Abstract
Acute kidney injury (AKI) is a major medical problem that is of particular concern after cardiac surgery. Perioperative AKI is independently associated with an increase in short-term morbidity, costs of treatment, and long-term mortality. In this review, we explore the definition of cardiac surgery-associated acute kidney injury (CSA-AKI) and identify diverse mechanisms and risk factors contributing to the renal insult. Current theories of the pathophysiology of CSA-AKI and description of its clinical course will be addressed in this review. Data on the most promising renal protective strategies in cardiac surgery, from well-designed studies, will be scrutinized. Furthermore, diagnostic tools such as novel biomarkers of AKI and their potential utility will be discussed.
Keywords: cardiac surgery, acute renal failure, acute kidney injury, prevention, therapy
Introduction
Acute kidney injury (AKI) after cardiac surgery occurs from a rapid deterioration in renal function following cardiac surgery expressed as a significant decrease in glomerular filtration rate (GFR). The reported prevalence of cardiac surgery-associated acute kidney injury (CSA-AKI) is up to 30%1 and is independently associated with an increase of morbidity and mortality. When CSA-AKI is defined in its most severe form as requiring dialysis, the prevalence is usually low, occurring up to 4%.2–4 With milder degrees of renal dysfunction, the incidence shows a wide variation in the reported literature. Even a small increase (0.3–0.5 mg/dL) in serum creatinine (sCr) after cardiac surgery may be independently associated with a significant increase in 30-day mortality.5 While mortality after open-heart surgery with no AKI, ranges between 1% and 8%, the odds of death increases more than fourfold.6 The need of renal replacement therapy (RRT) has been associated with an increase in mortality, up to 63%.7 CSA-AKI is the most common cause of AKI in intensive care unit (ICU) after sepsis.8 Regardless of whether there is a complete renal recovery or not, the 10-year mortality associated with AKI after cardiac surgery is still increased.9 In this narrative review article, pathogenesis, definition, risk prediction, early detection by biomarkers, promising prevention and treatment strategies for AKI after cardiac surgery will be discussed.
Defining acute kidney injury
More than 30 different definitions for ARF have been used in the past. The new diagnostic scales use variations in sCr values and urinary output to define the presence and severity of ARF and have been validated by numerous studies. In 2004, the risk-injury-failure-loss-end-stage kidney disease (RIFLE) definition by the Acute Dialysis Quality Initiative Group was introduced.10 The dysfunction criteria were based on a relative rise in sCr, the absolute level of urine output, or both. In 2007, the Acute Kidney Injury Network (AKIN) proposed a modification of the RIFLE classification.11,12 It occurred when the impact of small elevations of sCr on mortality (>0.3 mg/dL [>26 mmol/L]) was reported. This scale defines AKI as an abrupt reduction (within 48 hrs) of renal function with an absolute increase in sCr (≥0.3 mg/dL [≥26.4 mmol/L] or ≥50% [1.5 times compared to baseline]), or a reduction in urine output <0.5 mL/kg/hr for >6 consecutive hours. The application of AKIN and RIFLE criteria following cardiac surgery without correcting for sCr changes owing to fluid balance leads to AKI under-diagnosis.12 The Kidney Disease: Improving Global Outcomes (KDIGO) definition of AKI13 was associated with a higher sensitivity to diagnose AKI and to predict in-hospital mortality, compared to RIFLE or AKIN.14 The KDIGO definition, which is a combination of the RIFLE and AKIN classification, is the most commonly used definition for CSA-AKI. However, the main limitation of these definitions is that they rely on sCr, which is known to be affected by factors not-GFR related, including age, sex, race, body surface area, diet, diabetes, liver disease, different drugs and laboratory analytical methods.15 Furthermore, using sCr it is unknown whether the origin of the injury is tubular or glomerular. The three main definitions of AKI are slightly different. Therefore, using different definitions may lead to differences on the prevalence and prognosis of AKI after cardiac surgery.14 Criteria for the diagnosis of AKI are shown in Table 1.
Table 1.
Three criteria for the diagnosis of acute kidney injury
| Grade I | Grade II | Grade III | |
|---|---|---|---|
| RIFLE score | Increase creatinine x1.5 or GFR decreases >25% from baseline in 7 days or UO <0.5 mL/kg/hr for 6–12 hrs | Increase creatinine x2–2.9 or GFR decreases >50% from baseline in 7 days or UO <0.5 mL/kg/hr for >12 hrs | Increase creatinine >x3 or GFR decreases >75% from baseline in 7 days or creat >4 (with an acute rise of >0.5 mg/dL) or UO <0.3 mL/kg/hr for 24 hrs or anuria for >12 hrs |
| AKIN score | Increase creatinine x1.5 or by ≥0.3 mg/dL (≥26.5 μmol/L) from baseline in 48 hrs or UO <0.5 mL/kg/hr for 6–12 hrs | Increase creatinine x2-2.9 in 7 days from baseline or UO <0.5 mL/kg/hr for >12 hrs | Increase creatinine >x3 from baseline in 7 days or creatinine >4 (with an acute rise of >0.5 mg/dL) or UO <0.3 mL/kg/hr for 24 hrs or anuria for >12 hrs or initiation of RRT |
| KDIGO score | Increase creatinine by ≥0.3 mg/dL (≥26.5 μmol/L) in 48 hrs, or increase creatinine x1.5–1.9 from baseline within 7 days or UO <0.5 mL/kg/hr for 6–12 hrs | Increase creatinine x2-2.9 in 7 days from baseline or UO <0.5 mL/kg/hr for >12 hrs | Increase creatinine >x3 from baseline in 7 days or creatinie >4 (with no need for an acute rise of >0.5 mg/dL) or eGFR <35 mL/min if age <18 years old or UO <0.3 mL/kg/hr for 24 hrs or anuria for >12 hrs or initiation of RRT |
Abbreviations: RIFLE, risk-injury-failure-loss-end-stage kidney disease; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease: Improving Global Outcomes; GFR, glomerular filtration rate; RRT, renal replacement therapy; UO, urine output.
Pathogenesis of acute kidney injury after cardiac surgery
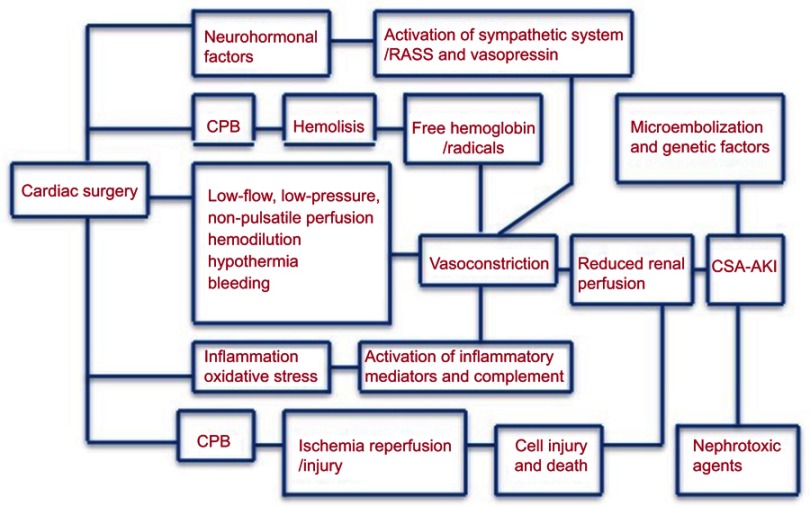
The pathophysiology of AKI after cardiac surgery is complex and multifactorial.16 The following mechanisms of injury might be involved: microembolization, neurohormonal activation, exogenous and endogenous toxins, metabolic as well as hemodynamic and inflammation factors, ischemia–reperfusion injury and oxidative stress. These mechanisms of injury may be interrelated and synergistic. The consequence of these insults is a cascade of reflex changes within the kidney leading to a common presentation of AKI manifesting as impairment of renal function, persistent renal vasoconstriction, an exaggerated response to exogenous vasoconstrictors, and vascular endothelial and tubular epithelial cell death due to necrosis and apoptosis.
