Abstract
Background
Osteosarcoma is a primary bone aggressive cancer, affecting adolescents worldwide. Increasing evidence suggests that dysfunction of microRNAs (miRNAs) plays a pivotal role in malignancies. The aim of this study was to evaluate the potential functions of miR-181c and verifying its regulatory effects on SMAD7 in osteosarcoma.
Material/Methods
The expressions of miR-181c and SMAD7 in osteosarcoma were detected by quantitative real-time polymerase chain reaction (qRT-PCR). Cell proliferation, invasion and migration abilities were assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) and Transwell assay. Bioinformatics analysis and luciferase reporter assay were used to explore the interaction between miR-181c and SMAD7. Western blot was performed to determine the functions of miR-181c on osteosarcoma cell epithelial-to-mesenchymal transition (EMT) and transforming growth factor-β (TGF-β) signaling pathway.
Results
Decreased expression levels of miR-181c and SMAD7 were identified in osteosarcoma using qRT-PCR. The downregulated miR-181c and SMAD7 expressions indicated poor prognosis of osteosarcoma patients. Moreover, miR-181c overexpression prominently repressed osteosarcoma cell proliferation, invasion, and migration abilities via modulating EMT and TGF-β signaling pathway. SMAD7 functioned as an important target for miR-181c in osteosarcoma cells. Furthermore, upregulation of miR-181c dramatically suppressed osteosarcoma tumorigenesis in vivo.
Conclusions
These findings indicated that miR-181c suppressed osteosarcoma progression, providing new insight into the pathogenesis and representing a potential therapeutic target for osteosarcoma.
MeSH Keywords: Epithelial-Mesenchymal Transition, Osteosarcoma, Smad7 Protein
Background
Osteosarcoma (OS) is a prevailing bone malignancy which typically occurs in adolescents, with high morbidity and great metastasis potentials [1,2]. Currently, surgery, adjuvant chemotherapy, radiotherapy, and neoadjuvant are the mainstay therapies for OS [3]. Although great improvement has been made in improving clinical outcomes, the prognosis of OS patients remains poor because of pulmonary metastasis and tumor recurrence after resection [4,5]. Additionally, the efficiencies of conventional treatments remain limited as the basic mechanism of OS is poorly understood. Hence, it is urgent to investigate the mechanisms of OS, which might be useful for developing promising therapeutic biomarkers.
Accumulated studies have indicated that abnormal expressions of miRNAs are implicated in the tumorigenesis of various tumors via binding to complementary sequence in 3′UTRs of their target mRNA [6]. MicroRNAs (miRNAs) serve as major regulators in the genetic networks which are involved in pathophysiological processes, such as the tumor initiation and development [7]. Recently, growing evidence has revealed that miRNA acts as a tumor suppressor or an oncogene in tumorigenesis of different malignancies. For example, Li et al. [8] found that miR-148a repressed breast cancer cell growth by regulating B-cell lymphoma 2 (Bcl-2); Liu et al. [9] reported that miR-495 inhibited gastric cancer cell proliferation and metastases by targeting Twist1; Liu et al. [10] showed that miR-18a suppressed cell growth through targeting IPMK and TRIAP1 in ovarian cancer. These findings provide strong evidence that manipulating the functions of specific miRNAs might be a promising approach in tumor therapy. Evidence from previous studies showed that miR-181c correlated with high grade OS recurrence, enhancing chemotherapeutic-induced cell death and reducing cell viability [11]. Therefore, in this study, we aimed to elucidate the molecular mechanism in the regulations of OS with a focus on miR-181c.
Epithelial-to-mesenchymal transition (EMT) is a cellular transdifferentiation process, whereby losing the epithelial polarities and junctions, and acquiring alterations in gene expressions and behaviors which are typically correlated with mesenchymal cells [12]. Emerging evidence has also indicated that EMT process is involved tumor progression. For instance, Jeong et al. [13] found that EMT in breast cancer was related to the triple-negative phenotype and high histological grade. Transforming growth factor β (TGF-β)/Smad signaling pathway, an important driver in carcinogenesis, is commonly dysregulated in the progression of various cancers, including cervical carcinoma [14], gastric carcinoma [15], and hepatocellular carcinoma [16]. TGF-β/Smad is a multi-functional regulator implicated in multiple processes such as cell metastases and growth [17,18]. TGF-β signaling pathway has been verified to function as an important cancer promoter via inducing EMT [19]. Therefore, it is hypothesized that EMT and the TGF-β/Smad pathway are involved in progression of OS. It is well-known that TGF-β1 can activate downstream mediators, such as SMAD2 and SMAD3, to exert its biological functions, while SMAD7 can negatively regulate its effects [20]. Previous studies have demonstrated that SMAD7 is one of the most vital factors in tumor development. Studies by De Simone et al. [21] showed that SMAD7 knockdown lead to colon cancer cell death via activating protein kinase RNA-associated eIF2alpha pathway. Another study by Javelaud et al. [22] revealed that stable SMAD7 overexpression inhibited the tumorigenicity in human melanoma cells. Hence, in this present study, we detected the association between SMAD7 and TGF-β signaling pathway in OS.
