Abstract
Background
The present study was performed to assess the effect of mechanical stretch on the proliferation and contractile function of hBSMCs.
Material/Methods
hBSMCs and ICCs were seeded at 8×104 cells/well in 6-well silicone elastomer-bottomed culture plates coated with type I collagen, and grown to 80% confluence in DMEM/10% FBS and a 5% CO2 humidified atmosphere at 37°C. Cells of hBSMCs and hBSMCs/ICCs of co-culture were then subjected to continuous cycles of stretch-relaxation using a computer-driven, stretch-inducing device. The treated concentration of imatinib was 10 μM. Mechanisms underlying observed hBSMCs contraction were examined using Western blotting and RT-PCR. The 0.1 μM carbachol was separately added to the experimental groups, and 300 s was recorded by laser scanning confocal microscope.
Results
We found that mechanical stretch increased contraction and proliferation of hBSMCs. Calcium ion activity increased significantly after mechanical stretch. The number of hBSMCs was significantly increased after the combination mechanical stretch with ICCs treatment. After combination mechanical stretch with hBSMCs/ICCs treatment, the mRNA and protein level of M2, M3, and c-kit were significantly increased. After combination of mechanical stretch with no imatinib treatment, the proliferation of hBSMCs was higher than others, and the mRNA and protein level of M2 and M3 were significantly increased.
Conclusions
We revealed that ICCs could promote hBSMC proliferation and contraction, and cyclic stretch could promote acetylcholine receptor M2 and M3 caused by c-kit in the ICCs, which promoted the contraction of hBSMCs.
MeSH Keywords: Cell Proliferation; Interstitial Cells of Cajal; Muscle Contraction; Stress, Mechanical; Urinary Bladder
Background
Every year, tens of thousands of patients worldwide undergo intestinal bladder replacement surgery [1]. Due to the inherent shortcomings of the current clinical application of intestinal bladder replacement, the exploration of the construction of bioactive bladder repair tissue by tissue engineering method has been a focus of urology research. Mechanical stretch is a necessary condition for bladder development [2]. In sheep embryos without stress, bladder development is blocked, the expression of apoptosis and migration genes is significantly decreased, the genes regulating cell adhesion and proliferation are significantly downregulated, and the development of bladder wall functionalization is blocked [3–5].
Interstitial cells of Cajal (ICCs) are a group of special mesenchymal cells first discovered in the gastrointestinal tract. They are the pacemaker of the slow wave of the gastrointestinal tract, transmitting electrical signals and mediating the signal regulation between the intestinal nerves and smooth muscle [6,7]. ICCs were also in found in the urinary system of guinea pigs [8–10], and they exist in the autonomic nerve endings and the bladder smooth muscle cells. They can be produced under the action of quasi choline medicine Ca2+ flow, resulting in spontaneous activity, suggesting that ICCs are involved in the spontaneity of bladder smooth muscle contractions. ICCs might be the pacemaker of bladder activity and nerve signal transmission to smooth muscle cells in the middle of the medium. In 2002, McClosky et al. first reported kit-positive cells were found in the guinea pig bladder [11]. c-kit is a proto-oncogene and its coding product kit is a tyrosine protein kinase receptor which is specifically expressed on interstitial cell membranes. In the gut, the functional role of kit-positive ICC has been better defined, and there is strong evidence now for a primary role for those kit-positive ICCs in generating and propagating spontaneous contractile activity in the gut [12]. An experiment by Gevaert et al. [13] showed that administration of imatinib in rats impaired the neonatal development of intramuscular interstitial cells in the bladder and resulted in altered contractile properties, as well as suggesting that c-kit is a key gene for ICC expression. The distribution of M receptors in various subtypes of mammals has a certain specificity, which is usually dominated by a single subtype, while other types coexist and play an auxiliary role [14]. The functions of each subtype of M receptor are different, and the degree of expression in each subtype in different cells is also different. The bladder detrusor muscle simultaneously expresses M2 and M3 subtypes of M receptors, but M3 is the only one directly related to detrusor contraction [15,16]. M2 synergizes with beta receptors to inhibit detrusor relaxation and assist in urination under the action of large doses of Ach [17,18].
Some researchers have proved that cyclic pressure can stimulate various intracellular signaling pathways, for example, Rho family GTPases, ERK1/2 MAPK, STAT3, p38/MAPK, and PI3K/Akt, which can initiate proliferation of bladder smooth muscle cells (BSMCs) [19,20]. Our research group previously conducted many studies [21–26] on the mechanism by which mechanical stress stimulates the proliferation and mechanism contraction of BSMCs. Chen et al. [25] were the first to report that cyclic hydrodynamic pressure stimulates the proliferation of human bladder smooth muscle cells (hBSMCs) cultured in scaffolds, and they found that the signal transduction mechanism for this process is involves PI3K/SGK1. Tian et al. [21] showed similar results, indicating that the PI3K-SGK1 is a novel therapy targeting specific urinary bladder diseases caused by excessive mechanical forces. In addition, Wu et al. [23] studied hBSMCs exposed to cyclic hydrodynamic pressures in vitro with defined parameters (static, 100 cmH2O, 200 cmH2O, and 300 cmH2O pressure) for 24 h, showing that Rac1 signaling plays a significant role in mechanotransduction and regulation of hBSMCs proliferation in response to cyclic hydrodynamic pressure. Sun et al. [26] showed that microRNA 4323 can induce hBSMC proliferation under cyclic hydrodynamic pressure by activation of the erk1/2 signaling pathway.
