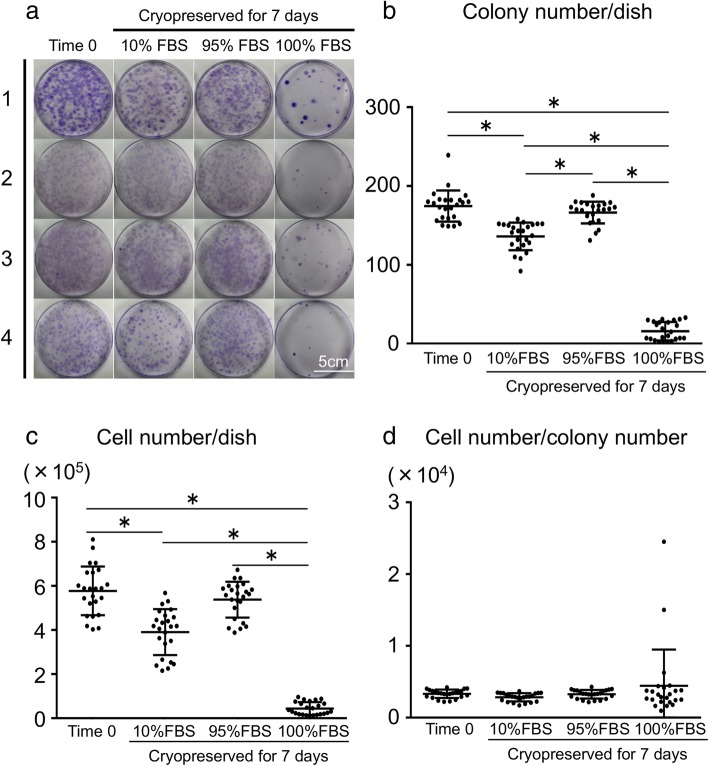
Colony formation was poor in the 100% FBS group (Fig. 3a). The colony numbers per dish were significantly higher in the Time 0 group and in the 95% FBS group than in the 10% FBS group (Fig. 3b). The colony numbers per dish were much lower in the 100% FBS group than in the other three groups. Similar differences were obtained for cell numbers per dish (Fig. 3c). No statistically significant differences were noted for cell numbers per colony among the four groups (Fig. 3d). Each donor analysis yielded similar results (Additional file 1: Figure S1).
Fig. 3.
Analysis of colony formation. a Representative dishes stained with crystal violet. Synovial mesenchymal stem cells (MSCs) were derived from four donors. b Colony numbers per dish. Data are shown as means ± SD (n = 4 for each donor). *p < .05 by the Friedman test followed by Dunn’s multiple comparisons. c Cell numbers per dish. d Cell numbers per colony
Chondrogenesis
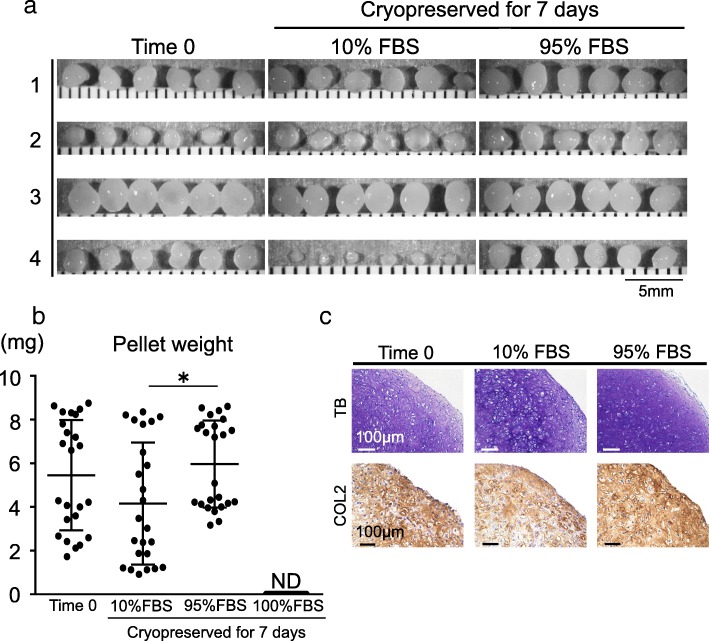
Cartilage pellets were obtained (Fig. 4a) for all except the 100% FBS group. The pellet weight was significantly heavier in the 95% FBS group than in the 10% FBS group, but no significant difference was noted between the Time 0 group and the 95% FBS group (Fig. 4b). The obtained cartilage pellets showed positive staining with toluidine blue and collagen type II (Fig. 4c). For each donor analysis, almost identical results were obtained, with no statistically significant difference (Additional file 2: Figure S2).