Common nephrotoxic agents are antibiotics, such as glycopeptides and aminoglycosides, and non-steroidal anti-inflammatory agents. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) may cause renal efferent arteriolar vasodilation and its use is associated with AKI.17
Regional blood flow and renal vasomotor tone are altered by the use of cardio-pulmonary bypass (CPB). Furthermore, systemic inflammatory response is activated, and microemboli may be generated, associated with the use of CPB. The systemic inflammatory response is activated mainly due to the direct contact of blood with the artificial surface of the bypass circuit.
The use of CPB has been associated with an alteration of vasomotor tone and a reduction in the renal parenchymal oxygen tension, and consequently, decreasing the renal perfusion pressure up to 30% and, hence, increasing the ischemia–reperfusion injury.18
The formation of microemboli may be increased by the use of CPB. It is well known that emboli smaller than 40 μm are not effectively filtered by CPB-system filters and can damage renal capillaries directly.18
The release of free hemoglobin secondary to hemolysis is associated with renal tubular damage as well as increased systemic vascular resistance, platelets and coagulation activity dysfunction and increased mortality.19 A summary of the different and complex pathophysiologic mechanisms is shown in Figure 1.
Figure 1.
Summary of the different and complex pathophysiologic mechanisms.
Risk factors and prediction of acute kidney injury after cardiac surgery
A plethora of risk factors have been identified over the last 45 years since the first report of ARF after cardiac surgery. AKI following cardiac surgery is the net result of several perioperative insults, some or all of which may occur in any given patient. Based on the current literature, the following risk factors are independently associated with AKI after cardiac surgery: i) elderly and female sex, ii) preoperative cardiac dysfunction (preoperative use of an intra-aortic balloon pump, New York Heart Association IV, cardiogenic shock, reduced left ventricular ejection fraction (LVEF), congestive heart failure), iii) emergent surgery, iv) peripheral arterial disease, v) reintervention, vi) insulin-requiring diabetes, vii) preoperative renal dysfunction [estimated glomerular filtration rate (eGFR) <60 mL/min, creatinine >2.1 mg/dL], viii) COPD, ix) intraoperative use of aprotinin.2–4,16,20 The risk factor preoperative renal dysfunction is the most predictive for AKI after cardiac surgery. Other risk factors described are related to the type of cardiac surgical procedure, regardless of bypass time. Patients undergoing valvular surgery, with or without coronary artery bypass graft (CABG) are at a greater risk for the development of AKI compared with those undergoing CABG alone. Furthermore, cross-clamp time and duration of CPB are important risk factor for CSA-AKI.4,20,21 Therefore, any adverse event during surgery causing longer cross-clamp time or bleeding will lead to an increased rate of AKI. Other important risk factors include transfusion of red blood cells (RBC) and haemodilution during CPB. Furthermore, preoperative anemia, defined as hemoglobin <12.5 mg/dL, is associated with an increased risk of AKI after cardiac surgery, regardless of intraoperative RBC transfusion.22
Early identification of patients at high risk of AKI after cardiac surgery using risk scores enables clinicians to initiate early preventive and therapeutic strategies to reduce the risk of AKI. Several risk scores to predict AKI have been developed, but no guidelines exist to recommend the use of a specific prediction model. The Cleveland Clinic score,4 the Mehta score,3 and the Simplified Renal Index (SRI) score2 have been used for predicting the need for dialysis initiation (c-statistics 0.75–0.79). However, their performance for predicting non-RRT AKI is generally poor (c-statistics 0.54–0.59).23 Among the prediction rules for AKI requiring dialysis after cardiac surgery, the Cleveland score has been the most widely tested thus far and has shown high discrimination in most of the tested populations.2,24,25 Mehta Score is not as easy to use than the SRI score and Cleveland score. Therefore, given its simplicity, the SRI score and the Cleveland score are the most commonly used in clinical practice. Moreover, the Cleveland score showed good performance to predict a composite end-point of severe AKI (defined as SCr rise >2.0 mg/dL or a twofold increase from preoperative values) in a cohort from the Mayo Clinic.25 New scores for predicting AKI after cardiac surgery, including intraoperative factors, have been recently published. The use of these new scores improves performance when compared with the previous scores.26,27
The main disadvantage of the modified Cleveland score is the large amount of variables included and the difficulty in calculating the score.
A summary assessing the predictive ability of different risk scores is shown in Table 2.
Table 2.
Risk prediction models for AKI after Cardiac surgery
| Variable | Cleveland score4 | Metha score3 | SRI2 | Modif Cleveland26 |
|---|---|---|---|---|
| Definition - Score | Definition - Score | Definition - Score | Definition - Score | |
| Age | Varies - varies | |||
| Race | Non-White - 2 | White Black Another |
||
| BMI | Varies - varies | |||
| Gender | Female - 1 | |||
| Preoperative Renal function | sCr 1.2-2.1 - 1 | sCr varies | eGFR 31–60 - 1 | eGFR - varies |
| sCr >2.1 - 5 | eGFR <31 - 2 | |||
| Preoperative Albumin | Varies - varies | |||
| Preoperative Sodium | Varies - varies | |||
| Preoperative Bicarbonate | Varies - varies | |||
| Preoperative sUrea Nitrogen | Varies - varies | |||
| Preoperative Hemoglobin | Varies - varies | |||
| Preoperative platelet | Varies - varies | |||
| Preoperative Bilirubin | Varies - varies | |||
| CHF | Yes - 1 | Varies - varies | ||
| HTN | Varies - varies | |||
| Diabetes | Requiring medication - 1 | Varies - varies | ||
| COPD | Yes - 1 | Yes - 3 | ||
| Pulmonary Disease | Varies - varies | |||
| Recent MI (<21 days) | Yes - 3 | |||
| LVEF | <35% - 1 | <40% - 1 | ||
| Previous surgery | Yes - 1 | Yes - 3 | Yes - 1 | |
| Previous IABP | Yes - 2 | Yes - 1 | ||
| Cardiogenic Shock | Yes - 7 | |||
| Timing of surgery | Emergence - 2 | Non-elective - 1 | Emergence - varies | |
| CPB time | <80 - varies | |||
| 81–120 - varies | ||||
| 121–150 - varies | ||||
| 151–180 - varies | ||||
| >180 - varies | ||||
| Intraop PRBC | None - varies | |||
| 1–2 - varies | ||||
| 3–4 - varies | ||||
| 5–6 - varies | ||||
| >6 - varies | ||||
| Intraop vasopressors | Varies - varies | |||
| Intraop UO | Varies - varies | |||
| Type of surgery | CABG only - 0 Valve only - 1 CABG + Valve - 2 |
CABG only - 0 Aortic Valve only - 2 AV + CABG - 5 Mitral Valve only - 4 MV + CABG - 7 |
Other than isolated CABG or ASD repair -1 | |
| Score range | 0–17 | 0–83 | 0–8 | 0–100 |
Note: In Thakar score, sCr <1.2 mg/dL and isolated CABG are considered as reference (0 points). In SRI score, an eGFR >60 mL/min and isolated CABG and correction of atrial septal defects are taken as reference (0 points).
