Abstract
Objective:
Microalbuminuria and hypertension are the risk factors for diabetic nephropathy, and increased levels of liver enzymes are prevalent among diabetic patients. The aim of this research was to examine the effects of Crocus sativus supplementation on nephropathy indices, liver enzymes, and blood pressure in patients with type 2 diabetes (T2D).
Materials and Methods:
This placebo-controlled, randomized clinical trial was performed among 80 T2D patients. Subjects were randomly assigned to either Crocus sativus (n = 40) or placebo (n = 40) groups and treated with C. sativus and or placebo for 12 weeks, respectively. Alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum urea, creatinine, 24-hr urine albumin, systolic blood pressure (SBP), diastolic blood pressure (DBP), physical activity, and dietary intakes were measured and blood samples were taken at baseline and after the 12‑week intervention to assess the differences between the two groups.
Results:
C. sativus supplementation compared with the placebo resulted in a significant reduction of SBP (P<0.005). However, changes in other indices including liver enzymes, serum creatinine, serum urea, and 24-hr urine albumin, and DBP were not significantly different between the two groups (p>0.05). Also, no significant changes in dietary intakes and physical activity were seen between the two groups.
Conclusion:
This report shows that daily supplementation with 100 mg C. sativus powder improved SBP. However, it did not considerably improve DBP, nephropathy indices and liver functions in T2D patients after 12 weeks of administration.
Key Words: Crocus sativus, Diabetes mellitus type 2, Herbal medicine, Blood pressure, Nephropathy
Introduction
Diabetes mellitus is a metabolic disorder which is related with severe hyperglycemia, impaired metabolism of carbohydrates, and fats, as well as proteins that give rise to relative or absolute insulin deficiency or insulin resistance that is divided into two major classes, type 1 and 2. 90% of the total prevalence of diabetes is type 2 diabetes (Larejani and Zahedi, 2001 ▶; Li et al., 2012 ▶). The prevalence of T2D is 6.4% in adults worldwide and it is anticipated that it reaches 4.4% in all groups in 2030 (Elshater et al., 2009 ▶; Shanmugam et al., 2009 ▶). The prevalence of diabetes according to the Esteghamati et al. study was 7.7% equal to 2 million cases when extrapolated to the Iranian population within the age range 25–64 years (Esteghamati et al., 2008 ▶).
Because of hyperglycemia in T2D, liver disorder including fatty liver disease is also very rampant in the diabetic population (Lavie et al., 2003 ▶). Resistance to insulin in patients with T2D has also been established as a predisposing factor of increased levels of liver enzymes such as ALT, ALP, AST, dyslipidemia, hypertension, and cardiovascular diseases (Esteghamati et al., 2008 ▶; Jyothirmayi and Kumar, 2011 ▶). Elevated oxidative stress and hypertension in diabetic patients may lead to some complications including retinopathy and nephropathy (Darko et al., 2002 ▶).
Hypertension and microalbuminuria are the risk factors for diabetic nephropathy that is observed in nearly 40 percent of all individuals with T2D and can lead to end-stage kidney disease (Parving et al., 2001 ▶).
Lack of proper control of diabetes complications (e.g. micro- and macro-vascular) causes a range of problems that diminish the life's quality, inflict high expenses to the healthcare system and increase mortality rate (Whiting et al., 2011 ▶). Therefore, it is very important for the health care systems to adopt appropriate strategies to reduce the complications of diabetes. In addition to the usual treatments for T2D such as insulin therapy and blood glucose lowering drugs, complementary and herbal medicine therapies have useful effects and ameliorate some risk factors in diabetic subjects (Azadmehr et al., 2014 ▶). Several clinical trials have shown that herbal medicine has positive effects on diabetes complications (Moradabadi et al., 2013 ▶). However, there is no convincing evidence for using herbal and complementary in previous works (Mirfeizi et al., 2016 ▶).
Saffron or Crocus sativus L. is a bulbous perennial of the iris family (Iridaceae) valued for its golden-colored, pungent stigmas, which are dried and used to color and flavor foods as well as a dye (Jelodar et al., 2018 ▶; Zheng et al., 2005 ▶). Crocus sativus contains crocetin that is effective in the control and treating some conditions such as dyslipidemia and hypertension (Xi et al., 2007 ▶). The possible lowering effect of C. sativus on blood pressure is related to some of its components include crocin, picrocrocin, safranal, and crocetin (Imenshahidi et al., 2010 ▶; Kaur and Khanna, 2012 ▶). Previous studies showed that C. sativus may protect the liver and kidneys against some toxic agents (Huang et al., 2014 ▶; Omidi et al., 2014 ▶; Tajadadi-Ebrahimi et al., 2014 ▶).