Material and Methods
Clinical tissues
Fifty pairs of OS tissue samples and matched non-tumor tissue samples were collected from 50 OS patients who underwent surgery resections at HwaMei Hospital, University of Chinese Academy of Sciences between July 2015 and October 2017. No OS patients enrolled in current study were subjected to chemotherapy or radiotherapy. All tissues were immediately frozen in liquid nitrogen and stored at −80°C until use. Informed consent was provided by all patients to approve the use of their tissues for research purposes. This study was approved by the Ethics Committee of HwaMei Hospital, University of Chinese Academy of Sciences.
Cell culture
A human osteoblast cell line hFOB 1.19 and OS cell lines (MG-63, Saos-2, and U2OS, HOS) were obtained from the ATCC (Manassas, VA, USA). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) at 37°C in a humidified incubator containing 5% CO2.
Cell transfection
MiR-181c mimics, inhibitors, and the negative controls (NC) were obtained from RiboBio (Guangzhou, China) and transfected into OS cell lines by Lipofectamine® 2000 (Invitrogen, Carlsbad, CA, USA) in strict line with the manufacturer’s protocol. After 48 hours of transfections, the OS cells were collected for further analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to isolate the total RNAs from the tissue samples and cultured cells. Reverse transcription reaction was performed to synthesize complementary deoxyribose nucleic acid (cDNA) using PrimeScript RT reagent kit (TaKaRa, Dalian, China). SYBR Premix Ex TaqII (TaKaRa Biotechnology Co., Ltd., Dalian, China) was used to perform qPCR on an ABI 7500 fast real-time PCR system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primer sequences for qRT-PCR are available in Table 1. The expression levels were normalized to U6 (for miRNA) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (for mRNA). The 2−ΔΔCt method was used to quantify the expressions.
Table 1.
Primer sequences for qRT-PCR.
| Primer | Sequence |
|---|---|
| miR-181c forward | 5′-GTGTGGGAACATTCAACCTGTCGGTG-3′ |
| miR-181c reverse | 5′-CCAGTCTCAGGGTCCGAGGTATTC-3′ |
| U6 forward | 5′-CTCGCTTCGGCAGCACA-3′ |
| U6 reverse | 5′-AACGCTTCACGAATTTGCGT-3′ |
| SMAD7 forward | 5′-TGGATGGCGTGTGGGTTTA-3′ |
| SMAD7 reverse | 5′-TGGCGGACTTGATGAAGATG-3′ |
| GAPDH forward | 5′-ATGGGGAAGGTGAAGGTCG-3′ |
| GAPDH reverse | 5′-GGGGTCATTGATGGCAACA-3′ |
U6 – small nuclear RNA, snRNA; SMAD7 – mothers against decapentaplegic homolog 7; GAPDH – glyceraldehyde-3-phosphate dehydrogenase.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay
MTT assays (Sigma-Aldrich, St. Louis, MO, USA) were performed to determine the cell proliferation ability. Briefly, the transfected cells were placed into 96-well plates and cultured for indicated hours (0, 24, 48, and 72 hours). Then, MTT solution was added into each well and then the cells were incubated for additional 4 hours at 37°C. Subsequently, dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) was added to each well to dissolve the crystals. Finally, the optical density (OD)490 was determined with a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Transwell assays
Transwell assays were conducted to detect the cell invasion and migration abilities using 8-μm pore size Corning chambers (Corning, Corning, NY, USA) with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) present or absent. Cells suspended in serum-free medium were placed into the top chambers while medium containing 10% FBS was added to bottom chambers as a chemoattractive factor. Then, cells were incubated at 37°C in a 5% CO2 atmosphere for 48 hours. Subsequently, cells remained on the top chambers were removed with cotton swabs, and on the other hand, cells invaded or migrated to the lower chambers were fixed and stained with paraformaldehyde and crystal violet respectively. Then, the cells were photographed and counted using an inverted microscope (Olympus, Tokyo, Japan).
