Abstract
Withdrawal from chronic alcohol drinking can cause depression, leading to an inability to function in daily life and an increased risk for relapse to harmful drinking. Understanding the causes of alcohol withdrawal-related depression may lead to new therapeutic targets for treatment. Epigenetic factors have recently emerged as important contributors to both depression and alcohol use disorder (AUD). Specifically, acetylation of the N-terminal tails of histone proteins that package DNA into nucleosomes is altered in stress-induced models of depression and during alcohol withdrawal. The goal of this study was to examine depression-like behavior during alcohol withdrawal and associated changes in histone acetylation and expression of histone deacetylase 2 (HDAC2) in the hippocampus, a brain region critical for mood regulation and depression. Male Sprague-Dawley rats were treated with the Lieber-DeCarli ethanol liquid diet for 15 days and then underwent withdrawal. Rats were treated with the HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA), during withdrawal and tested for depression-like behavior. In a separate group of rats, the hippocampus was analyzed for mRNA and protein expression of HDAC2 and levels of histone H3 lysine 9 acetylation (H3K9ac) during chronic ethanol exposure and withdrawal. Rats undergoing ethanol withdrawal exhibited depression-like behavior and had increased HDAC2 and decreased H3K9ac levels in specific structures of the hippocampus. Treatment with SAHA during withdrawal ameliorated depression-like behavior and normalized changes in hippocampal HDAC2 and H3K9ac levels. These results demonstrate that ethanol withdrawal causes an altered epigenetic state in the hippocampus. Treatment with an HDAC inhibitor can correct this state and alleviate depression-like symptoms developed during withdrawal. Targeting histone acetylation may be a novel strategy to reduce ethanol withdrawal-induced depression.
Keywords: Alcohol withdrawal, depression, epigenetic, HDAC, Vorinostat, hippocampus
Introduction
Alcohol use disorder (AUD) is highly comorbid with depression (Boden & Fergusson, 2011). One reason for this comorbidity is that abstinence from chronic alcohol drinking can cause depression (Petit, Luminet, Cordovil de Sousa Uva, Monhonval, et al., 2017; Petit, Luminet, Cordovil de Sousa Uva, Zorbas, et al., 2017). The functional link between AUD and depression is supported by preclinical studies demonstrating increased depression-like behavior in rodents during alcohol withdrawal (Anton et al., 2017; Getachew, Hauser, Csoka, Taylor, & Tizabi, 2017; Kang, Li, Bekker, & Ye, 2018; Li et al., 2017; Pang, Renoir, Du, Lawrence, & Hannan, 2013; Roni & Rahman, 2017; Walker et al., 2010; Yawalkar, Changotra, & Gupta, 2018). Depression during abstinence from alcohol also contributes to relapse to alcohol drinking, thus perpetuating the cycle of addiction (Greenfield et al., 1998; Oliva et al., 2018). Depression and alcohol abuse negatively impact overall health, productivity, and quality of life. A better understanding of the brain mechanisms contributing to alcohol withdrawal-induced depression would be extremely helpful in the development of novel therapeutic approaches effective in reducing comorbid alcohol abuse and depression.
The hippocampus is an important region of the brain for modulating emotional states such as depression (Akil et al., 2018; Fanselow & Dong, 2010). Chronic alcohol consumption is associated with decreased hippocampal volume, as measured using magnetic resonance imaging in alcohol-dependent subjects compared with controls (Beresford et al., 2006). Notably, this is also observed in patients with major depressive disorder (Malykhin, Carter, Seres, & Coupland, 2010), suggesting a link between alcohol-induced changes in the hippocampus and depression. Numerous molecules, signal transduction pathways, and basic cellular processes in the hippocampus are causally linked with depression. These include alterations in the serotonergic system, brain-derived neurotrophic factor (BDNF) expression, cyclic AMP response element binding protein (CREB) activity, neurogenesis, and neuroinflammation (Kubera, Obuchowicz, Goehler, Brzeszcz, & Maes, 2011; Rogers, Renoir, & Hannan, 2017).
Epigenetic mechanisms, such as acetylation of the N-terminal tails of histone proteins that package DNA into nucleosomes, also play an important role in depression (Misztak, Panczyszyn-Trzewik, & Sowa-Kucma, 2018). Levels of histone acetylation and the histone deacetylases (HDACs) that remove acetyl groups from the histone tails and thus decrease chromatin accessibility, are altered in the hippocampus of mice and rats in various models of stress-induced depression (Covington, Vialou, LaPlant, Ohnishi, & Nestler, 2011; Ferland & Schrader, 2011; Han, Sung, Chung, & Kwon, 2014; Kv et al., 2018; Liu et al., 2014; Tsankova et al., 2006). Of note, systemic or intra-hippocampal treatment of animals with HDAC inhibitors can reduce depression-like behavior (Covington et al., 2011; Gundersen & Blendy, 2009; Han et al., 2014; Liu et al., 2014; Yamawaki et al., 2012) and injection of MS-275 (Entinostat) directly into the hippocampus of mice subjected to chronic social defeat stress reversed the deficit in sucrose preference and increased social interaction and acetylated histone H3 levels in the hippocampus (Covington et al., 2011).
Similar to stress-induced models of depression, withdrawal from chronic alcohol exposure also has profound effects on histone acetylation and HDAC expression (Pandey, Ugale, Zhang, Tang, & Prakash, 2008; Simon-O’Brien et al., 2015; You, Zhang, Sakharkar, Teppen, & Pandey, 2014). Decreased histone H3 and H4 acetylation and elevated HDAC activity were observed in the amygdala of rats during withdrawal from chronic alcohol drinking, along with an associated increase in anxiety-like behavior (Pandey et al., 2008; You et al., 2014). Treatment of rats during ethanol withdrawal with the histone deacetylase inhibitor trichostatin A (TSA) attenuated anxiety-like behavior and restored normal levels of histone acetylation in the amygdala (Pandey et al., 2008; You et al., 2014). Additionally, in a rat model of dependence induced by ethanol vapor exposure, treatment with MS-275 decreased drinking only in ethanol-dependent rats and altered histone acetylation in various brain regions (Simon-O’Brien et al., 2015). In mice subjected to repeated ethanol injections and withdrawal, histone H3 lysine 9 acetylation (H3K9ac) was decreased and HDAC2 expression increased in the ventral tegmental area (Arora et al., 2013). Interestingly, adolescent alcohol exposure leads to an increase in HDAC activity and deficits in H3K9ac in rat hippocampus that are associated with decreased neurogenesis in adulthood. These changes were normalized by TSA treatment (Sakharkar et al., 2016). Together, these results indicate that alcohol withdrawal leads to dysregulated histone acetylation due to an increase in HDAC2 expression in several brain regions and that treatment with HDAC inhibitors can normalize this imbalance and alleviate negative affective states induced by alcohol withdrawal. However, histone acetylation and HDAC2 expression in the hippocampus and associated depression-like behavior during withdrawal after chronic ethanol exposure have not been examined.