Abbreviations: AKI, acute kidney injury; ASD, Atrial septal defect; BMI, body mass index; CABG, coronary artery bypass graft; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CPB, Cardio-pulmonary Bypass; eGFR, estimated GFR; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; sCr, serum creatinine; sUrea, serum urea; recent MI, recent myocardial infarction; UO, urine output.
Prognosis of acute kidney injury after cardiac surgery
The long-term consequences of AKI after cardiac surgery might be development of chronic kidney disease, increased mortality, reduced quality of life and increased risk of cardiovascular events.
Mortality after severe AKI requiring RRT was 52.6% at 60 days and 44.7% at 90 days in the RENAL study.28
Data from a retrospective study showed a 30-day mortality rate of 58.6% for patients with AKI requiring RRT after cardiac surgery.29
In the ATN study, which included patients undergoing cardiac surgery, complete recovery of kidney function (defined as sCr level ≤0.5 mg/dL (44 μmol/L) above the baseline value) by 28 days after randomization was seen in 16.9% of patients. Partial recovery (defined as a sCr level that remained at >0.5 mg/dL above baseline in patients who were not dependent on dialysis) was seen in 8.9% of patients. In the RENAL study, which also included patients undergoing cardiac surgery, 13.3% of patients were dependent on RRT at 28 days and 5.6% at 90 days after randomization. However, no specific data on renal recovery for patients undergoing cardiac surgery were provided in the RENAL trial.
Data from a meta-analysis on 35,021 patients in nine observational studies show a higher long-term mortality risk in those patients who have a persistent renal dysfunction compared to those who have a recovery of renal function to baseline levels before hospital discharge (HR 2.71, 95% CI 1.26–5.82).30
An important factor determining the prognosis of AKI after cardiac surgery is the duration of AKI. Data from an observational study on 4,987 patients showed that a duration of 7 days or longer were associated with both increased in-hospital mortality (15.3% vs 4.1%) and 5-year mortality (HR 3.40 vs 1.66) compared to AKI lasting 1–2 days.31 Data from a retrospective study on 10,275 consecutive patients showed that early renal recovery seems to offer a distinct survival benefit. The most significant predictor of 1-year survival is the percent decrease of sCr within 24 hr of its peak.32
Renal protective strategies
Currently, there is no pharmacological intervention that has consistently been associated with renal protection. This failure is likely related to the following reasons: i) AKI after cardiac surgery is pathophysiologically multifactorial and complex, thus simple strategies targeting single pathways will most likely fail, ii) strategies guided by creatinine rise will be initiated too late and consequently, they will also, most likely, fail, iii) most data come from clinical trials not enrolling a large sample size on low-risk patient population with lower prevalence of AKI. Therefore, most of them are inadequately powered to detect small benefits.
Preoperative strategies
Delaying elective surgery for renal function optimization in patients with reversible AKI should be considered. Preoperative decisions regarding chronic medication may influence perioperative renal risk. Renal function optimization by avoiding nephrotoxics (such as non-steroidal anti-inflammatory agents, aminoglycoside antibiotics, radiocontrast agents, ACE inhibitors and ARBs), avoiding intravascular volume depletion, by optimizing cardiac output and continuing congestive heart failure treatment, should be considered.
Current data show that preoperative aspirin therapy is associated with a significant decrease in 30-day mortality rate and postoperative AKI after cardiac surgery.33 Subgroup of patients with eGFR <60 mL/min may even have further benefit from using preoperative aspirin.34
Data from 2 studies show benefit from using exogenous albumin to correct hypoalbuminemia (level of <4 g/dL) in off-pump CABG surgery patients.35,36
Recent trials show no benefit from starting statins preoperatively.37,38
However, continuation of statins until the day of surgery should be considered.39
It is reasonable to use preoperative erythropoietin (EPO) plus iron (especially if ferritin is <100 mg/L), given several days before surgery, in patients with preoperative anemia (Hb <12.5) and patients who refuse transfusion, such as Jehovah’s Witness. However, chronic use of EPO is associated with thrombotic cardiovascular events in chronic kidney disease patients suggesting caution with the use of EPO in patients with unstable symptoms undergoing CABG surgery.
Intraoperative strategies
Preoperative as well as intraoperative anemia have been shown to be independently associated with AKI after cardiac surgery. However, it is also well known that intraoperative RBC transfusion is associated with a higher rate of AKI after cardiac surgery.6,20
Data from Toronto General Hospital database suggest that the change of hemoglobin from its baseline is more important than its absolute value.40 A decrease of hemoglobin of >50% from baseline, significantly increase the risk of AKI. Moreover, recent data from a Spanish multicenter cohort suggest that not only the change from baseline is important, but also the duration of anemia. A perioperative hemoglobin area under the curve (pHb-AUC) of <19 g/dL showed a higher association with AKI after cardiac surgery, compared to a single preoperative or intraoperative value of hemoglobin.41
2011 STS/SCA blood conservation guidelines suggest that transfusion to keep hemoglobin >7 g/dL while on CPB, on patients at risk for end-organ ischemia or injury, may be considered (IIb).42 TRICS-III trial showed no difference in primary outcome (a composite outcome of any cause-mortality, stroke, new-onset AKI requiring dialysis or myocardial infarction) comparing hemoglobin 7.5 vs 9.5 g/dL as a trigger for transfusion of RBC intraoperatively and postoperatively in the ICU.43
The initiation of a program on blood management decreases the rate of RBC, platelets and FFP transfusion as well as the rate of AKI (34% relative risk reduction; P=0.039) and the associated healthcare cost. This program consists of transfusion thromboelastography-guided, preoperative anemia optimization, restrictive threshold for intraoperative transfusion, retrograde autologous priming, reduced volume of the bypass circuit and increase use of cell saver and tranexamic acid.44
Despite new technologies, CPB is still contributing to the development of AKI after cardiac surgery. Modifying CPB strategies aimed at decreasing the renal effects have been investigated. Based on current data, use of mini-CBP may be considered,45,46 and zero-balanced ultrafiltration during CPB, especially for patients with eGFR <60, may be considered.47 Flow during CPB, rather than pressure, may be the important determinant of renal function. One of the main goals, if not the greatest, when conducting CPB is the delivery of oxygen to the tissues.
A goal-directed perfusion initiative aiming for oxygen delivery (DO2) >300 mL with mean arterial pressure >70 mmHg during CPB plus zero-balanced ultrafiltration, was associated with a significant reduction of AKI at 72 hrs (23.9% vs 9.1%).48 Hence, based on current data, MAP >70 mmHg and DO2 >300 mL O2/min/m during CPB should be considered.
