Abstract
Background
Evidence supports the fact that multicomponent exercise and HMB supplementation are, separately, effective in improving older adult’s health and palliate functional metabolic diseases in older people. However, the true effect of HMB supplementation combined with a tailored exercise program in frail older adults is still unknown. Thus, the aim of the HEAL (HMB + Exercise = Adults Living longer) study is to assess the effects of the combination of a daily multicomponent exercise and resistance training (VIVIFRAIL program) intervention in addition to HMB supplementation on older adults’ health.
Methods/design
A 24-week cluster randomized, double-blind, placebo-controlled study will be conducted on 104 adults ≥70 years. Nursing homes will be randomized to either of four groups: Ex-HMB (exercise intervention with HMB), Ex-Plac (exercise intervention with placebo), NoEx-HMB (no exercise intervention with HMB), and Controls (No exercise and no HMB). Intervention groups which include exercise will complete the individualized multicomponent (strength, balance and cardiovascular exercises) training program VIVIFRAIL. Intervention groups which include HMB supplementation will receive a 3 g/daily dose of free acid HMB in powder form. The primary outcome measure is the functional capacity. Secondary outcome measures are muscle strength and power, frailty and fall risk, body composition, biochemical analyses and cardiometabolic risk factor, disability and comorbidity, cognitive function and depression.
Discussion
The findings of the HEAL study will help professionals from public health systems to identify cost-effective and innovative actions to improve older people’s health and quality of life, and endorse exercise practice in older adults and people living in nursing homes.
Trial registration
NCT03827499; Date of registration: 01/02/2019.
Keywords: Healthy ageing, Physical activity, Physical fitness, Falls, Dynapenia
Background
To date, people are living more years than ever before in history and the world’s aging is rising at a staggering rate [1]. Thus, strategies focused on health maintenance for aging people through exercise and proper nutrition are required to contribute to lifelong wellbeing and prevent ageing diseases and chronic illness. Frailty, sarcopenia, dynapenia and sarcopenic obesity states are the main metabolic complications in older people and represent a major public health challenge [2–4]. A recent estimation from the Eurostat online database (28 European countries) suggests increments from 60 to 70% of individuals with sarcopenia in 2045 affecting 12.9 to 22.3% of people over 65 years old [5]. These diseases are caused by a degenerative loss of muscle mass (muscle wasting), strength and mobility. The combination of exercise with protein dietary supplementation is proven to be highly effective to increase muscle mass and strength in older adults [6–8].
Evidence states that exercise in older people is a main component in frailty prevention (increases strength and decreases falls incidence) and functional capacity preservation (increases mobility and autonomy) [8–10]. In turn, weak elders have greater risk of disability, hospitalization, morbidity and death [3, 11]. In addition to a better physical condition, exercise has a clear impact on psychological well-being in older people [12]. Although much remains to be done, the possibility of physical exercise as the new medication for the twenty-first century is truly inspiring. The first step of this revolution is that the question is turning from “may I prescribe physical activity for older people?” to “what kind of exercise must I prescribe?” [13]. Reducing sedentary behaviours and promoting exercise training in older adults living in nursing homes stands as a main global challenge [14, 15].
Very recently, the ERAMUS+ co-funding VIVIFRAIL project (http://www.vivifrail.com) has developed a multicomponent exercise program (strength, balance and cardiovascular exercises), carefully adapted, for improving functional capacity for older people above 70 years [16]. The program includes a practical guide for testing and prescribing the physical training according to each specific condition (serious, moderate, slight or no limitation, and with or without risk of falling). Furthermore, the VIVIFRAIL App allows individuals’ monitoring and provides clear instructions to effectively complete the program within the everyday environment. Now that long-term exercise interventions in older adults are more possible than ever [17], what is now required is to examine the effectiveness of this program on relevant health and functional outcomes for older adults and nursing homes residents [15].
The β-hydroxy-β-methylbutyrate (HMB) dietary is a bioactive metabolite formed from the decomposition of leucine, an essential branched-chain amino acid. The importance of leucine has anti-catabolic properties and plays an important role in protein metabolism, glucose homeostasis, insulin action and recovery from exercise [18–20]. A dose of 3 g of HMB dietary supplementation provides 60 g of leucine, which otherwise would imply 600 g of high biological value protein [21]. The HMB supplementation is affordable (around 50€/kg) and its consumption is safe with no adverse effects [22, 23]. In older adults > 60 years old, HMB is demonstrated to have anti-catabolic effect, enhance protein synthesis, attenuate proteolysis, increase muscle mass and decrease muscle damage [24–26]. Despite the fact that HMB efficacy varies [25], a meta-analysis concluded that HMB supplements contribute to the preservation of muscle mass in old age [27]. Based on these findings, the supplementation of HMB appears to be an effective strategy to prevent metabolic and physical complications in ageing (frailty, dynapenia, sarcopenia and sarcopenic obesity) and preserve health, functional capacity and strength in older people.
