Abstract
Introduction and hypothesis
Studies have yet to examine the impact of day-of-surgery voiding trials on post-operative urinary retention in women undergoing obliterative and apical suspension procedures for pelvic organ prolapse. Our objective was to evaluate if time to spontaneous void after these procedures is shorter when a voiding trial is performed on the day of surgery compared with our standard practice of post-operative day 1.
Methods
We conducted a randomized, parallel-arm trial in patients undergoing major pelvic floor reconstructive surgery. Women were randomized 1:1 to an early (4 h post-operatively on the day of surgery) or a standard (6 am on post-operative day 1) retrograde voiding trial.
Results
A total of 57 women consented. Mean age and BMI were 65 ±11 and 27.9 ± 4.4. Most women had stage III pelvic organ prolapse (77.2%). Groups had similar baseline characteristics. In the intention-to-treat analysis (n = 57), there was no difference in time to spontaneous void in the early versus standard voiding trial groups (15.9 ± 3.8 vs 28.4 ± 3.1 hours, p = 0.081). In the adjusted analysis using mutlivariable linear regression, an early voiding trial decreased the time to spontaneous void (abeta −2.00 h, p = 0.031) when controlling for vaginal packing and stage IV prolapse. In the per-protocol analysis, which excluded 4 patients for crossover, spontaneous void occurred 17 hours faster in the early voiding trial group (14.6 ± 3.7 vs 31.8 ± 2.9 hours; p = 0.022). Early voiding trial patients experienced ambulation sooner and more often than the standard group (p = 0.02).
Conclusions
A day-of-surgery voiding trial did not prolong catheter use after obliterative and apical suspension procedures.
Keywords: Pelvic organ prolapse, Post-operative urinary retention, Voiding trials
Introduction
Approximately 12% of all women will undergo pelvic floor reconstructive surgery for pelvic organ prolapse (POP) in their lifetime [1]. There is an elevated risk of transient post-operative urinary retention ranging from 15 to 45% [2–5]. Unaddressed urinary retention, or incomplete bladder emptying, can lead to bladder overdistension and elevated intravesicular pressures, with subsequent development of myogenic and neurogenic damage, ureteral reflux, and detrusor dysfunction [6–8].
The traditional care model of evaluating voiding function post-operatively is to perform either a retrograde or a spontaneous fill voiding trial on post-operative day 1 [1, 5, 9]. A retrograde voiding trial has a sensitivity of 94.4% and specificity of 58.1% for the detection of urinary retention compared with a spontaneous fill, which has a sensitivity and specificity of 100% and 25.8% respectively [4]. Studies have yet to examine the impact of a day-of-surgery voiding trial on voiding function in women undergoing apical suspension and obliterative procedures. As our institution developed a clinical pathway for same-day discharge in urogynecology patients, we sought to determine the effectiveness of two peri-operative catheter management strategies [10].
Our primary objective was to evaluate if conducting a voiding trial on the day of surgery would result in faster time to spontaneous void, compared with a standard voiding trial performed on post-operative day 1 in women undergoing major pelvic reconstructive surgery. We hypothesized that performing earlier evaluation would result in earlier catheter removal and consequently faster time to spontaneous void.
Materials and methods
Study design
We conducted a randomized, parallel-arm trial in 57 patients undergoing major pelvic floor reconstructive surgery by 6 FPMRS surgeons at a regional tertiary care center between February 2016 and March 2017. Women were randomized to an early voiding trial 4 hours post-operatively on the day of surgery or a standard voiding trial on post-operative day 1 at 6 am, which was common practice at our institution. Women were followed until the primary endpoint, return of spontaneous void, which was defined as the ability to void without the assistance of a catheter. The time to spontaneous void was compared between voiding trial groups for our primary outcome. This study was approved by the Institutional Review Board of the University of Pittsburgh (PRO15100653 approved 1/14/16) and is registered on www.clinicaltrials.gov (NCT02739256).
Study population
Patients were recruited from urogynecology faculty practices by co-investigators. Women were eligible for enrollment if they were scheduled for surgical repair of POP defined as any apical suspension or obliterative procedure with a planned overnight hospitalization. Exclusion criteria included preoperative incomplete bladder emptying defined as post-void residual (PVR) > 100 ml or neurological conditions known to have an impact on voiding function. After obtaining consent, past medical history, pelvic examination data, and PVR were ascertained from the electronic medical record (EMR). Patients completed baseline questionnaires that included a pain visual analog scale (VAS), State-Trait Anxiety Inventory state subscale (STAI-S), and questions related to catheter bother.
Randomization
Randomization was computer generated with 1:1 group allocation to an early or standard voiding trial in fixed blocks of 6. Randomization was concealed by a research assistant not involved in trial enrollment using consecutively numbered opaque envelopes. Patients were withdrawn from the study before randomization if their surgery was cancelled or if intra-operative events occurred that, in the surgeon’s judgment made them ineligible for a voiding trial 4 h after surgery, such as cystotomy or hemodynamic instability. Randomization assignment was performed after conclusion of the surgery in the operating room.