Western blot
The transfected cells were lysed using ice-cold radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with proteinase inhibitors. The protein concentrations were quantified using bicinchoninic acid (BCA) Protein assay kit (Beyotime, Beijing, China). Then, the protein was separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). After that, the protein was transferred onto the polyvinylidene fluoride (PVDF) membranes (Roche, Basel, Switzerland). Then 5%-skim milk in TBST was used to block the membranes at room temperature for 2 hours. Subsequently, the membrane was incubated overnight at 4°C with specific primary antibodies against: SMAD7 (1: 1000, Abcam, Cambridge, MA, USA), TGF-β1(1: 1000, Abcam, Cambridge, MA, USA), SMAD2 (1: 1000, Abcam, Cambridge, MA, USA), p-SMAD2 (1: 2000, Abcam, Cambridge, MA, USA), SMAD3 (1: 1000, Abcam, Cambridge, MA, USA), p-SMAD3 (1: 1000, Abcam, Cambridge, MA, USA), E-cadherin (1: 2000, Abcam, Cambridge, MA, USA), N-cadherin (1: 2000, Abcam, Cambridge, MA, USA), vimentin (1: 1000, Abcam, Cambridge, MA, USA) and GAPDH (1: 1000, Abcam, Cambridge, MA, USA). The membranes were then incubated with horse radish peroxidase (HRP)-conjugated secondary antibody (1: 3,000, Abcam, Cambridge, MA, USA) for 2 hours at room temperature. Electrochemiluminescence (ECL) detection reagent (Pierce, Thermo Fisher Scientific, Inc., Waltham, MA, USA) was utilized for the analysis of the signals on the membrane. GAPDH served as an internal control.
Dual-luciferase reporter assay
The SMAD7 3′UTR sequences containing wide-type (WT) or mutant (MUT) binding sites for miR-181c were inserted into the pGL3 reporter vectors (Promega, Madison, WI, USA). Then, OS cells were cotransfected with the WT or MUT SMAD7-3′UTR along with miR-181c mimics. The transfected cells were harvested 48 hours post the transfections, and Dual-Luciferase Assay Kit (Promega, Madison, WI, USA) was used to measure the luciferase activities.
In vivo xenograft tumor model
Female BALB/c nude mice (4- to 6-weeks old) were used for the in vivo investigation. MG63 cells stably expressing miR-181c (lenti-miR-181c) or a control (lenti-control) were injected subcutaneously into the dorsal flank of the nude mice. When the tumors were palpable, tumor volumes were examined every 3 days following the formula: volume (mm3)=(length×width2)/2.
Statistical analysis
All data in current study were from at least 3 independent experiments. Statistical analyses were carried out with Statistical Product and Service Solutions (SPSS) software version 17.0 (SPSS Inc., Chicago, IL, USA). Kaplan-Meier method and log-rank test were applied to estimate the survival rates. The significant differences between 2 groups were analyzed with Student’s t-test. Comparison between groups was done using one-way ANOVA test followed by post hoc test (least significant difference). P<0.05 indicated statistical significance.
Results
MiR-181c was downregulated in OS and indicated poorer prognosis
To determine the clinical relevance of miR-181c in OS, we firstly detected the expressions of miR-181c in OS tissue samples using qRT-PCR. Results demonstrated that miR-181c was obviously downregulated in OS tissues when compared to the matched normal tissues (Figure 1A). Additionally, we also performed Kaplan-Meier analysis to analyze the association between miR-181c expression level and the prognosis of OS patients. OS patients were assigned into a high miR-181c expressing group and a low miR-181c expressing group with the mean miR-181c expression level as the cutoff. Data revealed that OS patients with low miR-181c expressions presented poorer prognosis than patients with high miR-181c expression (Figure 1B). Moreover, we also analyzed the correlation between the clinicopathological characteristics and miR-181c expression of OS patients. Findings indicated that low miR-181c expressions were relevant with the adverse phenotypes of OS patients (Table 2).
Figure 1.
MiR-181c was downregulated in osteosarcoma (OS) and indicated poor prognosis of OS patients. (A) MiR-181c expressions in OS tissues were detected using quantitative real-time polymerase chain reaction. (B) Kaplan-Meier survival curves of miR-181c high- and low-expression groups. ** P<0.01.
Table 2.
Correlation of miR-181c expression with the clinicopathological characteristics of the OS patients.
| Clinicopathological features | Cases (n=50) | miR-181c# expression | P-value | |
|---|---|---|---|---|
| High (n=20) | Low (n=30) | |||
| Age (years) | 0.5463 | |||
| >60 | 28 | 12 | 16 | |
| ≤60 | 22 | 8 | 14 | |
| Gender | 0.3346 | |||
| Male | 26 | 9 | 17 | |
| Female | 24 | 11 | 13 | |
| Tumor size (cm) | 0.2134 | |||
| ≥5.0 | 24 | 6 | 18 | |
| <5.0 | 26 | 14 | 12 | |
| Lymph node metastasis | 0.0032* | |||
| Yes | 22 | 17 | 5 | |
| No | 28 | 3 | 25 | |
| TNM stage | 0.0019* | |||
| I+II | 23 | 15 | 8 | |
| III+IV | 27 | 5 | 22 | |
| Distant metastasis | 0.0025* | |||
| Yes | 30 | 14 | 16 | |
| No | 20 | 6 | 14 | |
OS – osteosarcoma; TNM – tumor-node-metastasis.