In this study, we hypothesized that withdrawal from chronic alcohol exposure would result in depression-like behavior and lead to a corresponding decrease in histone acetylation associated with an increase in HDAC2 expression in the hippocampus. Therefore, we investigated depression-like behavior and levels of HDAC2 and H3K9ac in the hippocampus during alcohol withdrawal. We also tested if treatment with the HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA, also known as Vorinostat), would be effective in attenuating depression-like behavior induced by alcohol withdrawal and normalize H3K9ac levels and HDAC2 expression. Our results provide preclinical evidence that using an HDAC inhibitor in individuals with comorbid AUD and depression, particularly alcohol-induced depression, might be an effective novel therapeutic strategy to treat these disorders.
Materials and methods
Animals
Adult male Sprague-Dawley rats (postnatal day 75–85) weighing ~250 g were purchased from Harlan (Indianapolis, IN) and individually housed in a temperature-controlled room with a 12/12 hour light/dark cycle, with food and water provided ad libitum prior to beginning the chronic ethanol treatment protocol described below. All procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Chronic ethanol treatment
Rats were fed with the nutritionally complete Lieber-DeCarli control liquid diet or ethanol liquid diet (Bio-Serv, Frenchtown, NJ) as their only source of food and fluid as previously described (Pandey et al., 2008). Rats were randomly assigned to three treatment groups: (i) control diet fed, (ii) ethanol diet fed (no withdrawal), and (iii) ethanol diet fed with a 24-hour withdrawal, referred to as the ethanol-withdrawn group. Briefly, rats were first fed 80 ml/day control diet for 3 days. The control group continued with control diet for 16 days, while the ethanol groups were gradually introduced to ethanol (1.8% to 8.1% within 7 days), and then maintained on 9% v/v ethanol diet for 15 days. Ethanol-withdrawn rats were switched to control liquid diet for 24 hours after removal of the ethanol liquid diet. One week before behavioral testing (described below), rats were handled for 3 minutes per day by the same individual to habituate them to handling. Rats were pair fed and their liquid diet intake and body weights were closely monitored. We have previously reported blood ethanol levels in the range of 172 to 198 mg % for ethanol diet fed (no withdrawal) rats, whereas the blood ethanol level after 24 hours of ethanol withdrawal was undetectable using this alcohol treatment paradigm (Pandey et al., 2008; You et al., 2014).
Drug treatment
Rats in the control and ethanol-withdrawn groups were treated intraperitoneally (IP) with either 50 mg/kg SAHA (Selleck Chemicals, Houston, TX) in 2% DMSO, 40% PEG 300, 5% Propylene glycol, and 1% Tween 80 (vehicle) or vehicle, 2 hours before behavioral testing or euthanasia for tissue collection at 24 hours of withdrawal. All rats were euthanized by decapitation under isoflurane anesthesia.
Sucrose splash test
Depression-like behavior was measured using the splash test as described by Marrocco et al. (Marrocco et al., 2014). A 10% sucrose solution was sprayed on the dorsal coat and abdomen of each rat in a clear cage to induce grooming behavior. The time the rat spent grooming and latency to groom was recorded for a period of 5 minutes after application of the sucrose solution. This test takes advantage of the natural propensity of the rats to groom, with a reduction in grooming and increased latency to groom indicating anhedonia and a lack of motivation for self-care. Decreased grooming time in the splash test is observed in rats after manipulations that induce multiple indices of depression-like behavior such as prenatal restraint stress, maternal separation, and chronic unpredictable mild stress (Hu et al., 2017; Marrocco et al., 2014; Masrour, Peeri, Azarbayjani, & Hosseini, 2018). Moreover, treatment with antidepressant medications such as fluoxetine normalizes grooming time in the splash test in mice and rats (Marrocco et al., 2014; Masrour et al., 2018; Santarelli et al., 2003; Surget et al., 2008; Yalcin, Belzung, & Surget, 2008).
Sucrose preference test
Bottles containing the control liquid diet were removed from the home cage at 24 hours of withdrawal (of both ethanol-withdrawn and control rats) and replaced with two standard water bottles containing either a 0.5% sucrose solution or water. Bottles were weighed after 1 hour and sucrose preference was calculated as the volume of sucrose solution consumed divided by the total volume of fluid consumed x 100. Decreased sucrose preference is indicative of anhedonia, a defining symptom of major depressive disorder, and is normalized by chronic treatment with antidepressants (Dichter, Damiano, & Allen, 2012; Papp, Moryl, & Willner, 1996; Willner, Towell, Sampson, Sophokleous, & Muscat, 1987).
In situ reverse transcription-polymerase chain reaction (RT-PCR)
In situ RT-PCR of Hdac2 mRNA was performed on 40 μm thick coronal brain sections, as described previously (Pandey et al., 2008; Zhang et al., 2010). Briefly, brain sections were treated with proteinase K and then digested with DNase. Sections were rinsed in PBS and transferred to PCR tubes containing 100 μl of PCR reaction mixture (Applied Biosystems, Foster City, CA) to reverse transcribe for 1 h at 42°C with oligo d(T)16 and reverse transcriptase (RT) enzyme. PCR was then performed as follows: 95°C for 5 minutes; 95°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds, for a total of 25 cycles; 72°C for 7 minutes using Taq DNA polymerase enzyme, 100 pmol each of Hdac2 forward and reverse primers (Hdac2 forward: 5′-CGGTGGCTCAGTTGCTGGGG; Hdac2 reverse: 5′-GGCCTCTGACTTCTTGGCGTGG) and 1 mM of each NTP (except that dTTP was replaced by digoxigenin (DIG)-11-dUTP) in 100 μl reaction mixture. Sections were then mounted on slides and Hdac2 mRNA-positive cells were detected using an alkaline phosphatase conjugated anti-DIG antibody with nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3’-indolyphosphate p-toluidine (NBT-BCIP) as the substrate, yielding a purple reaction product (Roche Molecular Biochemical, Mannheim, Germany). The intensity of Hdac2-positive cell bodies in the CA1, CA3 and dentate gyrus (DG) region of the dorsal hippocampus (approximately −2.3 to −3.3 mm posterior to bregma) was obtained by outlining the cell body layer and measuring the intensity using NIH ImageJ software from each section of 2–3 brain sections from each rat. Values were averaged and presented as mean intensity/100 μm2.