Study interventions
After randomization, all patients entered the post-anesthesia care unit with an indwelling catheter. Participants remained masked to assignment until completion of the first postoperative questionnaire had been administered 4 h after completion of surgery. Allocation concealment was no longer feasible beyond this time-point. Three additional questionnaires were administered 6 h after completion of surgery, 6 am on post-operative day 1, and 8 am on post-operative day 1.
Standardized voiding trials comprised a retrograde, gravity fill of the bladder with 300 mL or the maximum tolerated volume up to 300 mL performed by trained nurses. Once the catheter was removed, the patient immediately attempted to void. We considered patients to have successfully passed the voiding trial when the PVR < 100 mL as measured by the voided volume subtracted from the instilled volume [3]. Patients in either group with vaginal packing placed at the end of the surgery had the packing removed immediately before the voiding trial.
Patients in the early voiding trial group who failed their voiding trial had an indwelling catheter replaced overnight. A second voiding trial attempt was repeated at 6 am on postoperative day 1 to coincide with the timing of the standard voiding trial group. Patients in either group who failed the voiding trial on post-operative day 1 were discharged, performing clean intermittent self-catheterization (CISC) or with an indwelling catheter at the discretion of the surgeon based on patient ability. Voiding trial results were collected on post-operative day 1 by a research assistant or co-investigator.
Patients performing CISC at discharge reached spontaneous voiding when they reported two consecutive PVRs of ≤100 mL or one-third of the total bladder volume. These data were ascertained from participant-completed daily voiding diaries and post-operative nursing calls. Patients who had an indwelling catheter at the time of discharge returned to the office to repeat the voiding trial 2–7 days after surgery at the discretion of the surgeon. The time at which the voiding trial was successfully passed was considered time of spontaneous void. Post-operative outcomes were collected by a research assistant for 6 weeks.
Study outcomes
Our primary aim was to determine if conducting a voiding trial on the day of surgery compared with post-operative day 1 results in faster time to spontaneous void after major prolapse surgery. We hypothesized that a voiding trial conducted on the day of surgery would result in discontinuation of catheter use with overall faster time to spontaneous void. Our primary outcome was time to spontaneous void, as defined above, measured in hours from the end of surgery. In addition, we aimed to determine if the covariates, such as pre-operative PVR, post-operative complications, surgery end after 1 pm, pre-operative prolapse stage, anterior wall prolapse, prolapse procedure performed, concomitant procedures, vaginal packing, estimated blood loss (EBL), and duration of surgery were associated with time to spontaneous voiding. Important secondary outcomes included group comparisons for successful voiding by post-operative day 1, urinary retention management strategy, patient-reported outcomes, length of admission, post-operative complications, emergency department visits, and urinary tract infections. In addition, we assessed factors predictive of spontaneous voiding by the time of hospital discharge.
Post-operative patient-reported outcomes were collected in four questionnaires and included pain, anxiety, and ambulation assessments. Pain scores were measured using the visual analog score (VAS), a continuous scale comprising a horizontal line 10 cm in length, anchored by the verbal descriptors “no pain” and “worst imaginable pain” [11]. Anxiety was measured using a validated measure, the State-Trait Anxiety Inventory state subscale (STAI-S), a 20-item Likert scale assessing the current state of anxiety [12, 13]. Scoring ranges from 20 to 80, with higher scores reflecting situational anxiety and a 10-point change indicating clinical significance [14]. Patient outcomes were assessed by a randomization arm, including the presence of spontaneous void after the first voiding trial attempt and at the time of discharge. Last, we performed a cost-analysis to evaluate differences in cost by early and standard voiding trial groups.
Statistical analysis and sample size
Data are presented as means (± standard deviation) for continuous variables or medians (interquartile range) for data not normally distributed. Categorical data are presented as frequency (percentage). Group means that follow normal distribution were compared using Student’s t tests. Non-normal distributions were compared using Wilcoxon rank-sum tests. Chi-squared analyses or Fisher’s exact tests were used to compare proportional data.
For the primary outcome, time to spontaneous void, we planned to compare means between early and standard groups using Student’s t tests for all women randomized in an intention-to-treat (ITT) analysis. Given that time data can be skewed with a right tail, we planned a priori for a logarithmic transformation if necessary. Upon checking for normality, we found that our time to spontaneous void data was skewed, which in part was driven by the small number of patients who were discharged with retention and variation in time to spontaneous void in those with retention. Therefore, a logarithmic transformation of the primary outcome variable was performed before the primary outcome Student’s t test analysis. We report p values of this analysis and geometric means of the logarithmic scale (antilogs) for ease of interpretation. We did not use a time-to-event survival analysis for the primary outcome because the goal of the study was to determine time with the catheter, which would be more useful in clinical practice than hazard ratios or survival analysis. In addition to the ITT analysis, a per-protocol (PP) analysis was planned for the primary outcome owing to potential crossover between groups. This analysis excluded patients who did not have the voiding trial on the day assigned to assess the true treatment effect. Univariable and multivariable linear regression were used to determine effects on spontaneous voiding aside from the randomization arm. All baseline and surgical factors with p< 0.2 on univariable analysis were candidate variables for multivariable linear regression, which were fit with backward removal and confirmed with forward addition techniques. Results are presented as adjusted beta (abeta) coefficients and p values.