A recent investigation [28] reported for the first time a significant reduction of 50% in whole body plasma concentration of HMB and reductions of 25% on the conversion of leucine to HMB in older adults (~ 65 y-old). At the moment, there is limited understanding why this happens, but it seems clear than reductions in HMB conversion are associated with age [29]. These decrements on high quality protein synthesis importantly accounted for a decline in muscle weakness in older people [30]. Hence, the possibility of palliating muscle and functional losses in the ageing by HMB supplementation is truly inspiring and encourages further studies [26, 27].
To date, only five Randomized Control Trial (RCT) studies have examined the effectiveness of exercise training combined with HMB dietary supplementation in older adults > 60 years old [31–35]. Whereas it seems clear that HMB supplements contribute to the preservation of muscle mass in old age, contradictory evidence on its effects on strength increments [31–34] and functional performance [33–35] in older adults exists. These equivocal outcomes may be attributed to the protocols applied, with discrepancies in the training volume (number of sessions, time per session, number of reps), intensity (load monitoring and progression) and exercises. Besides, sample sizes explored are reduced (n < 32) and only one RCT [32] conducted an exercise intervention > 8 weeks. Consequently, there is a need for longer and larger studies to fully determine the potential effects of HMB supplementation on physical performance, translating to a functional benefit [34]. In this sense, the HEAL (HMB + Exercise = Adults Living longer) study will be the first RCT conducting a specific, individualized, multicomponent exercise intervention for the older adult population such as the VIVIFRAIL [16]. Because this evidence-based program has been proven as effective and safe in adults aged 65 years or over [17], it represents an excellent opportunity to determine the true effects of HMB supplementation in enhancing training performance. Furthermore, the promising results of HMB supplementation to mitigate age-related cognitive deficits [36, 37] and the lack of studies exploring its impact on people with very limited or no mobility [25, 38] encourage adopting this strategy in vulnerable people such as older nursing homes patients. Therefore, the aim of the HEAL study is to assess the effects of the combination of a daily multicomponent exercise and resistance training (VIVIFRAIL program) in addition to HMB supplementation on older adults’ health.
Methods/design
Study design and settings
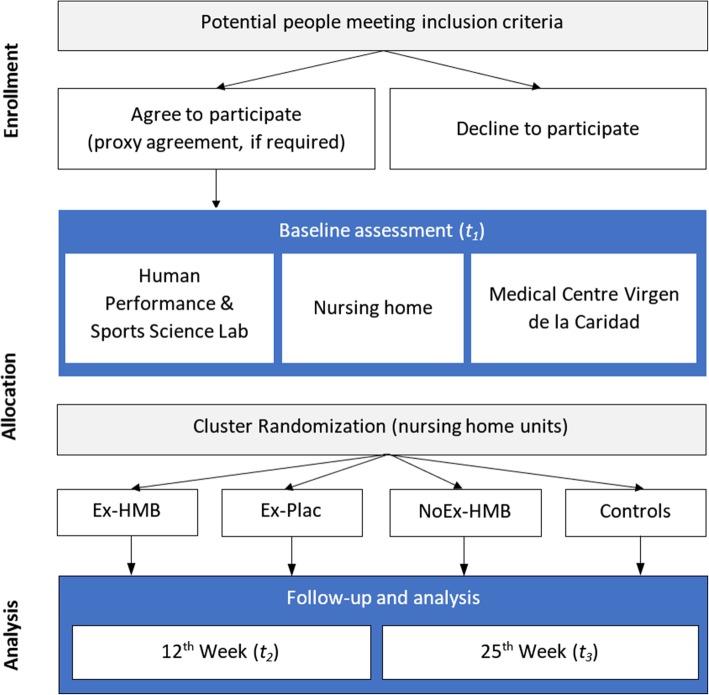
This is a cluster randomized, placebo-controlled study with four parallel groups. The study has been designed to determine the efficacy of HMB supplementation in addition to 24-weeks of multicomponent exercise and resistance training (the VIVIFRAIL program) in adults ≥70 years. Flowchart of the trial is shown in Fig. 1. Enrollment, intervention allocation, follow-up, and data analysis will be conducted according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement [39, 40].
Fig. 1.
Flowchart of the trial
Eligibility criteria
Inclusion criteria for enrollment will be: men and women aged ≥70 years, be able to follow an active physical rehabilitation program and voluntary participation. Enrollment of cognitively impaired older adults will require proxy permission (family member or caregiver) [41]. All potential participants will provide a medical history and undergo a medical examination to identify cardiovascular or metabolic conditions that would exclude participation (for full list, see Table 1).
Table 1.