Gold immunolabeling of HDAC2 and H3K9ac
HDAC2 and acetylated H3K9ac proteins were measured in the dorsal hippocampus of the rat brain using gold immunolabeling (Pandey et al., 2008; Sakharkar et al., 2014). Free floating 20 μm thick coronal sections were incubated with antibodies against HDAC2 (catalog number JM-3602–100, MBL International, Woburn, MA) and H3K9ac (catalog number 06–942, Millipore, Billerica, MA). After washing with PBS, the sections were incubated in gold particle-labeled goat anti-rabbit secondary antibodies (Nanoprobes, Yaphank, NY) and were counter-stained using the silver enhancement kit (Ted Pella, Redding, CA). Gold immunolabeling was quantified using an image analysis system (Loats Associates, Westminster, MD) at 100× magnification. We first set up a threshold for counting by outlining an area without staining, and then several object fields in each section were used for counting immunogold particles. Mean counts for each group were calculated and results are presented as the mean (± SEM) of the number of immunogold particles/100 μm2 area in the Cornu Ammonis (CA)1, CA3, and dentate gyrus (DG) regions of the hippocampus.
Statistical analysis
All data are presented as the mean ± SEM. Statistical testing was performed on the in situ RT-PCR and immunogold labeling data with a one-way analysis of variance (ANOVA) within each region of the hippocampus, followed by Tukey’s multiple comparisons test. Behavioral data was analyzed using a two-way ANOVA followed by post hoc comparisons using Sidak’s multiple comparisons tests. Statistical tests were performed using Prism software (GraphPad, San Diego, CA, version 7). A value of p < 0.05 was considered to be significant.
Results
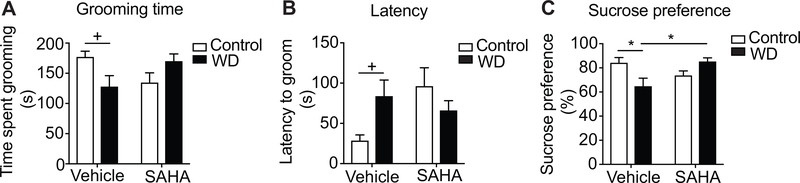
Withdrawal from chronic ethanol drinking causes depression-like behavior that is reversed by SAHA treatment
Withdrawal from chronic ethanol exposure is associated with a depression-like state that has been observed behaviorally in mice and rats (Anton et al., 2017; Getachew et al., 2017; Kang et al., 2018; Li et al., 2017; Pang et al., 2013; Roni & Rahman, 2017; Walker et al., 2010; Yawalkar et al., 2018). We examined depression-like behavior using the splash test in rats that were fed with a control or chronic ethanol Lieber DeCarli diet and underwent 24 hours of withdrawal. We also tested for the effect of the HDAC inhibitor SAHA, given acutely 2 hours prior to testing, since numerous studies have demonstrated that HDAC inhibitors, including SAHA, can reduce depression-like behavior in rats and mice (reviewed in (Misztak et al., 2018)). Rats withdrawn from chronic ethanol drinking groomed less and had increased latency to groom in the splash test, indicative of depression-like behavior, while treatment with SAHA normalized grooming time (Fig. 1A-B). A two-way ANOVA indicated a significant withdrawal by SAHA interaction in grooming time [F (1, 47) = 8.18, p = 0.006] and latency to groom [F (1, 47) = 5.86, p =0.019], but no main effects of ethanol withdrawal [grooming time: F (1, 47) = 0.21, p = 0.65; latency: F (1, 47) = 0.51, p = 0.48] or SAHA treatment [grooming time: F (1, 47) = 0.00028, P =0.99; latency: F (1, 47) = 2.0, p = 0.16]. Post-hoc multiple comparisons tests demonstrated that there was a nearly significant decrease in grooming time and increased latency to groom between control and ethanol-withdrawn rats within the vehicle-treated group (grooming time: p = 0.054; latency: p = 0.072), but no difference between control and ethanol-withdrawn rats within the SAHA-treated group (grooming time: p = 0.16; latency: p = 0.39). Latency to groom was highly variable, and SAHA-treated rats drinking control liquid diet exhibited an increased latency to groom compared with vehicle-treated controls, indicating that SAHA delays the initiation of behavior in the control rats. Nonetheless, SAHA-treated rats drinking control diet did not significantly differ in total grooming time when compared to vehicle-treated controls.
Fig. 1. Withdrawal from chronic ethanol drinking results in depression-like behavior that is reversed by SAHA treatment.
Rats drank control or ethanol liquid diet for 15 days. Ethanol diet was then removed and replaced with control diet for 24 hours prior to behavioral testing. SAHA (50 mg/kg, IP) or vehicle was administered 2 hours prior to testing. A. Time spent grooming over 5 minutes. Control/vehicle, n = 11; withdrawal/vehicle, n = 13; control/SAHA, n = 14; withdrawal/SAHA, n = 13. +p = 0.054 by post-hoc Sidak’s multiple comparisons test. B. Latency to groom. +p = 0.072. C. Sucrose preference. Control/vehicle, withdrawal/vehicle, and withdrawal/SAHA, n = 15; control/SAHA, n = 16; *p < 0.05. Abbreviations: WD, ethanol withdrawal. Data is shown as means ± SEM.
We next confirmed depression-like behavior during withdrawal from chronic ethanol drinking using the sucrose preference test. Ethanol-withdrawn rats had decreased sucrose preference compared with control rats and treatment with SAHA increased preference for sucrose to control levels (Fig. 1C). A two-way ANOVA indicated a significant ethanol withdrawal by SAHA interaction in sucrose preference [F (1, 57) = 11.18, p = 0.0015], but no main effects of ethanol withdrawal or SAHA treatment [withdrawal: F (1, 57) = 0.73, p = 0.398; SAHA: F (1, 57) = 1.16, p = 0.287]. Post-hoc multiple comparisons tests showed a significant difference between vehicle-treated control vs. ethanol-withdrawn rats (p = 0.028) and vehicle-treated, ethanol-withdrawn rats vs. SAHA-treated, ethanol-withdrawn rats (p = 0.018). These results indicate that depression-like behavior is observed in rats during withdrawal from chronic ethanol drinking and that acute treatment with SAHA during withdrawal attenuates the depression phenotype.