Although some reports indicated the effect of C. sativus on the metabolic indices, there is no complete documentation about the useful effects of C. sativus supplementation on T2D complications. The objective of our study was to determine the effects of C. sativus supplementation compared with placebo, on liver enzymes (Alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT)), nephropathy indices (serum urea, serum creatinine, and 24-hr urine albumin), systolic blood pressure (SBP) and diastolic blood pressure (DBP) in patients with T2D.
Materials and Methods
Study design
We conducted a prospective, double-blind, placebo-controlled, randomized study that was registered in the Iranian Registry of Clinical Trials (IRCT, www.irct.ir) under registration No. IRCT201510259472N9. The study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (registration No: IR.IUMS.REC.1394.26583), and a written informed consent was obtained from all subjects. Trial registration, baseline/eligibility testing, allocation, and follow-up were all conducted in accordance with Consolidated Standards of Reporting Trials guidelines (Schulz et al., 2010 ▶).
Participants
Ninety patients aged 30–70 years with T2D were recruited between December 2015 and October 2017.
The understudy subjects were diagnosed with T2D by an endocrinologist on the basis of the results of the hematologic tests and met the criteria required for being enrolled in this research work. These criteria included having a disease duration of at least 3 years, an HbA1c level of 6.5-10%, and a body mass index (BMI) of 20-35 kg/m2. Exclusion criteria were insulin therapy at baseline or during the study, changes in the type or dose of medications, changes in diet or daily physical activity, taking nutritional supplements during the last 3 months, smoking, and alcohol abuse during the last year, any acute illnesses or some chronic diseases including kidney, liver, cardiovascular, and gastrointestinal diseases, pregnancy and lactation, consumption of C. sativus or other botanical supplements, C. sativus hypersensitivity, and consumption of less than 80% of supplements during the study period.
We used the formula suggested for estimation of the sample size required for a randomized clinical trial ( ). The sample size was calculated 45 subjects in each group, considering a study power of 80%, type I error of 5% (α=0.05), type II error of 20% (β=0.20), and alanine aminotransferase (ALT) as a key variable. Totally, 90 individuals were selected at the baseline, and were followed for 12 weeks. Subjects were randomly allocated to either C. sativus supplemented group (n=45) or placebo-treated group (n=45). The randomization scheme was generated using a computer-based random-number generator.
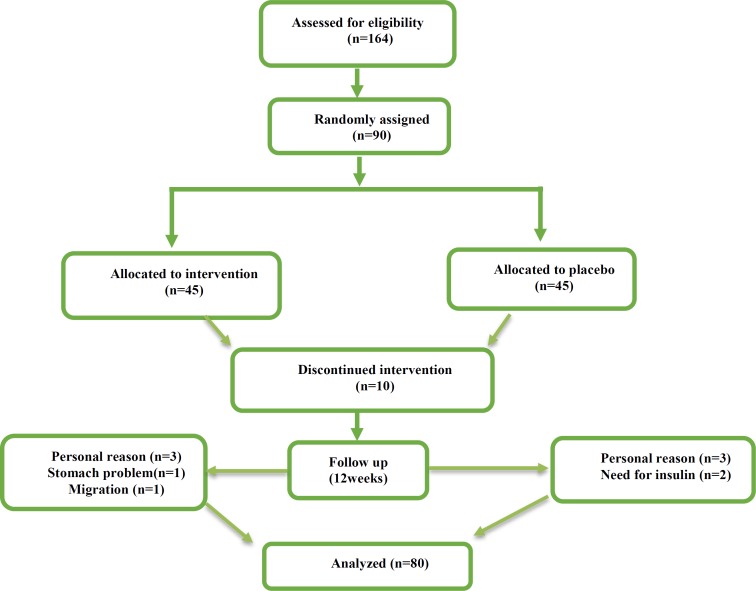
Although 90 T2D patients were deemed eligible for inclusion in the study after an initial visit, only 80 completed the study (five individuals in the saffron group either withdrew because of personal reasons, stomach problems or were lost to follow-up because they migrated elsewhere and five individuals in control group, due to personal reasons and need for insulin therapy). Of these, 40 were randomized to the saffron group and 40 were randomized to the control group (Figure 1). In this trial, 100% of powders were consumed in both groups and the rate of compliance in our study was high.