We conducted the present study to assess the effect of mechanical stretching on the proliferation and contractile function of hBSMCs. We assessed the effect of stretching on bladder stromal cells and explored the relevant signal pathways to provide a genetic basis for the clinical application of tissue engineering methods to construct bioactive bladder repair tissue.
Material and Methods
Cell culture and treatment
Following the method described previously by Park et al. [27], hBSMCs (catalog no. 4310; ScienCell, Carlsbad, CA, USA) and ICCs (FDCC, Shanghai, CHN) were seeded at 8×104 cells/well in 6-well silicone elastomer-bottomed culture plates coated with type I collagen (Bioflex; Flexcell, Hillsborough, NC) and grown to 80% confluence in DMEM/10% FBS and a 5% CO2 humidified atmosphere at 37°C. Cells of hBSMCs and hBSMCs/ICCs of co-culture were then subjected to continuous cycles of stretch-relaxation using a computer-driven, stretch-inducing device (Strain UnitFX-5000, Flexcell). Every cycle consisted of 5 s of stretch and 5 s of relaxation (0.1 Hz), and mechanical stretch was conducted under conditions of 5%, 10%, 15%, and 20% elongation (0.1 Hz) for 0, 2, 6, 12, and 24 h. Our results showed that mechanical stretch under conditions of 10% elongation (0.1 Hz) for 6 h is the best, so all subsequent experiments were carried out under those conditions. Imatinib (Monmouth Junction, NJ, USA) was dissolved in 0.01% dimethylsulfoxide. The treated concentration of imatinib was 10 μM, based on the method described by Kubota et al. [28].
Cell proliferation
The effects of mechanical stretch alone on cell proliferation of hBSMCs and ICCs were determined by CCK-8 assay. After treatment with 10% elongation (0.1 Hz) for 6 h, 1×104 cells were seeded into 96-well plate; unstretched cells served as controls. To determine the effect of mechanical stretching on hBSMCs proliferation and contraction ability of ICCs, the combination treatment was performed with mechanical stretch with hBSMCs alone, no stretch with hBSMCs/ICCs, and mechanical stretch with hBSMCs/ICCs, while unstretched with hBSMCs cells alone served as controls. To determine the effect of mechanical stretch on hBSMCs proliferation and contraction ability by c-kit, the combination treatment was performed with mechanical stretch with imatinib (10 μM) and hBSMCs/ICCs, no stretch with imatinib (10 μM) and hBSMCs/ICCs, and mechanical stretch with hBSMCs/ICCs, while mechanical stretch with hBSMCs cells alone served as controls. After incubation, 10 μl of CCK-8 solution (5 mg/ml) was added to the wells and incubated at 37°C for 4 h. The absorbance at 540 nm was recorded with a microplate reader (Thermo, Germany). Data are presented as mean ± standard deviation (SD) of 3 independent experiments.
Laser scanning confocal microscopy system
Co-cultured hBSMCs/ICCs pretreated and treated by the stretch-inducing device (Strain UnitFX-5000, Flexcell) were incubated with fluo-4 AM (5 μmol L) for 30 min, and then the plates were kept in the dark at room temperature for 30 min. The incubated hBSMCs/ICCs suspension was added into the cell pool, and a Leica TCS SP2 laser scanning confocal microscope (Leica, Germany) was used for observation. The spontaneous calcium activity of ICCs is directly related to the excitability of ICCs, so calcium ions are the most critical and primary entry point for ICCs excitability. According to McCloskey’s reports [11], the typical morphology of ICCs and SMCs were found to changes under the effect of calcium ions. Fluo-4 emitted fluorescence after binding with calcium ions. In order to more intuitively observe different values, we used relative fluorescence intensity after detection of calcium ion flux (F/F0: F=cellular calcium ion fluorescence intensity, F0=background fluorescence intensity) to observe the difference in excitability of cells. The imaging method of the laser scanning confocal microscope is line scanning, the sampling rate is 2 ms/line, the objective lens magnification is 40, the oil mirror is 1.25, the scanning frequency is 400 Hz, 1024 pixels/line, 512 lines/page. An Ar/ArKr laser tube was used, with excitation wavelength of 488 nm and emission wavelength of 498~510 nm.
