Abstract
Objective:
Hypertension is an important cause of cardiovascular disorders. The angiotensin converting enzyme (ACE) plays an important role in hypertension; therefore, inhibition of ACE in treatment of chronically elevated blood pressure is an important therapeutic approach. In the current review, we have provided information from Persian Traditional Plants described by Avicenna in the Canon of Medicine and a number of more current scientific databases, with a focus on angiotensin converting enzyme inhibitory activity of the following six plants: Allium sativum, Cinnamomum zeylanicum, Jasminum grandiflorum, Tribulus terrestris, Vaccinium myrtillus and Vitis vinifera.
Materials and Methods:
A literature search was conducted and information on different traditional plants used for hypertension was collected from the Canon of Medicine and several other databases including PubMed, Scopus, Google Scholar and Web of Science.
Results:
The present article highlights the antihypertensive potential of the above-noted six plants. Administered doses, manner of consumption, types of extracts, preparations and derivatives, personal habits, and other geographic and epidemiologic variables have an important role in the potential efficacy of these plants.
Conclusion:
Recent studies indicated a significant correlation between the traditional use of Persian plants to reduce blood pressure and angiotensin converting enzyme inhibitory activity.
Key Words: Hypertension, Angiotensin converting enzyme, Avicenna, Canon of medicine, Traditional plants
Introduction
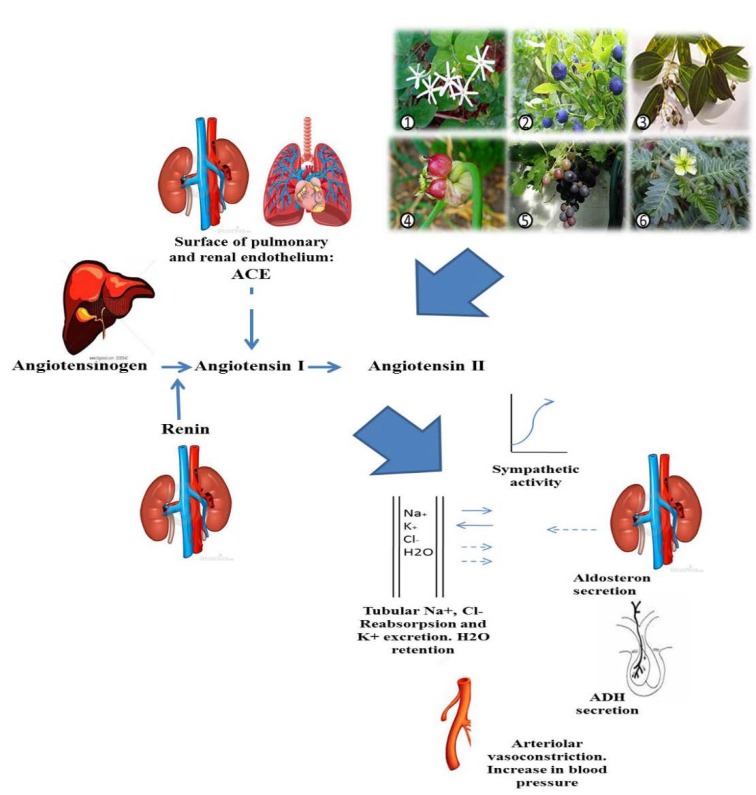
The eighth report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure categorized hypertension into three stages comprising pre-hypertension, and stages 1 and 2;a systolic blood pressure (BP) of 120-139mmHg or a diastolic BP of 80-89mmHg defined as pre-hypertension (Dennison-Himmelfarb et al., 2013 ▶).Hypertension affects about a billion people of the world population at some time-points during their lifetime and its prevalence increases with age(Emtiazy et al., 2014 ▶). One of every two persons over 65 years of age develop high BP (Chobanian et al., 2003 ▶). The angiotensin converting enzyme (ACE) plays an important role in hypertension via enhancing vasoconstriction and maintenance of peripheral resistance; therefore, inhibition of ACE is widely recommended as a therapeutic target for treatment of high BP (Barbosa-Filho et al., 2006 ▶) (Figure 1).
Figure 1.
Schematic diagram showing the proposed ACE – inhibitory activity of six Iranian traditional plants
1. Jasminum grandiflorum, 2. Vaccinium myrtillus, 3. Cinnamomum zeylanicum, 4. Allium sativum, 5. Vitis vinifera, and 6. Tribulus terrestris.
Avicenna was a famous Persian physician and one of the leading scientists of his time (Moosavi, 2009 ▶). In his well-known book ‘Canon of Medicine’, he dedicated a chapter to ‘Emtela’ which means ‘repletion’ or ‘plethora’. In this chapter, he described etiology, features and complications of emtela which is well-matched to high BP, although hypertension was not considered a disease in Persian medical textbooks (Emtiazy et al., 2014 ▶; Kardeh et al., 2014 ▶). Moreover, other records of Traditional Persian Medicine (TPM) have also mentioned emtela and its signs, symptoms and treatment(Jorjani, 1976 ▶).
Numerous adverse reactions related to the antihypertensive drugs may limit their usage and subsequently reduce treatment adherence. Therefore, developing new medications with limited adverse effects and higher efficacies is a research focus for the treatment of cardiovascular disorders (CVD).
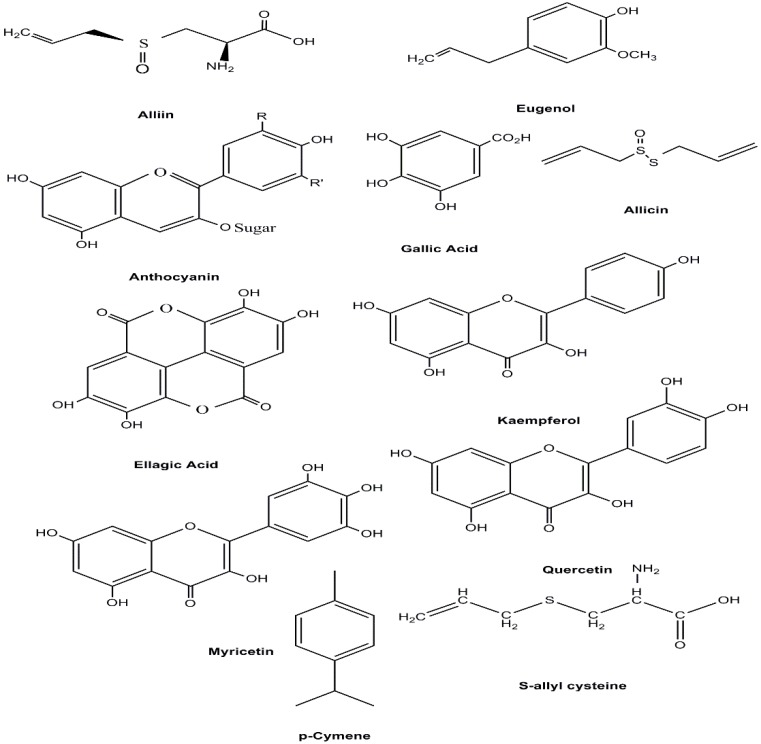
There are several researches on the anti-hypertensive properties of plants (Somanadhan et al., 1998 ▶; Somanadhan et al., 1999 ▶). Also, a number of reports showed the ACE inhibitory [ACEI] activity of medicinal plants (Meunier et al., 1987 ▶; Khan et al., 2001 ▶; Loizzo et al., 2008 ▶; Patten et al., 2016 ▶). Plants with some ACE inhibitor activity are listed in table 2 and IC50 or % inhibition (mg/ml) is shown in table 1. In Iran, about 40 plant species are used to treat hypertension (Baharvand-Ahmadi and Asadi-Samani, 2016 ▶). Plants with some ACE inhibitory activity are listed in Table 2. Among these, six were reported by TPM as ACE inhibitors and are currently used in Iran to treat BP. The chemical structures of some active components of these plants are shown in Figure 2.
Table 2.