The mean expression level of miR-181c was used as the cutoff;
statistically significant.
MiR-181c overexpression blocked OS cell proliferation
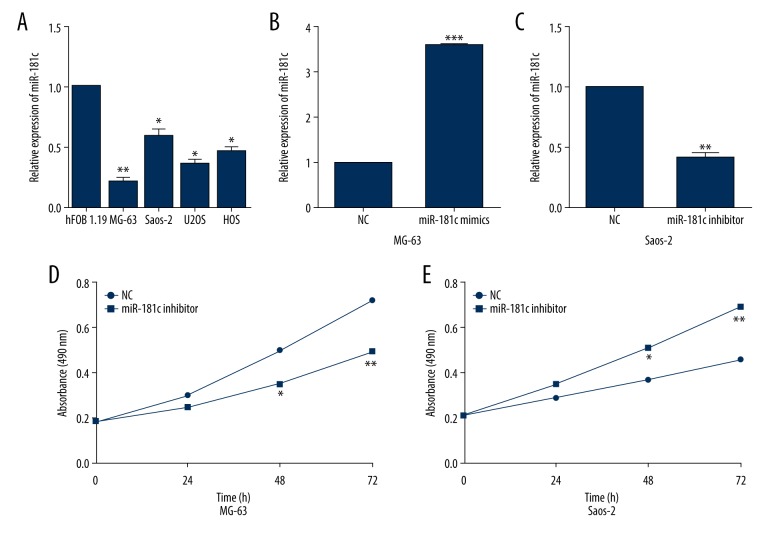
To further confirm the aforementioned results, OS cell lines was used to identify the functions of miR-181c in OS cell. We first measured the differential expressions of miR-181c in OS cells and normal osteoblast cell hFOB 1.19. As expected, miR-181c expressions in all OS cells were obviously decreased in comparison with that in hFOB 1.19 (Figure 2A). Then, MG-63 and Saos-2 cells were selected for further investigation due to their relatively low and high endogenous miR-181c expression. qRT-PCR analysis showed that miR-181c mimics successfully enhanced the miR-181c expressions in MG-63 cell (Figure 2B), whereas miR-181c inhibitor prominently reduced the miR-181c expressions in Saos-2 cells (Figure 2C). Subsequently, MTT assay was carried out to determine the proliferation capacity of miR-181c overexpressed MG-63 cells or miR-181c suppressed Saos-2 cells. We found that miR-181c overexpression dramatically repressed MG-63 cell proliferation (Figure 2D) whereas miR-181c suppression evidently facilitated Saos-2 cell proliferation (Figure 2E).
Figure 2.
MiR-181c overexpression dramatically inhibited osteosarcoma (OS) cell proliferation. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted to examine the expressions of miR-181c in OS cells; (B, C) qRT-PCR was carried out to validate the overexpression or knockdown of miR-181c in OS cells. (D, E) MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assays were used to determine the impact of miR-181c on OS cell proliferation. * P<0.05, ** P<0.01, *** P<0.001.
MiR-181c restoration repressed OS cell invasion and migration
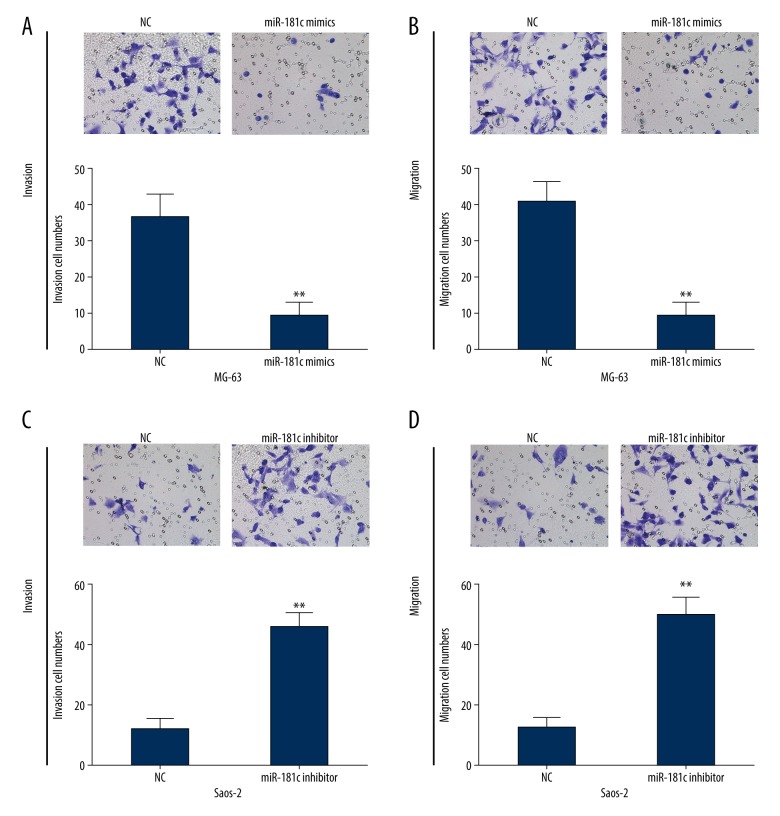
We then investigated whether miR-181c influenced OS cell migration and invasion by performing Transwell assay. The results indicated that cell invasion or migration ability was evidently inhibited following transfection with miR-181c mimics in MG-63 cell (Figure 3A, 3B). On the other hand, we also found that miR-181c inhibition caused by miR-181c inhibitor in Saos-2 cells could dramatically repress cell invasion and migration abilities (Figure 3C, 3D). All these results indicated that elevated miR-181c exerted anti-tumor functions in OS cells.