Reverse transcription and quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from cells using TRIzol (Takara, Japan), a Prime Script RT reagent kit (Takara, Japan) was used to generate cDNA, and reverse transcription was performed using the Prime Script RT reagent kit according to the manufacturer’s instructions. RT qPCR was performed on a CFX Connect qPCR system (Bio Rad Laboratory, USA) with a SYBR Premix Ex Taq II kit (Takara, Japan). The primer sequences of M2, M3, and c-kit were as follows:
M2 forward primer, AGCAAACATGCATCAGAATTGG and
reverse primer, GTGCACAAAAGGTGTTAATGAG;
M3 forward primer, ACCCAGCTCCGAGCAGATGGAC and
reverse primer, CGGCTGACTCTAGCTGGATGG;
c-kit forward primer, CGAGATTAGGCTGTTATGC and
reverse primer, ATCCATTCATTCTGCTTATTCT;
β-actin forward primer, 5′-GGGACCTGACTGACTACCTC-3′ and
reverse primer, 5′-ACGAGACCACCTTCAACTCCAC-3′.
The mRNA expression levels were evaluated by using the 2 ΔΔCq method, and β actin was used as a calibrator. All gene expression assays were repeated at least 3 times.
Protein extraction and Western blotting assay
The total protein of cells was extracted using RIPA reagent (Roche, Switzerland) containing protease inhibitor PMSF and phosphatase inhibitors NaF and Na3VO4. The protein was separated using protein extraction reagents (Beyotime, China). The protein concentration was determined using the BCA protein assay kit (Beyotime, China). The membranes were immersed in TBS-T blocking solution containing 5% non-fat milk for 2 h and then incubated with the primary antibodies (EMD Millipore, USA) at 4°C overnight. The antibodies used were as follows: M2 (rat monoclonal antibody, 1: 500, sc33712, Santa Cruz, USA), M3 (rabbit monoclonal antibody, 1: 1000 Ab154835, Abcam, Cambridge, UK), and c-kit (rabbit monoclonal antibody,1: 1000, Ab5505, Abcam, Cambridge, UK). The membranes were incubated with a goat anti-rabbit IgG secondary antibody (1: 2000; OriGene Technologies, USA) or a goat anti-rat IgG secondary antibody (1: 2000; OriGene Technologies, Inc.) for 1 h at 37°C. The bands of protein were exposed using the enhanced chemiluminescent (ECL) kit (Merck Millipore, USA). All Western blot assays were repeated at least 3 times.
The test of carbachol
We separately added 0.1 μM carbachol to the experimental groups [29], and then, to detect intracellular free calcium concentration in hBSMCs, 300 s was recorded by laser scanning confocal microscope.
Statistical analysis
All data are presented as means ±SEM. Comparison between groups was made using 1-way ANOVA; p<0.05 was considered statistically significant.
Results
Effect of mechanical stretch on hBSMCs’ proliferation and contraction
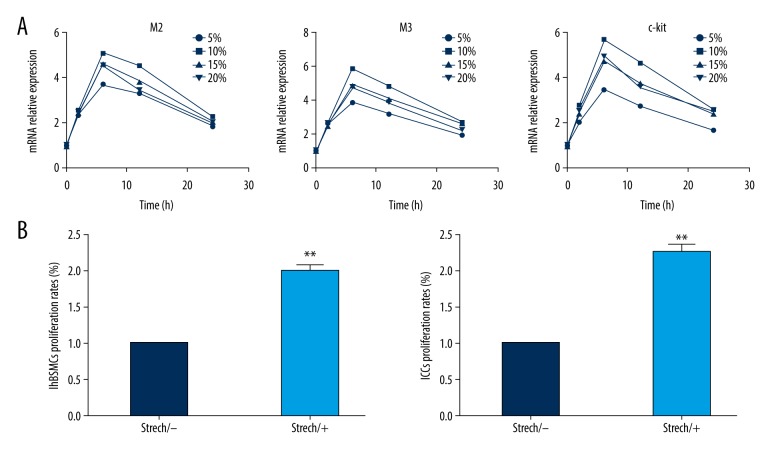
To determine whether M2 and M3 mRNA in hBSMCs and c-kit in ICCs were increased, hBSMCs were subjected to 5%, 10%, 15%, and 20% elongation (0.1 Hz) for 0, 2, 6, 12, and 24 h, and unstretched cells served as controls. Real-time RT-PCR was performed to assess the mRNA levels. As shown in Figure 1A, the highest peak was at 10% cyclic stretch (0.1 Hz) for 6 h after mechanical stretch treatment, at which point M2, M3, and c-kit mRNA were increased (5.071±0.732) (5.864±0.619) (5.683±0.927). The mRNA level of M2, M3, and c-kit in stretch-treated bladder hBSMCs and ICCs were increased in a time-dependent manner. They peaked at 6 h and returned to normal level at 24 h. Cell proliferation assay showed that mechanical stretch significantly increased the numbers of hBSMCs and ICCs at 10% elongation (0.1 Hz) at 6 h, as shown in Figure 1B. Taken together, our results show that mechanical stretch increased contraction and proliferation of hBSMCs.
Figure 1.
Effect of mechanical stretch on hBSMCs’ proliferation and contraction. (A) Real-time RT-PCR analysis of M2, M3, and c-kit expression in hBSMCs and ICCs. RNA samples were purified from mechanical stretch 5%, 10%, 15%, and 20% cyclic stretch (0.1 Hz) for 0, 2, 6, 12, and 24 h. (B) CCK-8 analysis of hBSMCs and ICCs. Cell samples were collected from mechanical stretch or no stretch hBSMCs and ICCs. Proliferation rates (%) were relative to control. N=3, ** p<0.01.