Fig. 4.
Analysis of chondrogenesis. a Representative macroscopic appearance of cartilage pellet. Synovial mesenchymal stem cells (MSCs) were derived from four donors. In the 100% fetal bovine serum (FBS) group, no cartilage pellets were formed. b Pellet weight. Data are shown as means ± SD (n = 4 for each donor). *p < .05 by the Friedman test followed by Dunn’s multiple comparisons. ND: not detected. c Representative histological sections stained with toluidine blue and immunostained for collagen type II
Discussion
We examined the effect of the cryopreservation medium composition on the maintenance of the colony formation and chondrogenic abilities of synovial MSCs. Cryopreservation of human synovial MSCs in 95% FBS with 5% DMSO maintained these abilities at the same level as that observed in the cells before cryopreservation. Preservation of human synovial MSCs in 100% FBS (without any DMSO) resulted in extensive loss of colony formation ability and a complete loss of chondrogenic ability.
The most common cellular damage caused by freezing occurs because of the formation of ice crystals, which form around 0 °C and destroy cell membranes [14]. Here, a higher concentration of serum in the cryopreservation medium resulted in a better preservation of the synovial MSCs. This is possibly due to a decrease in moisture in the cells in response to adjustments in osmotic pressure [15], as well as to a reduction in the occurrence of ice crystals due to the added FBS. More than half of the serum protein is albumin, which can buffer the pH of the solution and maintain the osmotic pressure [16], and thereby function as a cryoprotectant.
Another frequently used cryoprotectant is DMSO, but its use in mammals is limited because of its toxicity. In four species (mice, rats, cats, dogs), the LD50s are between 2.5 and 8.9 g/kg for DMSO administered intravenously. The symptoms at near lethal doses are similar and include spontaneous motor activity, tremors, myasthenia, prostration, transient convulsion, dyspnea, pulmonary edema, and hemorrhaging [17]. Yellowlees et al. reported a toxic reaction in two elderly people receiving an intravenous dosage of 100 g of 20% DMSO for three days for the treatment of arthritis. One patient experienced serious illness, including oliguria, hemolysis, tremor, and loss of consciousness, whereas the second patient did not become ill [18]. O’Donnell et al. reported a patient with myeloblastic leukemia received intravenous cryopreserved autologous marrow blood containing 35 g of DMSO as a 10% solution and suffered a reaction to the administration. In addition to a drop in hemoglobin, the patient became agitated, pyretic, hypotensive, and tachycardic and died ten days later [19]. High doses of DMSO are therefore potentially dangerous. In our case, 8 × 105 cells were suspended in 400 μL cryopreservation medium containing 5% DMSO. If 2.4 × 107 cells were to be administered, the 12 mL of cryopreservation medium would corresponds 66 mg of DMSO, as the specific gravity of DMSO is 1.1. This would be a safe amount for intravenous administration, but whether it would be safe for intra-articular administration is unknown.
Cryoprotectants are classified as either permeating (DMSO is this type) or non-permeating, depending on their ability to traverse the cell membrane [20]. Non-permeating cryoprotectants include polyvinylpyrrolidone [21], sugars such as trehalose [22], sucrose [23], lactose [24], glucose [25], sugar alcohols (such as mannitol [26] and sorbitol [27]), and the polymer hydroxyethyl starch (HES) [28]. In the future, our intention is to compare the optimal conditions we determined in the present study with those obtained using these non-permeating cryoprotectants.
In the present study, the culture medium for the 10% FBS group contained 1% antibiotics, whereas the media for the 95 and 100% FBS groups did not. We initially hypothesized that 100% FBS would function as a cryopreservation medium because 100% FBS worked as a preservation medium [7]. We also wanted to examine the additional effect of DMSO (i.e., in the 95% FBS group). We also desired to compare the 100% FBS group to the 5% FBS group as the latter was cultured in our standard cryopreservation medium (containing 1% antibiotics, 5% DMSO, and 84% α-MEM). Contrary to our hypothesis, cryopreservation in 100% FBS was not successful. Some cell culture studies have reported that antibiotics affected cellular DNA synthesis as well as protein synthesis [29], while others found that antibiotics had minimal detrimental effects on cultured cells [30]. Some microorganisms can withstand low temperatures, so the risk of bacterial contamination cannot be denied even during cryopreservation [31]. For cell transplantation in clinical situations, antibiotics may be needed in the cryopreservation medium for safety. We will investigate the effect of antibiotics in cryopreservation media with high serum concentrations in future work.