Hyperglycemia (>180 mg/dL) should be avoided during the perioperative period, with caution to avoid hypoglycemia also. The most important factor is to avoid large glucose variability throughout the entire perioperative time frame.49,50
Regarding the use of balanced hydroxyethyl starch (HES) 130/0.4 in cardiac surgery, data from two recent clinical trials in the context of critically ill patient population show that the use of HES increased the risk for the need of dialysis initiation.51,52 Furthermore, the 6S trial which focused on patients with severe sepsis found an 8% higher 90-day mortality associated with the use of HES.52 However, routine cardiac surgery patients were not included in these trials. Septic patient population differs from routine cardiac surgery patients. Vascular endothelium is differently affected,53 and therefore, large molecules are distributed differently.54
Data on the use of balanced HES 130/0.4 in cardiac surgery patients are limited. Data from a prospective multicenter cohort on 1,058 patients showed that the use of modern starch 6% HES 130/0.4 was not associated with a higher rate of AKI and dialysis after cardiac surgery.55 However, only 10% of this cohort presented with chronic kidney disease. Therefore, in author’s opinion, modern starch 6% HES 130/0.4 may be used in patients with normal renal function undergoing cardiac surgery.
Surgical strategies
The CORONARY trial found that there was no difference between off-pump and on-pump CABG, in terms of RRT requirement [HR, 1.04 (0.61–1.76), P=0.59]. However, the use of off-pump CABG was associated with a lower rate of mild AKI, defined by AKIN stage 1 [HR 0.87 (0.80–0.96), P=0.01] or RIFLE-risk [HR 0.87 (0.76–0.98), P=0.02]. In the CORONARY trial, the lack of definition for initiation of RRT leads to possible different managements in the same clinical scenario. No differences were found at 1 year and at 5 years in the CORONARY trial follow-up. Unfortunately, mild AKI was not analyzed.56,57
However, data from 5-year follow-up of the Veterans Affairs trial of on-pump versus off-pump CABG showed that off-pump CABG led to lower rates of 5-year survival and event-free survival than on-pump CABG.58 Consequently, the greater operative safety and possible prevention of AKI with OPCAB may come at the expense of long-term survival gains.
Catheter-based minimally invasive surgery, on the other hand, has been consistently associated with improved renal outcome, particularly in mitral valve surgery.59,60 Endovascular repair of aortic pathology may be beneficial with regard to postoperative renal function in comparison with open procedures.61
Pharmacological renal protection
Many drugs aiming for preventing the AKI after cardiac surgery have been studied. Up to date, none of them have conclusively showed to decrease the prevalence of AKI after cardiac surgery. However, some of them appeared promising: amino acids, dexmedetomidine and vasopressin.
Acute increases in overall amino acids blood concentration can be induced by a protein meal as well as intravenously infused amino acids.62 Amino acids affect the kidney through several mechanisms. First, their infusion in laboratory rats increases nephron plasma flow by decreasing afferent arteriole resistance.63 This effect appears mediated by reductions in signaling molecules of the tubule-glomerular feedback system, mitigating arteriolar constriction,64 or by increases in neuronal nitric oxide synthase in the kidney cortex, reducing afferent arteriolar tone.65 Second, in other animal models, high-protein diets, through vascular endothelial growth factor, lead to an increase in size and volume of glomeruli.66 Third, infused amino acids are filtered by the glomerulus and act directly on the kidney, stimulating proximal tubular sodium reabsorption, and may change the sensitivity of the macula densa sensing mechanism by altering cell permeability. Fourth, reduced tubular sodium chloride concentration induces release of endothelial growth factor and prostaglandins locally from the macula densa cells, which impairs voltage-dependent calcium channels in the smooth muscle cell membrane, resulting in afferent arteriolar vasodilatation.62 These effects provide the biologic rationale that infused amino acids may improve renal outcomes through increased renal plasma flow and an associated increase in GFR. Evidence from a randomized clinical trial published in the New England Journal of Medicine and including 53 critically ill patients demonstrated that a short-term infusion of amino acids led to faster recovery from severe acute renal failure.67 Moreover, a 2007 clinical trial demonstrated that patients randomized to receive short-term higher dose of intravenous amino acids were more likely to maintain diuresis and less furosemide to achieve a negative fluid balance were required.68 A RCT with 3,500 patients undergoing cardiac surgery testing the hypothesis of kidney protection by an amino acid infusion is underway (NCT03709264).
There has been some controversy regarding the best type of anesthesia used in cardiac surgery. A meta-analysis on 1,600 patients from 10 RCT shows that volatile anesthesia is associated with a decrease risk of AKI (RR 0.65; 0.43–0.97, P=0.04), compared to TIVA. Volatiles have a known effect of ischemic/reperfusion injury attenuation. Hence, based on current data, use of volatile anesthetics should be considered.69
The renal RIP trial showed a significant reduction on the incidence of major adverse kidney events at 90 days (death, RRT and persistent renal dysfunction) – 14.2% vs 25%, P=0.034, associated with the use of remote ischemic preconditioning (rIPC) in high-risk patients with Cleveland score ≥6. Moreover, AKI patients in rIPC group recover faster. They observed that the effect of rIPC is attenuated by propofol. Hence, based on current data, use of rIPC in high-risk patients with Cleveland score ≥6, without using propofol, may be considered.70,71
The LICRA trial on 1,136 patients showed no differences in AKI – KDIGO II and III at 5 days, comparing 0.9% saline with buffered salt solution.72 However, in our opinion, given the existing data showing increase risk of AKI with 0.9% saline in ICU patients, balanced crystalloid solutions should be used, unless hyponatremia is present.
It has been suggested that the use of fenoldopam, a selective dopamine receptor-1 agonist, might be associated with a lower rate of AKI after cardiac surgery. Unfortunately, a definitive recent multicenter RCT on 667 patients was stopped for futility in the interim analysis.73
Natriuretic peptide may induce natriuresis and vasodilatation by opposing the RAAS and vasopressin system. Several multicenter trials and meta-analyses show benefit by decreasing RRT and AKI. Data from 7 systematic reviews showed a significant decrease of RRT rate associated with atrial NP (NNT=22) and cerebral NP (NNT=11), with no effect on mortality.74 Hence, based on current data, use of natriuretic peptide, especially in high-risk patients, may be considered.
It has been suggested that the use of sodium bicarbonate might be renoprotective.75 A higher tubular pH could be protective by inhibiting the hydroxyl radical generation and lipid peroxidation. A multicentre RCT on 427 patients in 2013 was negative.76 More recently, a meta-analysis of 3 RCT, on 877 patients, observed a benefit in a sub-group analysis on patients undergoing CABG (adjusted OR for RRT 0.38 (0.25–0.58) and adjusted OR for AKIN grade 3 is 0.45 (0.43–0.48)). However, imbalances in prognostically important characteristics were observed.77 Hence, based on present data, prophylactic use of bicarbonate should not be considered.