Exclusion criteria for the HEAL study
| Exclusion criteria | |
|---|---|
|
- Acute heart attack (recent 3–6 months) or unstable angina - Uncontrolled atrial or ventricular arrhythmias - Aortic dissecting aneurysm - Severe aortic stenosis - Acute endocarditis / pericarditis - Uncontrolled high blood pressure (> 180/100 mmHg) - Acute thromboembolism - Acute or severe heart failure - Acute or severe respiratory failure - Uncontrolled postural hypotension - Uncontrolled acute decompensated diabetes mellitus or low blood sugar - A recent fracture in the last month. - Coincident participation in any intervention trial - HMB contraindication, intolerance, or allergy - Have regularly performed exercise (> 20 min > 3 days/week) in the last 3 months - Malignant diseases (exceptions: basal or squamous-cell skin carcinoma or carcinoma in situ of the uterine cervix) - Revascularization within 1 year - Severe loss of vision, hearing, or communicative ability - Conditions preventing cooperation |
Sample size
The required sample size will be determined on the basis on the functional capacity, using the Short Physical Performance Battery (SPPB) [42]. According to previous research on subjects with similar characteristics [17], a clinically relevant change is about 1.5 ± 1.0 points increments after 12-weeks. Differences of 2 point in total SPBB with a standard deviation of 3 points with a power of 80% and α of 0.05 can be estimated with 20 participants using the R software (v. 3.2.1) and the package samplesize. Assuming a maximum loss of follow-up of 30%, we will recruit 26 adults ≥70 years per group (n = 104). Similar interventions had an adherence rate of 75%, and a mean attendance of 80% to the sessions [17]. Therefore, the current estimation is realistic and affordable.
Recruitment process and measurements procedures
A schematic overview of the outcomes, measures and timeline is shown in Table 2. Recruitment will be carried out in nursing homes in Murcia (Spain) located within a radius of 15 km or less than 20 min by car from the assessment sites. There are over 15 nursing homes among this radius that ensure recruitment of enough participants. Initial assessment will be carried out in the Human Performance & Sports Science Lab, Faculty of Sport Sciences, University of Murcia (Murcia, Spain). Body composition and biochemical analyses will be performed in the Medical Centre Virgen de la Caridad (Murcia, Spain). Participants will be scheduled in small groups to be taken to the laboratory for the initial assessment and medical centre. Dependent people will be transported in adapted vehicles with a caregiver. All measurements will be performed under technical and medical supervision.
Table 2.
Schedule of enrollment, interventions, and assessments
| TIMEPOINT | Enrollment -t1 |
Allocation 0 |
Baseline t1 |
Intervention | Follow-up 12th week post baseline t2 |
Close-out 25th week post baseline t3 |
|---|---|---|---|---|---|---|
| ENROLLMENT | ||||||
| Eligibility screen | ✓ | |||||
| Informed consent | ✓ | |||||
| Randomized Allocation | ✓ | |||||
| INTERVENTION | ✓ | |||||
| Ex-HMB | ✓ | |||||
| NoEx-HMB | ✓ | |||||
| Ex-Plac | ✓ | |||||
| Control | ✓ | |||||
| ASSESSMENTS | ||||||
| Functional capacity (primary outcome) | ||||||
| SPPB: Gait speed, balance, and 5-sit-to-stand | ✓ | ✓ | ✓ | ✓ | ||
| Muscle strength and power | ||||||
| Grip strength | ✓ | ✓ | ✓ | |||
| 1RM seated leg press | ✓ | ✓ | ✓ | |||
| 1RM vertical bench press | ✓ | ✓ | ✓ | |||
| Sit-to-stand muscle power | ✓ | ✓ | ✓ | |||
| Frailty and fall risk | ||||||
| Frailty phenotype | ✓ | ✓ | ✓ | |||
| Falls history | ✓ | ✓ | ✓ | ✓ | ||
| Fall risk assessment | ✓ | ✓ | ✓ | ✓ | ||
| Body composition | ✓ | ✓ | ||||
| Blood pressure and resting heart rate | ✓ | ✓ | ✓ | |||
| Haematology | ✓ | ✓ | ✓ | |||
| Biochemical analyses | ✓ | ✓ | ✓ | |||
| Nutritional status | ✓ | ✓ | ✓ | |||
| Sarcopenia | ✓ | ✓ | ✓ | |||
| Disability and comorbidity | ||||||
| Barthel index | ✓ | ✓ | ✓ | |||
| Lawton index | ✓ | ✓ | ✓ | |||
| Comorbidity | ✓ | ✓ | ✓ | |||
| Cognitive function | ✓ | ✓ | ✓ | ✓ | ||
| Depression | ✓ | ✓ | ✓ | |||
Ex: 12-week of VIVIFRAIL multicomponent exercise program. HMB: dietary supplementation of HMB. Plac Placebo. SPPB Short Physical Performance Battery; 1RM one-repetition maximum
The VIVIFRAIL exercise program will be administered by a training team (experienced and qualified personal trainers and physical therapists), under nursing supervision. After the initial assessment and one week before the start of the intervention, participants will attend a familiarization session at the place in which the testing and training will be conducted.