Summary of Iranian plants with ACE inhibitory activities
|
Plant name
(common name) |
Family |
Main isolated
phytochemical constituents |
Part of plant | Action | References |
|
Allium sativum L. (garlic) |
Alliaceae | S-allyl cysteine, alliin, allicin, γ-glutamyl cysteine and ajoene | Cardioprotection Anti-oxidant Anti-hyperlipidemic Anti-hypertensive |
(Asdaq and Inamdar, 2010 ▶; Rastogi et al., 2016 ▶) (McRae, 2006 ▶; Duda et al., 2007 ▶) (Ried and Fakler, 2014 ▶) |
|
|
Cinnamomum zeylanicum Blume (true cinnamon or Ceylon) |
Lauraceae | P-cymene,eugenol, cinnzeylanine and cinnzeylanol | Bark | Anti-oxidant | (Jain, et al., 2015 ▶) (Sultana et al., 2016 ▶) (Patten et al., 2016 ▶) |
| “ | Cough suppressant | ||||
| “ | Anti-hypertensive | ||||
|
Jasminum grandiflorum L. (jasmine) |
Oleaceae | Secoiridoid, iridoid glycosides, saponins, terpenoids and flavonoids | Leaf | Dermal ulcer healing | (Chaturvedi et al., 2013 ▶) (Patten et al., 2016 ▶) (Chaturvedi and Tripathi, 2011 ▶; Arun et al., 2016 ▶) (Venkataiah et al., 2013 ▶) |
| Aerial | Anti-hypertensive | ||||
| Leaf “ “ |
Anti-inflammation Anti-oxidant nephroprotective |
||||
|
Tribulus terrestris L. (Gokhshura or puncture vine) |
Zygophyllaceae | Furostanol, spirostanol saponins, sulphated saponins of tigogenin and diosgenin | Fruit | Nephroprotective | (Kavitha and Jagadeesan, 2006 ▶) (Tuncer et al., 2009 ▶) (Phillips et al., 2006 ▶) |
| Anti-hyperlipidemic | |||||
| Whole plant | Anti-hypertensive | ||||
|
Vaccinium myrtillus L. (bilberry) |
Ericaceae | Different anthocyanins such as galactosides and glycosides of peonidin, petunidin, delphinidin, malvidin and cyanidin | Neuroprotactive | (Matsunagaet al., 2009 ▶) (Lee et al., 2013 ▶) |
|
| Fruit | Anti-oxidant Anti-hypertensive |
||||
|
Vitis vinifera L. (grapevine) |
Vitaceae | Phenolic components (myricetin, ellagic acid, kaempferol, gallic acid and quercetin), polyphenols (flavonoids, anthocyanins) | Seed | Radical scavenger | (Fauconneau et al., 1997 ▶; Facino et al., 1999 ▶; Aldini et al., 2003 ▶) |
| “ | Cardioprotective | ||||
| Fruit | Anti-oxidant | ||||
| Seed Stem bark |
Anti-hypertensive Alzheimer’s disease |
(Koo et al., 2008 ▶; Godse et al., 2010 ▶; Quiñones et al., 2013 ▶) | |||
| Seed | Vasorelaxant | (Gharib Naseri et al., 2010 ▶) | |||
| “ | Cardioprotective | (Afonso et al., 2013 ▶) | |||
| Seed Extract | Hypolipidemic | (Adisakwattana et al., 2010 ▶, Agrawal et al., 2010 ▶; Afonso et al., 2013 ▶) | |||
| Leaf Extract | Bronchodilator | (Gharib Naseri and Heidari, 2006 ▶) |
Table 1.
ACE inhibitor activity of Traditional Persian Medicine Plants
| Plant name | IC50 or % inhibition (mg/ml) | Part of plant | References |
|---|---|---|---|
| Allium sativum | 58% at 0.3 | Bulb | (Sendl et al., 1992 ▶) |
| Cinnamomum zeylanicum | 87% at 0.2 | Bark | (Inokuchi et al., 1984 ▶) |
| Jasminum grandiflorum | 78% at 0.33 | Aerial | (Somanadhan et al., 1999 ▶) |
| Tribulus terrestris | 50% at 0.33 | Aerial | (Somanadhan et al., 1999 ▶) |
| Vaccinium myrtillus | 0.0025 | Leaf | (Persson et al., 2009 ▶) |
| Vitis vinifera | 0.08 | Fruit | (Meunier et al., 1987 ▶) |
Figure 2.
Chemical structures of some active components of Traditional Persian Medicine Plants
The present study reports the anti-ACE activity of six medicinal plants recommended by TPM. In recent years, some of the plants suggested by Avicenna have been the subject of pharmacological and clinical trials. Herein, we searched
scientific databases to verify the effectiveness of the medicinal plants suggested by Avicenna for the treatment of hypertension. Moreover, major bioactive compounds of these plants are also discussed.
Materials and Methods
A literature search was conducted and information on the effect of six traditional plants used against hypertension, was collected from the Canon of Medicine and several other databases including PubMed, Scopus, Google Scholar and Web of Science using the following keyword: Iranian traditional plants, hypertension, ACE, Canon of medicine, Avicenna, Allium sativum, Jasminum grandiflorum, Cinnamomum zeylanicum, Tribulus terrestris, Vaccinium myrtillus and Vitis vinifera. No time restriction was considered for selection of published studies, in this review.
Results
Allium sativum L.
Plant description and distribution
Allium sativum, commonly known as garlic is a member of Alliaceae family (Patten et al., 2016 ▶). Garlic is a bulbous plant that is cultivated all around the world, but it originally was native to central and southern Asia (Elkayam et al., 2001 ▶; Rastogi et al., 2016 ▶). Various parts of the plant including green garlic leaves, seeds, stalks, flowers and bulbs are traditionally used as food and medicine. Different preparations of garlic such as oil, macerate, powder, aged garlic, allicin powder extract, etc. are used (Arzanlou and Bohlooli, 2010 ▶).
History of use
In different cultures, garlic has been used as a spice, food additive and as herbal medicine (Elkayam et al., 2001 ▶). The records of garlic consumption date back to 5000 years ago (Rastogi et al., 2016). It has been used for treatment of several ailments such as headache, tumors, and intestinal worms (Corzo-Martínez et al., 2007 ▶). Garlic has been tried as a complementary treatment for heart disorders and insect bites (Rastogi et al., 2016 ▶). In Sanskrit and TPM literature, garlic was reported as a beneficial remedy for the treatment of chronic cough, toothache, constipation and septic diseases (Rastogi et al., 2016 ▶). Moreover, the Zoroastrian holy record, dating back to the sixth century BC., mentioned culinary and medicinal properties of garlic (Bayan et al., 2014 ▶). In “The Canon of Medicine’’ oral use and topical application of garlic has been reported to possess analgesic and anti-inflammation properties (Mahdizadeh et al., 2015 ▶).In addition, several reports suggested aged garlic extract as an important remedy for heart diseases and arterial obstructive disorders (Aviello et al., 2009 ▶; Mahdizadeh et al., 2015 ▶).
Chemistry
Chemical studies on garlic showed the presence of several enzymes, amino acid, organosulfur compounds such as S-allyl cysteine, alliin, allicin, γ-glutamyl cysteine and ajoenein in various parts of the plant (Sendl et al., 1992 ▶; McRae, 2006 ▶; Rastogi et al., 2016 ▶). Powdered garlic contains about 1% alliin which is metabolized by the enzyme, alliinase, to allicin. This process occurs when its bulb is crushed or cut. Garlic oil and aged garlic contain different products of allicin transformation (Rastogi et al., 2016 ▶).
Pharmacology
Modern pharmacological studies confirmed that garlic has therapeutically useful properties against cardiovascular disorders, , chronic diseases related to aging and possibly cancer and for cancer prevention (Rastogi et al., 2016 ▶), as well as reducing blood glucose and inhibiting platelet aggregation (Elkayam et al., 2001 ▶). Other studies reported that S-allyl cysteine has anti-hepatotoxic and anti-oxidant effects and may reduce the incidence of stroke (Asdaq and Inamdar, 2010 ▶). According to McRae, allicin is responsible for inhibition of cholesterol synthesis (McRae, 2006 ▶). A number of pharmacological and clinical studies reported the anti-hypertensive activity of garlic and its major compounds (Duda et al., 2007 ▶; Ried et al., 2013 ▶; Ried et al., 2016 ▶).
