Abstract
Background: Previous studies have reported that the albumin-to-alkaline phosphatase ratio (AAPR), a novel blood biomarker-based index, is associated with clinical outcome in several cancers. However, data relating to lung cancer are rare. This study was performed to clarify the clinical significance of AAPR in patients with metastatic non-small-cell lung cancer (NSCLC).
Methods: In total, 290 stage IV NSCLC patients were enrolled in this retrospective study. Associations between serum enzyme levels and clinical characteristics were analyzed using the Mann–Whitney U-test or chi-squared test. Kaplan–Meier survival analysis and Cox’s proportional hazard regression model were adopted to assess the prognostic value of AAPR for overall survival (OS).
Results: The optimal cut-off points for AAPR and lactate dehydrogenase (LDH) were 0.36 and 265.5 U/L, respectively. Patients with AAPR ≤0.36 had apparently longer survival than those with AAPR >0.36 (13 vs 7 months, P<0.001). Furthermore, AAPR was an independent predictor of OS in metastatic NSCLC in multivariate analysis (HR=0.657, 95% CI=0.504–0.856, P<0.01). The prognostic impact of LDH for survival of NSCLC populations was also validated in this study (HR=1.462, 95% CI=1.070–1.999, P<0.05).
Conclusion: Elevated AAPR can be an independent favorable prognostic indicator in metastatic NSCLC.
Keywords: AAPR, LDH, NSCLC, prognosis
Introduction
Lung cancer remains one of the most prevalent malignancies and the leading cause of cancer-related death worldwide.1 Approximately 85% of primary lung cancers are non-small cell lung cancer (NSCLC), and more than half are metastatic NSCLC at the time of initial diagnosis, owing to a lack of typical symptoms in early disease.2 Patients with metastatic NSCLC can be treated with chemotherapy, radiotherapy, or both. Thanks to advances in treatment, they can benefit from targeted therapy as well.3 However, the prognosis for metastatic NSCLC is extremely poor, with a 5-year survival rate of 3.9%.2 Although the TNM classification system has the most marked discriminatory capacity for survival of cancers, it has reached its limits in metastatic NSCLC patients. Therefore, finding novel and efficient biomarkers to help predict clinical outcomes and guide clinical decision-making for metastatic NSCLC is of great importance.
Carcinogenesis and tumor progression always lead to abnormal serum enzyme synthesis, sometimes even before disease is clinically detectable.4 These enzymes can serve as useful indicators for tumor detection and monitoring. Alkaline phosphatase (ALP) is a hydrolase enzyme which is responsible for removing phosphate groups. Elevated serum ALP levels can be seen in liver, kidney, and bone diseases.5 ALP has been found to be associated with advanced cancer status.6,7 Lactate dehydrogenase (LDH) is another serum enzyme, which mainly participates in the conversion of pyruvate to lactate and is related to tumor metabolism. Numerous studies have found that LDH levels are elevated in various types of cancer, including NSCLC.8–11
Albumin (ALB), one of the most commonly used methods to assess nutritional status, usually shows no or only slight changes in the early stages of cancer. But as the disease progresses, malnutrition and inflammation lead to ALB synthesis being suppressed and degradation increasing, which result in ALB levels dropping significantly.12 The decreased ALB in cancer patients can result in shorter survival and increased cancer-related mortality.13,14 Besides ALB alone, several ALB-based markers, such as the Glasgow Prognostic Score,15,16 Advanced Lung Cancer Inflammation Index,17 and Prognostic Nutritional Index,18,19 have also been shown to correlate with survival in various cancers.
In the present study, we merge the parameters ALB and ALP to form a novel index called the albumin-to-alkaline phosphatase ratio (AAPR). The AAPR has been revealed as a prognostic indicator for hepatocellular carcinoma20 and nasopharyngeal carcinoma.21 However, whether AAPR can predict survival in NSCLC patients has not yet been investigated. Thus, we performed this single-center retrospective study to explore for the first time the prognostic impact of AAPR and its relationship with clinical characteristics in patients with metastatic NSCLC. The correlation between LDH and prognosis of metastatic NSCLC was also demonstrated.