Randomization and blinding
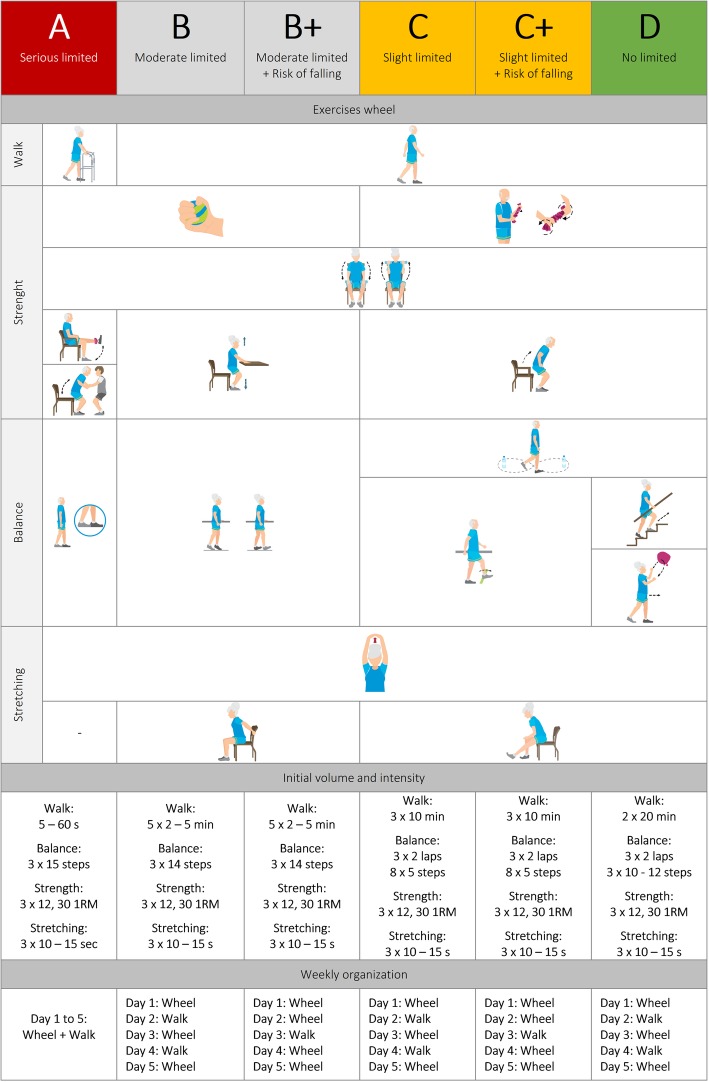
After recruitment and baseline measurements, nursing home will be randomized to either of the four groups in clusters, according to a computer-generated sequence using the Sealed Envelope Ltd. online system. The cluster design is chosen to prevent influences on participants’ behaviours within a given nursing home unit. A stratified randomization will be used according to their initial functional status (A, B, C or D, see Fig. 2) to reduce imbalance between groups. The allocation will be concealed in a password protected computer file. Given the nature of the treatment (i.e., daily exercise and dietary supplementation program), participants will be aware of their group allocation. Outcome assessors and data analysts will be blinded to the treatment group assignment. Assessors will not be involved in intervention activities. A dedicated protocol will be defined to protect the confidentiality of data.
Fig. 2.
Summary of the VIVIFRAIL multicomponent exercise intervention program. Individualization based on baseline testing scores. Full guidelines: www.vivifrail.com
Interventions
Dietary supplementation
Intervention groups including HMB supplementation (Ex-HMB and NoEx-HMB) will receive a 3 g daily dose of free acid HMB in powder form (myprotein.co.uk, Cheadle, Cheshire, UK) dissolved freely into 250 mL of water during a 24-week intervention [34, 43]. Nursing staff will supply the doses as a part of their daily diet routine. Ex-Plac and Control groups will receive stevioside. Supplements will be packaged in indistinguishable envelopes and boxes, with an identification code for each participant and group. The compliance of supplementation will be monitored and ensured by medical staff working at the nursing home. Oral supplement and Vitamin D will be provided to maintain an acceptable nutritional status.
Multicomponent physical exercise program
Intervention groups including exercise (Ex-HMB and Ex-Plac) will complete an individualized multicomponent training program, VIVIFRAIL [16], 5 days a week during 24 weeks. Free on-line resources and program guidelines are available online (http://www.vivifrail.com/resources). The VIVIFRAIL program has been carefully designed for people ≥70 years and includes six programs or “Passports” adapted for each participant’s condition according to their functional limitation (serious [A], moderate [B], slight [C] and no limitation [D]) and risk of falling (B+ and C+). Individualization is made based on the baseline testing scores (i.e., the VIVIFRAIL test). Each program combines strength, power, balance, walking, stretching and cardiovascular exercises, in the named “VIVIFRAIL Wheel”. Training sessions are daily and weekly organized (type of exercise, steps and reps) in individual “Passports”. A summary of the exercise program is shown in Fig. 2.
The VIVIFRAIL program has a free mobile app available on iOS and Android, the latest version of which, (launched in January, 2019) allows the recording of the results of the test to automatically assign each participant to a training program. The App includes a calendar with a daily progression, training monitoring and rate of perceiving effort assessment.
Outcome measures
Functional capacity (primary outcome): The Short Physical Performance Battery (SPPB) [42] scores from 1 (low mobility) and 12 (full mobility) points based on three tests: balance tests (tandem, semi-tandem and one foot next to the other), gait speed and 5-sit-to-stand test. The SPPB has been extensively administrated in older adults [17, 44]. The resulting scores are part of the VIVIFRAIL test to determine each individual’s physical exercise program [16].