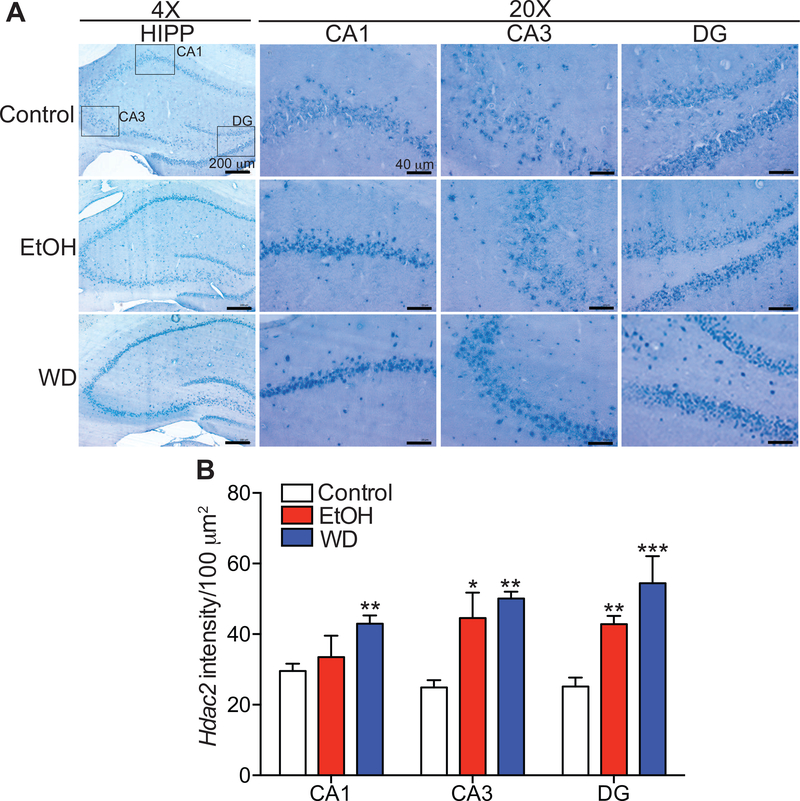
Increased Hdac2 mRNA expression in the hippocampus during chronic ethanol drinking and withdrawal
We next examined the expression of Hdac2 transcript in the hippocampus during withdrawal. Hdac2 mRNA expression was higher in the CA1, CA3 and DG regions of the hippocampus during ethanol drinking and withdrawal by in situ RT-PCR (Fig. 2). There were significant treatment effects by one-way ANOVA in all three regions [CA1, F (2, 17) = 6.38, p = 0.0086; CA3, F (2, 14) = 11.41, P = 0.0012; DG, F (2, 12) = 16.14, p = 0.0004]. Post-hoc multiple comparisons tests showed that Hdac2 expression increased in the CA1 (p = 0.0072), CA3 (p = 0.0012), and DG (p = 0.0005) during withdrawal when compared with the control group. Moreover, Hdac2 expression increased in the CA3 (p = 0.012) and DG (p = 0.0052) regions of the ethanol-drinking group (no withdrawal) compared with the control group. These results indicate that chronic ethanol drinking and withdrawal result in increased expression of Hdac2 mRNA in the hippocampus.
Fig. 2. Increased Hdac2 mRNA expression in the hippocampus during chronic ethanol drinking and withdrawal.
In situ RT-PCR for Hdac2 was performed on brain sections containing the hippocampus from rats that drank control or ethanol (EtOH) liquid diet and then underwent withdrawal (WD) from the EtOH diet for 24 hours. A. Representative images of the hippocampus (HIPP) in sections at 4× and 20× magnification with boxes in the 4× image showing the cornus ammonis (CA)1, CA3, and dentate gyrus (DG) regions that were analyzed at 20×. Scalebars: 4×, 200 μm; 20× 40 μm. B. Quantified intensity of Hdac2 RT-PCR digoxigenin signal in each region of the hippocampus using NIH ImageJ software (mean ± SEM). CA1: control, n = 8; EtOH, n = 4; WD, n = 8; CA3: control, n = 6; EtOH, n =5; WD, n = 6; DG: control, n = 6; EtOH, n =6; WD, n =3. *p < 0.05, **p < 0.01, and ***p < 0.001, by post-hoc Tukey’s test when compared with control group.
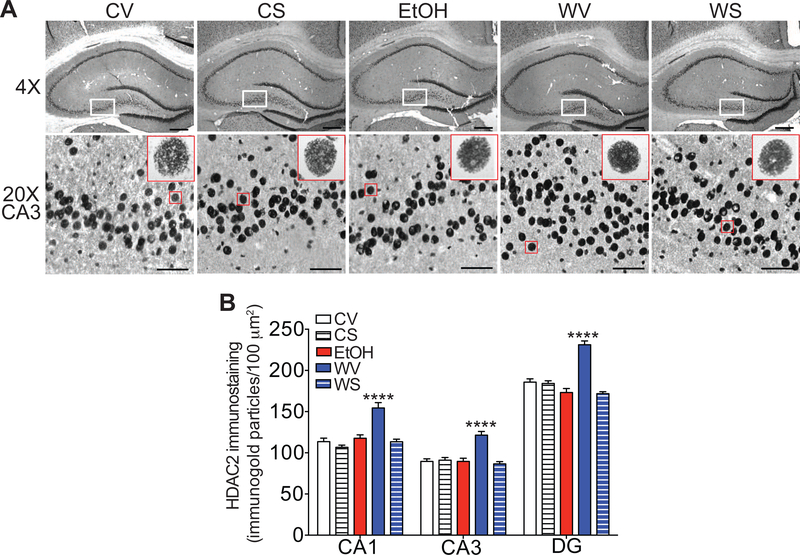
Increased HDAC2 protein in the hippocampus during withdrawal from chronic ethanol drinking is normalized by SAHA treatment
To determine if the increase in Hdac2 mRNA in the hippocampus during ethanol withdrawal results in an increase in HDAC2 protein, we next measured protein levels of HDAC2 in the hippocampus using the gold immunolabeling procedure. HDAC2 protein levels were significantly increased in the CA1, CA3, and DG regions of the hippocampus during withdrawal (Fig. 3). Notably, HDAC2 protein returned to control levels when rats were treated with SAHA during withdrawal (Fig. 3). One-way ANOVAs revealed significant effects of treatment in all three regions of the hippocampus [CA1, F (4, 20) = 19.29, p < 0.0001; CA3, F (4, 20) = 16.65, p < 0.0001; DG: F (4, 20) = 38.10, p < 0.0001], with post-hoc multiple comparisons tests showing a significant increase in HDAC2 expression in rats treated with vehicle during withdrawal compared with vehicle-treated control diet rats (p < 0.0001 for CA1, CA3, and DG), and a significant decrease in HDAC2 expression in rats treated with SAHA during withdrawal when compared with the withdrawal-vehicle group (p < 0.0001 for CA1, CA3, and DG). There were no significant differences in HDAC2 expression between rats that had the control diet (both vehicle- and SAHA-treated) and rats treated with SAHA during withdrawal, indicating that HDAC2 protein levels returned to normal after SAHA treatment in the ethanol-withdrawn rats. These results indicate that, in addition to increased Hdac2 transcript in the hippocampus during ethanol withdrawal, there is an associated increase in HDAC2 protein, which is normalized by SAHA treatment.
Fig. 3. Increased HDAC2 protein in the hippocampus during withdrawal from chronic ethanol drinking is normalized by SAHA treatment.