For our secondary outcomes of voiding trial success rates, emergency department visits, urinary tract infection rates, and post-operative complications, Student’s t, Wilcoxonrank sum, Chi-squared, and Fisher’s exact tests were used where indicated. To estimate the odds of spontaneous void by discharge on post-operative day 1, uni- and multi-variable logistic regression were used with similar methods to those mentioned for the primary outcome. Results are presented as adjusted odds ratios (aORs) with 95% confidence intervals (CIs). Lastly, generalized linear mixed models were used to analyze patient-reported outcomes over four time points postoperatively by voiding trial group. The cost analysis was performed using Student’s t tests to compare group means. All statistical analyses were performed using SAS (Version 9.0; SAS Institute, Cary, NC, USA), except for the cost analysis, which was performed using SPSS (Version 22; IBM, Armonk, NY, USA).
An a priori power calculation was estimated to determine an adequate sample size for our primary outcome. Pilot data from a small, unpublished, prospective cohort demonstrated a mean time to spontaneous void of 29.3 h after voiding trial 4 h post-operatively. The mean time to spontaneous void after a post-operative day 1 voiding trial was 41.5 h [9]. Given this, we estimated that the early voiding trial group would have a 12.2 ± 20-h decrease in time to spontaneous void. Therefore, to detect this difference, 43 patients were needed in each group with a two-sided alpha of 0.05 and a power of 0.8. No interim analyses were planned.
When we planned this trial, the timing of the voiding trial was a perceived barrier to early discharge in urogynecology patients. During the study period, the Department of Obstetrics and Gynecology implemented an initiative for same day discharge after all minimally invasive procedures. This had an impact on enrollment into this trial, leading us to terminate the study before achieving our estimated sample size.
Results
We approached 211 patients for study enrollment, of which 57 consented and were included in the ITT analysis (Fig. 1). Mean age and BMI were 65 ±11 years and 27.9 ± 4.4 kg/m2 respectively. Most women were Caucasian (93.0%, n = 53) and post-menopausal (n = 51, 89.5%). Almost half had undergone a previous hysterectomy (n = 27, 47.4%). Preoperative POP-Q prolapse stages included: 10.5% stage II (n = 6), 77.2% stage III (n = 44), and 12.3% stage IV (n = 7). Early and standard voiding trial groups had similar baseline characteristics, including anxiety levels (Table 1).
Fig. 1.
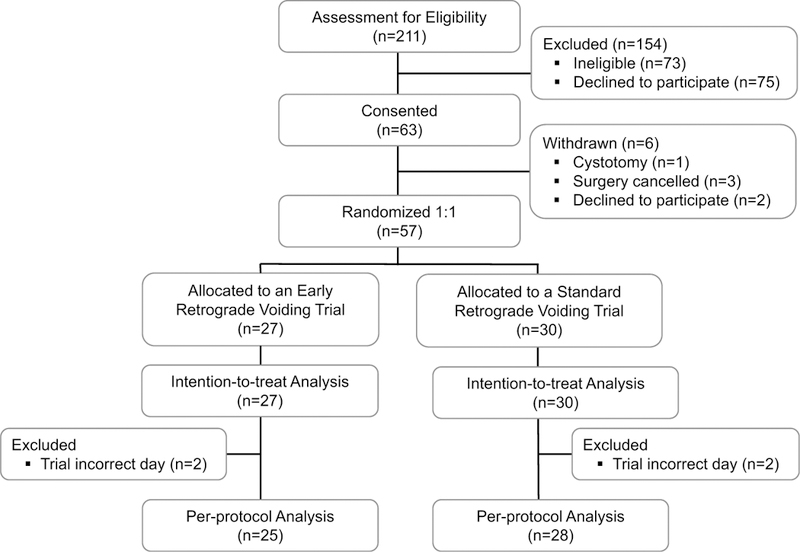
Consolidated Standards Of Reporting Trials (CONSORT) diagram
Table 1.