Maximal muscle strength and power output: grip strength measurement (Jamar digital dynamometer, NexGen ergonomics, Pointe Claire, Quebec, Canada) [45], one repetition maximum (1RM) seated leg press and vertical bench press strength (Salter Ltd., Barcelona, Spain) and muscle power (T-Force Dynamic Measurement System, Ergotech Consulting SL, Murcia, Spain) [46, 47], sit-to-stand muscle power [48], in the same order with a 3-min rest between tests in order to diminish fatigue [49].
Frailty and fall risk: frailty phenotype determination [50], complete falls history and fall risk assessment, physical examination [16].
Body composition: Body composition will be assessed using dual-energy x-ray absorptiometry - DXA (Hologic, Bedford, MA; Discovery A), between 6:00 AM and 9:00 AM after a ≥ 10-h fast and after participants had voided their bladders [51].
Blood pressure and resting heart rate: Systolic and diastolic blood pressure, as well as resting heart rate will be measured after 10 min of rest, two times 2 min apart (M6 upper arm blood pressure monitor Omron. Omron Health Care Europe B.V. Hoofddorp, The Netherlands).
Haematology: Erythrocyte count, haematocrit, haemoglobin, platelets, leukocytes and erythrocyte mean corpuscular volume will be quantified by Coulter Cell Counter.
Biochemical analyses: glucose, high-density lipoprotein (HDL), total cholesterol (TC), triglycerides (TG), glycosylated haemoglobin (HbA1c), thyroid-stimulating hormone (TSH), C-reactive protein (CRP), albumin, prealbumine, transferrin, insulin-like growth factors (IGF-1 and IGFBP-3), creatine phosphokinase (CPK) and 25-hydroxyvitamin D (25[OH]D). Blood analysis will be conducted with standard methods using an autoanalyzer. Insulin sensitivity will be derived from the homeostatic model assessment for insulin resistance (HOMA-IR).
Nutritional status: The Mini-Nutritional Assessment (MNA-SF) [52] will be used to evaluate nutrition status and malnutrition risk.
Sarcopenia: The SARC-F will be used to diagnose sarcopenia [53].
Disability and comorbidity: Barthel index [54] and Lawton index [55] will be used to assess disability in basic activities and instrumental activities of daily living, respectively. Given the limitations of comorbidity indexes in older people [56], we will consider comorbidity when a participant presents two or more geriatric syndromes from a list of selected geriatric syndromes, as previously proposed [57].
Cognitive function and depression: the validated Spanish version of the Mini-Mental State Examination (MMSE) [58] will be used to assess cognitive function. Depression will be assess with the Spanish version of the 15-item Yeasavage geriatric depression scale [59].
Statistical analysis
Analysis will be performed on participants who attended at least 80% the training sessions and completed all the measurements. Treatment effects will be tested using generalized linear models. All models will be adjusted for the baseline outcome value and repeated adjusting for gender, age, the group effect, and confounding factors.
Trial registration
The trial was registered on ClinicalTrials.gov (identifier: NCT03827499) on 01/02/2019.
Discussion
This paper outlines the protocol for a randomized, placebo-controlled study to determine the efficacy of HMB supplementation in addition to 24-weeks of multicomponent exercise and resistance training in adults ≥70 years old. At the time of writing, the study was ongoing (recruitment status). Baseline assessment is planned to started in March 2019.
Maintaining old people’s health and protecting them from frailty, muscle waist and cardiovascular diseases will save billions in public care costs by lengthening people’s healthy life, reducing the loss of income due to premature death and reducing nursing dependency [60]. Evidence supports that multicomponent exercise [8–10, 17, 61] and HMB supplementation [24–26] are effective in improving older adult’s health and palliating functional metabolic diseases in older people. However, the true effect of HMB supplementation combined with a tailored exercise program is still unknown. Just a few trials have investigated the combination of both [31–33], showing promising results. Moreover, the implementation of the new VIVIFRAIL multicomponent exercise program for frail old people in addition to HMB supplementation is still to be done.
The study results will be of high relevance to old people living in nursing homes and their health care providers. If the benefits of the combined VIVIFRAIL and HMB are proven, this could be an alternative management strategy to consider in nursing homes with older adults and people with functional metabolic diseases and muscle-wasting conditions. In addition, the current exercise intervention is inexpensive and freely available (http://www.vivifrail.com/), which permits its replication. The findings of the HEAL study will help professionals from public health systems to identify cost-effective and innovative actions to improve older people’s health and quality of life, and endorse exercise practice in older adults living in nursing homes.
Acknowledgements
*The HEAL study group:
Jesús García Pallarés –Human Performance and Sports Science Laboratory, Department of Physical Activity and Sport, Faculty of Sport Sciences, University of Murcia, Spain.
Javier Courel Ibáñez –Human Performance and Sports Science Laboratory, Department of Physical Activity and Sport, Faculty of Sport Sciences, University of Murcia, Spain.
Ricardo Morán Navarro –Human Performance and Sports Science Laboratory, Department of Physical Activity and Sport, Faculty of Sport Sciences, University of Murcia, Spain.
