Figure 1. An integrative model of the roles of mitochondrial metabolism in liver cancer stem cells.
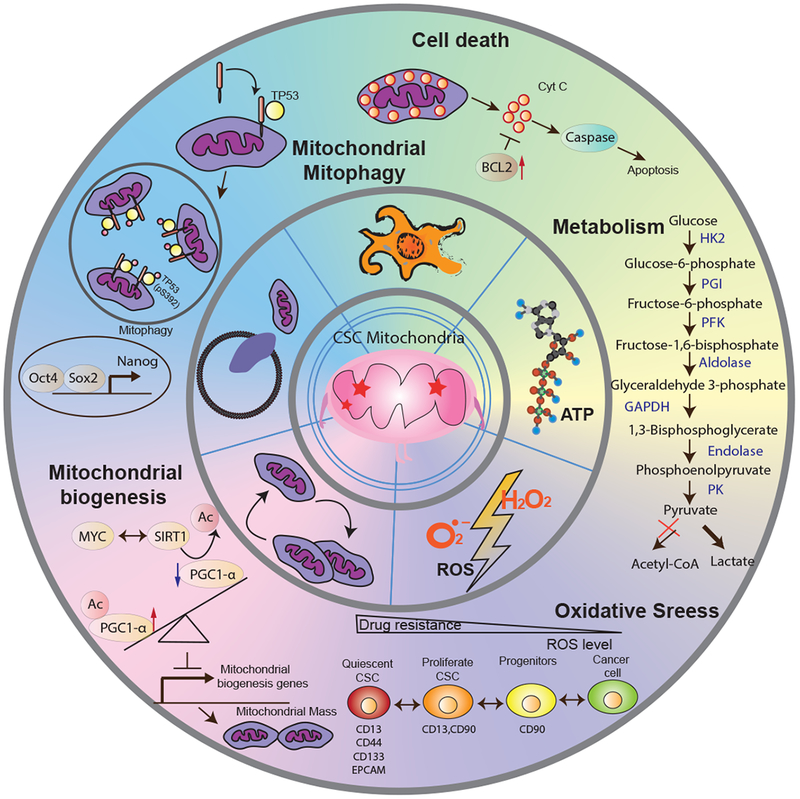
This illustration encompasses five key features of mitochondrial dysfunction needed for the maintenance of liver CSCs. In particular, Liver CSCs may preferentially (1) rely on glycolytic pathways to increase ATP production for biosynthesis; (2) reduce ROS levels to acquire a quiescent state in response to drug resistance; (3) decrease mitochondrial biogenesis through increased expression of acetylated PGC-1α to reduce mitochondrial respiration; (4) enhance mitophagy to block p53 mitochondrial translocation, which may bind to the NANOG promoter to inhibit the expression of NANOG, resulting in reduced stemness and self-renewal ability of liver CSCs; (5) acquire the ability for evading mitochondria-mediated death pathway by overexpressing BCL-2 family proteins thereby resistance to anticancer treatments. Inner circle: a dysfunctional CSC mitochondrium. Middle circle: five features of mitochondrial dysfunction. Outer circle: molecular signaling pathways of mitochondria, including mitochondrial metabolism, oxidative stress, mitochondrial biogenesis, mitochondrial mitophagy, and cell death, in the context of functional regulations of liver CSCs. Abbreviation: CSC: cancer stem cell; Cyt C: Cytochrome C; ROS: Reactive Oxygen Species; HK2: Hexokinase 2; PGI: Phosphoglucoisomerase; PFK: Phosphofructokinase; PK: Pyruvate kinase.