Baseline characteristics and intra-operative factors (N = 57)
| Early voiding trial (n = 27) |
Standard voiding trial (n = 30) |
p value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 64.9 ± 11.5 | 65.2 ± 10.3 | 0.915 |
| BMI (kg/m2) | 28.1 ±5.1 | 27.8 ± 3.8 | 0.852 |
| Race | |||
| Caucasian | 26 (96.3) | 27 (90) | |
| African-American | 1 (3.7) | 3(10) | 0.614 |
| Current smoker | 2 (7.4) | 2 (6.7) | 1.0 |
| Post-menopausal | 25 (92.6) | 26 (86.7) | 0.672 |
| Medical co-morbiditiesa | 15 (55.6) | 19 (63.3) | 0.550 |
| History of anxiety or depression | 7 (25.9) | 8 (26.7) | 0.949 |
| Previous abdominal or pelvic surgery | 21 (77.8) | 25 (83.3) | 0.596 |
| POP-Q point Ba | 2.4 ±2.5 | 2.7 ±2.6 | 0.683 |
| POP-Q point C | −0.1 ±4.3 | −0.7 ± 4.6 | 0.653 |
| Baseline POP-Q stage | |||
| Stage II | 2 (7.4) | 4(13.3) | |
| Stage III | 21 (77.8) | 23 (76.7) | |
| Stage IV | 4 (14.8) | 3 (5.3) | 0.689 |
| Baseline VAS pain score | 1.9 ±2.9 | 0.97 ± 1.7 | 0.145 |
| Baseline STAI-S score | 38.3 ± 14.7 | 39.6 ± 12.4 | 0.723 |
| Intra-operative factors | |||
| Length of surgery (hours) | 2.9 ±0.8 | 2.7 ± 0.9 | 0.641 |
| Intravenous fluids (mL) | 1,807.7 ±631.8 | 1,843.3 ± 552.4 | 0.823 |
| Estimated blood loss (mL) | 64.4 ± 57.8 | 66.7 ± 54.3 | 0.881 |
| Urine output (mL) | 210 ±234.1 | 159.6 ± 161.1 | 0.359 |
| Total local anesthesia used (mL) | 28.4 ±23.6 | 28.8 ± 32.3 | 0.951 |
| Spinal anesthesia | 9 (33.3) | 6 (20.0) | 0.254 |
| Major prolapse procedures | |||
| Uterosacral ligament suspension | 4 (14.8) | 7 (23.3) | |
| Sacrospinous ligament fixation | 2 (7.4) | 1 (3.3) | |
| Uphold™ LITE vaginal vault suspension | 1 (3.7) | 1 (3.3) | |
| Colpectomy/colpocleisis | 10 (37.0) | 10 (33.3) | |
| Laparoscopic sacrocolpopexy | 9 (33.3) | 10 (33.3) | |
| Robotic-assisted sacrocolpopexy | 1 (3.7) | 1 (3.3) | 0.975 |
| Concomitant hysterectomy | 13 (48.2) | 17 (56.7) | 0.520 |
| Transvaginal | 6 (22.2) | 8 (26.7) | |
| Laparoscopic supracervical | 4 (14.8) | 8 (26.7) | |
| Total laparoscopic | 3(11.1) | 1 (3.3) | |
| Concomitant perineorrhaphy | 8 (29.6) | 6 (20.0) | 0.399 |
| Concomitant mid-urethral sling | 0 | 2 (6.7) | 0.493 |
| Vaginal packing | 7 (25.9) | 8 (26.7) | 0.949 |
| Surgery end after 1pm | 12 (44.4) | 18(60.0) | 0.124 |
| Intra-operative complications b | 1 (3.7) | 1 (3.7) | 1.0 |
Data are n (%), mean ± standard deviation, or median (interquartile range)
BMI body mass index, POP-Q Pelvic Organ Prolapse quantification system, VAS Visual Analog Scale, STAI-S State-Trait Anxiety Inventory, State subscale
Medical co-morbidities, composite of hypertension, diabetes, chronic obstructive pulmonary disease, cerebrovascular accident, cardiac disease
Intra-operative complications include: hemorrhage (estimated blood loss > 300 mL) during rectocele repair and ureteral kinking during uterosacral ligament suspension requiring suture removal intra-operatively
Intra-operatively, most women received general anesthesia (n = 42, 73.7%); those who did not received spinal anesthesia. The mean duration of surgery was 2.8 ± 0.9 h. The most common prolapse procedure performed was sacrocolpopexy (33.3% laparoscopic [n = 19]; 3.5% robotic [n = 2]) followed by colpectomy/colpocleisis (35.1% [n = 20]), native tissue repairs (24.6% [n = 14]), and Uphold™ LITE vaginal vault suspension (3.5% [n = 2]). Those with a uterus in situ underwent concomitant hysterectomy (52.6% [n = 30]). Two women in the standard voiding trial group had a concomitant mid-urethral sling. One-quarter of women had vaginal packing placed at the end of the procedure at the discretion of the surgeon and removed before their voiding trial (26.3% [n = 15]). Intra-operative complications were low (3.5% [n = 2]). Early and standard voiding trial groups had similar intra-operative characteristics (Table 1).
In the ITT analysis, we were unable to detect a difference in time to spontaneous void in the early voiding trial group compared with the standard group (15.9 ± 3.8 vs 28.4 ± 3.1 h, p = 0.081). In the adjusted analysis using multivariable linear regression we found that an early voiding trial decreased the length of time to spontaneous void (abeta −2.00 h, p = 0.031) when controlling for vaginal packing use at the end of the case (abeta 2.82 h, p = 0.004) and baseline POP-Q stage IV (abeta 2.89 h, p= 0.022). In the PP analysis, which excluded 4 crossover patients, the early voiding trial group demonstrated spontaneous voiding 17.2 h sooner than the standard voiding trial group (14.6 ± 3.7 vs 31.8 ± 2.9 h, p = 0.022). In the adjusted per-protocol analysis, when controlling for vaginal packing use at the end of the procedure (abeta 2.40 h, p = 0.019) and baseline POP-Q stage IV (abeta 2.39 h, p = 0.021), patients in the early voiding trial group had faster return of spontaneous void than the standard group (abeta −2.31 h, p = 0.011). Candidate variables with p < 0.2 that were assessed but not included in the final ITT and PP analysis models were sacrocolpopexy as the major prolapse procedure, pre-operative PVR, and total local anesthetic used.