Levosimendan has predominant renal afferent vasodilatation, rather than efferent. It has been suggested that its use might be renoprotective. A metanalysis on 1,345 patients from 13 RCT, in which 87.5% have LVEF <40%, showed a reduction of AKI rate (OR 0.51, 95% CI 0.34–0.76) and AKI requiring RRT (OR 0.43, 95% CI 0.25–0.76), associated with the use of levosimendan.78 A recent meta-analysis on 2,243 patients from 14 RCT,79 which included 2 recent large RCT,80,81 showed that patients receiving levosimendan were less likely to die at 30 days (RR =0.71, 95% CI=0.53–0.95; P=0.023). It is suggested that this benefit may be limited to patients with low to moderate ejection fraction (RR=0.44, 95% CI=0.27–0.70; P<0.001), with no benefit for patients with preserved ejection fraction (RR=1.06, 95% CI=0.72–1.56; P=0.78). Patients receiving levosimendan were also less likely to require RRT (RR=0.66, 95% CI=0.47–0.92; P=0.015) and to have a low cardiac output syndrome (RR=0.40, 95% CI=0.22–0.73; P=0.003).
An updated 2017 expert opinion of the working group in AKI prevention from the European Society of Intensive Care Medicine, recommend against the use of levosimendan for renal protection in cardiac surgery patients with poor preoperative left ventricular function or needing postoperative hemodynamic support (Grade 1B).82 However, unpublished data from a subgroup analysis of all the patients undergoing CABG with LVEF <40% in the LEVO-CTS and the LICORN trial, presented at the 2018 European Association of Cardiothoracic Anesthesia (EACTA) meeting, showed a decreased risk of mortality at 1 and 3 months, associated with the use of levosimendan compared to placebo (7.7% vs 2.9%, OR 0.36, 95% CI 0.18–0.72). Based on current data, levosimendan still may have a role if used for CABG patients with LVEF <40%.83
Dexmedetomidine is a highly selective alpha-2 agonist with short half-life. Ratio of alpha-2 to alpha-1 is 1,600:1; inhibits renin release and attenuates sympathetic activity and vasoconstriction. It causes sympathetic stabilization, anti-inflammatory effect and ischemic/reperfusion injury attenuation. Data from an RCT on 200 patients showed a significant AKI reduction rate (14% vs 33%) associated with dexmedetomidine compared to placebo.84 A retrospective single-center on 1,133 patients, using propensity score matching and logistic regression, observed an association between dexmedetomidine and a decrease in AKI rate (26.1% vs 33.75%; adjusted OR 0.7; 95% CI 0.54–0.91, P=0.008).
Vasopressin binds to AVPR1a to promote vasoconstriction through several pathways, including modulation of ATP-sensitive K+ channel function and nitric oxide production and enhancement of the vascular response to catecholamines. Since vasopressin acts on renal efferent arterioles, as opposed to noradrenaline which acts mainly on the renal afferent arteriole, might have nephroprotective effects. A single double-blind RCT on 330 patients on vasoplejic shock after cardiac surgery showed a lower composite primary end-point (30-day mortality or stroke, AKI, Mech Vent >48 hrs, reoperation), with the use of vasopressin – 32% vs 49%, P=0.0014 – compared to norepinephrine. Use of vasopressin was also associated with less AKI (10.3% vs 35.8%, adjusted OR 0.26, 95% CI 0.15–0.46, P<0.0001), less AF with vasopressin (63.8% vs 82.1%; P=0.0004), no difference in digital or mesenteric ischemia and myocardial infarction.85 Hence, based on current data, use of vasopressin to protect the kidney in patients with vasoplejic syndrome after cardiac surgery should be considered.
Postoperative strategies
Based on current data, it is reasonable to maintain hemoglobin of >8 mg/dL during the postoperative period. The recent TITRe2 trial showed no differences in primary outcome at 3 months using postoperative hemoglobin of 7.5 vs 9 as a transfusion trigger for transfusion.86
Data from an RCT on 276 in high-risk patients showed that KDIGO bundle decrease AKI rate in high-risk patients undergoing cardiac surgery, defined by biomarkers, as urinary [TIMP-2]·[IGFBP7] >0.3. AKI defined by KDIGO 1 or worse at 72 hrs postoperatively (71% vs 55%, OR 0.48; 95% CI 0.29–0.79). KDIGO bundle consist of avoiding ACEi/ARB first 48 hrs postoperatively, avoiding hyperglycemia first 72 hrs postoperatively, using alternatives to radiocontrast agents and lastly optimizing hemodynamics guided by PiCCO first 48 hrs (consequently, more dobutamine and similar amount of fluid was used, compared to standard of care).87
The early use of RRT after cardiac surgery has been suggested to be associated with improved in-hospital survival in patients with CSA-AKI. Data from a meta-analysis on 847 patients, including 2 RCT and 9 observational cohorts, showed a decrease of 28-day mortality associated with use of early RRT after cardiac surgery (OR 0.29, 95% CI 0.16–0.52, P<0.0001).88
The ELAIN trial, a recent well-designed single center RCT on 231 patients (50% of them were post-cardiac surgery patients), showed that early use of RRT (within 8 hrs of diagnosis of KDIGO stage 2) was associated with a decrease in 90-day mortality (39.3% vs 54.7%), a decrease in RRT duration, mechanical ventilation time and length of hospital stay and increase rate of renal recovery by day 90 (53.6% vs 38.7%), compared to late use of RRT after cardiac surgery (within 12 hrs of AKIN stage 3).89 Therefore, based on current data, use of early RRT should be considered.
KDIGO guidelines suggest the use of continuous RRT in unstable patients (grade 2B). However, extended daily dialysis (EDD) may also be suitable for the treatment in this setting. EDD provides hemodynamic tolerability and is significantly cheaper. No difference in renal function, mortality, renal recovery and hospital stay has been shown. Hemodynamic measurements, including heart rate, systolic blood pressure and diastolic blood pressure were comparable.90
The 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group on Cardiac and Vascular Surgery-Associated Acute Kidney Injury suggests the following recommendations:91
Avoidance of glucose variability (1B)
Balanced crystalloid (vs saline) (1B)
Use of dexmedetomidine (2C)
Limited use of blood transfusion (1A)
Discontinuation of ACEIs and ARBs (1C)
Albumin in off-pump CABG patients with hypoalbuminemia (2C)
24-hr to 72-hr delay post-contrast before cardiac surgery (2C)
Pre-operative IABP in high-risk cardiac surgery patients (2C)
Volatile anesthetic agents (vs propofol) (2C)
Avoidance of hyperthermia (2C)
Pulsatile CPB in high-risk patients (2D)
Avoidance of hemodilution (2C)
Remote ischemic preconditioning in high-risk patients (2B)
KDIGO bundle in high-risk patients (1B)
We recommend not using natriuretic peptide, fenoldopam, diuretics, dopamine, or mannitol for the treatment of AKI after cardiac surgery (grade 1C).
The decision to start RRT should be individualized with consideration of the clinical context and not be based solely on renal function or stage of AKI. Once the decision to initiate RRT has been made, it should be started promptly (not graded).
The updated 2017 expert opinion of the working group on prevention, AKI section, from the European Society of Intensive Care Medicine, suggests the following recommendations regarding kidney protection during cardiac surgery:82
A summary of all measures for preventing AKI after cardiac surgery can be found in Table 3.
Table 3.