Materials and methods
Participants
We retrospectively reviewed newly diagnosed stage IV NSCLC patients in West China Hospital from March 2007 to December 2013. The inclusion criteria included the following: 1) pathologically confirmed NSCLC; 2) clinically diagnosed metastasis; 3) serum ALB and ALP measured at diagnosis; and 4) follow-up data were available. Patients with a history of a second primary malignancy, detectable infections, and known liver or renal or bone diseases were excluded from the study. In total, 290 NSCLC patients who met the inclusion criteria were eventually enrolled in the study. The West China Hospital Research Ethics Committee approved the study and signed informed consent from patients was waived because of the retrospective nature of the analysis. All patient data were treated with confidentiality, in accordance with the Declaration of Helsinki.
Clinical and follow-up data
We collected baseline and clinical characteristics of patients from the hospital medical system, including age, gender, smoking history, histology type, metastatic characteristics, and medication. Patients who had smoked fewer than 100 cigarettes in their lifetime were defined as non-smokers. Histology type was identified based on the WHO criteria established in 2004.22 Tumor stage was defined according to the seventh edition of the TNM classification.23 Furthermore, pretreatment laboratory data covering ALB, ALP, and LDH were extracted. The AAPR was calculated by dividing the serum ALB by the serum ALP level.
The primary outcome of the study was patients dead of lung cancer. Time from diagnosis to death was measured as overall survival (OS). We obtained survival information by interviewing patients on the telephone or by checking medical records. The last follow-up was in October 2017.
Statistical analysis
The optimal cut-off values for the AAPR and LDH levels were determined using a biostatistical tool called Cutoff Finder (http://molpath.charite.de/cutoff/).24 Statistical analyses were performed with SPSS software (version 22; IBM Corp., Armonk, NY, USA). Continuous variables were presented as medians and ranges, and later were transformed into dichotomous variables presented as frequencies and percentages. Comparison of clinicopathological characteristics between different groups was conducted by the Mann–Whitney U-test for continuous variables and chi-squared test for dichotomous variables. Associations between prognostic factors and OS were assessed by the Kaplan–Meier method and tested by the log-rank test, while Kaplan–Meier survival curves were drafted in Prism 5 (version 5.01; GraphPad Software, La Jolla, CA, USA). For univariate and multivariate survival analyses, Cox’s proportional hazard regression model was adopted. Candidate variables with a P-value less than 0.2 in univariate analysis were included in the multivariate model. A two-sided P-value less than 0.05 was considered statistically significant.
Results
Clinical characteristics of patients
Overall, 290 patients were involved in the study and their clinical characteristics are summarized in Table 1. Among these, 170 (60.7%) were males, 128 (44.1%) were aged older than 60 years (mean: 55 years; range: 25–90 years), and 139 (47.9%) had a current/past history of smoking. According to the histology, 215 out of 290 patients (74.1%) had an adenocarcinoma, while 60 patients (20.7%) had a squamous cell carcinoma and 15 (5.2%) had other types. At the time of diagnosis, the majority of the patients (242; 83.4%) had developed two or fewer metastasis sites and nearly half of the patients (140; 48.3%) had liver/bone metastasis. In addition, more than half of the patients (196; 67.6%) had received some form of systemic therapy, whereas the remaining patients (94; 32.4%) had not.
Table 1.