We found that 48% of patients randomized to an early voiding trial were spontaneously voiding 4 h after completing surgery. An additional 7 women (25.0%) in the early voiding trial group passed the voiding trial on post-operative day 1. Therefore, by the time of discharge there were no group differences in the rates of spontaneous voiding (74.1% vs 71%, p = 0.733; Table 2). After adjusting for vaginal packing use at the end of the case (aOR 0.16, 95%CI [0.04–0.72]), baseline POP-Q point Ba (aOR 0.74, 95%CI [0.56–0.98]), and surgery end after 1 pm (aOR 0.25, 95%CI [0.04–0.72]), the odds of spontaneous void by discharge on post-operative day 1 were no different by voiding trial timing (aOR 0.82, 95%CI [0.26–2.61]).
Table 2.
Primary and secondary outcomes (N = 57)
| Outcomes | Early voiding trial (n = 27) |
Standard voiding trial (n = 30) |
p value |
|---|---|---|---|
| Time to spontaneous void (ITT, h) | 15.9 ±3.8 | 28.4 ±3.1 | 0.081 |
| Time to spontaneous void (PP, h)a | 14.6 ±3.7 | 31.8 ±2.9 | 0.022 |
| Spontaneous void after 1st voiding trial attempt | 13 (48.2) | 22 (73.3) | 0.051 |
| Spontaneous void by POD 1b, c | 20 (74.1) | 21 (70.0) | 0.733 |
| Post-operative urinary retention management POD 1 | |||
| Performing CISC | 5/6 (83.3) | 5/8 (62.5) | 1.0 |
| Indwelling catheter | 1/6 (16.7) | 3/8 (37.5) | |
| VAS pain scores | |||
| 4 h post-operatively | 3.0 | 2.5 | |
| 6 h post-operatively | 3.2 | 3.1 | |
| 6 am POD 1 | 3.5 | 3.8 | |
| 8 am POD 1 | 3.8 | 4.4 | 0.090 |
| STAI-S scores | |||
| 4 h post-operatively | 37.1 | 37.7 | |
| 6 h post-operatively | 35.0 | 36.4 | |
| 6 am POD 1 | 32.8 | 35.1 | |
| 8 am POD 1 | 30.7 | 33.8 | 0.410 |
| Times ambulated | |||
| 4 h post-operatively | 1 (0) | 0 (0) | |
| 6 h post-operatively | 1 (1) | 1 (0) | |
| 6 am POD 1 | 3 (2) | 2 (1) | |
| 8 am POD 1 | 4 (2.5) | 2 (1) | 0.020 |
| Duration of admission (h) | 28.8 ± 12.0 | 25.7 ± 6.5 | 0.154 |
| ED visitsd | 2 (7.41) | 5 (16.67) | 0.427 |
| Post-operative complicationsd, e | 0 | 3 (10.0) | 0.093 |
| Post-operative UTId, f | 0 | 3 (10.0) | 0.239 |
Data are n (%), mean ± standard deviation, or median (interquartile range); values in italics indicate statistical significance
ITT intention-to-treat analysis, PP per-protocol analysis excluding 4 crossover patients, POD postoperative day, CISC clean intermittent self-catherization, ED emergency department, UTI urinary tract infections
Excluding 4 crossover patients
Patients randomized to the day-of-surgery voiding trial who failed the first attempt had the catheter replaced overnight and a second voiding trial repeated on post-operative day 1 (POD 1) at 6am, to coincide with the standard group.
One patient who initially passed the voiding trial at 6 am on POD 1, then developed urinary retention before discharge, accounting for the change in rates from first voiding trial attempt to POD 1.
Includes events within 30 days of surgery
Post-operative complications include acute post-operative urinary retention, constipation, and vaginal cuff hematoma.
Defined as a positive culture or symptoms and antibiotic treatment
Other surgical factors, including type of prolapse repair, concomitant hysterectomy, and concomitant mid-urethral sling, were not associated with spontaneous void at the time of discharge (Table 3). Length of admission did not differ by voiding trial group. When compared with those who were discharged using a catheter, those who passed their voiding trial had a 5-h shorter hospital admission (31.2 ± 13.0 vs 26.4 ± 5.8 h, p = 0.010). Of the 14 patients in both groups who required a catheter at the time of discharge on post-operative day 1 (24.6%), most (n = 12) were voiding spontaneously by post-operative day 6. The remaining 2 patients both in the standard group had prolonged urinary retention and required catheterization for 13 and 31 days respectively.
Table 3.