Our colony formation and chondrogenesis assays were conducted by adding cell suspensions directly to dishes or tubes, without first determining the numbers of viable cells, as we considered this a more accurate method for evaluating cell viability. Our previous studies indicated that the statistical dispersions of the live cell rate, as determined by live/dead assays, and the apoptosis rate, as determined by flow cytometry, were relatively wide for human synovial MSCs after 48 h of preservation in Ringer’s solution or in complete human serum at 4, 13, and 37 °C [7].
The condition of cryopreservation did not affect the cell numbers per colony, though it did affect the colony numbers per dish and the cell numbers per dish. This indicates the possibility that a fraction of the cells was resistant to poor culture conditions, and these cells had a comparable colony formation ability. However, the actual number of these cells was quite low.
The chondrogenesis potential of MSCs was evaluated based on the pellet weight. Our findings indicated that the pellet increased in diameter, weight, and amount of cartilage matrix synthesized during the in vitro chondrogenesis of MSCs. Contrary, the DNA yield per pellet decreased. The amount of DNA per cell, as assessed by pre-labeling with 3H-thymidine, was maintained during the in vitro chondrogenesis of MSCs [32]. Our results indicate that the increase in pellet weight can be caused by the production of extracellular matrix, rather than the proliferation of the cells. We also previously reported that pellet weight was correlated with the expression of chondrogenic-specific genes and the amounts of glycosaminoglycans [33–35]. We believe that the pellet weight is therefore a convincing indicator of in vitro chondrogenesis in a population of MSCs [1, 7, 36].
Our study had three main limitations. We cryopreserved cells in a freezer at − 80 °C, rather than in a liquid nitrogen tank at − 150 °C, and the period of cryopreservation was only 7 days. Had we conducted the cryopreservation in a liquid nitrogen tank for longer durations, our outcomes might have been different. Kim et al. reported that the viability of human umbilical cord MSCs cryopreserved in a liquid nitrogen tank for 1 year was comparable to that of cells cryopreserved for 7 days [37]. Abbruzzese at al. reported that hematopoietic stem cells cryopreserved in a medium containing 5% DMSO in a liquid nitrogen tank remained viable for 10 years [38]. Since cryopreservation seems to be less effective at − 80 °C than − 150 °C, this suggests that our cryopreservation medium containing 95% FBS and 5% DMSO might allow storage of synovial MSCs for quite long periods at − 150 °C.
A second limitation was that we examined the effect of DMSO only at a 5% concentration. Thirumala et al. reported that DMSO concentrations ranging between 2 and 10% had the same effect on the viability of cryopreserved hepatocytes, whereas DMSO at less than 2% decreased viability [39]. This suggests that we could possibly reduce the concentration of DMSO for cryopreservation of synovial MSCs. In forthcoming studies, we will examine variations in DMSO concentrations to determine the minimum amount of DMSO required for cryopreservation of synovial MSCs.
A third limitation was our use of FBS instead of autologous human serum, which we used in our clinical study [2]. In comparison with FBS, autologous human serum is safer in clinical cell transplantation because it can prevent immune reactions and contamination with pathogens, such as prions or zoonotic viruses [40–42]. Here we did not use autologous human serum because it requires additional work. Since human serum has shown effects comparable to FBS in terms of the proliferation, chondrogenesis [43], and preservation [7] of synovial MSCs, we expect that human serum would have the same effect as FBS with respect to cryopreservation.
Conclusions
Synovial MSCs cryopreserved in 95% FBS with 5% DMSO maintained their colony formation and chondrogenic abilities at the same level as that observed in the cells before cryopreservation. Synovial MSCs cryopreserved in 100% FBS (without any DMSO) lost their colony formation and chondrogenic abilities.
Additional files
Figure S1. Donor specific analysis of colony formation. (a) Colony number/dish. (b) Cell number/dish. (c) Cell number/colony number. (ZIP 507 kb)
Figure S2. Patient-specific analysis of chondrogenesis. (PDF 268 kb)
Acknowledgements
We thank Ms. Mika Watanabe and Ms. Kimiko Takanashi for the management of our laboratory and Ellen Roider for English editing.
Authors’ contributions
Research conception and design: MM and IS. Data collection: RF and MM. Analysis and interpretation of data: HKa, KO, NO, KT, and HKo. Drafting the manuscript: RF, MM, and IS. Manuscript review: HKa, KO, and NO. Study supervision: KT and HKo. All authors were involved in critical drafting of the article for important intellectual content and all authors approved the final version for publication. IS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) under Grant Number 16H06262 to MM for the design of the study, and by Japan Agency for Medical Research and Development (AMED) under Grant Number JP19bk0104012 to IS for data collection, analysis, and manuscript writing.