Figure 3.
MiR-181c upregulation significantly suppressed osteosarcoma (OS) cell invasion and migration. (A, B) Transwell assay was carried out to detect the invasion and migration capacities of MG-63 cells transfected with miR-181c mimics. (C, D) Invasion and migration abilities of miR-181c inhibited Saos-2 cells were determined by Transwell assays. ** P<0.01.
SMAD7 was an important direct target for miR-181c
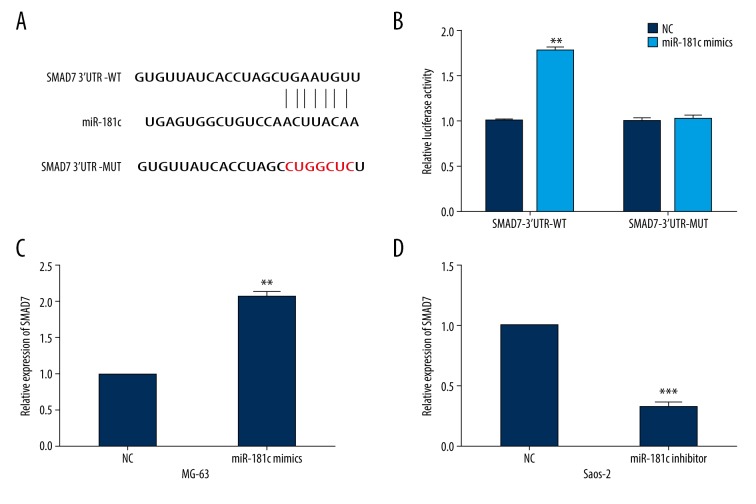
To investigate the underlying mechanism of miR-181c in OS progression, TargetScan was used to identify potential targets for miR-181c. We found that SMAD7 mRNA contained putative miR-181c binding sites in its 3′UTRs (Figure 4A). Luciferase assays were then performed to confirm the association between SMAD7 and miR-181c. As shown in Figure 4B, the luciferase activities of SMAD7-3′UTR-WT were markedly upregulated by miR-181c mimics. However, this intensive effects were abolished when the miR-181c binding sites in the SMAD7 3′UTR were mutated. Moreover, we detected the changes in SMAD7 expressions in miR-181c overexpressed MG-63 cells or miR-181c suppressed Saos-2 cells. We found that MG-63 cells with miR-181c overexpression showed significantly increased SMAD7 expressions, while miR-181c knockdown caused reduced SMAD7 expression in Saos-2 cells (Figure 4C, 4D).
Figure 4.
SMAD7 was a direct target of miR-181c. (A) The wild-type or mutated binding sequences between SMAD7 and miR-181c. (B) Luciferase activities of osteosarcoma (OS) cells transfected with miR-181c mimics and SMAD7-3′UTR-WT or MUT. (C, D) SMAD7 expressions in OS cells treated with miR-181c mimics or inhibitor. ** P<0.01, *** P<0.001.