Elena Saura Guillén – Endocrinology and Nutrition Service, University Hospital Virgen de la Arrixaca, Murcia, Spain.
Alejandro Martínez Cava –Human Performance and Sports Science Laboratory, Department of Physical Activity and Sport, Faculty of Sport Sciences, University of Murcia, Spain.
Alejandro Sánchez Pay –Human Performance and Sports Science Laboratory, Department of Physical Activity and Sport, Faculty of Sport Sciences, University of Murcia, Spain.
Ángel Buendía Romero – Faculty of Sport Sciences, University of Murcia, Spain.
Silverio García Conesa – Faculty of Sport Sciences, University of Murcia, Spain.
Abbreviations
- 1RM
One-repetition maximum
- CRP
C-reactive protein
- DXA
Dual-energy x-ray absorptiometry
- Ex-HMB
Exercise intervention with HMB
- Ex-Plac
Exercise intervention with placebo
- HDL
High-density lipoprotein
- HMB
β-hydroxy-β-methylbutyrate
- MMSE
Mini-Mental State Examination
- NoEx-HMB
No exercise intervention with HMB
- RCT
Randomized Control Trial
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
- SPPB
Short Physical Performance Battery
- TC
Total cholesterol
- TG
Triglycerides
- TSH
Thyroid-stimulating hormone
Authors’ contributions
JGP contributed in the conception of the idea for the study. JCI and JGP contributed in the development of the protocol, organization and writing the manuscript. All the authors read the draft, made contributions and approved the final manuscript.
Funding
This protocol has been peer-reviewed and funded by the Autonomous Community of the Region of Murcia, Regional Program for the Promotion of Scientific and Technical Research (Action Plan 2018), Seneca Foundation-Agency of Science and Technology, Region of Murcia (ID: 20872/PI/18).
Availability of data and materials
Data are not available due to EU General Data Protection Regulation. Please, contact the corresponding author if you are interested in study materials.
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Commission of the University of Murcia, Spain (code: 2131/2018). All participants will sign an informed consent according to the Declaration of Helsinki prior to data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Javier Courel-Ibáñez, Email: Javier.courel.ibanez@gmail.com.
Jesús G. Pallarés, Email: jgpallares@um.es.
on behalf of the HEAL study group:
Jesús G. Pallarés, Javier Courel Ibáñez, Ricardo Morán Navarro, Elena Saura Guillén, Alejandro Martínez Cava, Alejandro Sánchez Pay, Ángel Buendía Romero, and Silverio García Conesa
References
- 1.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2011;27:39–52. doi: 10.1016/j.cger.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Clark BC, Manini TM. Sarcopenia ≠ dynapenia. J Gerontol Ser A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 5.Ethgen O, Beaudart C, Buckinx F, Bruyère O, Reginster JY. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. 2017;100:229–234. doi: 10.1007/s00223-016-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LHT, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sport Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 7.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Pedro, Izquierdo Mikel, Radaelli Regis, Sbruzzi Graciele, Grazioli Rafael, Pinto Ronei Silveira, Cadore Eduardo Lusa. Effectiveness of Multimodal Training on Functional Capacity in Frail Older People: A Meta-Analysis of Randomized Controlled Trials. Journal of Aging and Physical Activity. 2018;26(3):407–418. doi: 10.1123/japa.2017-0188. [DOI] [PubMed] [Google Scholar]
- 9.Lopez P. Benefits of resistance training in physically frail elderly : a systematic review. Aging Clin Exp Res. 2017;0:0. doi: 10.1007/s40520-017-0863-z. [DOI] [PubMed] [Google Scholar]
- 10.Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16:105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DH, Buth KJ, Martin B-J, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 12.Veronese N, Stubbs B, Trevisan C, Bolzetta F, De Rui M, Solmi M, et al. Poor physical performance predicts future onset of depression in elderly people: Progetto Veneto Anziani longitudinal study. Phys Ther. 2017;97:659–668. doi: 10.1093/ptj/pzx017. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo M, Rodriguez-Mañas L, Sinclair AJ, Vivifrail Investigators Group What is new in exercise regimes for frail older people — how does the Erasmus Vivifrail project take us forward? J Nutr Heal Aging. 2016;20:736–737. doi: 10.1007/s12603-016-0702-5. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Global Strategy and Action Plan on Ageing and Health. 2015. [Google Scholar]
- 15.de Souto Barreto Philipe, Morley John E., Chodzko-Zajko Wojtek, H. Pitkala Kaisu, Weening-Djiksterhuis Elizabeth, Rodriguez-Mañas Leocadio, Barbagallo Mario, Rosendahl Erik, Sinclair Alan, Landi Francesco, Izquierdo Mikel, Vellas Bruno, Rolland Yves. Recommendations on Physical Activity and Exercise for Older Adults Living in Long-Term Care Facilities: A Taskforce Report. Journal of the American Medical Directors Association. 2016;17(5):381–392. doi: 10.1016/j.jamda.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Izquierdo M, Casas-Herrero A, Zambm-Ferraresi F, Martínez-Velilla N, Alonso-Bouzón C, Rodríguez-Mañas L, et al. A Practical Guide for Prescribing a Multi-Component Physical Training Program to prevent weakness and falls in People over 70. 2017. http://www.vivifrail.com/resources.