Effect of mechanical stretch on ICCs’ activity
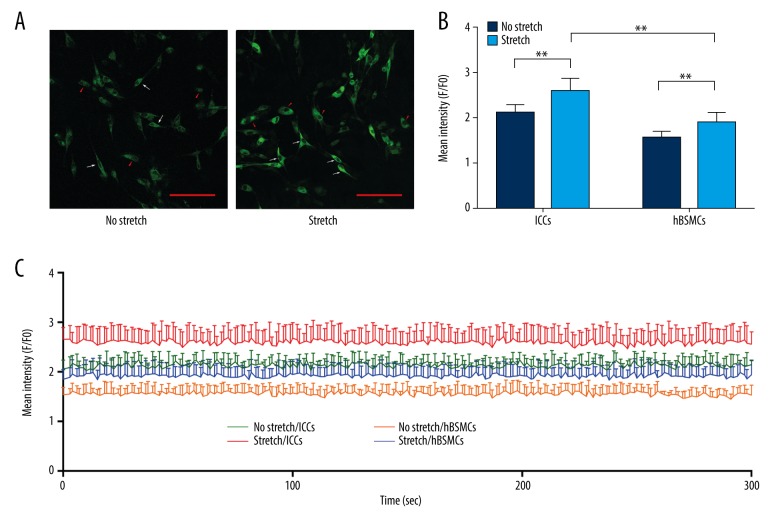
ICCs in the experimental and control groups showed slow and regular spontaneous periodic slow-wave calcium activity. After stress intervention, relative fluorescence intensity was 2.59±0.26 and 1.92±0.20, respectively. As shown in Figure 2, compared to the control group, calcium ion activity increased significantly after mechanical stretch. In addition, compared to ICCs, calcium activity in hBSMCs was much lower.
Figure 2.
Effect of mechanical stretch on ICCs’ activity. (A) Laser scanning confocal microscopy analysis of the calcium activity intensity of hBSMCs and ICCs (white arrows, ICC; red arrows, SMC; scale bar, 100 μm). (B, C) Relative fluorescence intensity of calcium ion flux were detected before and after mechanical stretch. ** p<0.01.
Mechanical stretch enhanced hBSMCs proliferation and contraction by ICCs
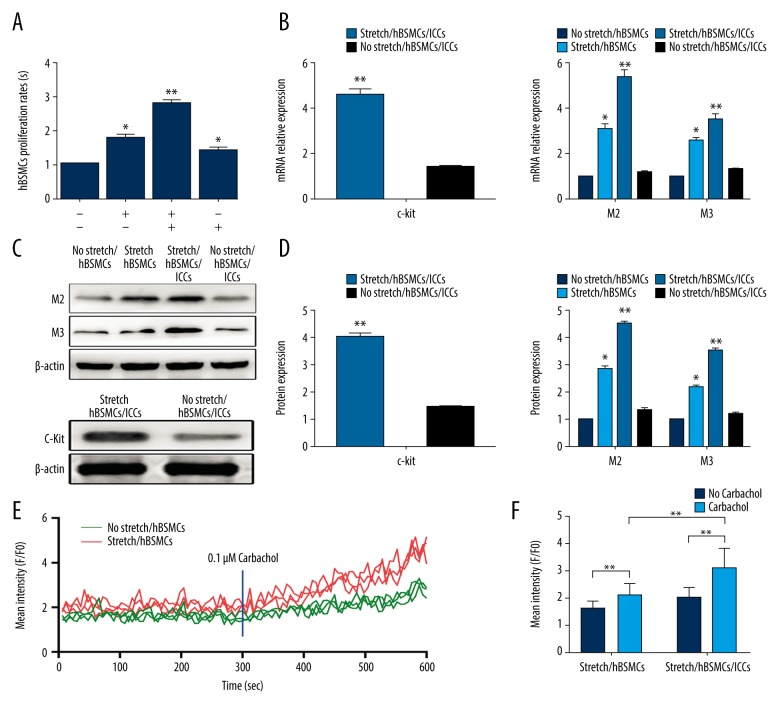
The hBSMC proliferation assay was performed, as shown in Figure 3A. Compared to the mechanical stretch group and ICCs treatment group, the number of hBSMCs was significantly increased after combination mechanical stretch with ICCs treatment, and the hBSMCs’ proliferation was also increased. As shown in Figure 3B, after combination mechanical stretch with hBSMCs/ICCs treatment, the mRNA levels of M2, M3, and c-kit were increased to 5.319±0.346, 3.517±0.245, and 4.581±0.288 (p<0.001), respectively, whereas the mRNA levels of M2 and M3 for combination mechanical stretch with hBSMCs treatment and combination no stretch with hBSMCs treatment were 3.079±0.273 and 2.574±0.157, and 1.183±0.072 and 1.325±0.061. Similarly, as shown in Figure 3C and 3D, the protein levels of M2, M3, and c-kit were increased to 4.512±0.201, 3.516±0.134, and 4.018±0.159 (p<0.001). After combination mechanical stretch with hBSMCs treatment, the protein levels of M2 and M3 were 2.845±0.143 and 2.209±0.113, respectively, and after combination no stretch with hBSMCs/ICCs treatment, M2, M3, and c-kit were 1.343±0.094, 1.207±0.077, and 1.433±0.073, respectively. Before adding carbachol, calcium fluorescence was detected in all groups of hBSMCs. After adding 0.1 μM carbachol separately to each experimental group, the relative fluorescence intensity of the group of stretch/hBSMCs/ICCs was significantly increased (3.09±0.74), and, compared with the relative fluorescence intensity of cyclic stretch acting on hBSMCs (2.10±0.42), there was a statistically significant difference, as shown in Figure 3E, 3F.