MiR-181c regulated EMT and TGF-β signaling pathway in OS cells
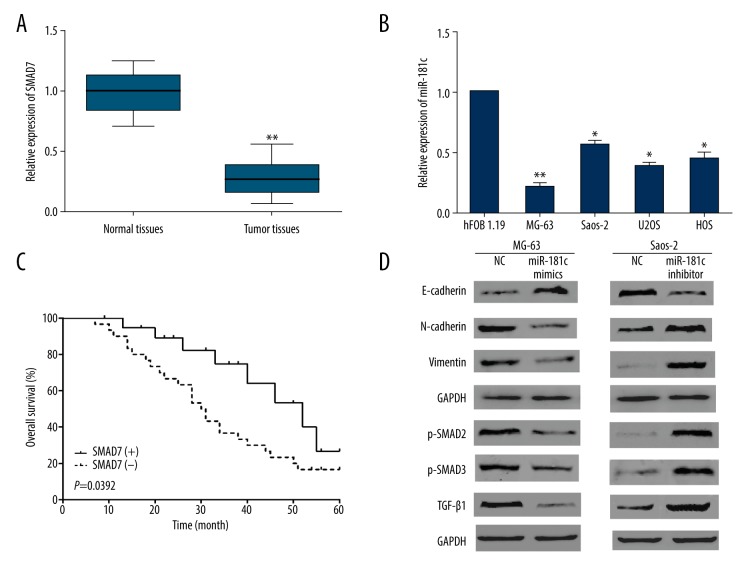
We further investigated the expression levels and the clinical significance of SMAD7 in OS patients. The qRT-PCR results demonstrated significant decrease of SMAD7 expressions not only in OS tissues but also in OS cells (Figure 5A, 5B). Subsequently, we analyzed the prognostic value of SMAD7 using Kaplan-Meier analysis. Results indicated that OS patients with low SMAD7 expressions presented significantly shorter overall survival than patients with high SMAD7 expressions (Figure 5C). Findings revealed that miR-181c targeted and upregulated SMAD7. Moreover, in the TGF-β signaling pathway SMAD7 was identified as a negative feedback loop. Hence, we proposed a hypothesis that miR-181c might regulate TGF-β pathway and EMT. Subsequently, we further determined the functions of miR-181c in OS cell EMT and TGF-β signaling pathway by performing western blots. Results showed that miR-181c overexpression could significantly downregulate N-cadherin and vimentin whereas upregulate E-cadherin (Figure 5D). Moreover, we examined the regulatory functions of miR-181c in TGF-β signaling pathway and the data indicated that p-SMAD2, p-SMAD3, and TGF-β1 were prominently downregulated by miR-181c upregulation while significantly enhanced by miR-181c downregulation (Figure 5D). Thus, these findings together indicated that miR-181c exhibited its inhibitory effect on OS progression by regulation of EMT and TGF-β signaling pathway.
Figure 5.
MiR-181c regulated osteosarcoma (OS) cell epithelial-to-mesenchymal transition (EMT) and transforming growth factor-β (TGF-β) signaling pathway via regulating SMAD7. (A, B) Quantitative real-time polymerase chain reaction was performed to determine SMAD7 expressions in OS tissues or cells. (C) Kaplan-Meier analysis was carried out to examine the correlation between SMAD7 and the overall survival of OS patients. (D) The impacts of miR-181c on OS cell EMT progress and TGF-β signaling pathway were detected by western blots. * P<0.05, ** P<0.01.
MiR-181c restoration inhibited OS growth in vivo
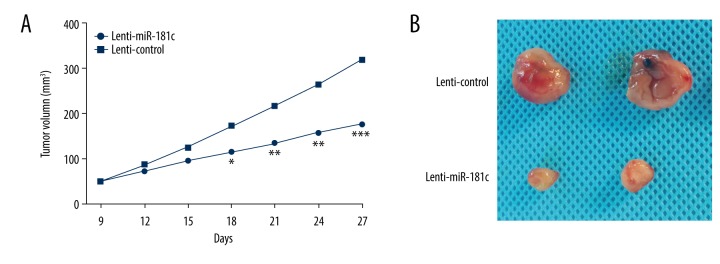
Finally, we wondered whether miR-181c might also functionally regulate OS development in vivo. An in vivo mouse xenograft model was established to determine the function of miR-181c in tumor growth. As shown in Figure 6A and 6B, both the tumor growth curve and the tumor volumes in the miR-181c mice when compared to the control group.
Figure 6.
MiR-181c overexpression inhibited osteosarcoma (OS) tumor growth in vivo. (A) The growth curve of subcutaneous xenograft. (B) Representative images of subcutaneous xenograft from the mice in different groups. * P<0.05, ** P<0.01, *** P<0.001.
Discussion
OS is a severe bone cancer characterized by a poor clinical outcome [23]. Therefore, exploring the potential mechanisms involved in OS development is urgently imperative to improve therapies for OS patients. Furthermore, mounting evidence has revealed that miRNAs take an important part in modulating OS initiation and progression, including metastases, invasion, apoptosis, and proliferation [24,25]. For example, Huang et al. [26] found that miR-124 acted as a tumor-suppressive miRNA in OS via inhibiting Snail2 expressions; Sun et al. [27] reported that miR-646 suppressed OS cell metastases through downregulating FGF2; Yuan et al. [28] proposed that miR-20a enhanced OS cell proliferation by upregulating TAK1. Herein, we determined biological function, the regulatory mechanisms and the clinical significance of miR-181c in OS progression.