Immunohistochemistry using gold-labeled antibodies to HDAC2 was performed on brain sections containing the hippocampus from rats that drank control or ethanol (EtOH) liquid diet and then underwent withdrawal from the EtOH diet for 24 hours. Rats were also treated with vehicle or SAHA 2 hours prior to tissue collection. A. Top panels, representative images of the hippocampus at 4× magnification. Scalebar: 200 μm. White box outlines area shown at higher magnification in bottom panels. Bottom panels, representative images of the CA3 region of the hippocampus at 20× magnification. Scalebar: 50 μm. The red box outlines a representative magnified cell shown in the inset at high magnification (100×). B. Quantification of immunogold particles per 100 μm2 area of each region. All data are shown as mean ± SEM, n = 5 rats per treatment group. ****p < 0.0001 when compared with all other groups by post-hoc Tukey’s test. Abbreviations: CV, control-vehicle; CS, control-SAHA; EtOH, ethanol group with no withdrawal; WV, withdrawal-vehicle; WS, withdrawal-SAHA; CA, cornus ammonis; DG, dentate gyrus.
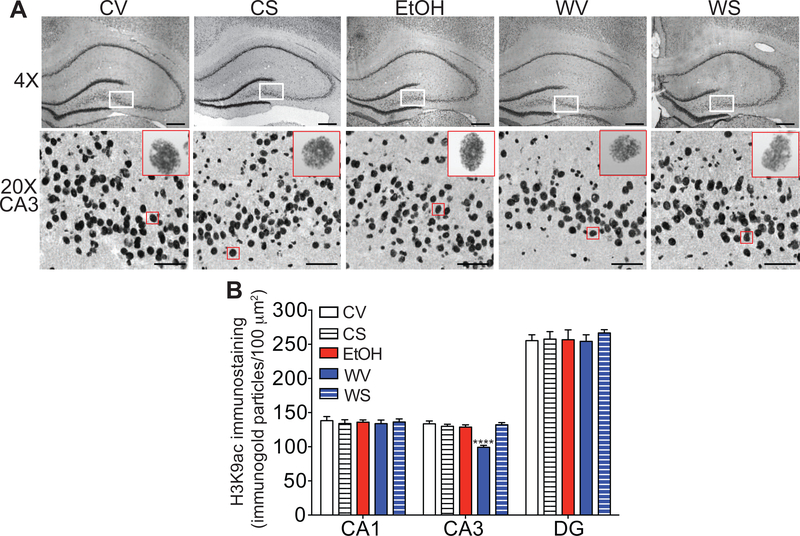
Decreased H3K9ac in the hippocampus during withdrawal from chronic ethanol drinking is normalized by SAHA treatment
Increased HDAC2 expression in the hippocampus during withdrawal from chronic ethanol drinking is predicted to cause a corresponding decrease in histone acetylation, while inhibition of HDACs with SAHA would increase histone acetylation. We measured global H3K9ac in the hippocampus during ethanol withdrawal using gold immunolabeling. There was a significant reduction in H3K9ac immunogold particles in the CA3 region during ethanol withdrawal that returned to control levels after SAHA treatment (Fig. 4). A one-way ANOVA showed significant treatment effects [F (4, 20) = 18.47, p < 0.0001], with post-hoc comparisons demonstrating a significant decrease in H3K9ac in withdrawal-vehicle rats compared with all other groups (p < 0.0001). H3K9ac was not altered in the DG or CA1 regions during withdrawal [one-way ANOVA, DG: F (4, 20) = 0.23, p = 0.92; CA1: F (4, 20) = 0.128, p = 0.97]. Together, these results indicate that hippocampal CA3 H3K9ac levels are decreased during withdrawal from chronic ethanol exposure and that normal levels can be restored by treatment with the HDAC inhibitor SAHA.
Fig. 4. Decreased H3K9ac in the hippocampus during withdrawal from chronic ethanol drinking is normalized by SAHA treatment.
Immnohistochemistry with gold-labeled antibodies to H3K9ac was performed on brain sections containing the hippocampus from rats that drank control or ethanol (EtOH) liquid diet and then underwent withdrawal from the liquid diet for 24 hours. Rats were also treated with vehicle or SAHA 2 hours prior to tissue collection. A. Top panels, representative images of the hippocampus at 4× magnification. Scalebar: 200 μm. White box outlines area shown at higher magnification in bottom panels. Bottom panels, representative images of the CA3 region of the hippocampus at 20× magnification. Scalebar: 50 μm. The red box outlines a representative magnified cell shown in the inset at high magnification (100×). B. Quantification of immunogold particles per 100 μm2 area of each region. All data are shown as mean ± SEM, n = 5 rats per treatment group. ****p < 0.0001 when compared with all other groups by post-hoc Tukey’s test. Abbreviations: CV, control-vehicle; CS, control-SAHA; EtOH, ethanol group with no withdrawal; WV, withdrawal-vehicle; WS, withdrawal-SAHA; CA, cornus ammonis; DG, dentate gyrus.
Discussion
Withdrawal from alcohol drinking causes negative mood states that contribute to relapse, including anxiety and depression. Several studies in rats and mice have shown that depression-like behavior is induced during ethanol withdrawal (Anton et al., 2017; Getachew et al., 2017; Kang et al., 2018; Li et al., 2017; Pang et al., 2013; Roni & Rahman, 2017; Walker et al., 2010; Yawalkar et al., 2018). In this study, we confirmed that rats exhibit depression-like behavior during withdrawal from an ethanol liquid diet using two behavioral measures and demonstrate that systemic treatment with the HDAC inhibitor SAHA during withdrawal alleviates depression-like behavior. In addition, we showed that ethanol withdrawal increases HDAC2 expression, with an associated decrease in H3K9ac in the hippocampus, alterations that are normalized by acute treatment with SAHA during ethanol withdrawal. This effect was apparent 2 hours following treatment with SAHA, which is consistent with previous studies demonstrating that acute treatment with the HDAC inhibitor TSA can reduce anxiety-like behavior, increase Npy, Bdnf, and Arc gene expression, and increase dendritic spine density in the central and medial nuclei of the amygdala in a similar time frame (Pandey et al., 2008; You et al., 2014). In addition, 2 hours after treatment with SAHA, H3K9ac and sensitivity to GABA in the VTA are increased (You et al., 2018). Collectively, these data indicate that SAHA can act fairly rapidly to alter both gene expression and behavior. Together, our results demonstrate that treatment with SAHA might be an effective strategy to reduce depression during alcohol withdrawal and identify an epigenetic change in the hippocampus as a potential molecular contributor to this condition.