Distribution of baseline characteristics of 290 metastatic NSCLC patients according to AAPR level
| Characteristics | Total, n (%) | AAPR ≤0.36, n (%) | AAPR >0.36, n (%) | P-value |
|---|---|---|---|---|
| Number of patients | 290 | 89 (30.7) | 201 (69.3) | |
| Age (years) | 0.898 | |||
| ≤60 | 162 (55.9) | 49 (55.1) | 113 (56.2) | |
| >60 | 128 (44.1) | 40 (44.9) | 88 (43.8) | |
| Gender | 0.696 | |||
| Male | 176 (60.7) | 56 (62.9) | 120 (59.7) | |
| Female | 114 (39.3) | 33 (37.1) | 81 (40.3) | |
| Smoking status | 1.000 | |||
| Current/past | 139 (47.9) | 43 (48.3) | 96 (47.8) | |
| Never | 151 (52.1) | 46 (51.7) | 105 (52.2) | |
| Histology | 0.502 | |||
| SCC | 60 (20.7) | 15 (16.9) | 45 (22.4) | |
| ADC | 215 (74.1) | 70 (78.7) | 145 (72.1) | |
| Others | 15 (5.2) | 4 (4.5) | 11 (5.5) | |
| Number of metastasis sites | 0.001* | |||
| ≤2 | 242 (83.4) | 64 (71.9) | 178 (88.6) | |
| >2 | 48 (16.6) | 25 (28.1) | 23 (11.4) | |
| Liver/bone metastasis | 0.011* | |||
| Positive | 140 (48.3) | 53 (37.9) | 87 (62.1) | |
| Negative | 150 (51.7) | 36 (24.0) | 114 (76) | |
| Systemic therapy | 0.007* | |||
| Yes | 196 (67.6) | 50 (56.2) | 146 (72.6) | |
| No | 94 (32.4) | 39 (43.8) | 55 (27.4) | |
| Albumin (g/L), median (range) | 37.97 (23.5–64.8) | 35.32 (23.5–45.9) | 39.14 (28.2–64.8) | 0.000* |
| ALP (U/L), median (range) | 121.73 (1.46–2,170.0) | 221.80 (79.0–2,170.0) | 77.43 (1.46–119.0) | 0.000* |
| LDH (U/L) | 0.001* | |||
| ≤265.5 | 234 (80.7) | 61 (68.5) | 173 (86.1) | |
| >265.5 | 56 (19.3) | 28 (31.5) | 28 (13.9) |
Note: *P<0.05.
Abbreviations: AAPR, albumin-to-alkaline phosphatase ratio; ADC, adenocarcinoma; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma.
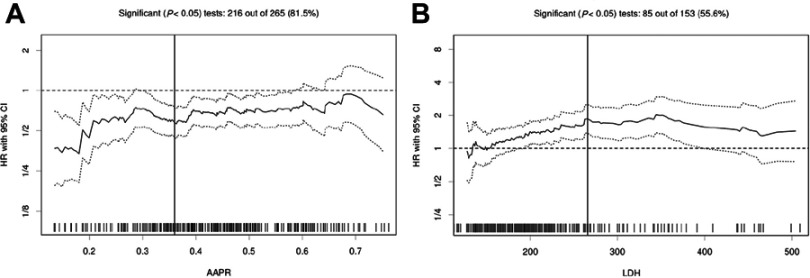
Using Cutoff Finder, an R-software engineered web-based system, we identified that the optimal cut-off values for AAPR and LDH were 0.36 and 265.5 U/L, respectively (Figure 1). Thus, 201 patients (69.3%) with AAPR >0.36 were categorized into the high AAPR group and the other 89 patients (30.7%) with AAPR ≤0.36 into the low AAPR group. When dividing the patients by level of LDH, 234 patients (80.7%) had LDH >265.5 U/L and 56 (19.3%) had LDH ≤265.5 U/L.
Figure 1.
Hazard ratios for overall survival independent of cut-off point for (A) albumin-to-alkaline phosphatase ratio (AAPR) and (B) lactate dehydrogenase (LDH) in patients with metastatic non-small-cell lung cancer. The vertical line shows the optimal cut-off point with the most significant split (log-rank test). The plots were generated using Cutoff Finder.
The distribution of clinical characteristics between the AAPR ≤0.36 and AAPR >0.36 groups is depicted in Table 1. Patients with elevated AAPR were more likely be those who harbored two or fewer metastasis sites, had undergone systemic therapy, had no liver/bone metastasis, and had LDH ≤265.5 U/L (all P<0.05). However, no significant differences were found regarding age, gender, smoking status, and tumor histology type between the two groups (all P>0.05).
A comparison of serum AAPR, ALP, LDH, and ALB levels in NSCLC patients with or without liver/bone metastasis is shown in Table 2. The results revealed that patients without liver/bone metastasis had higher AAPR and lower ALP than those with liver/bone metastasis (0.47 vs 0.40, P<0.001; 80.17 vs 95.40 U/L, P<0.001, respectively), while LDH and ALB levels in these two groups were not significantly different (both P>0.05).
Table 2.
Correlations between serum enzyme levels and liver/bone metastatic characteristics in NSCLC patients
| Characteristics | Liver/bone metastasis | P-value | |
|---|---|---|---|
| Median (range) | Positive | Negative | |
| AAPR | 0.40 (0.02–0.74) | 0.47 (0.02–44.38) | 0.000* |
| ALP (U/L) | 95.40 (47.00–1,573.00) | 80.17 (1.46–2,170.00) | 0.000* |
| LDH (U/L) | 201.50 (117.00–966.00) | 189.00 (46.00–691.00) | 0.078 |
| Albumin (g/L) | 38.40 (26.60–51.30) | 38.06 (23.50–64.80) | 0.641 |
Note: *P<0.05.