Figure 3.
Mechanical stretch enhanced hBSMCs’ proliferation and contraction by ICCs. (A) CCK-8 analysis of hBSMCs. First line ± means mechanical stretch and second line ± means ICCs. Proliferation rates (%) were relative to control. (B) Real-time RT-PCR analysis of M2, M3, and c-kit expression in hBSMCs. RNA samples were purified from no stretch/hBSMCs, stretch/hBSMCs, stretch/hBSMCs/ICCs, and no stretch/hBSMCs/ICCs. (C, D) Western blot analysis of M2, M3, and c-kit expression in hBSMCs and ICCs. Protein samples were purified from no stretch/hBSMCs, stretch/hBSMCs, stretch/hBSMCs/ICCs, and no stretch/hBSMCs/ICCs. (E, F) Calcium concentration curve showing the effects of carbachol intervention on the relative fluorescence intensity of hBSMCs in stretch/hBSMCs and stretch/hBSMCs/ICCs groups. * p<0.05, ** p<0.01.
Mechanical stretch enhances hBSMCs proliferation and contraction by c-kit
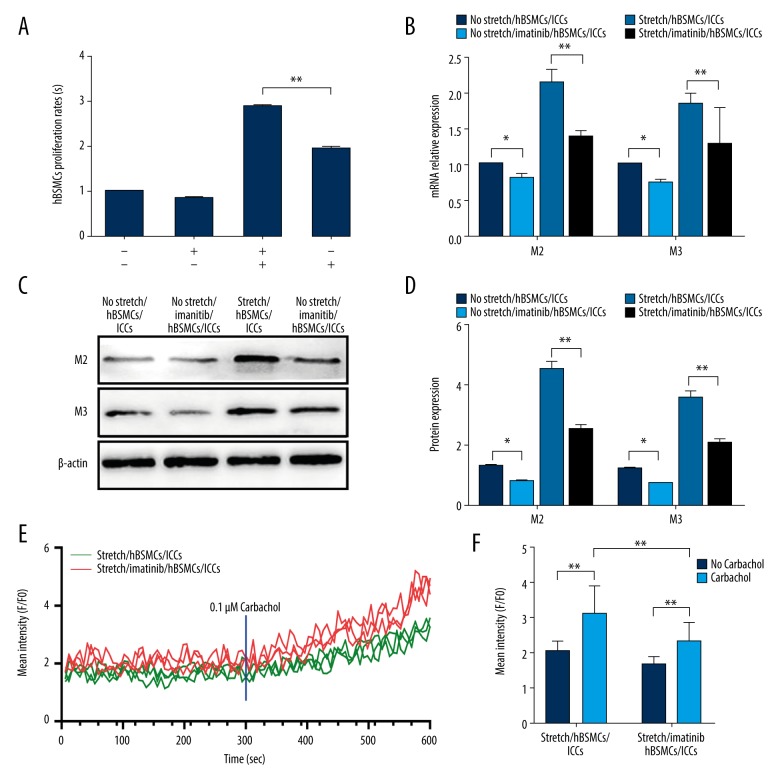
The results of hBSMC proliferation assay are shown in Figure 4A. After combination of mechanical stretch with no imatinib treatment, the number of hBSMCs was increased to 2.873±0.024 (p<0.0010), whereas the numbers after no stretch with imatinib and mechanical stretch with imatinib were 0.841±0.047 and 1.927±0.058, respectively. After combination of mechanical stretch with imatinib treatment, the proliferation of hBSMCs was higher than in hBSMCs alone. For further verification, we detected gene and protein expression of acetylcholine receptor M2, and M3. Real-time RT-PCR and Western blot analyses were performed and results are shown in Figure 4B. After combination mechanical stretch with no imatinib treatment, the mRNA levels of M2 and M3 were increased to 2.118±0.188 and 1.830±0.153, respectively (p<0.001), whereas after combination no stretch with imatinib treatment and combination mechanical stretch with imatinib treatment, the mRNA levels of M2 andM3 were 0.809±0.062 and 0.743±0.055, and 1.382±0.077 and 1.277±0.510, respectively. Similarly, after combination mechanical stretch with no imatinib treatment, the protein levels of M2 and M3 were increased to 4.511±0.283 and (3.573±0.229, respectively (p<0.001), whereas after combination mechanical stretch with imatinib treatment and combination no stretch with no imatinib treatment, the protein levels of M2 and M3 were 2.518±0.195 and 2.077±0.168, and 1.308±0.046 and 1.242±0.052, respectively, as shown in Figure 4C and 4D. Before adding 0.1 μM carbachol separately to each experimental group, the relative fluorescence intensity in hBSMCs of the stretch/hBSMCs/ICCs group and the stretch/imatinib/hBSMCs/ICCs group were 2.05±0.27 and 1.68±0.21, respectively. After adding 0.1 μM carbachol, the relative fluorescence intensity in hBSMCs was 3.10±0.75 and 2.33±0.50, respectively, and the difference was statistically significant (p<0.01).