MiR-181c plays crucial roles in many physiological and pathological progresses. Recent studies have documented the indispensable role of miR-181c in various cancers including gastric carcinoma [29], colorectal carcinoma [30], and breast carcinoma [31]. Owing to its potential use as a biomarker for diagnosis and therapy, it is of importance to study the mechanisms of miR-181c in OS. In the present study, miR-181c was found to be downregulated in OS tissues and the decreased miR-181c was related to the poor prognosis and malignant clinicopathologic features. Moreover, we performed functional assays to determine the effects of miR-181c on OS cell proliferation, invasion, and migration. Results showed that upregulation of miR-181c dramatically repressed OS cell proliferation, invasion, and migration abilities.
It is well known that miRNAs usually modulate tumorigenesis by regulation of their target mRNAs. Hence, bioinformatics analysis and luciferase assays were carried out to explore the potential target genes of miR-181c. Data showed that SMAD7 was a direct functional target of for miR-181c in OS cells. Increasing studies have indicated that SMAD7 are important signal transducers and key regulators of cellular processes including apoptosis [32], invasion [33], and proliferation [34]. SMAD7 might negatively regulate the TGF-β signaling pathway. As results demonstrated that miR-181c targeted SMAD7 in OS cells, we further explored whether miR-181c mediated TGF-β pathway and EMT. Hence, the mechanisms underlying the repressive roles of miR-181c in OS cells were further investigated by performing western blots. We found that miR-181c overexpression could repress EMT and TGF-β signaling pathway in OS cells. Altogether, these results implied that miR-181c suppressed OS cell EMT and TGF-β signaling pathway via regulating SMAD7.
Conclusions
In conclusion, the decreased miR-181c demonstrated adverse clinical phenotypes of OS patients. Findings of functional assays showed that miR-181c restoration significantly impaired OS cell proliferation, invasion, and migration abilities. Moreover, it was found that miR-181c directly targeted SMAD7, the expression level of which was significantly upregulated by miR-181c. Furthermore, we detected the impact of miR-181c on EMT and TGF-β signaling pathway in OS cells using western blot, and results showed that miR-181c could obviously inhibit the EMT progress and TGF-β signaling pathway. In addition, we also found that miR-181c overexpression prominently suppressed OS cell growth in vivo.
Footnotes
Source of support: HwaMei Research Foundation (No. 2019HMKY66) and Key Program of Medical Disciplines of Ningbo, China (No. 2016-F06)
Conflict of interest
None.
References
- 1.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–18. [PubMed] [Google Scholar]
- 2.Zhang ZF, Li GR, Cao CN, et al. MicroRNA-1294 targets HOXA9 and has a tumor suppressive role in osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22:8582–88. doi: 10.26355/eurrev_201812_16621. [DOI] [PubMed] [Google Scholar]
- 3.Xie C, Chen B, Wu B, et al. LncRNA TUG1 promotes cell proliferation and suppresses apoptosis in osteosarcoma by regulating miR-212-3p/FOXA1 axis. Biomed Pharmacother. 2018;97:1645–53. doi: 10.1016/j.biopha.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 4.El-Naggar AM, Veinotte CJ, Cheng H, et al. Translational activation of HIF1alpha by YB-1 promotes sarcoma metastasis. Cancer Cell. 2015;27:682–97. doi: 10.1016/j.ccell.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–35. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei Q, Li X, Guo M, et al. The miRNA network: Micro-regulator of cell signaling in cancer. Expert Rev Anticancer Ther. 2014;14:1515–27. doi: 10.1586/14737140.2014.953935. [DOI] [PubMed] [Google Scholar]
- 7.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Ren P, Shi P, et al. MicroRNA-148a promotes apoptosis and suppresses growth of breast cancer cells by targeting B-cell lymphoma 2. Anticancer Drugs. 2017;28:588–95. doi: 10.1097/CAD.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Jian M, Qi H, Mao WZ. MicroRNA 495 inhibits proliferation and metastasis and promotes apoptosis by targeting Twist1 in gastric cancer cells. Oncol Res. 2019;27:389–97. doi: 10.3727/096504018X15223159811838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Liu P, Qi X, Bian C, et al. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett. 2017;13:4039–46. doi: 10.3892/ol.2017.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori F, Sacconi A, Canu V, et al. MiR-181c associates with tumor relapse of high-grade osteosarcoma. Oncotarget. 2015;6(16):13946–61. doi: 10.18632/oncotarget.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannito S, Novo E, di Bonzo LV, et al. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383–430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 13.Jeong H, Ryu YJ, An J, et al. Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology. 2012;60:E87–95. doi: 10.1111/j.1365-2559.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Chen X, Peng R, et al. Let7a suppresses cell proliferation via the TGFbeta/SMAD signaling pathway in cervical cancer. Oncol Rep. 2016;36:3275–82. doi: 10.3892/or.2016.5160. [DOI] [PubMed] [Google Scholar]