Epidemiological studies demonstrated a significant correlation between garlic consumption and the reduction of systolic and diastolic blood pressure (Xiong et al., 2015 ▶). A meta-analysis conducted by Xiong and colleagues, demonstrated a correlation between garlic consumption and reduction of systolic and diastolic blood pressure. In these trials, patients with SBP≥140mmHg and/or DBP≥90mmHg received six garlic preparations including a dried garlic homogenate (188 mg), processed garlic capsules, garlic powder, aged garlic extract (960 mg), regular garlic pills (900 mg) and time-released garlic powder tablets (600 or 2400 mg/day). Mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) were decreased by all of the garlic preparations indicating that garlic is an effective approach for lowering BP (Xiong et al., 2015 ▶).In a clinical trial conducted by McMahon and his colleagues, it was found that BP was significantly reduced5-14 h after consumption of 2400mg garlic tablets containing 31.2mg allicin in nine patients with severe hypertension (DBP≥115mmHg)(McMahon and Vargas, 1993 ▶). Another study carried out by Reid et al. in 50 patients with uncontrolled hypertension (SBP≥140 mmHg) showed that following consumption of aged garlic capsules (960 mg/day containing 2.4 mg S-allyl cystein) for 12 weeks, mean SBP was dramatically decreased (Ried et al., 2010 ▶). Controversially, Capraz et al.reported no significant BP-lowering effect for consumption of garlic cloves or garlic tablets (Cirkulin®) compared to control group (Capraz et al., 2007 ▶). These discrepancies may be attributed to differences in administered doses of garlic tablets, number of participants, personal habits and other geographic and epidemiologic variables.
A majority of reports, however, suggest that blood pressure lowering effect of garlic is attributed to its vasorelaxant activity and is mediated by release of endothelium-derived relaxing factor (EDRF) or muscle-derived relaxing factor (MDRF). Ozturk and his colleagues compared the relaxant effects of garlic and acetylcholine on the rat aorta, in vitro. The results demonstrated that garlic has a dose-dependent relaxant effect which is attenuated in the absence of endothelium but is not completely abolished. Therefore, it is speculated that EDRF may be responsible for the relaxant effects of garlic on the arterial smooth muscle tone (Öztürk et al., 1994 ▶).
Various reports suggested different mechanisms for garlic BP lowering effects including production of hydrogen sulfide (H2S), stimulation of nitric oxide (NO) (Ried et al., 2013 ▶;Shouk et al., 2014 ▶), inhibition of ACE (Hosseini et al., 2007 ▶; Oboh et al., 2013 ▶; Ried et al., 2013 ▶; Shouk et al., 2014 ▶; Jain et al., 2015 ▶) and blockage of α adrenergic receptors and calcium channels (Shouk, et al., 2014 ▶). Reid et al. (2014) ▶ reported that allicin has a low sustained bioavailability in human tissues, so its activity via inhibition of angiotensin-II production and vasodilation effects are less plausible than its NO- and H2S-mediated mechanisms (Ried and Fakler, 2014 ▶).
Animal experiments showed that administration of S-allyl cysteine and captopril can synergistically reduce BP via inhibition of ACE (Shouk et al., 2014 ▶). Sharifi et al also demonstrated ACEI effects of allicin in reduction of blood pressure (Sharifi et al., 2003 ▶). Oboh et al. (2013) ▶ studied the effect of phenolic extract of garlic on BP and reported that it can strongly act as an inhibitor of ACE, in vitro. In this study, evaluation of the free and bound phenolic inhibitory effects on ACE revealed that bound phenolics have more potent effect than the free phenolics in reduction of ACE activity; however, both inhibited malondialdehyde production (Asdaq and Inamdar, 2010 ▶) in a dose-dependent manner (Oboh et al., 2013 ▶).
Cinnamomum zeylanicum Blume
Plant description and distribution
Cinnamomum zeylanicum, commonly known as cinnamon tree, is a member of the Lauraceae family (Patten et al., 2016 ▶). The genus Cinnamomum has about 250 species, 20 of which grow in India (Jayaprakasha and Rao, 2011 ▶). Various parts of the cinnamon tree including the bark, leaves, flowers, fruits and roots, are used as medicine or food additive (Ranasinghe et al., 2013 ▶).
History of use
cinnamon has been considered a medicinal plant in different countries and also has been widely exploited as a common spice for thousands of years (Jayaprakasha and Rao, 2011 ▶; Ranasinghe et al., 2013 ▶). Cinnamon oil is extensively used in flavors and foods as well as beverages, perfumery and pharmaceutical industries as a preservative (Jayaprakasha and Rao, 2011 ▶; Saleem et al., 2015 ▶). C. zeylanicum is largely found in tropical Asia and Sri Lanka. The fruits grow from May to August (Jayaprakasha and Rao, 2011 ▶). As a folk remedy, C. zeylanicum has been considered for the treatment of digestive, respiratory and gynecological ailments (Ranasinghe et al., 2013 ▶)and nervous stress(Malik et al., 2015 ▶).
Chemistry
Analysis of the volatile oil from the leaves of C. zeylanicum showed the presence of p-cymene and eugenol as the main components. Additional studies reported the presence of α-pinene, lomonen, cinnamaldehyde, copaene, β-cadinene, δ-cadinene, calamenene, 3,7 (II)-salinadiene, amorphene and O-methoxy cinnamaldehyde in C. zeylanicum essential oil (Saleem et al., 2015 ▶). Moreover, cinnzeylanine and cinnzeylanol were isolated from the dried bark of C. zeylanicum (Jayaprakasha and Rao, 2011 ▶).
Pharmacology
Modern pharmacological reports suggested that C. zeylanicum improves cognitive impairment and oxidative stress (Jain et al., 2015 ▶), prevents carbon tetrachloride-induced damages on the male reproductive system (Yüce et al., 2014 ▶) and ameliorates inflammation and arthritis (for polyphenolic fraction of the bark)(Rathi et al., 2013 ▶).
C. zeylanicum was reported to have useful effects on blood pressure following short-term administration to patients with diabetes (Akilen et al., 2013 ▶) An extract of the bark was suggested as a potential anti-hypertensive agent (Kesari et al., 2014 ▶). It was also reported that a methanol extract of C. zeylanicum stem bark has both acute and chronic anti-hypertensive potential. At concentrations of 5, 10 and 20mg/kg, cinnamon was reported to reduce the mean arterial blood pressure (MABP) 12.5, 26.6 and 30.6%, respectively (Nyadjeu et al., 2013 ▶).
A methanol extract of C. zeylanicum reduced the plasma level of triglycerides (TG) and total cholesterol up to 38.1% and 32.1%, respectively (also, decreases LDL-cholesterol (75.3%) and increases HDL-cholesterol (58.4%) in rats) (Nyadjeu, et al. 2013 ▶). A similar extract of dried bark also inhibited ACE in experimental animals (Barbosa-Filho et al., 2006 ▶). The anti-hypertensive mechanism was speculated to be mediated hrough elevation of endothelial NO and activation of the K-ATP channel in vascular smooth muscle (Nyadjeu et al., 2011 ▶).
In 2016, Ranjini and his colleagues determined the inhibitory effects of methanolic extract of C. zeylanicum on ACE activity in sheep tissues. In the presence of the extract, tissue ACE activity was reduced and these effects were more significant in the kidney than in the testis and lung tissues (Ranjini et al., 2016 ▶).
Jasminum grandiflorum L.
Plant description and distribution
Jasminum grandiflorum, commonly known as jasmine is a member of the Oleaceae family (Sadhu et al., 2007 ▶; Sandeep, 2009 ▶; Ferreres et al., 2014 ▶). Jasminum is a genus of shrubs and vines containingaround200 species found all over the world (Arun et al., 2016 ▶). J. grandiflorum is domestic to temperate and tropical areas including parts of Asia, Kashmir, Philippines, Myanmar and Sri Lanka (Sandeep, 2009 ▶; Arun et al., 2016 ▶). It is distributed across the west coast of India from south Canara to the low elevations of Kerala and is also cultivated in Italy, China, India, France, Egypt and Morocco (Somanadhan et al., 1998 ▶; Sandeep, 2009 ▶). The flower are harvested from July to November and from May to December in North and South India (Sandeep, 2009 ▶). Various parts of the plant including stem, bark, leaves, flowers and roots are used for medicinal purposes (Arun et al., 2016 ▶).
History of use
It has been extensively used by Indian tribes as a popular remedy for different ailments including body and stomach pain and toothaches (Sandeep, 2009 ▶; Arun et al., 2016 ▶). It was also reported to have beneficial properties in treating amenorrhea, chronic constipation and dysmenorrhea (Sandeep, 2009 ▶; Arun et al., 2016 ▶). A decoction from leaves and roots was reported to be useful against headache and edema, as well as giddiness (Somanadhan et al., 1998 ▶).