Figure 4.
Mechanical stretch enhanced hBSMCs proliferation and contraction by c-kit. (A) CCK-8 analysis of hBSMCs. The first line ± means mechanical stretch and the second line ± means imatinib. (B) Real-time RT-PCR analysis of M2 and M3 expression in hBSMCs. RNA samples were purified from no stretch/hBSMCs/ICCs, no stretch/imatinib/hBSMCs/ICCs, stretch/hBSMCs/ICCs, and stretch/imatinib/hBSMCs/ICCs. (C, D) Western blot analysis of M2 and M3 expression in hBSMCs. Protein samples were purified from no stretch/hBSMCs/ICCs, no stretch/imatinib/hBSMCs/ICCs, stretch/hBSMCs/ICCs, and stretch/imatinib/hBSMCs/ICCs. (E, F) Calcium concentration curve. The effect of carbachol intervention on the relative fluorescence intensity of hBSMCs in the stretch/hBSMCs/ICCs group and the stretch/imatinib/hBSMCs/ICCs group. * p<0.05, ** p<0.01.
Discussion
According to the literature [21–25], the cells of organs and tissues depend on or are regulated by a certain mechanics environment. Mechanical forces from different sources, such as bone pressure caused by exercise and stress exerted by blood flow on endothelial cells, lead to biological changes at the cellular and subcellular levels and promote adaptive tissue reconstruction. Biological stress profoundly affects gene expression, protein synthesis, proliferation and differentiation of cells, and tissue growth, maintenance, and reconstruction. It is an important factor in tissue engineering. Our previous studies [21–25] have found that MAPK, PI3K, and integrin signaling pathways regulate the number and function of bladder smooth muscle cells under mechanical stress. To promote the full differentiation and functional reconstruction of seed cells and promote the organic combination of cells and matrix materials, appropriate stimulation of biological stress is very important and necessary. ICCs were first found in a special group of interstitial cells in the gastrointestinal tract. They can generate spontaneous slow wave potential, which is also the signature potential of ICCs, and it is the origin of the basic electrical rhythm of the gastrointestinal tract. Some studies [8–10] have found that ICCs exist in the urinary system of guinea pigs, and are involved in spontaneity of bladder smooth muscle contractions. Therefore, we conducted the present study to discuss the role of interstitial cells of Cajal in promoting the proliferation of smooth muscle cells in the optimization of tissue-engineered bladder structure induced by mechanical stretch. We found that mechanical stretch enhanced hBSMCs proliferation and contraction, and mechanical stretch enhanced BSMCs proliferation and contraction by ICCs and c-kit.
Some researchers have reported that mechanical stimulation affects smooth muscle cells [19–24,28–30]. Nagatomi et al. [30] reported that when rat BSMCs are exposed to cyclic pressure (0.1 Hz, 40 cmH2O) for 1 h, multiple signaling pathways respond to mechanical stimuli. Lee et al. [31] also reported that the expression of prolyl 4-hydroxylase is significantly increased when BSMCs are exposed to pressures of 40 cmH2O or less for up to 72 h. In other words, cyclic pressure represents mechanical pressure, and mechanical stretch stimulates the proliferation of BSMCs. In addition, Wang et al. [32] conduct a study on the effect of mechanical stretch on ICCs in guinea pig bladders. They examined the number and morphology of ICCs using a specific ICCs marker, c-kit, and immunochemistry staining was used in vivo to assess the effect of stretch load in detrusor overactivity (DO) models produced by 4 week of partial bladder outlet obstruction (PBOO). They found that mechanical sensitivity and interaction with BSMCs of ICCs contributed to the mechanosensitive conductances in bladder regulation. However, Chester et al. [33] reached a different conclusion in a study in which medical-grade silicon bands were wrapped around the distal colon to induce partial obstruction in wild-type and ICCs-deficient (W/Wv) mice, demonstrating COX-2 mRNA level and circular muscle contractility, indicating that ICCs deficiency limits the effect on stretch-induced expression of COX-2 and suppression of smooth muscle contractility in obstruction, which is consistent with our finding that mechanical stretch enhances BSMCs proliferation and contraction.