Summary of all measures that might protect the kidney in cardiac surgery
| Preoperative strategies | Intraoperative strategies | Postoperative strategies |
|---|---|---|
| -General measures: avoid intravascular volume depletion, optimize cardiac output, avoid nephrotoxic drugs -Aspirin33,34 -Statins continuation39 -Iron if Hb <12.5 and ferritin is <100 mg/L -Using exogenous albumin to correct hypoalbuminemia (level of <4 g/dL) in off-pump CABG surgery35,36 |
-Zero-balanced ultrafiltration during CPB for patients with eGFR <6047 -DO2 >300 ml with MAP >70 mmHg during CPB48 -Avoid RBC unless Hb <7 g/dL42 -Volatile anesthesia69 -Avoid glycemia >180 mg/dL and large glucose variability49,50 -Program on blood management (TEG-guided transfusion, cell saver and use of tranexamic acid)44 -rIPC in patients with Cleveland score ≥6, with no use of propofol70,71 -Levosimendan for CABG if LVEF <40%83 -Use of vasopressin/terlipressin85 |
-Keep Hb >8 mg/dL86 -Use of early RRT88,89 -Avoid ACEi/ARB87 -Avoid nephrotoxic drugs -Use of dexmedetomidine84 -Avoid glycemia >180 mg/dL and large glucose variability49,50 -To optimize hemodynamics individually guided by transpulmonary thermodilution first 2 days87 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CPB, cardio-pulmonary bypass; DO2, oxygen delivery; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; LVEF, left ventricle ejection fraction; RBC, red blood cells; RRT, renal replacement therapy; TEG, thromboelastography.
Early detection of acute kidney injury: the role of new biomarkers
RIFLE, AKIN and KDIGO definitions have an important prognostic impact.92 However, they are based on sCr, and its use may delay the diagnosis of AKI for 24–72 hrs, compared to new biomarkers. This could be explained by its late increase. The loss of half of the renal function is required in order for the sCr to increase. Therefore, it may take days to increase. Several other factors may affect the concentration of sCr.15
The combination of two tubular stress biomarkers – insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) – is very promising for early detection of tubular damage. Recent data show that these two biomarkers combined may diagnose AKI in a very early phase.
Data from a prospective cohort study on 150 patients showed an independent association between [TIMP-2*IGFBP7] at ICU admission and postoperative AKI at 48 hrs (OR 11.83; P<0.001, aROC=0.74) after logistic regression adjusting for EuroSCORE II and CBP-time.93 A recent meta-analysis, with 10 prospective cohorts studies on 747 patients undergoing cardiac surgery, published from 2014 to 2017, showed an aROC of 0.83, indicating high predictive accuracy.94 Based on current data, the combination of these two tubular stress biomarkers (Nephrocheck®), IGFBP7 plus TIMP-2, is the most promising biomarkers for early diagnosis of AKI after cardiac surgery.
Patients with an early higher urinary and plasma NGAL levels are most likely to be diagnosed of AKI (aROC 0.78).95 Therefore, NGAL levels may detect AKI 24–72 hrs earlier than sCr. Furthermore, NGAL has also prognostic impact. Whether other confounding factors may affect NGAL is still unknown.
Data from a meta-analysis on 2,322 patients suffering from AKI related to cardiac disease, suggest that patients with a higher NGAL, are most likely to develop adverse outcomes, even when sCr is normal. Hence, it is suggested that subclinical AKI may be diagnosed by this biomarker.96 However, several issues should be clarified before using NGAL routinely in clinical practice, mainly a large variability in its predictive capacity (aROC) as shown in published data.97–99
Predictive capacity of several other biomarkers has been studied (Table 4). Cystatin C is a potential marker of GFR. It also appears to be independent of age, sex and lean muscle mass. Because of controversial results in early studies, its predictive capacity and prognostic impact in AKI after cardiac surgery needs to be further studied.100
Table 4.
New biomarkers for detecting AKI post injury after cardiac surgery
| Biomarker | Sample source | Features | Heart surgery |
|---|---|---|---|
| NGAL | Urine and plasma | It is filtered freely by the glomerulus It is reabsorbed in the DT |
Increase 2 hrs post-CPB |
| IL-18 | Urine | Proinflammatory cytokine produced after the injury of the epithelial cells of DT | Increase 4–6 hrs post-CPB |
| KIM-1 | Urine | Transmembrane glycoprotein expressed after the injury of the epithelial cells of DT | Increase 12–24 hrs post-CPB |
| L-FABP | Urine | Cytosol protein synthesized in the liver | Increase 4 hrs post-CPB |
| Cystatin C | Urine and plasma | It filters freely through the glomerulus without reabsorbing | Increase 12 hrs post-CPB |
Abbreviations: CPB, cardio-pulmonary bypass; DT, distal tubule; NGAL, neutrophil gelatinase-associated lipocalin; IL-18, interleukin 18; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid-binding protein.
Acute tubular injury is associated with an increase in urinary IL-18. Given that urinary IL-18 levels have a stronger association with CPB time rather than AKI itself, it is suggested that urinary IL-18 levels are more a marker of inflammation rather than a marker of kidney injury in patients undergoing cardiac surgery.100
The use of a combination of several markers has been suggested to improve diagnostic performance of AKI after cardiac surgery. Data from a prospective observational study on 77 patients suggest that a combination of urinary biomarkers is associated with a higher discriminatory capacity, compared to a single biomarker (aROC 0.81 vs aROC 0.75 for NAG alone or aROC 0.72 for L-FABP alone).101 Furthermore, the inclusion of a combination of biomarkers to clinical factors was associated with an improvement in diagnostic performance, compared to clinical factors alone (aROC 0.86 vs 0.79).
Data from a prospective cohort on 380 patients showed that biomarkers may predict worsening of AKI after cardiac surgery.102 Biomarkers also improved predictive performance, compared to clinical factors alone. Included biomarkers were urinary IL-18, urinary albumin to creatinine ratio and urinary and plasma NGAL.
Stress biomarkers are promising tools for early diagnosis of AKI after cardiac surgery. However, important issues need to be better understood before using these biomarkers in our clinical practice. These issues include, first, the large variations in diagnostic performance reported. Second, factors not kidney-related affecting individual biomarkers are not well understood. Lastly, data reporting the diagnostic performance of biomarkers, excluded patients with chronic kidney diasease, which are high-risk group of patients for developing renal injury after cardiac surgery. Based on current data, however, the most promising biomarker for early prediction of CSA-AKI is the combination of two tubular stress biomarkers (urinary IGFBP7 and TIMP-2 levels) measured at ICU admission.94
Conclusion
AKI after cardiac surgery is independently associated with a significant increase in morbidity, mortality and health-care costs.
Kidney protection strategies to prevent AKI after cardiac surgery are not yet conclusive. These strategies include delaying elective surgery to enable full kidney function optimization, individualized hemodynamic optimization by the use of inotropics or fluid therapy and judicious use of RBC.
Prolonged aortic cross-clamping, intravascular hemolysis or contrast dye exposure should be avoided.
The current most promising pharmacological prevention strategies are dexmedetomidine, vasopressin and amino acids, but much more data are needed.
As the knowledge of the pathophysiology of AKI after cardiac surgery improves, our aim of preventing and treating as early as possible, will be also improved.