Various parts of J. grandiflorum are traditionally used in Indian medicine. For instance, jasmine leaves were applied to eliminate corns, fresh jasmine flowers and oil were used to cure sores and jasmine root extract was used to embrocate the eyes (Patnaik, 1993 ▶).
Chemistry
A number of studies detected secoiridoids, terpenoids, avonoids, tannins, saponins and flavonoids in various parts of the jasmine plant(Arun et al., 2016 ▶). Different compounds including phenolics, protocatechuic acid, triterpene and oleanolic acid were isolated from methanol extracts of the dried aerial parts of the jasmine plant (Sadhu et al., 2007 ▶).
Pharmacology
J. grandiflorum was reported to possess various pharmacological activities including cytoprotective, anti-convulsant, anti-cancer (Sandeep, 2009 ▶; Arun et al., 2016 ▶) and dermal ulcers healing properties (Chaturvedi et al., 2013 ▶). Anethanolic leaves extract of J. grandiflorum was evaluated for its antiulcer, anti-oxidant, anti-nociceptive and anti-inflammatory activities while amethanolic extract was reported to have anti-inflammatory and anti-oxidant activities in both in vitro and In vivo models (Chaturvedi and Tripathi, 2011 ▶; Arun et al., 2016 ▶).
Invitro enzymatic assays showed strong ACE inhibitory activity for the extracts obtained from the aerial parts of J. grandiflorum (Somanadhan et al., 1998 ▶; Arun et al., 2016 ▶; Patten et al., 2016 ▶). The ACE inhibitory activity of the aqueous, ethanol and acetone extracts of J. grandiflorum (whole plant) was 46, 60 and 78%, respectively (Somanadhanet al., 1999 ▶).
Arun and his colleagues reported the half maximal inhibitory concentration (IC50) values of jasmine to be 26-36μM (Arun et al., 2016 ▶). The IC50 values for ACE inhibition of secoiridoid aglycones of jasmine were 20-25µM (Kiss et al., 2008 ▶). Patten et al. reported relatively high ACE inhibitory activity )IC50 30μM) for Sambacein I-III isolated from J. grandiflorum (Patten et al., 2016 ▶).
Tribulu sterrestris L.
Plant description and distribution
Tribulus is a genus of 20 species belonging to Zygophyllaceae (Ukani et al., 1997 ▶). T. terrestr is commonly known as caltrop, is an annual species with opposite and pinnate leaves and yellow petals that grow up to 10-60cm in height (Ganzera Bedir et al., 2001 ▶; Chhatre Nesari et al., 2014 ▶). This plant is widely distributed in tropical, mild temperate areas and desert climates such as Asia, the Mediterranean region and Mexico (Dinchev Janda et al., 2008 ▶; Hussain et al., 2009 ▶; Martino-Andrade et al., 2010 ▶; Hashim et al., 2014 ▶).
History of use
Ayurvedic medical documents record some characteristics of the plant including a sweet taste and its use to help digestion and cooling tempers. There is a document which shows that T. terrestris was used for bladder disorders and urinary stone (Chhatre Nesari et al., 2014 ▶). T. terrestris is used in Persian and Chinese folk medicine as a remedy for various disorders (Chhatre et al., 2014 ▶) including cough, polyuria, and dysuria and as a gastric stimulant and aphrodisiac (Ukani et al., 1997 ▶; Hussain et al., 2009 ▶).
Currently, T. terrestris is used as a food supplement (“Tribocard”) and in veterinary medicine to improve reproductive activity and fertilization. In addition, supplements containing T. terrestis are applied in cases of libido disorder (Evstatieva and Tchorbanov, 2011 ▶).
Various parts of the herb such as leaves, stem and roots are utilized as appetite suppressing and as astringents, cathartic, and anodyne (Ukani et al., 1997 ▶).
Chemistry
Several phytochemical studies showed the presence of various chemical classes in T. terrestris (Ukani et al., 1997 ▶; Dinchev et al., 2008 ▶; Abirami and Rajendran, 2011 ▶). For example, saponins, flavonoids, alkaloids, cinammic acid amides and lignin amides were found in T. terrestris. Furostanol, spirostanol saponins and four sulphated saponins of tigogenin and diosgenin were also isolated from this plant (Ukani et al., 1997 ▶; Kostova and Dinchev, 2005 ▶; Hashim et al., 2014 ▶). The fruit and root of T. terrestris are rich in flavonoids, alkaloids, phytosteroids and glycosides (Ukani et al., 1997 ▶; Hashim et al., 2014 ▶) and its leaves contain diosgenin, gitogenin and chlorogenin (Hashim et al., 2014 ▶).
Pharmacology
In vitro data showed that the methanolic fraction of T. terrestris fruit extract decreases the level of reactive oxygen species (ROS) and protects against kidney cellular damage caused by mercuric chloride(Kavitha and Jagadeesan, 2006 ▶). It was shown that T. terrestris has anti-hypertensive effects mediated via inhibition of ACE especially in the kidneys (Tuncer et al., 2009 ▶).
Sharifi et al. (2003b) ▶ in their evaluation of an aqueous extract of T. terrestris suggested that the BP lowering effect of the extract resulted from its ACE inhibitory activity (Sharifi et al., 2003 ▶). Anethnopharmacological investigation on Indian medical herbs reported ACE inhibitory activities for aqueous, ethanol and acetone extracts of T. terrestris (aerial parts). The inhibitory effect was dependent on the type of the extract with the aqueous extract having the highest ACEI activity (Somanadhan et al., 1999 ▶).
Vaccinium myrtillus L.
Plant description and distribution
Vaccinium myrtillus, commonly known as bilberry, is a medicinal plant belonging to the Ericaceae family (Matsunaga et al., 2009 ▶). It is also known as whortleberry, huckleberry, blueberry and European blueberry (Chu et al., 2011 ▶). V. myrtillus is a low-growing shrub with dark red or blue fruit that grows in forests, moors and mountainous regions of Asia, North America and Europe (Matsunaga et al., 2009 ▶; Persson et al., 2009 ▶; Song et al., 2010 ▶).
History of use
Berries have a long history of medicinal use and it was also used in food and pharmaceutical products (Puupponen-Pimiä et al., 2008 ▶; Song et al., 2010 ▶). Wild berries are used in daily diet in Nordic regions (Puupponen-Pimiä et al., 2008 ▶). Also, extracts of the fruit are used as coloring agents in wine, jams and syrups (Ulbricht et al., 2009 ▶).
As a folk remedy, bilberry is used for treatment of ailments such as diarrhea (Puupponen-Pimiä et al., 2008 ▶), vascular disorders (Song et al., 2010 ▶), and mucus inflammation (Ulbricht et al., 2009 ▶). The dried fruit is also used for treatment of various eye disorders including eyestrain, and myopia and to promote night vision (Puupponen-Pimiä et al., 2008 ▶;Ulbricht et al., 2009 ▶).
Chemistry
The berries contain high levels of phenolic acid, flavonoid, lignin and phenolic polymers (e.g. polymeric tannins) (Puupponen-Pimiä et al., 2008 ▶). Chemical studies on the bilberry fruit and its extracts showed the presence of water-soluble polyphenolic flavonoids especially anthocyanins that are considered to be responsible for the health-promoting properties of bilberry (Matsunaga et al., 2009 ▶; Ulbricht et al., 2009 ▶; Song et al., 2010 ▶; Chu et al., 2011 ▶). V. myrtillus contains different anthocyanins such as galactosides and glycosides of peonidin, petunidin, delphinidin, malvidin and cyaniding (Ichiyanagi et al., 2004 ▶; Persson et al., 2009 ▶; Lee et al., 2013 ▶).
Pharmacology
It was reported that the anthocyanins of bilberry have beneficial properties such as anti-oxidant (Matsunaga et al., 2009 ▶; Persson et al., 2009 ▶; Ulbricht et al., 2009 ▶), anti-platelet, the ability to facilitate collagen biosynthesis, vasoprotection (Matsunaga et al., 2009 ▶), anticancer and antibacterial effects (Persson et al., 2009 ▶). In addition, the bilberry was shown to possess anti-inflammatory, hypolipidemic and hypoglycemic effects (Ulbricht et al., 2009 ▶). Beside such properties, the bilberry was reported to have ocular and neuroprotective effects (Cravotto et al., 2010 ▶; Chu et al., 2011 ▶).