Availability of data and materials
All the data supporting our findings are contained within the manuscript.
Ethics approval and consent to participate
This study was approved by the institutional review board of Tokyo Medical and Dental University (reference number: M2017–142). Written informed consent forms were submitted by all four participating patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis & Rheumatism. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 2.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473(7):2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohno Y, Mizuno M, Ozeki N, Katano H, Otabe K, Koga H, et al. Comparison of mesenchymal stem cells obtained by suspended culture of synovium from patients with rheumatoid arthritis and osteoarthritis. BMC Musculoskelet Disord. 2018;19(1):78. doi: 10.1186/s12891-018-1998-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuno M, Katano H, Otabe K, Komori K, Matsumoto Y, Fujii S, et al. Platelet-derived growth factor (PDGF)-AA/AB in human serum are potential indicators of the proliferative capacity of human synovial mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):243. doi: 10.1186/s13287-015-0239-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo S, Muneta T, Nakagawa Y, Koga H, Watanabe T, Tsuji K, et al. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J Orthop Res. 2017;35(6):1274–1282. doi: 10.1002/jor.23211. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr Cartil. 2015;23(6):1007–1017. doi: 10.1016/j.joca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno M, Katano H, Otabe K, Komori K, Kohno Y, Fujii S, et al. Complete human serum maintains viability and chondrogenic potential of human synovial stem cells: suitable conditions for transplantation. Stem Cell Res Ther. 2017;8(1):144. doi: 10.1186/s13287-017-0596-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant. 2000;25(12):1299–1301. doi: 10.1038/sj.bmt.1702452. [DOI] [PubMed] [Google Scholar]
- 9.Hoyt R, Szer J, Grigg A. Neurological events associated with the infusion of cryopreserved bone marrow and/or peripheral blood progenitor cells. Bone Marrow Transplant. 2000;25(12):1285–1287. doi: 10.1038/sj.bmt.1702443. [DOI] [PubMed] [Google Scholar]
- 10.Bauwens D, Hantson P, Laterre P-F, Michaux L, Latinne D, De Tourtchaninoff M, et al. Recurrent seizure and sustained encephalopathy associated with dimethylsulfoxide-preserved stem cell infusion. Leuk Lymphoma. 2009;46(11):1671–1674. doi: 10.1080/10428190500235611. [DOI] [PubMed] [Google Scholar]
- 11.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75(3):781–786. [PubMed] [Google Scholar]
- 12.Shioda M, Muneta T, Tsuji K, Mizuno M, Komori K, Koga H, et al. TNFalpha promotes proliferation of human synovial MSCs while maintaining chondrogenic potential. PLoS One. 2017;12(5):e0177771. doi: 10.1371/journal.pone.0177771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Prickett RC, Marquez-Curtis LA, Elliott JAW, McGann LE. Effect of supercooling and cell volume on intracellular ice formation. Cryobiology. 2015;70(2):156–163. doi: 10.1016/j.cryobiol.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Laroche C, Gervais P. Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl Microbiol Biotechnol. 2003;60(6):743–747. doi: 10.1007/s00253-002-1167-5. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg HF. Osmotic pressure of the serum proteins. Annals of Clinical & Laboratory Science. 1978;8(2):155–164. [PubMed] [Google Scholar]
- 17.Rubin LF. Toxicologic update of dimethyl sulfoxide. Ann N Y Acad Sci. 1983;411:6–10. doi: 10.1111/j.1749-6632.1983.tb47278.x. [DOI] [PubMed] [Google Scholar]
- 18.Yellowlees P, Greenfield C, McIntyre N. Dimethylsulphoxide-induced toxicity. Lancet. 1980;2(8202):1004–1006. doi: 10.1016/S0140-6736(80)92158-3. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell J.R., Burnett A.K., Sheehan T., Tansey P., Mcdonald G.A. SAFETY OF DIMETHYLSULPHOXIDE. The Lancet. 1981;317(8218):498. doi: 10.1016/S0140-6736(81)91879-1. [DOI] [PubMed] [Google Scholar]
- 20.Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Smillie JA, Munro AC, Wood GC, Mitchell R. Cryopreservation of human platelets with polyvinylpyrrolidone. Transfusion. 1981;21(5):552–556. doi: 10.1046/j.1537-2995.1981.21582040818.x. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, Pyatt DW. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004;13(3):295–305. doi: 10.1089/154732804323099226. [DOI] [PubMed] [Google Scholar]