Abbreviations: AAPR, albumin-to-alkaline phosphatase ratio; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; NSCLC, non-small-cell lung cancer.
Kaplan–Meier survival analysis of AAPR for OS
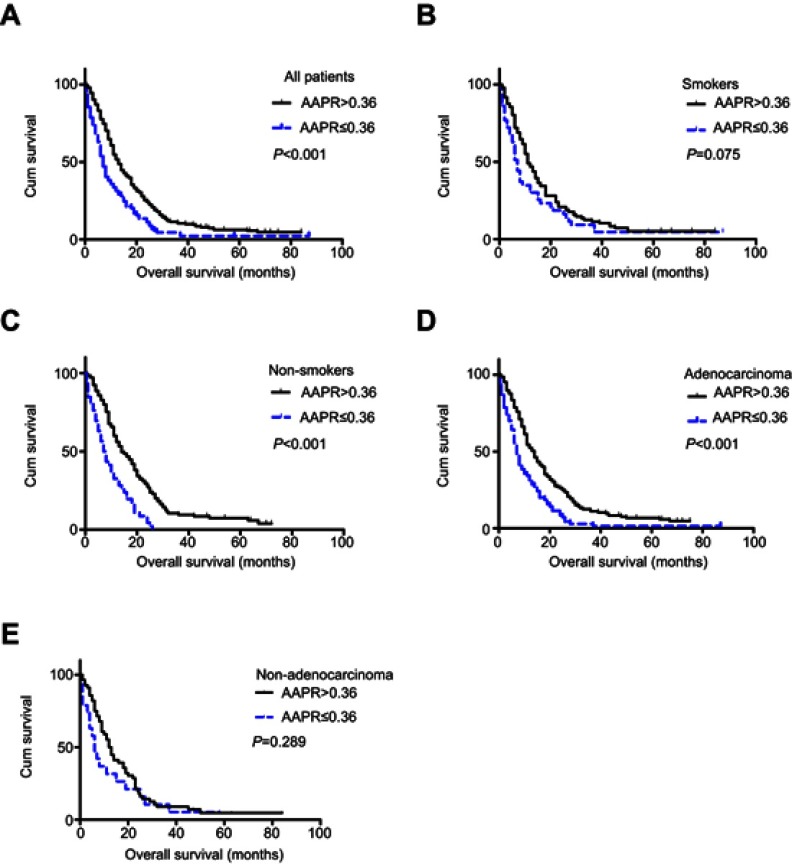
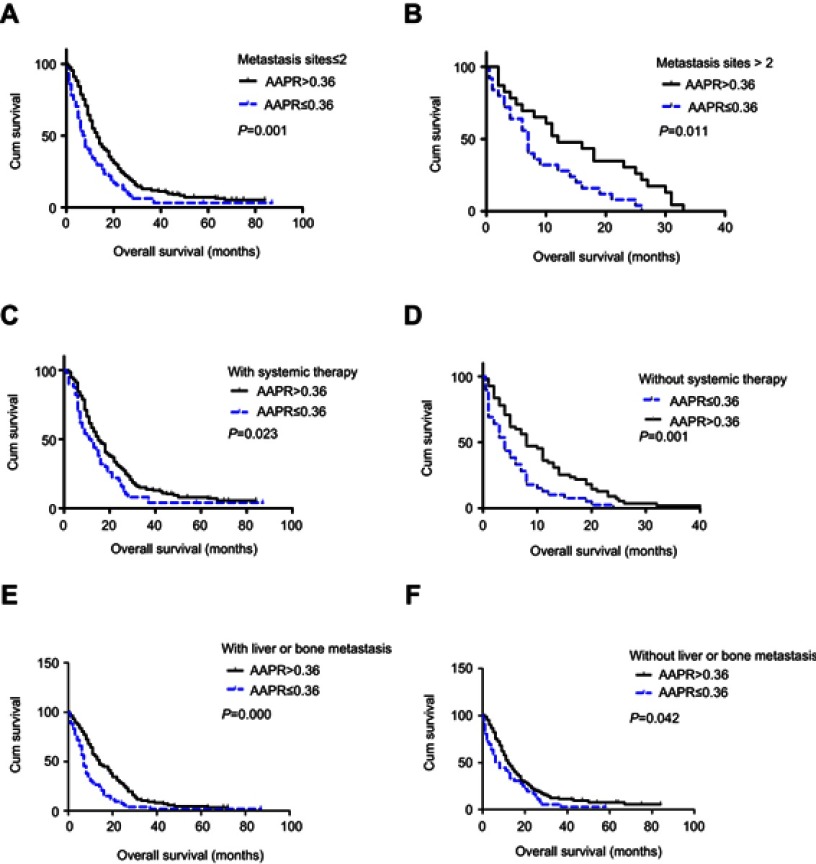
The median follow-up time of the 290 NSCLC patients was 16 months (range: 1–84 months). Altogether, 277 patients died during the follow-up period. A difference in prognosis was apparent between patients in the high AAPR group and patients in the low APPR group (13 months vs 7 months, P<0.001) (Figure 2A). Later, stratified analyses were conducted. No significant difference was found in patients with current/past smoking divided by level of AAPR (P>0.05) (Figure 2B), while in patients without smoking history, those with high AAPR showed significantly better survival than those with low AAPR (P<0.001) (Figure 2C). A similar survival trend was observed when patients were stratified into adenocarcinoma (P<0.001) (Figure 2D), but not in non-adenocarcinoma (P>0.05) (Figure 2E). Furthermore, we analyzed the relationship between AAPR and OS in patients with two or fewer metastasis sites and those with more than two, patients with and those without systemic therapy, and patients with and those without liver/bone metastasis, respectively. All of the results indicated that patients with elevated AAPR survived longer (all P<0.05) (Figure S1).