Procedures and outcomes by voiding status on post-operative day 1 (n = 57)
| Outcomes | Spontaneous void (n = 41) |
Urinary retention (n =16) |
p value |
|---|---|---|---|
| Prolapse repair procedures | 0.463 | ||
| Native tissue or transvaginal mesh repair | 10 (24.4) | 6 (37.5) | |
| Colpectomy/colpocleisis | 14(34.1) | 6 (37.5) | |
| Sacrocolpopexy | 17(41.5) | 4 (25.0) | |
| Concomitant hysterectomy | 20 (48.8) | 7 (43.8) | 0.733 |
| Concomitant perineorrhaphy | 9 (22.0) | 5 (31.3) | 0.505 |
| Concomitant mid-urethral sling | 0 | 2(12.5) | 0.075 |
| VAS pain score POD 1 8 am a | 3.1 ±2.3 (n = 32) | 4.1 ±2.6 (n =14) | 0.153 |
| STAI-S score POD 1 8am b | 28.5 ±10.1 (n = 31) | 37.9 ± 13.7 (n = 15) | 0.012 |
| Length of admission (h) | 26.4 ±5.8 | 31.2 ± 13.0 | 0.010 |
| ED visitsc | 5 (12.3) | 2(12.5) | 1.0 |
| Post-operative complicationsc,d | 8 (19.5) | 3 (18.8) | 1.0 |
| Post-operative UTIc,e | 3 (7.3) | 0 | 0.552 |
Data are n (%), mean ± standard deviation, or median (interquartile range); values in italics indicate statistical significance
On post-operative day 1 at 8 am
On post-operative day 1 at 8am
Includes events within 30 days post-operatively
Post-operative complications include: acute post-operative urinary retention, constipation, and vaginal cuff hematoma
Defined as a positive culture or symptoms and antibiotic treatment
For additional secondary outcomes, we found that length of admission, rates of urinary tract infection, post-operative complications, and emergency department visits up until 6 weeks post-operatively were similar by voiding trial groups (Table 2). Rates of urinary tract infection were low and not different between early and standard groups (0% vs0%, p = 0.552). Post-operative situational anxiety, pain scores, and meals eaten during the hospital admission were similar over time by voiding trial group (Table 2). However, we found that patients in the early voiding trial group ambulated sooner and more often than those in the standard group during the hospital admission (p = 0.02, Table 2).
Situational anxiety in both groups was similar, decreasing throughout the admission (Table 2). The highest scores were at baseline, indicating more anxiety, and lowest scores were on post-operative day 1 at 8 am. At the time of discharge on post-operative day 1, we found patients who were spontaneously voiding regardless of randomization group had significantly lower situational anxiety (STAI-S scores) than those who required catheterization (28.5 ± 10.1 vs 37.9 ± 13.7, p = 0.012). At discharge, women who were spontaneously voiding had less anxiety than at their pre-operative baseline (38.0 ± 13.1 vs 28.5 ± 10.1, p = 0.029). Women with retention did not experience a decrease in anxiety levels at discharge; their baseline and discharge scores were similar (41.4 ± 14.1 vs 37.9 ± 13.7, p = 0.482).
We found that baseline anxiety did not differ for patients with or without a history of anxiety or depression (39.3 ± 2.8 vs 38.9 ± 2.3, p = 0.916). However, women with a history of anxiety or depression had higher baseline pre-operative VAS pain scores than women with no history (2.5 ± 2.9 vs 0.95 ±2.0, p = 0.026).
Lastly, there was no difference in total, direct, or indirect costs by voiding trial group (Table 4). For cost categories, the early voiding trial group had higher post-anesthesia care unit costs by $121 (p = 0.033) compared with the standard voiding trial group. Medical/surgical floor costs were reciprocally higher with the standard voiding trial group by $71, but this was not statistically significant.
Table 4.
Costs associated with hospital admission (n = 57)
| Cost $US | Early voiding trial (n = 27) |
Standard voiding trial (n = 30) |
p value |
|---|---|---|---|
| Total costs | $5,732.58 (± 1,437.40) | $5,659.68 (± 2,082.24) | 0.877 |
| Indirect costs (unit operating) | $579.50 (±356.51) | $460.56 (±474.41) | 0.294 |
| Indirect costs (unit supporting) | $2,419.60 (±728.72) | $2,550.13 (±861.25) | 0.542 |
| Direct costs | $2,733.48 (± 1,067.54) | $2,648.99 (± 1,234.29) | 0.784 |
| Total costs by category | |||
| Operating room | $4,193.54 (± 1,434.72) | $4,169.98 (± 1,882.57) | 0.959 |
| Anesthesia | $125.74 (± 108.06) | $107.42 (±29.91) | 0.377 |
| Post-anesthesia care unit | $381.68 (±249.32) | $260.22 (±161.99) | 0.033 |
| Medical/surgical floor care | $647.23 (± 153.42) | $718.10 (±244.30) | 0.231 |
| Pharmacy | $321.63 (±299.09) | $249.42 (± 176.88) | 0.287 |
Data are mean ± standard deviation; value in italics indicates statistical significance
Discussion
This parallel-arm, randomized effectiveness trial evaluated early catheter removal after major pelvic floor reconstructive surgery. Our goal was to determine if an early voiding trial would result in a shorter duration of catheter use. In the ITT analysis, we did not detect a difference in time to spontaneous void by group, which is likely because the trial was underpowered owing to early study termination. However, in the multivariable regression using the ITT data, we found that the day-of-surgery voiding trial was associated with decreased time to spontaneous voiding when controlling for potential confounding factors. This decrease was also seen in the PP-unadjusted and -adjusted analyses, which excluded crossover patients. The PP analyses represent the true effect of the intervention, which is important for assessing the two catheter management strategies. We can conclude from our findings that a day-of-surgery voiding trial is feasible and does not prolong catheter use, which has yet to be demonstrated in the literature for women undergoing major POP surgery. Our research has led to a universal buy-in for same-day discharge within our urogynecology division, shifting an outdated paradigm of postoperative day 1 catheter management after major pelvic floor reconstructive surgery. This is particularly important as many institutions move toward same-day discharge for minimally invasive and vaginal procedures.