In a randomized controlled clinical trial on hypertensive cases, an adverse correlation between a combination of polyphenols [green tea (100mg), grape seed (330mg), resveratrol (60mg), quercetin, ginkgo biloba and bilberry (60mg)] and diastolic blood pressure was observed. This observation may be related to the potential of the polyphenols in activation and production of NO (Biesinger et al., 2016 ▶).
Persson et al. in their study on bilberry and its polyphenols, incubated the endothelial cells isolated from umbilical veins with bilberry 25E extract (containing the chloride salt of the anthocyanidins and myrtillin chloride) for 10 min. The results showed that V. myrtillus extract (0.0062, 0.0125, 0.025, 0.05 and 0.1mg/ml) could inhibit ACE activity in a dose-dependent manner. Proanthocyanidins (e.g. tannins) isolated from bilberry decreased fluid retention, inhibited the renin-angiotensin-aldosterone system and induced an anti-hypertensive effect (Persson et al., 2009 ▶).
In a randomized placebo-controlled clinicaltrial on71 participants, two portions of berries were consumed daily by 35 participants for 8 weeks. Berry consumption reduced SBP by about 1.5mmHg (Cravotto et al., 2010 ▶).Moreover, treatment of spontaneously hypertensive stroke-prone rats with 3% blueberries for 2 weeks, decreased the level of ACE activity in the blood. However, it had no effect on ACE activity in the testis, lung, kidney or aorta (Wiseman et al., 2010 ▶).
Vitis vinifera L.
Plant description and distribution
Vitis (grapevine) is a genus with around 80 species belonging to the Vitaceae family. Vitis vinifera is a species native to southwestern Asia, Central Europe and North America. These plants grow under subtropical and Mediterranean conditions (Terral et al., 2010 ▶). The history of domestication of grapevine dates back to the first millennium BC in a region between the black sea and Iran. Anatolia in the Asia part of Turkey has a notable role in the diversification of grape varieties (Terral et al., 2010 ▶; Yilancioglu and Cetiner, 2013 ▶). The wild grapevine is a heliophilous liana that possesses flaky bark and fruits – known as a grape- with green, red or purple (black) color (Terral et al., 2010 ▶).
History of use
Consumption of grapes has a history of more than 6,000 years. Greek philosophers reported the healing properties of grape wine (Ismail et al., 2014 ▶). Consumption of grapes was mentioned in ‘‘the Canon of Medicine” by Avicenna as a natural remedy for oral disorders especially gingival health and loose teeth (Faridi et al., 2015 ▶). In Eber papyrus, there are documents describing the usage of grapes for urinary problems (Inoue and Craker, 2014 ▶). Other applications of grapes are treatment of diarrhea, hemorrhage and varicose vein (Ismail et al., 2014 ▶). Indeed, today, grape is cultivated for its fruit and juice worldwide (Terral et al., 2010 ▶).
Chemistry
Grape seeds are a rich source of proteins, high-value fatty oils, procyanidins and phenolic components (Zhou et al., 2011 ▶). The grape seeds also contains oxidative derivatives of catechin and epicatechin and viniferone A, B and C (Fan et al., 2004 ▶).The phenolic components found in all parts of grape are responsible for wine color, its bitter taste and astringency (Basha et al., 2004 ▶). Major phenolic components in the leaves are myricetin, ellagic acid, kaempferol, gallic acid and quercetin (Ismail et al., 2014 ▶). The leaves also contain a wide range of polyphenols such as flavonoids, anthocyanins and organic acids, mainly oxalic, malic and tartaric acid; fumaric, succinic acid are also found in trace amounts (Ismail et al., 2014 ▶).
Pharmacology
Several studies suggested that V. vinifera has therapeutic applications such as reduction of ischemic/reperfusion damage (Bombardelli et al., 1997 ▶; Facino et al., 1999 ▶), anti-inflammation (Ismail et al., 2014 ▶), anti-oxidant (Bombardelli et al., 1997 ▶; Fauconneau et al., 1997 ▶; Pourghassem-Gargari et al., 2011 ▶), vasorelaxant (Gharib Naseri et al., 2010 ▶), bronchodilatory (Gharib Naseri and Heidari, 2006 ▶), hypolipidemic (Adisakwattana et al., 2010 ▶) and hypoglycemic effects (Ismail et al., 2014 ▶).
Procyanidins protect endothelial cells from peroxynitrite damage and induce relaxation in human arteries, suggesting their cardioprotective activity (Aldini et al., 2003 ▶).
Various studies reported the anti-hypertensive effects of grape potentially through ACE inhibition (Godse et al., 2010 ▶; Borde et al., 2011 ▶; Afonso et al., 2013 ▶). The antihypertensive and antioxidant effects were observed after chronic administration of myricetin (100 and 300mg/kg, per oral, for 4 weeks) –an important flavonol of grapes - to deoxycorticosterone acetate -induced hypertensive rats. Following myricetin treatment using strips of ascending colon, the cumulative concentration-response curve of angiotensin II and serotonin shifted to right (Borde et al., 2011 ▶).
Discussion
Medicinal plants and natural products have long been used for treatment of a broad range of diseases. Many Traditional Medical Systems including Persian Medicine have used herbal medicines to manage cardiovascular disorders (Sobhani et al., 2017 ▶). In the present article, we discussed medicinal plants with ACE inhibitory activities suggested by TPM, that are currently used in Iran for the treatment of BP. These plants also act through other BP lowering mechanisms. Garlic and its major compounds allicin and S-allylcystein were found to lower BP in human clinical trials and animal experiments with a number of mechanisms including inhibition of ACE, production of H2S, stimulation of NO and blockage of α adrenergic receptors and calcium channels. Cinnamon was reported to possess antihypertensive effects through inhibition of ACE, elevation of the endothelial NO and activation of the K-ATP channels in vascular smooth muscles. Jasmine and its compounds sambaceins I-III and T. terrestris also demonstrated remarkable ACE inhibitory activities. Bilberry and its proanthocyanidins could also serve as a potential antihypertensive medicine through ACE inhibition along with reducing fluid retention and blocking the renin-angiotensin-aldosterone system. Grapes also demonstrated BP lowering effects potentially via ACEI activities. All the extracts and their active compounds seem to act at relatively low concentrations which are theoretically applicable in human studies based on the principles of allometric scaling of experimental doses(Shakeri et al., 2016 ▶). Importantly, most of the mentioned plants and natural products demonstrated a wide spectrum of cardiovascular protective activities such as reducing blood glucose, inhibiting platelet aggregation, inhibition of cholesterol synthesis, antidiabetic effects, anti-inflammatory and antioxidant activities, reduction of ischemic/ reperfusion damage, etc. (Elkayam et al., 2001 ▶; Bombardelli et al., 1997 ▶; Facino et al., 1999 ▶; Sobhani et al., 2017 ▶). These activities along with BP lowering effects would potentially enhance the applicability of the plants.
Although a number of studies supported the traditional use of the discussed plants for the treatment of BP, the exact indications of use and doses are to be studied in future clinical trials. Fortunately, the mentioned plants are generally considered safe as they are mostly consumed as foods and food additives. However, further studies on potential adverse effects of such plants or herb-drug reactions are necessary. For instance, it is established that hydroxybenzoic and hydroxycinnamic acids which are present in many fruits including grapes can inhibit CYP3A4 activity in human liver microsomes by noncompetitive inhibition (Basheer and Kerem, 2015 ▶). Co-administration of garlic and warfarin was reported to increase international normalized ratio (INR) which leads to bleeding (Fugh-Berman, 2000 ▶). This effect is due to the presence of allicin which can interact with CYP3A4 (Rosenkranz et al., 2012 ▶). Accordingly, caution has to be given with respect to the co-administration of these plants with other prescribed medications.
Use of traditional remedies and medicinal plants in management of hypertension has become popularized in recent decades. The present article highlights the antihypertensive potential of six plants namely, garlic (Allium sativum), cinnamon (Cinnamomum zeylanicum), jasmine (Jasminum grandiflorum), caltrop (Tribulus terrestris), bilberry (Vaccinium myrtillus) and grape (Vitis vinifera).It is important to note that the differences in administered doses, manner of consumption, types of extracts, preparations and derivatives, personal habits, and other geographic and epidemiologic variables have an important role in the potential efficacy of these plants. The results of current and future animal and human studies can provide a better understanding of the mechanisms of actions by which extracts of these plants serve to ameliorate systolic and diastolic blood pressure. Moreover, further clinical trials are needed to evaluate the exact dosage of these plants and their active compounds, pharmacokinetic aspects, potential adverse effects and herb-drug interactions.