- 17.García-Molina R, Ruíz-Grao MC, Noguerón-García A, Martínez-Reig M, Esbrí-Víctor M, Izquierdo M, et al. Benefits of a multicomponent falls unit-based exercise program in older adults with falls in real life. Exp Gerontol. 2018;110:79–85. doi: 10.1016/j.exger.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Garlick Peter J. The Role of Leucine in the Regulation of Protein Metabolism. The Journal of Nutrition. 2005;135(6):1553S–1556S. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 19.Layman Donald K. Role of Leucine in Protein Metabolism During Exercise and Recovery. Canadian Journal of Applied Physiology. 2002;27(6):646–662. doi: 10.1139/h02-038. [DOI] [PubMed] [Google Scholar]
- 20.Mero A. Leucine supplementation and intensive training. Sport Med. 1999;27:347–358. doi: 10.2165/00007256-199927060-00001. [DOI] [PubMed] [Google Scholar]
- 21.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab (Lond). 2008;5(1). 10.1186/1743-7075-5-1. [DOI] [PMC free article] [PubMed]
- 22.GALLAGHER PHILIP M., CARRITHERS JOHN A., GODARD MICHAEL P., SCHULZE KIMBERLEY E., TRAPPE and SCOTT W. ??-hydroxy-??-methylbutyrate ingestion, Part II: effects on hematology, hepatic and renal function. Medicine and Science in Sports and Exercise. 2000;32(12):2116–2119. doi: 10.1097/00005768-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Nissen S., Sharp R. L., Panton L., Vukovich M., Trappe S., Fuller J. C. β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Humans Is Safe and May Decrease Cardiovascular Risk Factors. The Journal of Nutrition. 2000;130(8):1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 24.Fitschen PJ, Wilson GJ, Wilson JM, Wilund KR. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29:29–36. doi: 10.1016/j.nut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8:529–541. doi: 10.1002/jcsm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi AP, D’Introno A, Rubele S, Caliari C, Gattazzo S, Zoico E, et al. The potential of β-Hydroxy-β-Methylbutyrate as a new strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging. 2017;34:833–840. doi: 10.1007/s40266-017-0496-0. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2015;61:168–175. doi: 10.1016/j.archger.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Deutz NEP, Thaden JJ, ten Have GAM, Walker DK, Engelen MPKJ. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism. 2018;78:167–178. doi: 10.1016/j.metabol.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shreeram S, Ramesh S, Puthan JK, Balakrishnan G, Subramanian R, Reddy MT, et al. Age associated decline in the conversion of leucine to β-Hydroxy-β-Methylbutyrate in rats. Exp Gerontol. 2016;80:6–11. doi: 10.1016/j.exger.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vukovich Matthew D., Stubbs Nancy B., Bohlken Ruth M. Body Composition in 70-Year-Old Adults Responds to Dietary β-Hydroxy-β-Methylbutyrate Similarly to That of Young Adults. The Journal of Nutrition. 2001;131(7):2049–2052. doi: 10.1093/jn/131.7.2049. [DOI] [PubMed] [Google Scholar]
- 32.Stout JR, Smith-Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, et al. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol. 2013;48:1303–1310. doi: 10.1016/j.exger.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Deutz NEP, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704–712. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Din USU, Brook MS, Selby A, Quinlan J, Boereboom C, Abdullah H, et al. A double-blind placebo controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin Nutr. 2018. 10.1016/j.clnu.2018.09.025. [DOI] [PMC free article] [PubMed]
- 35.Berton L, Bano G, Carraro S, Veronese N, Pizzato S, Bolzetta F, et al. Effect of Oral Beta-Hydroxy-Beta-Methylbutyrate (HMB) supplementation on physical performance in healthy old women over 65 years: an open label randomized controlled trial. PLoS One. 2015;10:e0141757. doi: 10.1371/journal.pone.0141757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munroe M, Mahmassani ZS, Dvoretskiy S, Reid JJ, Miller BF, Hamilton K, et al. Cognitive function is preserved in aged mice following long-term β-hydroxy β-methylbutyrate supplementation. Nutr Neurosci. 2018:1–13. 10.1080/1028415X.2018.1483101. [DOI] [PubMed]
- 37.Hankosky ER, Sherrill LK, Ruvola LA, Haake RM, Kim T, Hammerslag LR, et al. Effects of β-hydroxy-β-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr Neurosci. 2017;20:379–387. doi: 10.1080/1028415X.2016.1145376. [DOI] [PubMed] [Google Scholar]
- 38.Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SDR, Hart N, et al. Beta-Hydroxy-beta-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. Am J Clin Nutr. 2019;109:1119–1132. doi: 10.1093/ajcn/nqy373. [DOI] [PubMed] [Google Scholar]