Figure 1.
Schematic diagram of the study; individuals in the intervention group received powder containing 100 mg of saffron per day during the study; those in the placebo group received 100 mg placebo (maltodextrin) powder at the same times
C. sativus preparation and dose of intervention
Crocus sativus was supplied by the Novin Saffron Co. (Mashhad, Iran). It was formulated as a dried powder of C. sativus in the Faculty of Pharmacy at Tehran University of Medical Sciences. Placebo powder (maltodextrin) matched saffron powder in size and volume of content and was manufactured by the same company.
Different doses of Crocus sativus have been used in different studies (from 20 to 400 mg/day), and we selected a dose of 100 mg/day which is a safe dose for intervention (Ayatollahi et al., 2014 ▶; Broadhead et al., 2019 ▶; Moazen-Zadeh et al., 2018 ▶; Shemshian et al., 2014 ▶).
Measurements
Detailed information about sex, age, anthropometric indices, medical history, taking medical drugs, and supplements use were collected. Anthropometric measurements of weight, height, and BMI were recorded for each subject at the outset and end of the study. We measured weights without shoes and with minimal clothes to the nearest 0.5 kg. Height was measured by using a non-stretched tape measure without shoes, while the shoulders were in a normal position. BMI was determined as weight in kilograms divided by height in meters squared.
At the beginning and end of the study, physical activity level was determined by using International Physical Activity Questionnaire (IPAQ), for which reliability and validity were evaluated in Iran (Vasheghani-Farahani et al., 2011 ▶). This questionnaire assesses walking time, moderate and vigorous intensity physical activities, and the amount of time spent sitting in a typical week. We asked all participants not to change the physical activity during the research time. However, the differences between the two groups in physical activity were adjusted in the ultimate analysis.
All individuals were permitted to take their regular medications based on their physician᾽s prescription. We obtained 3-day dietary records at the beginning, in the middle and at the end of the study. The mean values from all three time points were considered regular dietary intake of participants during the intervention period. Participants were asked to record everything they take during the day, including the between-meal, supplements, and late-evening snacks as accurately as possible. For dietary records, we used values in household measurements (Ghaffarpour et al., 1999 ▶).
To obtain macro and micronutrient consumption of the individuals according to dietary records, we used Nutritionist IV software (based on US National Nutrient Databank) modified for Iranian foods. The reliability and validity of 3-day food record for Iranian adults had been confirmed previously (Mohammadifard et al., 2015 ▶).
Blood samples (10 ml) were taken in 10-12 hours fasting state at the beginning and after 12 weeks of intervention. The serum was obtained by high-speed centrifugation (3000 rpm) at 4°C for 10 min and frozen immediately at −80°C until assay.
Serum urea, serum creatinine, 24-hr urine albumin, ALT, AST, and ALP were measured using Hitachi 911 automatic analyzer (Hitachi Ltd, Tokyo, Japan). SBP and DBP were measured three times in every session after a 15-min rest sitting down by the mercury sphygmomanometer (Zyklusmed Co, Germany).
Intervention
After assignment of subjects to C. sativus or placebo group, participants in the C. sativus group received daily a powder containing 100 mg C. sativus in the morning, and those in the placebo group daily received the same amount of placebo (maltodextrin) for 12 weeks. According to previous studies, maltodextrin at a dose of 100 mg/day has no effect on cardiovascular and liver functions (Hofman et al., 2016 ▶). The appearance of the placebo powders (including color and packaging) was similar to the C. sativus powder. All subjects were recommended not to change the common dietary intake during the study, and not to take any supplements (vitamins, minerals, proteins carbohydrates, etc.).
Statistical analysis
We analyzed all data by means of SPSS software version 18 (SPSS, Inc., Chicago, IL, USA). We used the Kolmogorov–Smirnov test to examine the normal distribution of variables. Log transformation was conducted for non-normally distributed variables. Independent-samples t-test was applied to examine the homogeneity of general characteristics, anthropometric measurements, and dietary intakes in two groups. We used paired samples t-test (Wilcoxon in non-parametric distribution) to assess the effects of both C. sativus and placebo supplements on nephropathy indices, hepatic enzymes, and blood pressure measurements.