Acknowledgment
The authors are thankful to Mashhad University of Medical Sciences for financial support.
Conflicts of interest
The authors report no conflict of interest.
References
- Abirami P, Rajendran A. GC-MS analysis of tribulus terrestris. Asian J Plant Sci Res. 2011;1:13–16. [Google Scholar]
- Adisakwattana S, Moonrat J, Srichairat S, Chanasit C, Tirapongporn H, Chanathong B, Ngamukote S, Mauml K, Sapwarobol S. Lipid-lowering mechanisms of grape seed extract (Vitis vinifera L) and its antihyperlidemic activity. J Med Plant Res. 2010;4:2113–2120. [Google Scholar]
- Afonso J, Passos CP, Coimbra MA, Silva C M, Soares-Da-Silva P. Inhibitory effect of phenolic compounds from grape seeds (Vitis vinifera L) on the activity of angiotensin I converting enzyme. LWT-food Sci Technol. 2013;54:265–270. [Google Scholar]
- Agrawal M, Nandini D, Sharma V, Chauhan N. Herbal remedies for treatment of hypertension. Int J Pharm Sci Res. 2010;1:1–21. [Google Scholar]
- Akilen R, Pimlott Z, Tsiami A, Robinson N. Effect of short-term administration of cinnamon on blood pressure in patients with prediabetes and type 2 diabetes. Nutrition. 2013;29:1192–1196. doi: 10.1016/j.nut.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Aldini G, Carini M, Piccoli A, Rossoni G, Facino RM. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 2003;73:2883–2898. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- Arun APM, Satish S, Anima P. Phytopharmacological profile of Jasminum grandiflorum Linn (Oleaceae) Chin J Integr Med. 2016;22:311–320. doi: 10.1007/s11655-015-2051-3. [DOI] [PubMed] [Google Scholar]
- Arzanlou M, Bohlooli S. Introducing of green garlic plant as a new source of allicin. Food chem. 2010;120:179–183. [Google Scholar]
- Asdaq S, Inamdar M. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17:1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Aviello G, Abenavoli L, Borrelli F, Capasso R, Izzo AA, Lembo F, Romano B, Capasso F. Garlic: empiricism or science? Nat prod commun. 2009;4:1785–1796. [PubMed] [Google Scholar]
- Baharvand-Ahmadi B, Asadi-Samani M. A mini-review on the most important effective medicinal plants to treat hypertension in ethnobotanical evidence of Iran. J Nephropharmacol. 2016;6:3. [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Filho JM, Martins VK, Rabelo LA, Moura MD, Silva MS, Cunha EV, Souza MF, Almeida RN, Medeiros IA. Natural products inhibitors of the angiotensin converting enzyme (ACE): A review between 1980-2000. Rev Bras Farmacogn. 2006;16:421–446. [Google Scholar]
- Basha SM, Musingo M, Colova VS. Compositional differences in the phenolics compounds of muscadine and bunch grape wines. Afr J Biotechnol. 2004;3:523–528. [Google Scholar]
- Basheer L, Kerem Z. Interactions between CYP3A4 and dietary polyphenols. Oxid Med Cell Longev. 2015;2015:15. doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1–14. [PMC free article] [PubMed] [Google Scholar]
- Biesinger S, Michaels H, Quadros A, Qian Y, Rabovsky A, Badger R, Jalili T. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur J Clin Nutr. 2016;70:10–16. doi: 10.1038/ejcn.2015.88. [DOI] [PubMed] [Google Scholar]
- Bombardelli E, Morazzoni P, Carini M, Aldini G, Maffei Facino R. Biological activity of procyanidins from Vitis vinifera L. BioFactors. 1997;6:429–431. [Google Scholar]
- Borde P, Mohan M, Kasture S. Effect of myricetin on deoxycorticosterone acetate (DOCA)-salt-hypertensive rats. Nat Prodt Res. 2011;25:1549–1559. doi: 10.1080/14786410903335190. [DOI] [PubMed] [Google Scholar]
- Capraz M, Dilek M, Akpolat T. Garlic, hypertension and patient education. Int J Cardiol. 2007;121:130–131. doi: 10.1016/j.ijcard.2006.08.060. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AP, Kumar M, Tripathi Y B. Efficacy of Jasminum grandiflorum L leaf extract on dermal wound healing in rats. Int Wound J. 2013;10:675–682. doi: 10.1111/j.1742-481X.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AP, Tripathi YB. Methanolic extract of leaves of Jasminum grandiflorum Linn modulates oxidative stress and inflammatory mediators. Inflammopharmacology. 2011;19:273–281. doi: 10.1007/s10787-011-0087-3. [DOI] [PubMed] [Google Scholar]
- Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev. 2014;8:45. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chu WK, Cheung SC, Lau RA, Benzie IF, Wachtel-Galor S. Herbal medicine: biomolecular and clinical aspects. Boca Raton (FL): CRC Press; 2011. p. 55. [PubMed] [Google Scholar]
- Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. [Google Scholar]
- Cravotto G, Boffa L, Genzini L, Garella D. Phytotherapeutics: an evaluation of the potential of 1000 plants. J Clin Pharm Ther. 2010;35:11–48. doi: 10.1111/j.1365-2710.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- Dennison-Himmelfarb C, Handler J, Lackland DT, Lefevre ML, Mackenzie TD, Ogedegbe O, Smith Jr, SC , Svetkey LP, Taler SJ, Townsend RR. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2013;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Dinchev D, Janda B, Evstatieva L, Oleszek W, Aslani MR, Kostova I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry. 2008;69:176–186. doi: 10.1016/j.phytochem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Duda G, Suliburska J, Pupek-Musialik D. Effects of short-term garlic supplementation on lipid metabolism and antioxidant status in hypertensive adults. Pharmacol Rep. 2007;60:163–170. [PubMed] [Google Scholar]
- Elkayam A, Mirelman D, Peleg E, Wilchek M, Miron T, Rabinkov A, Sadetzki S, Rosenthal T. The effects of allicin and enalapril in fructose-induced hyperinsulinemic hyperlipidemic hypertensive rats. Am J Hypertens. 2001;14:377–381. doi: 10.1016/s0895-7061(00)01298-x. [DOI] [PubMed] [Google Scholar]
- Emtiazy M, Choopani R, Khodadoost M, Tansaz M, Dehghan S, Ghahremani Z. Avicenna’s doctrine about arterial hypertension. Acta Med Hist Adriat. 2014;12:157–162. [PubMed] [Google Scholar]
- Evstatieva L, Tchorbanov B. Complex investigations of Tribulus terrestris L for sustainable use by pharmaceutical industry. Biotechnol Biotechnol Equip. 2011;25:2341–2347. [Google Scholar]
- Facino RM, Carini M, Aldini G, Berti F, Rossoni G, Bombardelli E, Morazzoni P. Diet enriched with procyanidins enhances antioxidant activity and reduces myocardial post-ischaemic damage in rats. Life Sci. 1999;64:627–642. doi: 10.1016/s0024-3205(98)00605-5. [DOI] [PubMed] [Google Scholar]
- Fan P, Lou H, Yu W, Ren D, Ma B, Ji M. Novel flavanol derivatives from grape seeds. Tetrahedron Lett. 2004;45:3163–3166. [Google Scholar]
- Faridi P, Moatamedi M, Zarshenas MM, Abolhassanzadeh Z, Mohagheghzadeh A. Natural remedies in the Canon of Medicine for dentistry and oral biology. Trends Pharm Sci. 2015;1:4–9. [Google Scholar]
- Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- Ferreres F, Grosso C, Gil-Izquierdo A, Valentão P, Andrade PB. Assessing Jasminum grandiflorum L authenticity by HPLC-DAD-ESI/MS n and effects on physiological enzymes and oxidative species. J Pharm Biomed anal. 2014;88:157–161. doi: 10.1016/j.jpba.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- Ganzera M, Bedir E, Khan I. Determination of steroidal saponins in Tribulus terrestris by reversed‐phase high‐performance liquid chromatography and evaporative light scattering detection. J Pharm Sci. 2001;90:1752–1758. doi: 10.1002/jps.1124. [DOI] [PubMed] [Google Scholar]
- Gharib Naseri MK, Heidari A. Bronchodilatory activity of Vitis vinifera leaf hydroalcoholic extract in rat. Iran Biomed J. 2006;10:79–83. [Google Scholar]
- Gharib Naseri MK, Navid Hamidi M, Heidari A. Vasorelaxatory effect of Vitis vinifera extract on rat aorta. Iran J Pharm Res. 2010;4:93–99. [Google Scholar]