However, there are differences between the present study and those mentioned above. We used imatinib (a c-kit kinase-specific inhibitor) to carry out the experiment, and we measured growth rates using CCK-8 assays and measured mRNA and protein levels of M2 and M3 in hBSMCs in the experiment. Imatinib mesylate (Glivec) is a selective inhibitor of c-kit receptor tyrosine kinase and the oncogene Bcr-Abl, and has Food and Drug Administration approval for the treatment of chronic myeloid leukemia and gastrointestinal stromal tumor. Several researchers have demonstrated that inhibition of c-kit reduces bladder activity via c-kit receptor in bladder ICCs [34–36]. Moro et al. [37] and Sui et al. [38] found that acetylcholine M receptor is widely expressed on the surface of bladder smooth muscle cells, which promotes cells to contract after binding with acetylcholine, and c-kit kinase promotes M2 and M3 binding to acetylcholine. Ng et al. [29] reported that activation of M2 and M3 receptors by exogenous carbachol enhanced spontaneous contractions in whole bladder preparations from normal 1- to 2-week-old rats. In our study, that conclusion is further confirmed. Our experiments show that mechanical stretch enhanced hBSMCs proliferation and contraction by c-kit, promoting M2 and M3 binding to acetylcholine. Further research is needed to verify these relationships and mechanisms.
Conclusions
We found that ICCs effectively promoted hBSMC proliferation and contraction, and cyclic stretch effectively promoted acetylcholine receptor M2 and M3 caused by c-kit in the ICCs, which promoted the contraction of hBSMCs.
Footnotes
Source of support: This work was supported by the Natural Science Foundation of Sichuan Provincial Department of Education (16ZB0227), the Scientific Research Foundation of Health and Family Planning Commission of Sichuan Province (17PJ155), and the City of Nanchong Strategic Cooperation with Local Universities Foundation of Technology (grants NSMC20170421, NSMC20170111, 18SXHZ0581, and 18SXHZ0128)
Conflicts of interest
None.
References
- 1.PDQ Adult Treatment Editorial Board. Bladder Cancer Treatment (PDQ®) National Cancer Institute; USA: Jan 17, 2019. [Google Scholar]
- 2.Galvin DJ, Watson RW, Gillespie JI, et al. Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am J Physiol Renal Physiol. 2002;283:F1192–99. doi: 10.1152/ajprenal.00168.2002. [DOI] [PubMed] [Google Scholar]
- 3.Daga M, Pizzimenti S, Dianzani C, et al. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine. 2018;56:156–64. doi: 10.1016/j.phymed.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Li W, Ning J, et al. Long noncoding RNA UCA1 targets miR-582-5p and contributes to the progression and drug resistance of bladder cancer cells through ATG7-mediated autophagy inhibition. Onco Targets Ther. 2019;12:495–508. doi: 10.2147/OTT.S183940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Li X, Jiang F, Wang X, Gu X. SPAG9 regulates HEF1 expression and drives EMT in bladder transitional cell carcinoma via rac1 signaling pathway. Am J Cancer Res. 2018;8:2467–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S, Kang HG, Wu MJ, et al. Effects of Ca2+-activated Cl− channel ANO1inhibitors on pacemaker activity in interstitial cells of Cajal. Cell Physiol Biochem. 2018;51:2887–99. doi: 10.1159/000496041. [DOI] [PubMed] [Google Scholar]
- 7.Sun T, Li D, Hu S, et al. Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging (Albany, NY) 2018;10:3851–65. doi: 10.18632/aging.101677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin MJ, Chen L, Huang ZP, et al. Neutrophils injure gallbladder interstitial Cajal-like cells in a guinea pig model of acute cholecystitis. J Cell Physiol. 2019;234:4291–301. doi: 10.1002/jcp.27197. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZP, Qiu H, Yu BP. Acute cholecystitis reduces interstitial cells of Cajal in porcine gallbladder through decreased mRNA synthesis. Cell Physiol Biochem. 2018;47:535–44. doi: 10.1159/000489987. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y, Wu SD, Fu BB, et al. Decreased number of interstitial cells of Cajal play an important role in the declined intestinal transit during cholesterol gallstone formation in guinea pigs fed on high cholesterol diet. Int J Clin Exp Med. 2014;7:1262–68. [PMC free article] [PubMed] [Google Scholar]
- 11.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–36. [PubMed] [Google Scholar]
- 12.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gevaert T, Hutchings G, Everaerts W, et al. Administration of imatinib mesylate in rats impairs the neonatal development of intramuscular interstitial cells in bladder and results in altered contractile properties. Neurourol Urodyn. 2014;33:461–68. doi: 10.1002/nau.22415. [DOI] [PubMed] [Google Scholar]
- 14.Cai YQ, Chen SR, Han HD, et al. Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J Neurochem. 2009;111:1000–10. doi: 10.1111/j.1471-4159.2009.06396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama T, Nakamura T, Obara K, Inoue H. Up-regulated PAR-2-mediated salivary secretion in mice deficient in muscarinic acetylcholine receptor subtypes. J Pharmacol Exp Ther. 2007;320:516–24. doi: 10.1124/jpet.106.113092. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Matsui M, Uchida K, et al. M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558:561–75. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar-Fajardo PD, Aréchiga-Figueroa IA, López-Serrano AL, et al. The voltage-sensitive cardiac M2 muscarinic receptor modulates the inward rectification of the G protein-coupled, ACh-gated K+ current. Pflugers Arch. 2018;470:1765–76. doi: 10.1007/s00424-018-2196-y. [DOI] [PubMed] [Google Scholar]