To compare the changes between groups, we applied independent-samples t-test (Mann-Whitney U test in case of non-parametric distribution). We used a chi-squared test for physical activity analyses. To find if the magnitude of the change depends on baseline values, physical activity, and usual dietary intake, we adjusted these variables by using analysis of covariance (ANCOVA) to obtain the independent effect of C. sativus supplementation on liver enzymes, blood pressure, and nephropathy indices. A p<0.05 was considered significant. All values are reported as mean±SD.
Results
The baseline data of subjects are shown in Table 1. There was no statistically significant difference in sex, age, weight, height, waist circumference, BMI, disease duration, and type of consumed oral hypoglycemic drugs between the two groups.
Table 1.
General baseline characteristics of subjects who received either C. sativus or placebo
| Variables |
Crocus sativus groupa (n=40) |
Placebo groupb (n=40) |
p-valuec | |
|---|---|---|---|---|
| Age (year) | 55.2±7.3 | 53±10.6 | 0.61 | |
| Sex | Male n(%) |
20 (50) | 16 (40) | 0.51 |
| Female n(%) |
20 (50) | 24 (60) | ||
| Height (cm) | 162±9.9 | 163.7±8.3 | 0.41 | |
| Weight (kg) | 75.3±12.8 | 80.3±12.8 | 0.08 | |
| BMI (kg/m2) | 29.3±4.9 | 30.5±4.7 | 0.25 | |
| WC (cm) | 106±9.5 | 107.8±12.9 | 0.75 | |
| Duration of T2D (year) | 7.8±5.4 | 6.6±6.1 | 0.13 | |
| Types of hypoglycemic drugs | Metformin n(%) |
4 (10) | 6 (15) | 0.454 |
| Metformin+ glibenclamide n (%) |
8 (20) | 13 (32.5) | ||
| Metformin+ glibenclamide n(%) |
10 (25) | 8 (20) | ||
| Other drugs n (%) |
18 (45) | 13 (32.5) | ||
Abbreviations: BMI, Body Mass Index; WC, Waist Circumference. All quantitative values are expressed as means±SDs. All qualitative values are expressed as numbers and percentages (%).
Received 100 mg Crocus sativus per day during the study.
Received 100 mg placebo per day during the study.
Obtained from independent-samples t test for quantitative values and Chi-square for qualitative values.
The usual dietary intake of participants throughout the intervention on the basis of 3-day dietary records is presented in Table 2. No significant differences were found in usual intake of energy, protein, carbohydrate, and fat between C. sativus and placebo groups.
Table 2.
Usual dietary intake of participants who received either C. sativus or placebo during the study
| Variables |
Crocus sativus groupa (n=40) |
Placebo groupb (n=40) |
p-valuec |
|---|---|---|---|
| Energy Intake (Kcal) | 1848±164 | 1890±160 | 0.608 |
| Carbohydrate (g per day) | 230.6±38.8 | 279.4±277.7 | 0.275 |
| Protein Intake (g per day) | 59.4±12.5 | 80.2±158.5 | 0.411 |
| Fat (g per day) | 83.2±14.2 | 84.2±17.2 | 0.799 |
All values are presented as mean±SD.
Received 100 mg C. sativus per day during study.
Received 100 mg placebo per day during the study.
As evaluated by independent-samples t test.
Moreover, physical activity level was not different before and after the study (p>0.05) (Table 3).
Table 3.
Physical activity measurements at the baseline and 12 weeks after intervention in the two groups
| p-value b | Control Group (n=40) |
Crocus sativus Group (n=40) |
Group |
||||
|---|---|---|---|---|---|---|---|
| Severe | Moderate | Low | Severe | Moderate | Low | Intensity of PA a Stage of Intervention |
|
| 0.124 | 3 (7.5) |
5 (12.5) |
32 (80) |
4 (10) |
12 (30) |
24 (60) |
Before Intervention
n (%) |
| 0.124 | 3 (7.5) |
5 (12.5) |
32 (80) |
4 (10) |
12 (30) |
24 (60) |
After Intervention
n (%) |
. Physical activity
. As assessed by Chi-squared test
Baseline and post-intervention values of ALT, AST and ALP are presented in Table 4. In this study, supplementation with 100 mg C. sativus per day compared with placebo (100 mg maltodextrin) for 12 weeks had no effect on liver enzymes (p>0.05).