Another important finding was that half of women who failed the voiding trial on the day of surgery went on to pass the voiding trial the next morning, resulting in similar rates of spontaneous voiding at discharge in both groups. In addition, by the day of discharge, women in the early group had ambulated twice as often as those in the standard group. These findings, in conjunction with shorter duration of catheter use, provide data on early voiding trial outcomes that may be important to the patient, yet are lacking in the literature.
Post-anesthesia care unit (PACU) costs were higher in the early voiding trial group by $121. Women in the early group commonly had the voiding trial performed in the PACU, as opposed to the standard voiding trial group, who had the voiding trial on the floor post-operative day 1. The time needed to perform the test likely represents the difference in PACU costs. Reciprocally, the medical/surgical floor costs were higher in the standard group.
We found that a few hours of bladder rest after a failed early voiding trial resulted in passing a subsequent voiding trial the next morning. It is hypothesized that an attenuated response to normal bladder filling prevents initiation of the micturition reflex. Short-term abnormalities in bladder sensation are influenced by post-surgical factors such as pain, inflammation, edema, and anesthesia [6, 15]. Modifying these factors through adequate pain control, anti-inflammatory medications, and decompression in the immediate post-operative period may allow normal sensation to return overnight and lead to spontaneous voiding. It is possible that better pain and nausea control contributed to the early return of bladder sensation and spontaneous void in our study patients who had an early voiding trial. Pre- and post-operative anti-emetics and analgesics use was not recorded as part of this study.
When we dichotomized patients by voiding status at the time of discharge we found that women with urinary retention had a significant 5-h increase in the length of admission. It is intuitive that women who require education for catheterization techniques may need more time in the hospital. We found that women with more advanced anterior wall prolapse, later surgery end, and vaginal packing use were more likely to have urinary retention at the time of discharge. If we can identity women with these high-risk characteristics, there may be an opportunity for education earlier than the morning of postoperative day 1. Other studies have demonstrated conflicting results on predictors of post-operative urinary retention with severity of anterior wall prolapse, anesthesia type, higher EBL, concomitant midurethral sling, and levator myorrhaphy being both associated and not associated with retention [3–5, 8]. To our knowledge, our study is one of the first to examine the impact of a day-of-surgery voiding trial on women undergoing major apical suspension and obliterative prolapse procedures.
There are several considerations that limit the interpretation of our findings. The first was our inability to meet the estimated sample size, increasing the risk of bias due to a type II error. However, in our adjusted ITT analysis and PP analyses we did find a decrease in time to spontaneous void. In addition, our findings may be pertinent for hospitals that have not yet adopted same-day discharge. The perceived roadblock of voiding trial timing still exists in other locations, especially after pelvic floor reconstructive surgery. Our research question is timely, as many surgeons and hospital systems are moving toward expedited recovery to decrease costs and complications associated with hospitalization, such as venous thromboembolic events and infection [16–19]. Notably, we did not find a decrease in length of admission in the early voiding trial group. This reflects their pre-planned inpatient status regardless of their voiding status. After completion of this study, the parameters for discharge home have changed and patients are being discharged on the day of surgery. Our study population was representative of a diverse sample of prolapse procedures to increase generalizability. The procedure breakdown was also reflective of our surgical practice patterns. However, we were not powered to detect differences between procedure types and thus our findings should be interpreted broadly. We did attempt to control for prolapse procedure type in the regression analyses, as all prolapse procedures were candidate variables. After backward removal techniques, prolapse procedures did not remain in the final models. Lastly, our findings are limited to a single institution and a mostly Caucasian, post-menopausal patient population.
Strengths of the present trial include the study design and strict definition of spontaneous voiding. We excluded women with pre-operative retention and randomized women undergoing a variety of procedures for pelvic organ prolapse. We used stringent criteria to define post-operative retention. The best PVR cut-off remains unknown, as there is little evidence supporting any one value. A recent American College of Obstetrics and Gynecology committee opinion, published after our trial ended, recommended PVR < 150 mL on three consecutive self-catheterization attempts or after a voiding trial with an indwelling catheter to define resolution of retention after mesh-augmented procedures [20]. We chose a conservative threshold, as that was standard practice at our institution, and to reduce unplanned visits for acute retention after discharge. In addition, we collected patient-centered outcome measures including pain, anxiety, and ambulation. We used an active comparator for our control group, which reflected a historical and national standard of care.