- 39.Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prusaczyk B, Cherney SM, Carpenter CR, DuBois JM. Informed consent to research with cognitively impaired adults: transdisciplinary challenges and opportunities. Clin Gerontol. 2017;40:63–73. doi: 10.1080/07317115.2016.1201714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 43.Fuller JC, Baier S, Flakoll P, Nissen SL, Abumrad N, Rathmacher JA. Vitamin D status affects strength gains in older adults supplemented with a combination of β-hydroxy-β-methylbutyrate, arginine, and lysine: a cohort study. J Parenter Enter Nutr. 2011;35:757–762. doi: 10.1177/0148607111413903. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Gomez D, Bandinelli S, Del-Panta V, Patel KV, Guralnik JM, Ferrucci L. Three-year changes in physical activity and decline in physical performance over 9 years of follow-up in older adults: the Invecchiare in chianti study. J Am Geriatr Soc. 2017;65:1176–1182. doi: 10.1111/jgs.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mora-Rodríguez R, Pallarés JG, López-Samanes Á, Ortega JF, Fernández-Elías VE. Caffeine ingestion reverses the circadian rhythm effects on neuromuscular performance in highly resistance-trained men. PLoS One. 2012;7:e33807. doi: 10.1371/journal.pone.0033807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morán-Navarro Ricardo, Martínez-Cava Alejandro, Sánchez-Medina Luis, Mora-Rodríguez Ricardo, González-Badillo Juan José, Pallarés Jesús G. Movement Velocity as a Measure of Level of Effort During Resistance Exercise. Journal of Strength and Conditioning Research. 2019;33(6):1496–1504. doi: 10.1519/JSC.0000000000002017. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Medina L, González-Badillo JJ, Pérez CE, Pallarés JG. Velocity- and power-load relationships of the bench pull vs bench press exercises. Int J Sports Med. 2014;35:209–216. doi: 10.1055/s-0033-1351252. [DOI] [PubMed] [Google Scholar]
- 48.Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, Alfaro-Acha A, Rodriguez-Mañas L, Ara I, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol. 2018;112:38–43. doi: 10.1016/j.exger.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Alcazar J, Guadalupe-Grau A, García-García FJ, Ara I, Alegre LM. Skeletal muscle power measurement in older people: a systematic review of testing protocols and adverse events. J Gerontol Ser A. 2018;73:914–924. doi: 10.1093/gerona/glx216. [DOI] [PubMed] [Google Scholar]
- 50.Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W. J., Burke G., McBurnie M. A. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 51.Jakubowski JS, Wong EPT, Nunes EA, Noguchi KS, Vandeweerd JK, Murphy KT, et al. Equivalent hypertrophy and strength gains in HMB- or leucine-supplemented men. Med Sci Sport Exerc. 2018;51(1). 10.1249/MSS.0000000000001752. [DOI] [PMC free article] [PubMed]
- 52.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol Ser A Biol Sci Med Sci. 2001;56:M366–M372. doi: 10.1093/gerona/56.6.M366. [DOI] [PubMed] [Google Scholar]
- 53.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;4:61–65. [PubMed] [Google Scholar]
- 55.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 56.Martínez-Velilla N, Cambra-Contin K, Ibáñez-Beroiz B. Comorbidity and prognostic indices do not improve the 5-year mortality prediction of components of comprehensive geriatric assessment in hospitalized older patients. BMC Geriatr. 2014;14:64. doi: 10.1186/1471-2318-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutiérrez-Valencia M, Izquierdo M, Lacalle-Fabo E, Marín-Epelde I, Ramón-Espinoza MF, Domene-Domene T, et al. Relationship between frailty, polypharmacy, and underprescription in older adults living in nursing homes. Eur J Clin Pharmacol. 2018;74:961–970. doi: 10.1007/s00228-018-2452-2. [DOI] [PubMed] [Google Scholar]
- 58.Lobo A, Escobar V, Ezquerra J, Seva Díaz A. “El Mini-Examen Cognoscitivo” (Un test sencillo, práctico, para detectar alteraciones intelectuales en pacientes psiquiátricos). [The “Mini-Examen Cognoscitiuo”: A simple and practical test to detect intellectual dysfunctions in psychiatric patients.] Rev Psiquiatr y Psicol Médica. 1980;14:39–57. [Google Scholar]
- 59.Martínez de la Iglesia J, Onís Vilches MC, Dueñas Herrero R, Albert Colomer C, Aguado Taberné C, Luque Luque R. Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Medifam. 2002;12:26–40. doi: 10.1109/PROC.1963.2309. [DOI] [Google Scholar]
- 60.Cavill N, Kahlmeier S, Racioppi F. Physical activity and health in Europe: evidence for action. Copenhagen: WHO Regional Office for Europe; 2006. http://www.euro.who.int/__data/assets/pdf_file/0011/87545/E89490.pdf.
- 61.Casas-Herrero A, Cadore EL, Zambom-Ferraresi F, Idoate F, Millor N, Martínez-Ramirez A, et al. Functional capacity, muscle fat infiltration, power output, and cognitive impairment in institutionalized frail oldest old. Rejuvenation Res. 2013;16:396–403. doi: 10.1089/rej.2013.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not available due to EU General Data Protection Regulation. Please, contact the corresponding author if you are interested in study materials.