Table 4.
liver functional tests measurements at baseline and 12 weeks after the intervention in subjects who received either Crocus sativus or placebo
| Variables | Crocus sativus groupa (n=40) | p-valueb | Placebo groupc (n=40) | P-valueb | p-valued | ||
|---|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | ||||
| AST (U/L) | 22.4±10.3 | 21.2±8.8 | 0.248 | 22.1±8.5 | 19.8±7.5 | 0.02 | 0.448 |
| ALT (U/L) | 22.35±10.7 | 21±11.9 | 0/308 | 23.6±11.1 | 20.8±11 | 0.046 | 0.438 |
| ALP (U/L) | 177.1±49.6 | 172.3±45.8 | 0.419 | 167.1±46.6 | 166.4±45.2 | 0.871 | 0.582 |
Abbreviations: AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; ALP, Alkaline phosphatase. All values are presented as means±SD.
Received 100 mg C. sativus per day during study.
As assessed by paired-samples t test.
Received 100 mg placebo per day during the study.
As assessed by independent-samples t test.
Intake of C. sativus compared with placebo did not significantly affect nephropathy indices including serum urea, serum creatinine, 24-hr urine albumin, and DBP (Table 5). Consumption of C. sativus, compared with placebo, resulted in a significant decrease in SBP (P=0.005) (Table 5).
Table 5.
Blood pressure and nephropathy indices measured at baseline and 12 weeks after the intervention in subjects who received either Crocus sativus or placebo
| Variables | Crocus sativus groupa (n=40) | p-valueb | Placebo groupc (n=40) | p-valueb | p-valued | ||
|---|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | ||||
| SBP (mmHg) | 132.7±21.3 | 124.5±13.2 | 0.004 | 127.4±15.3 | 128.3±12.4 | 0.604 | 0.005 |
| DBP (mmHg) | 79.5±10.8 | 76.7±9.9 | 0.104 | 79.7±11.1 | 75.9±14 | 0.091 | 0.621 |
| Serum creatinine (mg/dl) |
1.09±0.17 | 1.1±0.2 | 0.430 | 1.1±0.19 | 1.1±0.2 | 0.964 | 0.505 |
| Serum urea (mg/dl) | 32.6±8.6 | 30.4±8.5 | 0.067 | 31.4±7.4 | 31.2±7.7 | 0.817 | 0.24 |
| Baseline | After | p-valuef | Baseline | After | p-valuef | p-valuee | |
| Albumin (mg/24 hr) | 38.4±22.8 | 35.7±26.7 | 0.332 | 29.8±26.7 | 29.9±25.4 | 0.828 | 0.401 |
Abbreviations: SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
Received 100 mg C. sativus per day during the study.
As assessed by paired-samples t test.
Received 100 mg placebo per day during the study.
As assessed by independent-samples t test.
As assessed by Wilcoxon signed rank test.
As assessed by Mann-Whithney U test.
In addition, analysis following adjustment of baseline values, physical activity and usual dietary intake throughout the study revealed no significant changes in AST, ALT, ALP, DBP, serum creatinine, serum urea, and 24-hr urine albumin (p>0.05), however, changes in SBP were significant (p<0.05) (Table 6).
Table 6.
Adjusted changes in the liver functional tests, blood pressure and nephropathy indices measurement in subjects who received either Crocus sativus or placebo
| Variables |
Crocus sativus groupa (n=40) |
Placebo groupb (n=40) |
p-valuec |
|---|---|---|---|
| AST (U/L) | -1.2±6.6 | -2.3±5.9 | 0.326 |
| ALT (U/L) | -1.3±8.3 | -2.8±8.6 | 0.522 |
| ALP (U/L) | -4.8±37.1 | -0/7±28.1 | 0.858 |
| SBP (mmHg) | -7.2±14.6 | 0.8±9.9 | 0.006 |
| DBP (mmHg) | -2.7±10.3 | -3.8±10.2 | 0.631 |
| Serum creatinine (mg/dl) |
0.02±0.18 | -0.001±0.13 | 0.583 |
| Serum urea (mg/dl) | -2.25±7.54 | -0.27±7.3 | 0.334 |
| Albumin (mg/24 hr) | -0.65±7.01 | 0.05±5.8 | 0.585 |
Abbreviations: AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; ALP, Alkaline phosphatase; SBP, systolic blood pressure; DBP, diastolic blood pressure. All values are expressed as means±SD.
Received 100 mg C. sativus per day during study.
Received 100 mg placebo per day during the study.
As assessed by AVCOVA test.