Several behavioral tests have been used to measure depression-like behavior in rats and mice. These include the forced swim test (FST), sucrose preference test, learned helplessness test, and splash test (Akil et al., 2018). Studies of depression-like behavior during ethanol withdrawal in rats have employed the FST and found increased immobility time in animals during withdrawal (Anton et al., 2017; Getachew et al., 2017; Kang et al., 2018; Li et al., 2017; Walker et al., 2010; Yawalkar et al., 2018). In this study, we used the splash test because it is a straightforward measure of depression that takes advantage of the natural propensity of the animal to groom, with less grooming time indicating decreased motivation for self-care. This characteristic is similar in humans diagnosed with depression. In addition, the splash test does not require any prior habituation session, which is needed when performing the FST with rats (Slattery & Cryan, 2012), thus allowing the investigation of depression-like behavior after 24 hours of withdrawal without prior training during the intoxication period. Decreased grooming in the splash test is observed after chronic unpredictable mild stress, a well-validated model of depression (Hu et al., 2017; Isingrini et al., 2010; Surget et al., 2008; Yalcin et al., 2008). The splash test has also been pharmacologically validated using fluoxetine, with fluoxetine treatment increasing grooming time (Marrocco et al., 2014; Masrour et al., 2018; Santarelli et al., 2003; Surget et al., 2008; Yalcin et al., 2008). To our knowledge, this is the first time the splash test has been used to measure depression-like behavior during ethanol withdrawal. We propose that it is an easily employed measure of ethanol withdrawal-induced depression that does not cause undue stress to the animal. Importantly, our findings in the splash test were confirmed using another simple test, the sucrose preference test, which verified that ethanol withdrawal-induced depression-like behavior is ameliorated by SAHA treatment.
Our finding that treatment with SAHA during withdrawal alleviates depression-like behavior is consistent with the effect of SAHA in reducing depression in stress-induced models of depression. Systemic treatment of mice with SAHA during chronic mild unpredictable stress sessions reduced depression- and anxiety-like behavior as measured using the social interaction test, sucrose preference test, novelty-suppressed feeding, and FST (Uchida et al., 2011). Microinjection of SAHA into the nucleus accumbens reversed stress-induced social aversion and decreased immobility time in the FST (Covington et al., 2009). In a different model of depression involving repeated injections of corticosterone in mice, SAHA corrected several measures of anxiety and depression, including grooming time in the splash test, and normalized hypothalamic-pituitary-adrenal axis reactivity and inflammatory-related gene expression in the hippocampus (Kv et al., 2018). SAHA was also able to decrease depression-like behavior exhibited by mice with a knockout of the CREB-regulated transcription coactivator-1 (Crtc1) gene when mice were tested in a repeated open-space forced swim procedure, but not in the novelty-suppressed feeding test (Meylan, Halfon, Magistretti, & Cardinaux, 2016). Overall, SAHA appears to be an effective treatment for anxiety and depression in multiple animal models of these psychopathological conditions.
Previous work found that treatment with the HDAC inhibitor TSA alleviated ethanol withdrawal-induced anxiety, as measured using the light-dark box and elevated plus maze (Pandey et al., 2008; You et al., 2014). Our results with the splash and sucrose preference tests add another dimension to the negative affective state observed during ethanol withdrawal using the same treatment paradigm and suggest that these rats undergoing withdrawal not only demonstrate anxiety-like behaviors but also show depression-like behaviors as indicated in the current study. The effect of SAHA on depression-like behavior during withdrawal is consistent with the effect of TSA on anxiety-like behavior. Both SAHA and TSA are pan- HDAC inhibitors, so the specific HDAC target of these compounds in decreasing anxiety and depression is not known. We demonstrate here that HDAC2 transcript and protein expression are higher in the hippocampus during ethanol withdrawal. Previous studies have found that the innately anxious and high alcohol-preferring P rats have increased HDAC2 in the amygdala, and infusion of an HDAC2 siRNA into the amygdala or treatment with TSA decreased anxiety-like and alcohol drinking behaviors (Moonat, Sakharkar, Zhang, Tang, & Pandey, 2013; Sakharkar et al., 2014). HDAC2 expression in the amygdala is also observed after binge alcohol exposure in rats (Lopez-Moreno et al., 2015) and in adulthood after adolescent alcohol exposure (Pandey, Sakharkar, Tang, & Zhang, 2015). Interestingly, treatment with TSA was able to attenuate adolescent alcohol exposure-induced anxiety-like and alcohol drinking behaviors in adulthood (Pandey et al., 2015). These results implicate HDAC2 as a putative target of SAHA involved in depression-like as well as anxiety-like behavior during withdrawal. We measured mRNA expression by qPCR of Hdac1–10 in the hippocampus during withdrawal from chronic ethanol drinking and found that Hdac6 was the only other HDAC family member increased during withdrawal (unpublished results). Since SAHA is a pan-HDAC inhibitor, it is possible that the effect of SAHA on reducing depression-like behavior during withdrawal could be due to inhibition of either HDAC6 or HDAC2. Both of these HDACs have been implicated in depression (Jochems et al., 2014; Uchida et al., 2011). Future work will explicitly address whether increased HDAC2, specifically in the hippocampus, is responsible for depression induced by ethanol withdrawal.
Associated with the increase in HDAC2 was a corresponding decrease in H3K9ac in the CA3 region of the hippocampus during alcohol withdrawal, and given that H3K9ac is an HDAC2 substrate, our results are consistent with higher levels of HDAC2 directly acting to deacetylate H3K9ac during ethanol withdrawal. Treatment with SAHA increased total levels of H3K9ac in the CA3 region of the hippocampus. Although we observed an overall increase in HDAC2 in the CA1, CA3, and DG regions of the hippocampus during ethanol withdrawal, the associated change in H3K9ac was only observed in the CA3 region. It is possible that H3K9ac was reduced in the CA1 and DG, but occurred at the level of individual gene promoters, which would not be detectable when measuring total H3K9ac. One intriguing finding is that treatment of control rats with SAHA did not increase total H3K9ac in the hippocampus. It is possible that HDAC2 activity is very low under control conditions and that the increase in HDAC2 during withdrawal provides more active HDAC2 for inhibition by SAHA. Indeed, Guan et al did not observe a significant increase in acetylated lysines in the hippocampus of control mice treated with SAHA (Guan et al., 2009), and treatment with another pan-HDAC inhibitor, TSA, also does not alter H3K9ac in the amygdala of control rats (Pandey et al., 2008; Sakharkar, Zhang, Tang, Shi, & Pandey, 2012; You et al., 2014).