- Godse S, Mohan M, Kasture V, Kasture S. Effect of myricetin on blood pressure and metabolic alterations in fructose hypertensive rats. Pharm biol. 2010;48:494–498. doi: 10.3109/13880200903188526. [DOI] [PubMed] [Google Scholar]
- Hashim S, Bakht T, Khan B, Jan J. Medicinal properties, phyto-chemistry and pharmacology of Tribulus terrestris L. (Zygophyllaceae). Pak J Bot. 2014;46:399–404. [Google Scholar]
- Hosseini M, Shafiee SM, Baluchnejadmojarad T. Garlic extract reduces serum angiotensin converting enzyme (ACE) activity in nondiabetic and streptozotocin-diabetic rats. Pathophysiology. 2007;14:109–112. doi: 10.1016/j.pathophys.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Hussain AA, Mohammed AA, Ibrahim HH, Abbas AH. Study the biological activities of tribulus terrestris extracts. World Acad Sci Eng Technol. 2009;57:433–435. [Google Scholar]
- Ichiyanagi T, Hatano Y, Matsugo S, Konishi T. Structural dependence of HPLC separation pattern of anthocyanins from bilberry (Vaccinium myrtillus L) Chem Pharm P Bull. 2004;52:628–630. doi: 10.1248/cpb.52.628. [DOI] [PubMed] [Google Scholar]
- Inokuchi J, Okabe H, Yamauchi T, Nagamatsu A. Inhibitors of angiotensin converting enzyme in crude drugs. I. Chem pharm bull. 1984;32:3615–3619. doi: 10.1248/cpb.32.3615. [DOI] [PubMed] [Google Scholar]
- Inoue M, Craker LE. Medicinal and aromatic plants—uses and functions. Horticulture: Plants for People and Places . 2014;2:645–669. [Google Scholar]
- Ismail EH, Khalil MM, Al Seif FA, El-Magdoub F. Biosynthesis of gold nanoparticles using extract of grape (vitis vinifera) leaves and seeds. Prog Nanotechnol Nanomater. 2014;3:1–12. [Google Scholar]
- Jain S, Sangma T, Shukla SK, Mediratta PK. Effect of Cinnamomum zeylanicum extract on scopolamine-induced cognitive impairment and oxidative stress in rats. Nutr Neur. 2015;18:210–216. doi: 10.1179/1476830514Y.0000000113. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G, Rao LJM. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51:547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- Jorjani S, Zakhirehkharazmshahi . Treasure of Kharazmshahi), Saeedisirjani AA. Photo print of the manuscript dated 1206 A.D Tehran: The lranian Culture Foundation; 1976. p. 3. [Google Scholar]
- Kardeh S, Choopani R, Mahmoudi NG, Zargaran A. The urinary catheter and its significant applications described by Avicenna (980-1037 AD) in the canon of medicine. Urology. 2014;84:993–996. doi: 10.1016/j.urology.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Kavitha A, Jagadeesan G. Role of Tribulus terrestris (Linn) (Zygophyllacea) against mercuric chloride induced nephrotoxicity in mice, Mus musculus (Linn) J Environ Biol. 2006;37:397–400. [PubMed] [Google Scholar]
- Kesari A, Mahto PK, Kishan S, Singh S. Herbal anti hypertension drugs. Pharma Tutor. 2014;2:49–61. [Google Scholar]
- Khan M, Matsui T, Matsumoto Y, Jabbar S. In vitro ACE inhibitory effects of some Bangladeshi plant extracts. Die Pharmazie. 2001;56:902–903. [PubMed] [Google Scholar]
- Kiss AK, Mańk M, Melzig M. Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. J Ethnopharmacol. 2008;120:220–225. doi: 10.1016/j.jep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Koo M, Kim S, Lee N, Yoo M, Ryu S, Kwon D, Kim Y. 3-Hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitory effect of Vitis vinifera. Fitoterapia. 2008;79:204–206. doi: 10.1016/j.fitote.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kostova I, Dinchev D. Saponins in Tribulus terrestris–chemistry and bioactivity. Phytochem Rev. 2005;4:111–137. [Google Scholar]
- Lee C, Han D, Kim B, Baek N, Baik BK. Antioxidant and anti‐hypertensive activity of anthocyanin‐rich extracts from hulless pigmented barley cultivars. Int J Food Sci Technol. 2013;48:984–991. [Google Scholar]
- Loizzo MR, Saab AM, Tundis R, Menichini F, Bonesi M, Piccolo V, Statti GA, De Cindio B, Houghton PJ, Menichini F. In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J Ethnopharmacol. 2008;119:109–116. doi: 10.1016/j.jep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Mahdizadeh S, Ghadiri MK, Gorji A. Avicenna's Canon of Medicine: a review of analgesics and anti-inflammatory substances. Avicenna J phytomed. 2015;5:182. [PMC free article] [PubMed] [Google Scholar]
- Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer’s type. J Basic Clinic Physiol Pharmacol. 2015;26:275–285. doi: 10.1515/jbcpp-2014-0012. [DOI] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Morais RN, Spercoski KM, Rossi SC, Vechi MF, Golin M, Lombardi NF, Greca CS, Dalsenter PR. Effects of Tribulus terrestris on endocrine sensitive organs in male and female Wistar rats. J Ethnopharmacol. 2010;127:165–170. doi: 10.1016/j.jep.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Imai S, Inokuchi Y, Shimazawa M, Yokota S, Araki Y, Hara H. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol Nutr Food Res. 2009;53:869–877. doi: 10.1002/mnfr.200800394. [DOI] [PubMed] [Google Scholar]
- Mcmahon FG, Vargas R. Can garlic lower blood pressure? A pilot study. Pharmacotherapy: Hum Gene Ther Clin Dev. 1993;13:406–407. [PubMed] [Google Scholar]
- Mcrae MP. A review of studies of garlic (Allium sativum) on serum lipids and blood pressure before and after 1994: does the amount of allicin released from garlic powder tablets play a role? J Chiropr Med. 2006;4:182–190. doi: 10.1016/S0899-3467(07)60149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier MT, Villié F, Jonadet M, Bastide J, Bastide P. Inhibition of angiotensin I converting enzyme by flavanolic compounds: in vitro and in vivo studies. Planta med. 1987;53:12–15. doi: 10.1055/s-2006-962606. [DOI] [PubMed] [Google Scholar]
- Moosavi J. The place of avicenna in the history of medicine. Avicenna J Med Biotechnol. 2009;1:3–8. [PMC free article] [PubMed] [Google Scholar]
- Nyadjeu P, Dongmo A, Nguelefack TB, Kamanyi A. Antihypertensive and vasorelaxant effects of Cinnamomum zeylanicum stem bark aqueous extract in rats. J Complement Integr Med. 2011:8. doi: 10.2202/1553-3840.1490. [DOI] [PubMed] [Google Scholar]
- Nyadjeu P, Nguelefack-Mbuyo EP, Atsamo AD, Nguelefack TB, Dongmo AB, Kamanyi A. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Alternat Med. 2013;13:27. doi: 10.1186/1472-6882-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboh G, Akinyemi AJ, Ademiluyi AO. Inhibitory effect of phenolic extract from garlic on angiotensin-1 converting enzyme and cisplatin induced lipid peroxidation-in vitro. Int J Biomed Sci. 2013;9:98–106. [PMC free article] [PubMed] [Google Scholar]
- Öztürk Y, Aydin S, Koşar M, Başer KHC. Endothelium-dependent and independent effects of garlic on rat aorta. J Ethnopharmacol. 1994;44:109–116. doi: 10.1016/0378-8741(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Patnaik N. The garden of life: an introduction to the healing plants of India. New York: Doubleday 195p: 1993. ISBN. [Google Scholar]
- Patten GS, Abeywardena MY, Bennett LE. Inhibition of angiotensin converting enzyme, angiotensin II receptor blocking, and blood pressure lowering bioactivity across plant families. Crit Rev Food Sci Nutr. 2016;56:181–214. doi: 10.1080/10408398.2011.651176. [DOI] [PubMed] [Google Scholar]
- Persson IAL, Persson K, Andersson RG. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J Agric Food Chem. 2009;57:4626–4629. doi: 10.1021/jf900128s. [DOI] [PubMed] [Google Scholar]
- Phillips OA, Mathew KT, Oriowo MA. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J Ethnopharmacol. 2006;104:351–355. doi: 10.1016/j.jep.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Pourghassem-Gargari B, Abedini S, Babaei H, Aliasgarzadeh A, Pourabdollahi P. Effect of supplementation with grape seed (Vitis vinifera) extract on antioxidant status and lipid peroxidation in patient with type diabetes. J Med Plant Res. 2011;5:2029–2034. [Google Scholar]