Finally, early treatment by RRT of patients early diagnosed by a combination of biomarkers may improve outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoste EA, Cruz DN, Davenport A, et al. The epidemiology of cardiac surgery-associated acute kidney injury. Int J Artif Organs. 2008;31:158–165. [DOI] [PubMed] [Google Scholar]
- 2.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RH, Grab JD, O’Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16;quiz. [DOI] [PubMed] [Google Scholar]
- 4.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. [DOI] [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. [DOI] [PubMed] [Google Scholar]
- 6.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. [DOI] [PubMed] [Google Scholar]
- 7.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. [DOI] [PubMed] [Google Scholar]
- 8.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. [DOI] [PubMed] [Google Scholar]
- 9.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative w. acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Critical Care. 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englberger L, Suri RM, Li Z, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Critical Care. 2011;15:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Critical Care. 2014;18:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 16.Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs. 2008;31:166–178. [DOI] [PubMed] [Google Scholar]
- 17.O’Neal JB, Shaw AD, Billings F. Acute kidney injury following cardiac surgery: current understanding and future directions. Critical Care. 2016;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002;90:133–138. [DOI] [PubMed] [Google Scholar]
- 19.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: a review in search of a treatment algorithm. J Extra Corpor Technol. 2008;40:257–267. [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Valdivieso JR, Monedero P, Vives M, Garcia-Fernandez N, Bes-Rastrollo M. Cardiac-surgery associated acute kidney injury requiring renal replacement therapy. A Spanish retrospective case-cohort study. BMC Nephrol. 2009;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis. 2003;41:76–83. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Wijeysundera DN, Yau TM, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology. 2011;115:523–530. [DOI] [PubMed] [Google Scholar]
- 23.Ranucci M, Aloisio T, Cazzaniga A, Di Dedda U, Gallazzi C, Pistuddi V. Validation of renal-risk models for the prediction of non-renal replacement therapy cardiac surgery-associated acute kidney injury. Int J Cardiol. 2018;272:49–53. [DOI] [PubMed] [Google Scholar]
- 24.Vives M, Monedero P, Perez-Valdivieso JR, et al. External validation and comparison of three scores to predict renal replacement therapy after cardiac surgery: a multicenter cohort. Int J Artif Organs. 2011;34:329–338. [DOI] [PubMed] [Google Scholar]
- 25.Englberger L, Suri RM, Li Z, et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis. 2010;56:623–631. [DOI] [PubMed] [Google Scholar]
- 26.Demirjian S, Schold JD, Navia J, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis. 2012;59:382–389. [DOI] [PubMed] [Google Scholar]
- 27.Echarri G, Duque-Sosa P, Callejas R, et al. External validation of predictive models for acute kidney injury following cardiac surgery: a prospective multicentre cohort study. Eur J Anaesthesiol. 2017;34:81–88. [DOI] [PubMed] [Google Scholar]
- 28.Investigators RRTS, Bellomo R, Cass A, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Sun J, Liu S, et al. Relationship among mortality of patients with acute kidney injury after cardiac surgery, fluid balance and ultrafiltration of renal replacement therapy: an observational study. Blood Purif. 2017;44:32–39. [DOI] [PubMed] [Google Scholar]
- 30.Corredor C, Thomson R, Al-Subaie N. Long-term consequences of acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2016;30:69–75. [DOI] [PubMed] [Google Scholar]
- 31.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan M, Hudson CC, Phillips-Bute BG, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg. 2010;89:1098–1104. [DOI] [PubMed] [Google Scholar]
- 33.Cao L, Young N, Liu H, et al. Preoperative aspirin use and outcomes in cardiac surgery patients. Ann Surg. 2012;255:399–404. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg. 2015;261:207–212. [DOI] [PubMed] [Google Scholar]
- 35.Lee EH, Kim WJ, Kim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. 2016;124:1001–1011. [DOI] [PubMed] [Google Scholar]
- 36.Lee EH, Baek SH, Chin JH, et al. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012;38:1478–1486. [DOI] [PubMed] [Google Scholar]
- 37.Billings F, Brown NJ. Statins to reduce acute kidney injury after cardiac surgery – reply. JAMA. 2016;316:349–350. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Jayaram R, Jiang L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–1753. [DOI] [PubMed] [Google Scholar]
- 39.Molnar AO, Parikh CR, Coca SG, et al. Association between preoperative statin use and acute kidney injury biomarkers in cardiac surgical procedures. Ann Thorac Surg. 2014;97:2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, van Rensburg A, Beattie WS. The influence of baseline hemoglobin concentration on tolerance of anemia in cardiac surgery. Transfusion. 2008;48:666–672. [DOI] [PubMed] [Google Scholar]
- 41.Duque-Sosa P, Martinez-Urbistondo D, Echarri G, et al. Perioperative hemoglobin area under the curve is an independent predictor of renal failure after cardiac surgery. Results from a Spanish Multicenter Retrospective Cohort Study. PloS One. 2017;12:e0172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraris VA, Brown JR Despotis GJ, et al. 2011 update to the society of thoracic surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 91;2011:944–982. [DOI] [PubMed] [Google Scholar]
- 43.Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017;377:2133–2144. [DOI] [PubMed] [Google Scholar]
- 44.Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55:1075–1081. [DOI] [PubMed] [Google Scholar]
- 45.Asteriou C, Antonitsis P, Argiriadou H, et al. Minimal extracorporeal circulation reduces the incidence of postoperative major adverse events after elective coronary artery bypass grafting in high-risk patients. A single-institutional prospective randomized study. Perfusion. 2013;28:350–356. [DOI] [PubMed] [Google Scholar]
- 46.Anastasiadis K, Antonitsis P, Haidich AB, Argiriadou H, Deliopoulos A, Papakonstantinou C. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2013;164:158–169. [DOI] [PubMed] [Google Scholar]
- 47.Matata BM, Scawn N, Morgan M, et al. a single-center randomized trial of intraoperative zero-balanced ultrafiltration during cardiopulmonary bypass for patients with impaired kidney function undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:1236–1247. [DOI] [PubMed] [Google Scholar]
- 48.Magruder JT, Crawford TC, Harness HL, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–25 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song JW, Shim JK, Yoo KJ, Oh SY, Kwak YL. Impact of intraoperative hyperglycaemia on renal dysfunction after off-pump coronary artery bypass. Interact Cardiovasc Thorac Surg. 2013;17:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal B, Carvalho P, Mehta Y, et al. Prognostic significance of glycemic variability after cardiac surgery. J Diabetes Complications. 2016;30:613–617. [DOI] [PubMed] [Google Scholar]
- 51.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 52.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 53.Steppan J, Hofer S, Funke B, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165:136–141. [DOI] [PubMed] [Google Scholar]
- 54.Bansch P, Nelson A, Ohlsson T, Bentzer P. Effect of charge on microvascular permeability in early experimental sepsis in the rat. Microvasc Res. 2011;82:339–345. [DOI] [PubMed] [Google Scholar]