- 18.Randáková A, Rudajev V, Doležal V, et al. Novel long-acting antagonists of muscarinic ACh receptors. Br J Pharmacol. 2018;175:1731–43. doi: 10.1111/bph.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halachmi S, Aitken KJ, Szybowska M, et al. Role of signal transducer and activator of transcription 3 (STAT3) in stretch injury to bladder smooth muscle cells. Cell Tissue Res. 2006;326:149–58. doi: 10.1007/s00441-006-0204-6. [DOI] [PubMed] [Google Scholar]
- 20.Drumm MR, York BD, Nagatomi J. Effect of sustained hydrostatic pressure on rat bladder smooth muscle cell function. Urology. 2010;75:879–85. doi: 10.1016/j.urology.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Yue X, Luo D, et al. Increased proliferation of human bladder smooth muscle cells is mediated by physiological cyclic stretch via the PI3K-SGK1-Kv1.3 pathway. Mol Med Rep. 2013;8:294–98. doi: 10.3892/mmr.2013.1473. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Wei T, Liao B, et al. Physiological stretch induced proliferation of human urothelial cells via integrin α6-FAK signaling pathway. Neurourol Urodyn. 2018;37:2114–20. doi: 10.1002/nau.23572. [DOI] [PubMed] [Google Scholar]
- 23.Wu T, Chen L, Wei T, et al. Effect of cyclic hydrodynamic pressure-induced proliferation of human bladder smooth muscle through Ras-related C3 botulinum toxin substrate 1, mitogen-activated protein kinase kinase 1/2 and extracellular regulated protein kinases 1/2. Int J Urol. 2012;19:867–74. doi: 10.1111/j.1442-2042.2012.03043.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Wang K, Li H, et al. Effects of different sustained hydrostatic pressures on connexin 43 in human bladder smooth muscle cells. Urol Int. 2013;90:75–82. doi: 10.1159/000338924. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Wei TQ, Wang Y, et al. Simulated bladder pressure stimulates human bladder smooth muscle cell proliferation via the PI3K/SGK1 signaling pathway. J Urol. 2012;188:661–67. doi: 10.1016/j.juro.2012.03.112. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Luo D, Zhu Y, Wang K. MicroRNA 4323 induces human bladder smooth muscle cell proliferation under cyclic hydrodynamic pressure by activation of erk1/2 signaling pathway. Exp Biol Med (Maywood) 2017;242(2):169–76. doi: 10.1177/1535370216669837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JM, Borer JG, Freeman MR, Peters CA. Stretch activates heparin-binding EGF-like growth factor expression in bladder smooth muscle cells. Am J Physiol Cell Physiol. 1998;275:C1247–54. doi: 10.1152/ajpcell.1998.275.5.C1247. [DOI] [PubMed] [Google Scholar]
- 28.Kubota Y, Biers SM, Kohri K, et al. Effects of imatinib mesylate (Glivec®) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourology and Urodynamics. 2006;25:205–10. doi: 10.1002/nau.20085. [DOI] [PubMed] [Google Scholar]
- 29.Ng Y-K, De Groat WC, Wu H-Y. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1049–59. doi: 10.1152/ajpregu.00236.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagatomi J, Wu YN, Gray M. Proteomic analysis of bladder smooth muscle cell response to cyclic hydrostatic pressure. Cell Mol Bioeng. 2009;2:166–73. [Google Scholar]
- 31.Lee SD, Akbal C, Miseeri R, et al. Collagen prolyl 4-hydroxylase is upregulated in an acute bladder outlet obstruction. J Pediatr Urol. 2006;2:225–32. doi: 10.1016/j.jpurol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Fang Q, Lu Y, et al. Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res. 2010;164:e213–19. doi: 10.1016/j.jss.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Wu CC, Lin YM, Gao J, et al. Are interstitial cells of Cajal involved in mechanical stress-induced gene expression and impairment of smooth muscle contractility in bowel obstruction? PLoS One. 2013;8:e76222. doi: 10.1371/journal.pone.0076222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui GP, Rothery S, Dupont E, et al. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–29. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 35.Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–16. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- 36.Vahabi B, McKay NG, Lawson K, Sellers DJ. The role of c-kit positive interstitial cells in mediating phasic contractions of bladder strips from streptozotocin – induced diabetic rats. BJU Int. 2011;107:1480–87. doi: 10.1111/j.1464-410X.2010.09507.x. [DOI] [PubMed] [Google Scholar]
- 37.Moro C, Uchiyama J, Chess-Williams R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology. 2011;78:1442.e9–15. doi: 10.1016/j.urology.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 38.Wu C, Gui GP, Fry CH. Intracellular Ca (2+) regulation and electrophysiological properties of bladder urothelium subjected to stretch and exogenous agonists. Cell Calcium. 2011;49:395–99. doi: 10.1016/j.ceca.2011.03.008. [DOI] [PubMed] [Google Scholar]