- Puupponen-Pimiä R, Nohynek L, Ammann S, Oksman-Caldentey KM, Buchert J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem. 2008;56:681–688. doi: 10.1021/jf072001h. [DOI] [PubMed] [Google Scholar]
- Quiñones M, Guerrero L, Suarez M, Pons Z, Aleixandre A, Arola L, Muguerza B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res Int. 2013;51:587–595. [Google Scholar]
- Ranasinghe P, Pigera S, Premakumara GS, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Alternat Med. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjini H, Udupa PE, Kamath SU, Setty MM, Hadapad BS, Kamath A. An in vitro study of cinnamomum zeylanicum as natural inhibitor of angiotensin-converting enzyme (ace) on sheep (ovis aries) tissues. Asian J Pharm Clin Res. 2016;9:249–252. [Google Scholar]
- Rastogi S, Pandey MM, Rawat A. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2016;23:1082–11089. doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Rathi B, Bodhankar S, Mohan V, Thakurdesai P. Ameliorative effects of a polyphenolic fraction of Cinnamomum zeylanicum L Bark in animal models of inflammation and arthritis. Sci Pharm. 2013;81:567–89. doi: 10.3797/scipharm.1301-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K, Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: mechanisms of action and clinical relevance. Integr Blood Press Control. 2014;7:71–82. doi: 10.2147/IBPC.S51434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K, Frank O, Stocks N. Aged garlic extract reduces blood pressure in hypertensives: a dose–response trial. Eur J Clin Nutr. 2013;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K, Frank OR, Stocks NP. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas. 2010;67:144–150. doi: 10.1016/j.maturitas.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Ried K, Travica N, Sali A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: the age at heart trial. Integr Blood Press Control. 2016;9:9–21. doi: 10.2147/IBPC.S93335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz B, Fasinu P, Bouic P. An overview of the evidence and mechanisms of herb–drug interactions. Front Pharmacol. 2012;30:69. doi: 10.3389/fphar.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu SK, Khan MS, Ohtsuki T, Ishibashi M. Secoiridoid components from Jasminum grandiflorum. Phytochemistry. 2007;68:1718–1721. doi: 10.1016/j.phytochem.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Saleem M, Bhatti HN, Jilani MI, Hanif MA. Bioanalytical evaluation of Cinnamomum zeylanicum essential oil. Nat prodres. 2015;29:1857–1859. doi: 10.1080/14786419.2014.1002088. [DOI] [PubMed] [Google Scholar]
- Sandeep PP. Jasminum grandiflorum Linn (Chameli): ethnobotany, phytochemistry and pharmacology–a review. Pharma-cologyonline. 2009;2:586–595. [Google Scholar]
- Sendl A, Elbl G, Steinke B, Redl K, Breu W, Wagner H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta med. 1992;58:1–7. doi: 10.1055/s-2006-961378. [DOI] [PubMed] [Google Scholar]
- Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L–A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2016;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Sharifi AM, Darabi R, Akbarloo N. Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat. J Ethnopharmacol. 2003a;86:219–224. doi: 10.1016/s0378-8741(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Sharifi AM, Darabi R, Akbarloo N. Study of antihypertensive mechanism of Tribulus terrestris in 2K1C hypertensive rats: role of tissue ACE activity. Life Sci. 2003b;73:2963–2971. doi: 10.1016/j.lfs.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Shouk R, Abdou A, Shetty K, Sarkar D, Eid AH. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res. 2014;34:106–115. doi: 10.1016/j.nutres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Sobhani Z, Reza Nami S, Ahmad Emami S, Sahebkar A, Javadi B. Medicinal plants targeting cardiovascular diseases in view of Avicenna. Curr Pharm Des. 2017;23:2428–2443. doi: 10.2174/1381612823666170215104101. [DOI] [PubMed] [Google Scholar]
- Somanadhan B, Smitt UW, George V, Pushpangadan P, Rajasekharan S, Duus JØ, Nyman U, Olsen CE, Jaroszewski JW. Angiotensin converting enzyme (ACE) inhibitors from Jasminum azoricum and Jasminum grandiflorum. Planta med. 1998;64:246–250. doi: 10.1055/s-2006-957419. [DOI] [PubMed] [Google Scholar]
- Somanadhan B, Varughese G, Palpu P, Sreedharan R, Gudiksen L, Smitt UW, Nyman U. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J Ethnopharmacol. 1999;65:103–112. doi: 10.1016/s0378-8741(98)00201-3. [DOI] [PubMed] [Google Scholar]
- Song J, Li Y, Ge J, Duan Y, Sze SCW, Tong Y, Shaw PC, Ng TB, Tsui KC, Zhuo Y. Protective effect of bilberry (Vaccinium myrtillus L) extracts on cultured human corneal limbal epithelial cells (HCLEC) Phytother Res. 2010;24:520–524. doi: 10.1002/ptr.2974. [DOI] [PubMed] [Google Scholar]
- Sultana S, Khan A, Alhazmi MMSHA. Cough suppressant herbal drugs: a review. Int J Pharm Sci Invent. 2016;5:15–28. [Google Scholar]
- Terral J, Fabre L. Evolution and history of grapevine (Vitis vinifera) under domestication: new morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient European cultivars. Ann Bot. 2010;105:443–455. doi: 10.1093/aob/mcp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncer MA, Yaymaci B, Sati L, Cayli S, Acar G, Altug T, Demir , R Influence of Tribulus terrestris extract on lipid profile and endothelial structure in developing atherosclerotic lesions in the aorta of rabbits on a high-cholesterol diet. Acta histochem. 2009;111:488–500. doi: 10.1016/j.acthis.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Ukani M, Nanavati D, Mehta N. A review on the ayurvedic herb tribulus terrestris L. Anc Sci Life. 1997;17:144. [PMC free article] [PubMed] [Google Scholar]
- Ulbricht C, Basch E, Basch S, Bent S, Boon , Burke D, Costa D, Falkson C, Giese N, Goble M. An evidence-based systematic review of bilberry (Vaccinium myrtillus) by the natural standard research collaboration. J Diet Suppl. 2009;6:162–200. doi: 10.1080/19390210902861858. [DOI] [PubMed] [Google Scholar]
- Venkataiah G, Kumar CP, Rejeena D. Effect of methanolic extract of Jasminum grandiflorum linn leaves on gentamicin induced nephrotoxicity in rats. Indo Am J Pharm Res. 2013;3:7462–7467. [Google Scholar]
- Wiseman W, Egan JM, Slemmer JE, Shaughnessy KS, Ballem K, Gottschall-Pass KT, Sweeney MI. Feeding blueberry diets inhibits angiotensin II-converting enzyme (ACE) activity in spontaneously hypertensive stroke-prone rats. Can J Physiol Pharmacol. 2010;89:67–71. doi: 10.1139/y10-101. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wang P, Li S, Li X, Zhang Y, Wang J. Garlic for hypertension: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2015;22:352–361. doi: 10.1016/j.phymed.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Yilancioglu K, Cetiner S. Rediscovery of historical Vitis vinifera varieties from the South Anatolia region by using amplified fragment length polymorphism and simple sequence repeat DNA fingerprinting methods. Genome. 2013;56:295–302. doi: 10.1139/gen-2012-0175. [DOI] [PubMed] [Google Scholar]
- Yüce A, Türk G, Çeribaşı S, Güvenç M, Çiftçi M, Sönmez M, Özer Kaya Ş, Cay M, Aksakal , M Effectiveness of cinnamon (Cinnamomum zeylanicum) bark oil in the prevention of carbon tetrachloride‐induced damages on the male reproductive system. Andrologia. 2014;46:263–272. doi: 10.1111/and.12072. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhang T, Liu W, Zhao G. Physicochemical characteristics and functional properties of grape (Vitis vinifera L) seeds protein. Int J Food Sci Technol. 2011;46:635–641. [Google Scholar]