Figure 2.
Kaplan–Meier curves of overall survival according to albumin-to-alkaline phosphatase ratio (AAPR) level in (A) all patients, (B) current/past smokers, (C) non-smokers, (D) adenocarcinoma, and (E) non-adenocarcinoma.
Univariate and multivariate analyses for OS
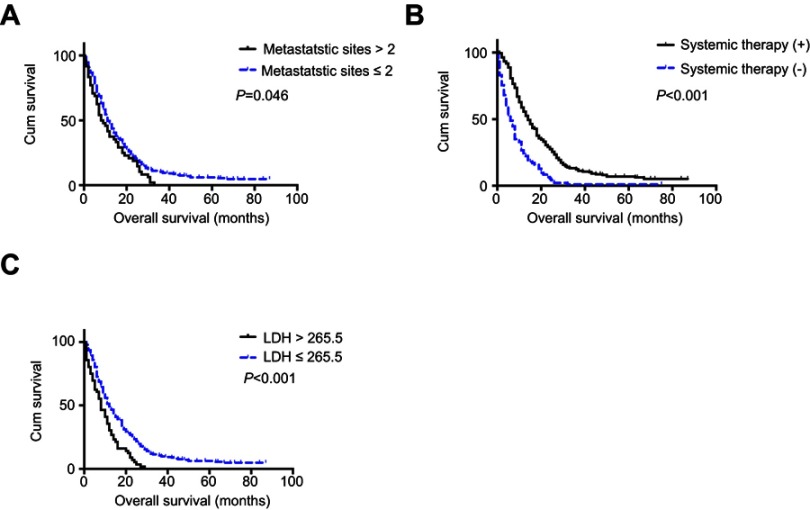
APPR level, LDH level, and systemic therapy were found to be significantly correlated with OS of metastatic NSCLC patients in univariate analysis (all P<0.001), while age, gender, smoking status, and tumor histology type were not (all P>0.05). In particular, the association between number of metastatic sites at diagnosis and OS was at the very edge of significance (P=0.053). Four variables with a P-value less than 0.2 in univariate analysis were enrolled in multivariate analysis. The result revealed that AAPR was an independent prognosis predictor for metastatic NSCLC patients (HR=0.657, 95% CI=0.504–0.856, P<0.05). The number of metastatic sites (HR=1.418, 95% CI=1.026–1.960, P<0.05), systemic therapy (HR=0.441, 95% CI=0.338–0.577, P<0.001), and LDH level (HR=1.462, 95% CI=1.070–1.999, P<0.05) were also significantly related to OS in multivariate analysis (Table 3), and their Kaplan–Meier survival curves are shown in Figure 3.