Discussion
To the best of our knowledge, this study is one of few studies that examined the effect of C. sativus supplementation on blood pressure, nephropathy indices and liver enzymes levels in patients with T2D. The results of the present study indicated that 12-week supplementation with 100 mg daily of C. sativus significantly decreases SBP in T2D patients compared with baseline and placebo group. This effect remained significant even after adjustment of primary values, physical activity and usual dietary intake of subjects throughout the study. We did not find any significant effect for C. sativus on serum AST, ALT, and ALP levels as well as DBP, serum urea and creatinine, and 24-hr urine albumin. Patients with T2D are very susceptible to nephropathy, increased activity of liver enzymes, and elevated blood pressure. Hypertension and increased liver enzymes among patient with T2D can lead to vascular disease and insulin resistance (Beckman et al., 2002 ▶; Hanley et al., 2007 ▶).
Earlier studies have reported beneficial effects of C. sativus on blood pressure. The majority of these studies were performed in animal models. The findings of our study were consistent with several studies done in animals. In a study done by Fatehi et al., consumption of 50 mg/100 g C. sativus resulted in decreased blood pressure in anaesthetized rats (Fatehi et al., 2003 ▶). The blood pressure lowering effect of C. sativus in hypertensive rats may be mediated through blocking calcium channels by crocetin (Imenshahidi et al., 2014 ▶). In one clinical trial conducted on T2D patients, C. sativus 15 mg administered twice a day for 8-weeks could not affect the blood pressure, These results are inconsistent with our findings probably due to differences in study duration, and the low dose of C. sativus supplementation administered (Milajerdi et al., 2017 ▶). Endothelial dysfunction can play an important role in the pathogenesis of the vascular complications of diabetes and leads to hypertension (De Vriese et al., 2000 ▶). The exact mechanisms explaining the effect of C. sativus on blood pressure are unknown, but significant effect of C. sativus may be explained by its high content of crocetin that can decrease the expression of soluble intercellular adhesion molecule-1 (sICAM-1) protein and probably decrease arterial stiffness and blood pressure (Xiang et al., 2006 ▶). In Modaghegh et al. study, supplementation with 400 mg C. sativus in healthy individuals can reduce SBP during 7 days (Modaghegh et al., 2008 ▶). The results of this study is in line with our results, however, considering the limited evidence of the effect of C. sativus consumption on blood pressure, further studies are required to reach a certain conclusion.
The study results showed that C. sativus could not decrease liver enzymes (AST, ALT, and ALP) levels, compared to placebo. In contrast to our findings, in one animal study, 20 mg/kg C. sativus had been able to decrease ALT and AST in Wistar rats with fatty liver. These effects of C. sativus may be due to modulation of liver enzymes in parallel with major normalization of liver size and structure as well as a decrease of fatty infiltration in hepatocytes (Mashmoul et al., 2016 ▶). In another study, injection of 40 mg/kg C. sativus in rats reduced oxidative stress and improved liver and kidney functions (Pitsikas et al., 2008 ▶). These results are inconsistent with our findings probably due to differences in study duration and dose of C. sativus used. Milajerdi et al. found that administration of 30 mg/day C. sativus extract to T2D patients have no significant effects on ALT, AST, ALP, and nephropathy indices following weeks intervention. These findings are in line with the results of our study (Milajerdi et al., 2017 ▶). More clinical trials using safe dose of C. sativus should be performed to approve the present results and find the exact mechanism(s) underlying such effects.
Diabetic nephropathy is the usual reason for end-stage kidney disease and structural injury develops over years before clinical and laboratory manifestations such as albuminuria, hypertension, or reduced glomerular filtration rate (GFR), appear (Caramori et al., 2002 ▶; Tavafi et al., 2011 ▶). Although the effect of the C. sativus extract on kidney function is rarely investigated in the human studies, a new animal study showed significant reductions of blood urea nitrogen (BUN), and creatinine in the presence of crocin (isolated from C. sativus) in diabetic rats (Naghizadeh et al., 2010 ▶). The authors of this study suggested that this kidney protective effect is probably due to the antioxidant effects of crocin. In contrast to our findings, supplementation of high-dose C. sativus (400 mg/day) in healthy participants, could increase some kidney hematological indices such as BUN and creatinine in the normal ranges (Modaghegh et al., 2008 ▶). Differences in the dosage of C. sativus supplements and health conditions of participants may be reasons for these conflicting results.
As a result of limited funding, we did not measure the serum crocetin levels at the beginning and end of the study. Thus, these limitations should be considered in future studies.
In conclusion, C. sativus supplementation in T2D patients had beneficial effects on blood pressure as it decreased SBP after 12 weeks; however, it did not affect serum urea and creatinine, 24-hr urine albumin, liver enzymes, and DBP. Therefore, further clinical trial studies are needed to explain how C. sativus affects vascular functions.