Previous studies have yet to demonstrate the effectiveness of an early or day-of-surgery voiding trial in women undergoing major pelvic reconstructive surgery. Although we were underpowered for our primary aim, we found in the multivariable regression ITT analysis that an early voiding trial did decrease time with a catheter and that regardless of voiding trial timing, the probability of spontaneous voiding by the morning of post-operative day 1 was over 70%. This trial shifted an outdated catheter management paradigm and consequently removed a considerable barrier to same-day discharge in a urogynecology population. Ultimately, we can conclude that early catheter removal is a feasible, safe, and acceptable alternative to the standard timing on post-operative day 1 in women undergoing major pelvic reconstructive surgeries.
Acknowledgements
Kristine Ruppert of the University of Pittsburgh for statistical support. Her work was supported by the National Institutes of Health through grant number UL1TR001857.
Financial support This project was supported by the Clinical Research Trainee Award from Magee-Womens Research Institute and the National Institutes of Health through grant number UL1-TR-000005.
Footnotes
Compliance with ethical standards
Conflicts of interest None.
References
- 1.Geller EJ, Siddiqui NY, Wu JM, Visco AG. Short-term outcomes of robotic sacrocolpopexy compared with abdominal sacrocolpopexy. Obstet Gynecol. 2008;112(6):1201–6. [DOI] [PubMed] [Google Scholar]
- 2.Book NM, Novi B, Novi JM, Pulvino JQ. Postoperative voiding dysfunction following posterior colporrhaphy. Female Pelvic Med Reconstr Surg. 2012;18(1):32–4. [DOI] [PubMed] [Google Scholar]
- 3.Foster RT, Borawski KM, South MM, Weidner AC, Webster GD, Amundsen CL. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197(6):627.e1–4. [DOI] [PubMed] [Google Scholar]
- 4.Geller EJ, Hankins KJ, Parnell BA, Robinson BL, Dunivan GC. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118(3):637–42. [DOI] [PubMed] [Google Scholar]
- 5.Hakvoort RA, Dijkgraaf MG, Burger MP, Emanuel MH, Roovers JP. Predicting short-term urinary retention after vaginal prolapse surgery. Neurourol Urodyn. 2009;28(3):225–8. [DOI] [PubMed] [Google Scholar]
- 6.Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health. 2014;6:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bross S, Schumacher S, Scheepe JR, Zendler S, Braun PM, Alken P, et al. Effects of acute urinary bladder overdistension on bladder response during sacral neurostimulation. Eur Urol. 1999;36(4): 354–9. [DOI] [PubMed] [Google Scholar]
- 8.Madersbacher H, Cardozo L, Chapple C, Abrams P, Toozs-Hobson P, Young JS, et al. What are the causes and consequences of bladder overdistension? ICI-RS 2011. Neurourol Urodyn. 2012;31(3):317–21. [DOI] [PubMed] [Google Scholar]
- 9.Turner LC, Kantartzis K, Shepherd JP. Predictors of postoperative acute urinary retention in women undergoing minimally invasive sacral colpopexy. Female Pelvic Med Reconstr Surg. 2015;21(1): 39–42. [DOI] [PubMed] [Google Scholar]
- 10.Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and out-patient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000–2014. JAMA Surg. 2016;151(9):876–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–19. [DOI] [PubMed] [Google Scholar]
- 12.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 13.Spielberger CD, Gorsuch RL, Lushene RE, Vagg P, Jacobs G. State-Trait Anxiety Inventory. Palo Alto: Mind Garden; 1983. [Google Scholar]
- 14.Spielberger CD, Gorsuch RL. Assessment of state and trait anxiety. New York: Hemisphere; 1990. p. 69–82. [Google Scholar]
- 15.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary 18. retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–57. [DOI] [PubMed] [Google Scholar]
- 16.Barber EL, Neubauer NL, Gossett DR. Risk of venous thromboembolism in abdominal versus minimally invasive hysterectomy for benign conditions. Am J Obstet Gynecol. 2015;212(5):609.e1–7. [DOI] [PubMed] [Google Scholar]
- 17.Graves N, Janda M, Merollini K, Gebski V, Obermair A; LACE trial committee. The cost-effectiveness of total laparoscopic hysterectomy compared to total abdominal hysterectomy for the treatment of early stage endometrial cancer. BMJ Open 2013;3(4):e001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahdi H, Goodrich S, Lockhart D, DeBernardo R, Moslemi-Kebria M. Predictors of surgical site infection in women undergoing hysterectomy for benign gynecologic disease: a multicenter analysis using the national surgical quality improvement program data. J Minim Invasive Gynecol. 2014;21(5):901–9. [DOI] [PubMed] [Google Scholar]
- 19.Mueller MG, Pilecki MA, Catanzarite T, Jain U, Kim JY, Kenton K. Venous thromboembolism in reconstructive pelvic surgery. Am J Obstet Gynecol. 2014;211(5):552.e1–6. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785–94. [DOI] [PubMed] [Google Scholar]