Table 3.
Prognostic factors for overall survival of metastatic NSCLC patients in univariate and multivariate Cox regression analyses
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) (>60/≤60) | 1.199 (0.945–1.520) | 0.143 | 1.107 (0.868–1.411) | 0.413 |
| Gender (female/male) | 1.059 (0.831–1.350) | 0.643 | ||
| Smoking status (current or past/never) | 1.037 (0.819–1.314) | 0.763 | ||
| Histology (ADC/non-ADC) | 1.006 (0.768–1.317) | 0.996 | ||
| Number of metastasis sites (>2/≤2) | 1.362 (0.995–1.864) | 0.053 | 1.418 (1.026–1.960) | 0.034* |
| Systemic therapy (yes/no) | 0.426 (0.329–0.551) | 0.000* | 0.441 (0.338–0.577) | 0.000* |
| LDH (>265.5/≤265.5 U/L) | 1.829 (1.354–2.470) | 0.000* | 1.462 (1.070–1.999) | 0.017* |
| AAPR (>0.36/≤0.36) | 0.587 (0.454–0.758) | 0.000* | 0.65 7(0.504–0.856) | 0.002* |
Note: *P<0.05.
Abbreviations: AAPR, albumin-to-alkaline phosphatase ratio; ADC, adenocarcinoma; non-ADC, non-adenocarcinoma; LDH, lactate dehydrogenase; NSCLC, non-small-cell lung cancer.
Figure 3.
Kaplan–Meier curves of overall survival according to (A) metastatic sites, (B) systemic therapy, and (C) lactate dehydrogenase (LDH).
Discussion
The main aim of the present retrospective study was to elucidate the associations between the AAPR and survival in patients with metastatic NSCLC. We found that elevated AAPR was correlated with longer survival in metastatic NSCLC patients, especially for patients who were non-smokers and had confirmed adenocarcinoma. Later, we identified AAPR as an independent favorable prognostic indicator for metastatic NSCLC in multivariate analysis. We also proved that LDH had a prognostic effect in metastatic NSCLC patients.
By multivariate analysis, the high AAPR group was found to be independently related to better survival in metastatic NSCLC. The novel APPR index, calculated from two routine blood parameters, namely ALB and ALP, was first derived in a cohort of hepatocellular carcinoma patients receiving curative surgery, where Chan et al identified that a lower AAPR level increased the risk of early death 3.3-fold and the risk of tumor relapse 2.3-fold.20 A later study retrospectively reviewed 209 metastatic nasopharyngeal carcinoma patients treated with cisplatin-based regimens, and found that higher AAPR indicated longer OS and progression-free survival compared with lower AAPR (24.3 months vs 17.3 months, 8.4 months vs 5.9 months, respectively).21 All of these results were consistent with our findings.
We also conducted subanalyses stratified by clinical characteristics, considering that some of the baseline characteristics were uneven between the AAPR >0.36 and AAPR ≤0.36 groups. When it comes to subanalyses divided by smoking status and tumor histology types, the discriminatory power of AAPR for OS stayed strong in patients who were non-smokers and had adenocarcinomas, while, in contrast, it became insignificant in those who were smokers and non-adenocarcinoma patients. This result may be explained by the fact that adenocarcinoma was a predominant histological type among lung cancer in non-smokers, while distant metastases such as bone or liver metastases developed earlier and were more prevalent among adenocarcinomas compared to non-adenocarcinomas.25 During subanalyses among patients with two or fewer metastasis sites and those with more than two, patients with or without systemic therapy, and those with absent or existing liver/bone metastasis, we found that the tendency remained unchanged, which demonstrates that the AAPR predicted prognosis independently of therapies, number of metastatic sites, and liver/bone metastatic status at diagnosis.
Moreover, in multivariate analysis, we found that having two or fewer metastatic sites, the presence of systemic therapy, and low LDH level were significant related to better survival. Although it is generally acknowledged that the first two conditions predict longer survival than those harboring more than metastatic sites and without systemic therapy, they are not as cheap and convenient as AAPR in predicting prognosis. Regarding LDH, we validated its prognostic value for survival, as indicated in previous studies.10,11 AAPR and LDH could supplement each other in clinical application.