Acknowledgment
The authors wish to thank all participants, Iranian Diabetes Society and Institute of Endocrinology and Metabolism, Firouzgar Hospital and from Deputy of Research Center, Iran University of Medical Sciences. This paper was adapted from an MSc dissertation, which was supported by Department of Nutrition, School of Public Health, Iran University of Medical Sciences (IR.IUMS.REC.1394.26583).
Conflicts of interest
The authors declare no conflict of interests.
References
- Ayatollahi H, Javan AO, Khajedaluee M, Shahroodian M, Hosseinzadeh H. Effect of Crocus sativus L (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother Res. 2014;28:539–543. doi: 10.1002/ptr.5021. [DOI] [PubMed] [Google Scholar]
- Azadmehr A, Ziaee A, Ghanei L, Huseini HF, Hajiaghaee R, Tavakoli-far B, Kordafshari G. A randomized clinical trial study: anti-oxidant, anti-hyperglycemic and anti-hyperlipidemic effects of olibanum gum in type 2 diabetic patients. Iran J Pharm Res. 2014;13:1003–1009. [PMC free article] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Broadhead GK, Grigg JR, McCluskey P, Hong T, Schlub TE, Chang AA. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefes Arch Clin Exp Ophthalmol. 2019;257:31–40. doi: 10.1007/s00417-018-4163-x. [DOI] [PubMed] [Google Scholar]
- Caramori ML, Kim YH, Chunmei F, Alfred J, Rich SS, Miller ME, Mauer M. Cellular basis of diabetic nephropathy: 1 Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51:506–513. doi: 10.2337/diabetes.51.2.506. [DOI] [PubMed] [Google Scholar]
- Darko D, Dornhorst A, Kelly FJ, Ritter JM, Chowienczyk PJ. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin Sci. 2002;103:339–344. doi: 10.1042/cs1030339. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de V, Johan L, Norbert H, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshater AE, Salman Muhammad MA, Moussa Mahrous MA. Effect of ginger extract consumption on levels of blood glucose, lipid profile and kidney functions in alloxan induced-diabetic rats. Egypt Acad J Biosci. 2009;2:153–162. [Google Scholar]
- Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, Gregg EW. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran. Diabetes care. 2008;31:96–98. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- Fatehi M, Rashidabady T, Fatehi-Hassanabad Z. Effects of Crocus sativus petals’ extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J Ethnopharmacol. 2003;84:199–203. doi: 10.1016/s0378-8741(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 1999;7 [Google Scholar]
- Hanley AJG, Wagenknecht LE, Festa A, D'agostino RB, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort. Diabetes care. 2007;30:1819–1827. doi: 10.2337/dc07-0086. [DOI] [PubMed] [Google Scholar]
- Hofman DL, Van Buul VJ, Brouns Fred JPH. Nutrition, health, and regulatory aspects of digestible maltodextrins. Crit Rev Food Sci Nutr. 2016;56:2091–2100. doi: 10.1080/10408398.2014.940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Chen YC, Wang PC, Tsai MA, Yeh SC, Liang HJ, Chen SC. Efficacy of a formalin-inactivated vaccine against Streptococcus iniae infection in the farmed grouper Epinephelus coioides by intraperitoneal immunization. Vaccine. 2014;32:7014–7020. doi: 10.1016/j.vaccine.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran J Basic Med Sci. 2014;17:9–13. [PMC free article] [PubMed] [Google Scholar]
- Jelodar G, Javid Z, Sahraian A, Jelodar S. Saffron improved depression and reduced homocysteine level in patients with major depression: A Randomized, double-blind study. Avicenna J Phytomed. 2018;8:43–50. [PMC free article] [PubMed] [Google Scholar]
- Jyothirmayi B, Kumar JS. Insulin resistance and alanine amino transaminase (ALT) levels in first degree relatives of type 2 diabetes mellitus. Diabetes & Metab-Res. 2011;5:143–147. doi: 10.1016/j.dsx.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Kaur R, Khanna N. Pathophysiology and risk factors related to hypertension and its cure using herbal drugs. Spatula DD. 2012;2:245–256. [Google Scholar]
- Larejani B, Zahedi F. Epidemiology of diabetes mellitus in Iran. IJDLD. 2001;1:1–8. [Google Scholar]
- Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Tran VH, Duke CC, Roufogalis BD. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta medica. 2012;78:1549–1555. doi: 10.1055/s-0032-1315041. [DOI] [PubMed] [Google Scholar]
- Mashmoul M, Azlan A, Mohtarrudin N, Yusof BNM, Khaza’ai H, Khoo HE, Boroushaki MT. Protective effects of saffron extract and crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement Altern Med. 2016;16:401–407. doi: 10.1186/s12906-016-1381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milajerdi A, Jazayeri S, Bitarafan V, Hashemzadeh N, Shirzadi E, Derakhshan Z, Akhondzadeh S. The effect of saffron (Crocus sativus L) hydro-alcoholic extract on liver and renal functions in type 2 diabetic patients: A double-blinded randomized and placebo control trial. J Nutr Intermed Metab. 2017;9:6–11. [Google Scholar]
- Mirfeizi M, Mehdizadeh TZ, Mirfeizi SZ, Asghari Jafarabadi M, Rezvani HR, Afzali M. Controlling type 2 diabetes mellitus with herbal medicines: A triple‐blind randomized clinical trial of efficacy and safety. J Diabetes. 2016;8:647–656. doi: 10.1111/1753-0407.12342. [DOI] [PubMed] [Google Scholar]
- Moazen-Zadeh E, Abbasi SH, Safi-Aghdam H, Shahmansouri N, Arjmandi-Beglar A, Hajhosseinn Talasaz A, Akhondzadeh S. Effects of saffron on cognition, anxiety, and depression in patients undergoing coronary artery bypass grafting: a randomized double-blind placebo-controlled trial. J Altern Complement Med. 2018;24:361–368. doi: 10.1089/acm.2017.0173. [DOI] [PubMed] [Google Scholar]
- Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. J Phymed. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Mohammadifard N, Sajjadi F, Maghroun M, Alikhasi H, Nilforoushzadeh F, Sarrafzadegan N. Validation of a simplified food frequency questionnaire for the assessment of dietary habits in Iranian adults: Isfahan Healthy Heart Program, Iran. ARYA atheroscler. 2015;11:139. [PMC free article] [PubMed] [Google Scholar]
- Moradabadi L, Kouhsari SM, Sani MF. Hypoglycemic effects of three medicinal plants in experimental diabetes: inhibition of rat intestinal α-glucosidase and enhanced pancreatic insulin and cardiac glut-4 mrnas expression. Iran J Pharm Res. 2013;12:387–397. [PMC free article] [PubMed] [Google Scholar]
- Naghizadeh B, Mansouri SM, Mashhadian NV. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem Toxicol. 2010;48:2650–2655. doi: 10.1016/j.fct.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Omidi A, Riahinia N, Montazer Torbati MB. 2014. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J Phytomed. Behdani MA;4:330–336. [PMC free article] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, Sakellaridis N. Effects of the active constituents of Crocus sativus L crocins, in an animal model of anxiety. J Phymed. 2008;15:1135–1139. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam KR, Ramakrishana Ch, Mallikarjuna K, Reddy KS. The impact of ginger on kidney carbohydrate metabolic profiles in STZ induced diabetic rats. Asian J Exp Sci. 2009;23:127–134. [Google Scholar]
- Shemshian M, Mousavi SH, Norouzy A, Kermani T, Moghiman T, Sadeghi A, Ferns GA. Saffron in metabolic syndrome: its effects on antibody titers to heat-shock proteins 27, 60, 65 and 70. J Complement Integr Med. 2014;11:43–49. doi: 10.1515/jcim-2013-0047. [DOI] [PubMed] [Google Scholar]
- Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65:34–41. doi: 10.1159/000365153. [DOI] [PubMed] [Google Scholar]
- Tavafi M, Ahmadvand H, Khalatbari A, Tamjidipoor A. Rosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. IJBMS. 2011;14:275–283. doi: 10.1016/j.tice.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Vasheghani-Farahani A, Tahmasbi M, Asheri , Hossein A, Haleh Nedjat S, Kordi R. The Persian, last 7-day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian J Sports Med. 2011;2:106–116. doi: 10.5812/asjsm.34781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, Zhang Y 2007. J Nutr Biochem. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats;18:64–72. doi: 10.1016/j.jnutbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107:25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Zheng S, Qian Z, Tang F, Sheng L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem Pharmacol. 2005;70:1192–1199. doi: 10.1016/j.bcp.2005.07.034. [DOI] [PubMed] [Google Scholar]