- 15.Wu TT, Lu J, Zheng PQ, et al. Yiqi Huayu Jiedu decoction inhibits the invasion and metastasis of gastric cancer cells through TGF-beta/Smad pathway. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/1871298. 1871298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Liu W, Meng W, et al. Downregulation of miR-542-3p promotes cancer metastasis through activating TGF-beta/Smad signaling in hepatocellular carcinoma. Onco Targets Ther. 2018;11:1929–39. doi: 10.2147/OTT.S154416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YP, He Q, Wu F, et al. [Effects of Wnt3a on proliferation, activation and the expression of TGFb/Smad in rat hepatic stellate cells]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:111–15. doi: 10.3760/cma.j.issn.1007-3418.2013.02.009. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 18.Bao RF, Shu YJ, Hu YP, et al. MiR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and Smad pathways in gallbladder carcinoma. Oncotarget. 2016;7:22339–54. doi: 10.18632/oncotarget.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015 doi: 10.1155/2015/587193. 587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He W, Li AG, Wang D, et al. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J. 2002;21:2580–90. doi: 10.1093/emboj/21.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Simone V, Bevivino G, Sedda S, et al. Smad7 knockdown activates protein kinase RNA-associated eIF2alpha pathway leading to colon cancer cell death. Cell Death Dis. 2017;8:e2681. doi: 10.1038/cddis.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javelaud D, Delmas V, Moller M, et al. Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene. 2005;24:7624–29. doi: 10.1038/sj.onc.1208900. [DOI] [PubMed] [Google Scholar]
- 23.Misaghi A, Goldin A, Awad M, Kulidjian A. Osteosarcoma: A comprehensive review. SICOT J. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S, Shao NN, Fan L, et al. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technolog Med Sci. 2014;34:889–95. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Sun X, Wu J, Li Z. MicroRNA-613 suppresses proliferation, migration and invasion of osteosarcoma by targeting c-MET. Am J Cancer Res. 2016;6:2869–79. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Huang J, Liang Y, Xu M, et al. MicroRNA-124 acts as a tumor-suppressive miRNA by inhibiting the expression of Snail2 in osteosarcoma. Oncol Lett. 2018;15:4979–87. doi: 10.3892/ol.2018.7994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Sun XH, Geng XL, Zhang J, Zhang C. miRNA-646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2) Tumour Biol. 2015;36:2127–34. doi: 10.1007/s13277-014-2822-z. [DOI] [PubMed] [Google Scholar]
- 28.Yuan G, Zhao Y, Wu D, et al. MiRNA-20a upregulates TAK1 and increases proliferation in osteosarcoma cells. Future Oncol. 2018;14:461–69. doi: 10.2217/fon-2017-0490. [DOI] [PubMed] [Google Scholar]
- 29.Zabaglia LM, Bartolomeu NC, Dos SM, et al. Decreased MicroRNA miR-181c expression associated with gastric cancer. J Gastrointest Cancer. 2018;49:97–101. doi: 10.1007/s12029-017-0042-7. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki N, Koga Y, Taniguchi H, et al. High expression of miR-181c as a predictive marker of recurrence in stage II colorectal cancer. Oncotarget. 2017;8:6970–83. doi: 10.18632/oncotarget.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang WL, Zhang JH. MiR-181c promotes proliferation via suppressing PTEN expression in inflammatory breast cancer. Int J Oncol. 2015;46:2011–20. doi: 10.3892/ijo.2015.2896. [DOI] [PubMed] [Google Scholar]
- 32.Yao W, Wang X, Xiao K. Protective effect of berberine against cardiac ischemia/reperfusion injury by inhibiting apoptosis through the activation of Smad7. Mol Cell Probes. 2018;38:38–44. doi: 10.1016/j.mcp.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Yu Z, Xiao Q, et al. Expression of BAMBI and its combination with Smad7 correlates with tumor invasion and poor prognosis in gastric cancer. Tumour Biol. 2014;35:7047–56. doi: 10.1007/s13277-014-1962-5. [DOI] [PubMed] [Google Scholar]
- 34.Lin N, Ji Z, Huang C. Smad7 alleviates glomerular mesangial cell proliferation via the ROS-NF-kappaB pathway. Exp Cell Res. 2017;361:210–16. doi: 10.1016/j.yexcr.2017.10.003. [DOI] [PubMed] [Google Scholar]