- 23.Rumsey SC, Galeano NF, Arad Y, Deckelbaum RJ. Cryopreservation with sucrose maintains normal physical and biological properties of human plasma low density lipoproteins. J Lipid Res. 1992;33(10):1551–1561. [PubMed] [Google Scholar]
- 24.Bedford SJ, Varner DD, Meyers SA. Effects of cryopreservation on the acrosomal status of stallion spermatozoa. J Reprod Fertil. 2000;(56):133–40. [PubMed]
- 25.Kotelba-Witkowska B, Schiffer CA. Cryopreservation of platelet concentrates using glycerol-glucose. Transfusion. 1982;22(2):121–124. doi: 10.1046/j.1537-2995.1982.22282177117.x. [DOI] [PubMed] [Google Scholar]
- 26.Diettrich B, Popov A, Pfeiffer B, Neumann D, Butenko R, Luckner M. Cryopreservation of Digitalis lanata cell cultures. Planta Med. 2007;46(10):82–87. doi: 10.1055/s-2007-970026. [DOI] [PubMed] [Google Scholar]
- 27.Gray GD, Phillips RS. Use of sorbitol in the cryopreservation of babesia. Res Vet Sci. 1981;30(3):388–389. doi: 10.1016/S0034-5288(18)32567-0. [DOI] [PubMed] [Google Scholar]
- 28.Stolzing A, Naaldijk Y, Fedorova V, Sethe S. Hydroxyethylstarch in cryopreservation – mechanisms, benefits and problems. Transfus Apher Sci. 2012;46(2):137–147. doi: 10.1016/j.transci.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kuhlmann I. The prophylactic use of antibiotics in cell culture. Cytotechnology. 1996;19(2):95–105. doi: 10.1007/bf00749764. [DOI] [PubMed] [Google Scholar]
- 30.Parker RA, Clegg PD, Taylor SE. The in vitro effects of antibiotics on cell viability and gene expression of equine bone marrow-derived mesenchymal stromal cells. Equine Vet J. 2012;44(3):355–360. doi: 10.1111/j.2042-3306.2011.00437.x. [DOI] [PubMed] [Google Scholar]
- 31.Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24(10):2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]
- 32.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci. 2002;99(7):4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohno Y, Mizuno M, Ozeki N, Katano H, Komori K, Fujii S, et al. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Res Ther. 2017;8(1). 10.1186/s13287-017-0572-8. [DOI] [PMC free article] [PubMed]
- 34.Mizuno M, Katano H, Mabuchi Y, Ogata Y, Ichinose S, Fujii S, et al. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Res Ther. 2018;9. 10.1186/s13287-018-0870-9. [DOI] [PMC free article] [PubMed]
- 35.Nagase T, Muneta T, Ju Y-J, Hara K, Morito T, Koga H, et al. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis & Rheumatism. 2008;58(5):1389–1398. doi: 10.1002/art.23418. [DOI] [PubMed] [Google Scholar]
- 36.Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis & Rheumatism. 2006;54(3):843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 37.Kim S-M, Yun C-K, Park J-H, Hwang JW, Kim ZH, Choi Y-S. Efficient cryopreservation of human mesenchymal stem cells using silkworm hemolymph-derived proteins. J Tissue Eng Regen Med. 2017;11(8):2181–2192. doi: 10.1002/term.2116. [DOI] [Google Scholar]
- 38.Abbruzzese L, Agostini F, Durante C, Toffola RT, Rupolo M, Rossi FM, et al. Long term cryopreservation in 5% DMSO maintains unchanged CD34+cells viability and allows satisfactory hematological engraftment after peripheral blood stem cell transplantation. Vox Sang. 2013;105(1):77–80. doi: 10.1111/vox.12012. [DOI] [PubMed] [Google Scholar]
- 39.Thirumala S, Gimble JM, Devireddy RV. Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose-derived adult stem cells. Stem Cells Dev. 2010;19(4):513–522. doi: 10.1089/scd.2009.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink DW. FDA regulation of stem cell-based products. Science. 2009;324(5935):1662–1663. doi: 10.1126/science.1173712. [DOI] [PubMed] [Google Scholar]
- 41.Halme DG, Kessler DA. FDA regulation of stem-cell–based therapies. N Engl J Med. 2006;355(16):1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 42.Knoepfler PS. From bench to FDA to bedside: US regulatory trends for new stem cell therapies. Adv Drug Deliv Rev. 2015;82-83:192–196. doi: 10.1016/j.addr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis & Rheumatism. 2008;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Donor specific analysis of colony formation. (a) Colony number/dish. (b) Cell number/dish. (c) Cell number/colony number. (ZIP 507 kb)
Figure S2. Patient-specific analysis of chondrogenesis. (PDF 268 kb)
Data Availability Statement
All the data supporting our findings are contained within the manuscript.