- 55.Vives M, Callejas R, Duque P, et al. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: a prospective multicentre cohort. Br J Anaesth. 2016;117:458–463. [DOI] [PubMed] [Google Scholar]
- 56.Lamy A, Devereaux PJ, Prabhakaran D, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013;368:1179–1188. [DOI] [PubMed] [Google Scholar]
- 57.Lamy A, Devereaux PJ, Prabhakaran D, et al. Five-year outcomes after off-pump or on-pump coronary-artery bypass grafting. N Engl J Med. 2016;375:2359–2368. [DOI] [PubMed] [Google Scholar]
- 58.Shroyer AL, Hattler B, Wagner TH, et al. Five-year outcomes after on-pump and off-pump coronary-artery bypass. N Engl J Med. 2017;377:623–632. [DOI] [PubMed] [Google Scholar]
- 59.Valdez GD, Mihos CG, Santana O, et al. Incidence of postoperative acute kidney injury in patients with chronic kidney disease undergoing minimally invasive valve surgery. J Thorac Cardiovasc Surg. 2013;146:1488–1493. [DOI] [PubMed] [Google Scholar]
- 60.Tang P, Onaitis M, Desai B, et al. Minithoracotomy versus sternotomy for mitral surgery in patients with chronic renal impairment: a propensity-matched study. Innovations. 2013;8:325–331. [DOI] [PubMed] [Google Scholar]
- 61.Radak D, Neskovic M, Otasevic P, Isenovic E. Renal dysfunction following elective endovascular aortic aneurysm repair. Curr Vasc Pharmacol. 2019;17(2):133–140. [DOI] [PubMed] [Google Scholar]
- 62.Woods LL. Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Int. 1993;44:659–675. [DOI] [PubMed] [Google Scholar]
- 63.Meyer TW, Ichikawa I, Zatz R, Brenner BM. The renal hemodynamic response to amino acid infusion in the rat. Trans Assoc Am Physicians. 1983;96:76–83. [PubMed] [Google Scholar]
- 64.Seney FD Jr., Persson EG, Wright FS. Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol. 1987;252:F83–F90. [DOI] [PubMed] [Google Scholar]
- 65.Yao B, Xu J, Qi Z, Harris RC, Zhang MZ. Role of renal cortical cyclooxygenase-2 expression in hyperfiltration in rats with high-protein intake. Am J Physiol Renal Physiol. 2006;291:F368–F74. [DOI] [PubMed] [Google Scholar]
- 66.Schrijvers BF, Rasch R, Tilton RG, Flyvbjerg A. High protein-induced glomerular hypertrophy is vascular endothelial growth factor-dependent. Kidney Int. 2002;61:1600–1604. [DOI] [PubMed] [Google Scholar]
- 67.Abel RM, Beck CH Jr., Abbott WM, Ryan JA Jr., Barnett GO, Fischer JE. Improved survival from acute renal failure after treatment with intravenous essential L-amino acids and glucose. Results of a prospective, double-blind study. N Engl J Med. 1973;288:695–699. [DOI] [PubMed] [Google Scholar]
- 68.Singer P. High-dose amino acid infusion preserves diuresis and improves nitrogen balance in non-oliguric acute renal failure. Wien Klin Wochenschr. 2007;119:218–222. [DOI] [PubMed] [Google Scholar]
- 69.Cai J, Xu R, Yu X, Fang Y, Ding X. Volatile anesthetics in preventing acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2014;148:3127–3136. [DOI] [PubMed] [Google Scholar]
- 70.Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. Jama. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 71.Zarbock A, Kellum JA, Van Aken H, et al. Long-term effects of remote ischemic preconditioning on kidney function in high-risk cardiac surgery patients: follow-up results from the RenalRIP trial. Anesthesiology. 2017;126:787–798. [DOI] [PubMed] [Google Scholar]
- 72.McIlroy D, Murphy D, Kasza J, Bhatia D, Wutzlhofer L, Marasco S. Effects of restricting perioperative use of intravenous chloride on kidney injury in patients undergoing cardiac surgery: the LICRA pragmatic controlled clinical trial. Intensive Care Med. 2017;43:795–806. [DOI] [PubMed] [Google Scholar]
- 73.Bove T, Zangrillo A, Guarracino F, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. Jama. 2014;312:2244–2253. [DOI] [PubMed] [Google Scholar]
- 74.Patel NN, Angelini GD. Pharmacological strategies for the prevention of acute kidney injury following cardiac surgery: an overview of systematic reviews. Curr Pharm Des. 2014;20:5484–5488. [DOI] [PubMed] [Google Scholar]
- 75.Haase M, Haase-Fielitz A, Bellomo R, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med. 2009;37:39–47. [DOI] [PubMed] [Google Scholar]
- 76.McGuinness SP, Parke RL, Bellomo R, Van Haren FM, Bailey M. Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med. 2013;41:1599–1607. [DOI] [PubMed] [Google Scholar]
- 77.Bailey M, McGuinness S, Haase M, et al. Sodium bicarbonate and renal function after cardiac surgery: a prospectively planned individual patient meta-analysis. Anesthesiology. 2015;122:294–306. [DOI] [PubMed] [Google Scholar]
- 78.Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2016;67:408–416. [DOI] [PubMed] [Google Scholar]
- 79.Tena MA, Urso S, Gonzalez JM, et al. Levosimendan versus placebo in cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 80.Landoni G, Lomivorotov VV, Alvaro G, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. 2017;376:2021–2031. [DOI] [PubMed] [Google Scholar]
- 81.Mehta RH, Leimberger JD, van Diepen S, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med. 2017;376:2032–2042. [DOI] [PubMed] [Google Scholar]
- 82.Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on prevention, AKI section, European Society of intensive care medicine. Intensive Care Med. 2017;43:730–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guarracino F, Heringlake M, Cholley B, et al. Use of levosimendan in cardiac surgery: an update after the LEVO-CTS, CHEETAH, and LICORN trials in the light of clinical practice. J Cardiovasc Pharmacol. 2018;71:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho JS, Shim JK, Soh S, Kim MK, Kwak YL. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89:693–700. [DOI] [PubMed] [Google Scholar]
- 85.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: the VANCS randomized controlled trial. Anesthesiology. 2017;126:85–93. [DOI] [PubMed] [Google Scholar]
- 86.Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- 87.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Davari-Farid S, Arora P, Porhomayon J, Nader ND. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2014;28:557–563. [DOI] [PubMed] [Google Scholar]
- 89.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. Jama. 2016;315:2190–2199. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. [DOI] [PubMed] [Google Scholar]
- 91.Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the ADQI (Acute disease quality initiative) group. J Am Heart Assoc. 2018;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the RIFLE and acute kidney injury network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–1376. [DOI] [PubMed] [Google Scholar]
- 93.Oezkur M, Magyar A, Thomas P, et al. TIMP-2*IGFBP7 (Nephrocheck(R)) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hrs. Kidney Blood Press Res. 2017;42:456–467. [DOI] [PubMed] [Google Scholar]
- 94.Su LJ, Li YM, Kellum JA, Peng ZY. Predictive value of cell cycle arrest biomarkers for cardiac surgery-associated acute kidney injury: a meta-analysis. Br J Anaesth. 2018;121:350–357. [DOI] [PubMed] [Google Scholar]
- 95.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; Group NM-aI. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. [DOI] [PubMed] [Google Scholar]
- 96.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–433. [DOI] [PubMed] [Google Scholar]
- 98.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perry TE, Muehlschlegel JD, Liu KY, et al. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth Analg. 2010;110:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McIlroy DR, Wagener G, Lee HT. Biomarkers of acute kidney injury: an evolving domain. Anesthesiology. 2010;112:998–1004. [DOI] [PubMed] [Google Scholar]
- 101.Katagiri D, Doi K, Honda K, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93:577–583. [DOI] [PubMed] [Google Scholar]
- 102.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]