A putative target gene modified by H3K9ac and HDAC2 that might be involved in depression during alcohol withdrawal is Bdnf. Numerous evidence supports the hypothesis that low levels of BDNF in the hippocampus are associated with depression (Bjorkholm & Monteggia, 2016) and that Bdnf expression is regulated by H3K9ac through the histone acetyltransferase CREB binding protein (CBP) (Hing, Sathyaputri, & Potash, 2018) and HDAC2 (Guan et al., 2009). Decreased BDNF in the hippocampus is observed during alcohol withdrawal in rats, which is normalized by antidepressant treatment (Hauser, Getachew, Taylor, & Tizabi, 2011). In addition, adolescent rats exposed to a binge ethanol exposure protocol had decreased BDNF in the hippocampus and exhibited depression-like behavior during withdrawal, which was ameliorated by intra-hippocampal infusion of an agonist to the BDNF receptor TrkB (Briones & Woods, 2013). However, other genes involved in depression might also be regulated by histone acetylation during ethanol withdrawal. Increased inflammation-related genes such as cytokines and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling molecules are also observed in the hippocampus during ethanol withdrawal (Doremus-Fitzwater et al., 2014; Gano, Doremus-Fitzwater, & Deak, 2016; He & Crews, 2008; Kane et al., 2014; Pascual, Balino, Aragon, & Guerri, 2015), and HDAC inhibitors reduce expression of neuroimmune genes in glial cells in culture and in the brain (Faraco et al., 2009; Kazantsev & Thompson, 2008; Patnala, Arumugam, Gupta, & Dheen, 2017). It is likely that multiple genes expressed in the hippocampus are under epigenetic control and contribute to the negative affective state during alcohol withdrawal. Investigation into these epigenetic mechanisms will no doubt lead to potential new therapeutic targets for the treatment of comorbid AUD and depression.
Highlights.
SAHA treatment reduces depression-like behavior during ethanol withdrawal.
HDAC2 expression increases in the hippocampus during ethanol withdrawal.
Acetylated histone H3 decreases in the hippocampus during ethanol withdrawal.
Acknowledgments
Funding
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (Center for Alcohol Research in Epigenetics, P50 AA022538 to SCP and AWL, and the INIA Consortium U01 AA020912 to AWL). SCP is also supported by Senior Research Career Scientist Award by the Department of Veterans Affairs.
Footnotes
Conflicts of Interest
No biomedical financial interests or potential conflicts of interest were reported by authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, . . . Nestler EJ (2018). Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev, 84, 272–288. doi: 10.1016/j.neubiorev.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton M, Alen F, Gomez de Heras R, Serrano A, Pavon FJ, Leza JC, . . . Orio L (2017). Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addict Biol, 22(3), 724–741. doi: 10.1111/adb.12365 [DOI] [PubMed] [Google Scholar]
- Arora DS, Nimitvilai S, Teppen TL, McElvain MA, Sakharkar AJ, You C, . . . Brodie MS (2013). Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors. Neuropsychopharmacology, 38(9), 1674–1684. doi: 10.1038/npp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, . . . Davatzikos C (2006). Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res, 30(11), 1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x [DOI] [PubMed] [Google Scholar]
- Bjorkholm C, & Monteggia LM (2016). BDNF - a key transducer of antidepressant effects. Neuropharmacology, 102, 72–79. doi: 10.1016/j.neuropharm.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, & Fergusson DM (2011). Alcohol and depression. Addiction, 106(5), 906–914. doi: 10.1111/j.1360-0443.2010.03351.x [DOI] [PubMed] [Google Scholar]
- Briones TL, & Woods J (2013). Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience, 254, 324–334. doi: 10.1016/j.neuroscience.2013.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, . . . Nestler EJ (2009). Antidepressant actions of histone deacetylase inhibitors. J Neurosci, 29(37), 11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Vialou VF, LaPlant Q, Ohnishi YN, & Nestler EJ (2011). Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett, 493(3), 122–126. doi: 10.1016/j.neulet.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, & Allen JA (2012). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord, 4(1), 19. doi: 10.1186/1866-1955-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, & Deak T (2014). Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res, 38(8), 2186–2198. doi: 10.1111/acer.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. doi: 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Pittelli M, Cavone L, Fossati S, Porcu M, Mascagni P, . . . Chiarugi A (2009). Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis, 36(2), 269–279. doi: 10.1016/j.nbd.2009.07.019 [DOI] [PubMed] [Google Scholar]
- Ferland CL, & Schrader LA (2011). Regulation of histone acetylation in the hippocampus of chronically stressed rats: a potential role of sirtuins. Neuroscience, 174, 104–114. doi: 10.1016/j.neuroscience.2010.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, & Deak T (2016). Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res, 1646, 62–72. doi: 10.1016/j.brainres.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Csoka AB, Taylor RE, & Tizabi Y (2017). Role of cortical alpha-2 adrenoceptors in alcohol withdrawal-induced depression and tricyclic antidepressants. Drug Alcohol Depend, 175, 133–139. doi: 10.1016/j.drugalcdep.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, & Michael J (1998). The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry, 55(3), 259–265. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, . . . Tsai LH (2009). HDAC2 negatively regulates memory formation and synaptic plasticity. Nature, 459(7243), 55–60. doi: 10.1038/nature07925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, & Blendy JA (2009). Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology, 57(1), 67–74. doi: 10.1016/j.neuropharm.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Sung YB, Chung SY, & Kwon MS (2014). Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology, 81, 292–302. doi: 10.1016/j.neuropharm.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, & Tizabi Y (2011). Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav, 100(2), 253–258. doi: 10.1016/j.pbb.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, & Crews FT (2008). Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol, 210(2), 349–358. doi: 10.1016/j.expneurol.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing B, Sathyaputri L, & Potash JB (2018). A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am J Med Genet B Neuropsychiatr Genet, 177(2), 143–167. doi: 10.1002/ajmg.b.32616 [DOI] [PubMed] [Google Scholar]
- Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, . . . Yang J (2017). Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS One, 12(9), e0185129. doi: 10.1371/journal.pone.0185129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, & Belzung C (2010). Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One, 5(4), e10404. doi: 10.1371/journal.pone.0010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, . . . Berton O (2014). Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology, 39(2), 389–400. doi: 10.1038/npp.2013.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, . . . Drew PD (2014). Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res, 38(2), 384–391. doi: 10.1111/acer.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Li J, Bekker A, & Ye JH (2018). Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology, 129, 47–56. doi: 10.1016/j.neuropharm.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, & Thompson LM (2008). Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov, 7(10), 854–868. doi: 10.1038/nrd2681 [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, & Maes M (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry, 35(3), 744–759. doi: 10.1016/j.pnpbp.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Kv A, Madhana RM, Js IC, Lahkar M, Sinha S, & Naidu VGM (2018). Antidepressant activity of vorinostat is associated with amelioration of oxidative stress and inflammation in a corticosterone-induced chronic stress model in mice. Behav Brain Res, 344, 73–84. doi: 10.1016/j.bbr.