There are several shortcomings of our study. First, as this was a retrospective and single-center study, multicenter and well-designed prospective studies are needed to validate our findings. Second, although we excluded patients with known liver, renal, and bone diseases to restrict confounding factors, patients with undiscovered liver, renal, or bone diseases could have been mistakenly enrolled in the study. Third, there should be little difference between AAPRs of 0.35 and 0.37, but they fell on the side of high risk and low risk, respectively, based on the current study, owing to our transforming AAPR into a dichotomous variable when it was actually a continuous one. Moreover, the optimal cut-off value for AAPR requires external validation. Fourth, the mutation status of genes, eg, EGFR, KROS, and EML4-ALK, and performance status of patients were unknown owing to information loss or unavailable data. Further studies are required to assess correlations between AAPR level and specific gene mutations, and to explore the prognostic impact of the AAPR in NSCLC treated with targeted therapy. Finally, whether dynamic changes in AAPR could be applied to monitor cancer progression and response to therapy in NSCLC remains to be solved in future research.
Conclusion
AAPR, a simple, inexpensive, and easily gained biomarker-based index, can be an independent predictor for OS in metastatic NSCLC. Further large-scale prospective studies are needed to validate and expand our findings.
Acknowledgments
This work was supported by the Transformation Projects of Sci-Tech Achievements of Sichuan Province (2016CZYD0001), and the Sci-Tech Support Program of Science and Technology Department of Sichuan Province (2016SZ0073).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii27–39. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira LM, Hebrant A, Dumont JE. Metabolic reprogramming of the tumor. Oncogene. 2012;31(36):3999–4011. [DOI] [PubMed] [Google Scholar]
- 5.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73(9):989–991. doi: 10.1038/ki.2008.104 [DOI] [PubMed] [Google Scholar]
- 6.Hung HY, Chen JS, Chien Y, et al. Preoperative alkaline phosphatase elevation was associated with poor survival in colorectal cancer patients. Int J Colorectal Dis. 2017;32(12):1775–1778. doi: 10.1007/s00384-017-2907-4 [DOI] [PubMed] [Google Scholar]
- 7.Namikawa T, Ishida N, Tsuda S, et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2019 Jul;22(4):684–691. doi: 10.1007/s10120-018-0897-8. [DOI] [PubMed] [Google Scholar]
- 8.Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15(8):2336–2344. . [DOI] [PubMed] [Google Scholar]
- 9.Marmorino F, Salvatore L, Barbara C, et al. Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer. 2017;116(3):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomarker. 2016;16(3):415–23. [DOI] [PubMed] [Google Scholar]
- 11.Lee DS, Park KR, Kim SJ, et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: predictive value of metastases and relation to survival outcomes. Tumor Biol. 2016;37(1):619–625. [DOI] [PubMed] [Google Scholar]
- 12.McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8 [DOI] [PubMed] [Google Scholar]
- 13.Deme D, Telekes A. [Prognostic importance of albumin in oncology]. Orv Hetil. 2018;159(3):96–106. [DOI] [PubMed] [Google Scholar]
- 14.Fruchtenicht AV, Poziomyck AK, Kabke GB, et al. Nutritional risk assessment in critically ill cancer patients: systematic review. Rev Bras Ter Intensiva. 2015;27(3):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142(6):1285–1297. doi: 10.1007/s00432-015-2113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang PA-O, Yeh KY, Wang CH, et al. Impact of the pretreatment glasgow prognostic score on treatment tolerance, toxicities, and survival in patients with advanced head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck. 2017;39(10):1990–1996. [DOI] [PubMed] [Google Scholar]
- 17.Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58(11):1048–1057. doi: 10.1097/DCR.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 19.Li D, Yuan X, Liu J, Li C, Li W. Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis. J Thorac Dis. 2018;10(9)5298–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AW, Chan SL. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie M, Sun P, Chen C, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cisplatin-based chemotherapy-treated patients with metastatic nasopharyngeal carcinoma. J Cancer. 2017;8(5):809–815. doi: 10.7150/jca.17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90–97. [DOI] [PubMed] [Google Scholar]
- 23.Vallieres E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(9):1049–1059. doi: 10.1097/JTO.0b013e3181b27799 [DOI] [PubMed] [Google Scholar]
- 24.Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers–a review. Eur J Cancer. 2012;48(9):1299–1311. doi: 10.1016/j.ejca.2012.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.