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Li J, Kang S, Fu R, Wu L, Wu W, Liu H, . . . Ye JH (2017). Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology, 126, 108–120. doi: 10.1016/j.neuropharm.2017.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Qiu HM, Fei HZ, Hu XY, Xia HJ, Wang LJ, . . . Zhou QX (2014). Histone acetylation and expression of mono-aminergic transmitters synthetases involved in CUS-induced depressive rats. Exp Biol Med (Maywood), 239(3), 330–336. doi: 10.1177/1535370213513987 [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Marcos M, Calleja-Conde J, Echeverry-Alzate V, Buhler KM, Costa-Alba P, . . . Gine E (2015). Histone Deacetylase Gene Expression Following Binge Alcohol Consumption in Rats and Humans. Alcohol Clin Exp Res, 39(10), 1939–1950. doi: 10.1111/acer.12850 [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, & Coupland NJ (2010). Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci, 35(5), 337–343. doi: 10.1503/jpn.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, Reynaert ML, Gatta E, Gabriel C, Mocaer E, Di Prisco S, . . . Mairesse J (2014). The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J Neurosci, 34(6), 2015–2024. doi: 10.1523/JNEUROSCI.4131-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masrour FF, Peeri M, Azarbayjani MA, & Hosseini MJ (2018). Voluntary Exercise During Adolescence Mitigated Negative the Effects of Maternal Separation Stress on the Depressive-Like Behaviors of Adult Male Rats: Role of NMDA Receptors. Neurochem Res, 43(5), 1067–1074. doi: 10.1007/s11064-018-2519-6 [DOI] [PubMed] [Google Scholar]
- Meylan EM, Halfon O, Magistretti PJ, & Cardinaux JR (2016). The HDAC inhibitor SAHA improves depressive-like behavior of CRTC1-deficient mice: Possible relevance for treatment-resistant depression. Neuropharmacology, 107, 111–121. doi: 10.1016/j.neuropharm.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misztak P, Panczyszyn-Trzewik P, & Sowa-Kucma M (2018). Histone deacetylases (HDACs) as therapeutic target for depressive disorders. Pharmacol Rep, 70(2), 398–408. doi: 10.1016/j.pharep.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, & Pandey SC (2013). Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry, 73(8), 763–773. doi: 10.1016/j.biopsych.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva F, Nibbio G, Vizzuso P, Jaretti Sodano A, Ostacoli L, Carletto S, & Picci RL (2018). Gender Differences in Anxiety and Depression before and after Alcohol Detoxification: Anxiety and Depression as Gender-Related Predictors of Relapse. Eur Addict Res, 24(4), 163–172. doi: 10.1159/000490046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, & Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis, 82, 607–619. doi: 10.1016/j.nbd.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, & Prakash A (2008). Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci, 28(14), 3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, & Hannan AJ (2013). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci, 37(11), 1803–1810. doi: 10.1111/ejn.12195 [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E, & Willner P (1996). Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol, 296(2), 129–136. doi: 10.1016/0014-2999(95)00697-4 [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM, & Guerri C (2015). Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology, 89, 352–359. doi: 10.1016/j.neuropharm.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Patnala R, Arumugam TV, Gupta N, & Dheen ST (2017). HDAC Inhibitor Sodium Butyrate-Mediated Epigenetic Regulation Enhances Neuroprotective Function of Microglia During Ischemic Stroke. Mol Neurobiol, 54(8), 6391–6411. doi: 10.1007/s12035-016-0149-z [DOI] [PubMed] [Google Scholar]
- Petit G, Luminet O, Cordovil de Sousa Uva M, Monhonval P, Leclercq S, Spilliaert Q, . . . de Timary P (2017). Gender Differences in Affects and Craving in Alcohol-Dependence: A Study During Alcohol Detoxification. Alcohol Clin Exp Res, 41(2), 421–431. doi: 10.1111/acer.13292 [DOI] [PubMed] [Google Scholar]
- Petit G, Luminet O, Cordovil de Sousa Uva M, Zorbas A, Maurage P, & de Timary P (2017). Differential spontaneous recovery across cognitive abilities during detoxification period in alcohol-dependence. PLoS One, 12(8), e0176638. doi: 10.1371/journal.pone.0176638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Renoir T, & Hannan AJ (2017). Gene-environment interactions informing therapeutic approaches to cognitive and affective disorders. Neuropharmacology. doi: 10.1016/j.neuropharm.2017.12.038 [DOI] [PubMed] [Google Scholar]
- Roni MA, & Rahman S (2017). Lobeline attenuates ethanol abstinence-induced depression-like behavior in mice. Alcohol, 61, 63–70. doi: 10.1016/j.alcohol.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, & Pandey SC (2016). A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct, 221(9), 4691–4703. doi: 10.1007/s00429-016-1196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, & Pandey SC (2014). Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol, 17(8), 1207–1220. doi: 10.1017/S1461145714000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, & Pandey SC (2012). Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res, 36(1), 61–71. doi: 10.1111/j.1530-0277.2011.01581.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, . . . Hen R (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, 301(5634), 805–809. doi: 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Simon-O’Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, & Vilpoux C (2015). The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals. Addict Biol, 20(4), 676–689. doi: 10.1111/adb.12161 [DOI] [PubMed] [Google Scholar]
- Slattery DA, & Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc, 7(6), 1009–1014. doi: 10.1038/nprot.2012.044 [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, . . . Belzung C (2008). Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry, 64(4), 293–301. doi: 10.1016/j.biopsych.2008.02.022 [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, & Nestler EJ (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci, 9(4), 519–525. doi: 10.1038/nn1659 [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, . . . Watanabe Y (2011). Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron, 69(2), 359–372. doi: 10.1016/j.neuron.2010.12.023 [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, & Ehlers CL (2010). Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol, 44(6), 487–493. doi: 10.1016/j.alcohol.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, & Muscat R (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl), 93(3), 358–364. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Belzung C, & Surget A (2008). Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res, 193(1), 140–143. doi: 10.1016/j.bbr.2008.04.021 [DOI] [PubMed] [Google Scholar]
- Yamawaki Y, Fuchikami M, Morinobu S, Segawa M, Matsumoto T, & Yamawaki S (2012). Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J Biol Psychiatry, 13(6), 458–467. doi: 10.3109/15622975.2011.585663 [DOI] [PubMed] [Google Scholar]
- Yawalkar R, Changotra H, & Gupta GL (2018). Protective influences of N-acetylcysteine against alcohol abstinence-induced depression by regulating biochemical and GRIN2A, GRIN2B gene expression of NMDA receptor signaling pathway in rats. Neurochem Int, 118, 73–81. doi: 10.1016/j.neuint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- You C, Vandegrift BJ, Zhang H, Lasek AW, Pandey SC, & Brodie MS (2018). Histone Deacetylase Inhibitor Suberanilohydroxamic Acid Treatment Reverses Hyposensitivity to gamma-Aminobutyric Acid in the Ventral Tegmental Area During Ethanol Withdrawal. Alcohol Clin Exp Res, 42(11), 2160–2171. doi: 10.1111/acer.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, & Pandey SC (2014). Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol, 17(2), 313–322. doi: 10.1017/S1461145713001144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, & Pandey SC (2010). Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res, 34(3), 451–461. doi: 10.1111/j.1530-0277.2009.01109.x [DOI] [PMC free article] [PubMed] [Google Scholar]