Abstract
Introduction
Systematic patient education has been reported to improve adherence to treatment, leading to better clinical outcomes. This cluster randomized real-world study investigated the effect of a systematic education program and telephone support on self-reported adherence to oral glucose-lowering treatment in patients with type 2 diabetes mellitus (T2DM).
Methods
Centers were randomized (1:1) to provide either standard-of-care (control group) or standard-of-care along with the education program and telephone support (empowerment group). Adherence to treatment and satisfaction with treatment were assessed using the four-item Morisky Medication Adherence Scale (MMAS-4) and the Diabetes Treatment Satisfaction Questionnaire (DTSQ). The study population included 457 patients (258/199 male/female) with T2DM and non-optimal glycemic control, on oral antidiabetic treatment (age 62.7 [11.4]; disease duration 8.5 [6.5] years).
Results
MMAS-4 high adherence rates for the control and empowerment groups were increased by 3.8% and 16.8% at 4 months (Breslow-Day test p = 0.04) and by 8.5% and 18.8% at 8 months of follow-up, respectively (Breslow-Day test p = 0.09), compared to baseline. Intense physical activity was increased in both control and empowerment groups by 2.3% and 13.9% at 4 months (Breslow-Day test p = 0.082) and by 4.0% and 22.5% at 8 months of follow-up (Breslow-Day test p < 0.001). Baseline mean (SD) HbA1c was significantly lower in the control group compared with the empowerment group [7.7% versus 8.0%, p = 0.001] and decreased in both groups at 4 months by 0.7% and 0.9%, respectively. The change from baseline in the mean DTSQ status score at 4 months was greater in the empowerment group, and the effect was sustained at 8 months (control group: 29.1, 30.5, and 30.9; empowerment group: 25.0, 28.7, and 29.4 at baseline, 4 and 8 months, respectively, p < 0.001).
Conclusion
Systematic education combined with telephone support delivered by physicians might be associated with improvement in treatment adherence and treatment satisfaction in patients with T2DM.
Funding
MSD, Greece.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-0648-9) contains supplementary material, which is available to authorized users.
Keywords: Oral glucose-lowering agents, Systematic education program, Telephone support, Treatment adherence, Treatment satisfaction, Type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease which is associated with significant premature mortality and morbidity. Diabetes patient care is a complex process, mainly aiming to attain euglycemia with glycated hemoglobin (HbA1c) levels at 7% [1, 2]. Current treatment guidelines encourage healthcare providers and patients to co-develop a “patient-centered” diabetes care plan [2], with the responsibility for the daily self-management activities transferred from the healthcare provider to the patient [3, 4].
National and international diabetes associations recognize patient education delivered in the context of standard care, or in a structured way, as a key component of diabetes care and recommend that it should be provided to all patients [2, 5, 6]. In standard-of-care, patient education comprises basic information on lifestyle modification and disease management. In contrast, structured patient education addresses a multitude of patient characteristics and other factors that influence each person’s ability to perform the required self-management activities [6]. Structured patient education can be provided to groups of patients or individually, with each mode having advantages and disadvantages [7–9].
Adherence to the complex T2DM treatment regimen may be overwhelming for patients. In fact, adherence rates have been reported to range from 36% to 93% in patients that remained on treatment with oral glucose-lowering agents for 6–24 months [10]. Adherence to treatment is influenced by a variety of factors, including patient characteristics, characteristics of the treatment regimen, features of the disease, prescriber-level factors (including patient–physician relationship), and the clinical setting [11]. Rubin [12] proposed that “education is a useful resource for addressing all barriers to treatment adherence.” Furthermore, the author concluded that self-management education can ultimately lead to improved self-care behavior, glycemic control, and positive patient outcomes.
In Greece, the prevalence of T2DM is estimated at approximately 7.0% [13], and the prevalence of undiagnosed cases is possibly even higher [14]. The level of diabetes education appears to be poor among patients who are treated with oral glucose-lowering agents [15]. However, Greek patients have been shown to benefit from diabetes education; a relatively recent study reported that a structured 6-h education program delivered in small groups of patients was more effective than standard-of-care in improving glycemic control [16]. Furthermore, a non-experimental study reported that patients adhered to certain aspects of self-management (e.g., diet, blood test exams, regarding self-care etc.) while adherence to exercise and foot management were low [17]. Currently, no studies could be identified assessing the effect of patient education on adherence in Greek patients with T2DM.
This study aimed at evaluating the results of a systematic education program and telephone support on self-reported adherence to treatment with oral glucose-lowering agents in patients with T2DM. In addition, the study sought to investigate the impact of education on other biomedical variables, health-related quality of life, and diabetes treatment satisfaction patient-reported outcomes.
Methods
Study Design and Participants
ADVICE was a non-interventional, cluster randomized, parallel-group study conducted in 45 primary and secondary outpatient diabetes care centers throughout Greece. Eligible patients were 18 years of age or older, with uncontrolled T2DM (HbA1c > 7%), treated with oral glucose-lowering medications for at least 1 year prior to enrollment. Key exclusion criteria were type 1 diabetes mellitus (T1DM), gestational diabetes, hospitalized patients, and history of alcohol or drug abuse within the year preceding enrolment; pregnant, breastfeeding, or female patients with childbearing potential were also excluded.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013, and with the standards of Good Pharmacoepidemiology Practice; all applicable local laws, rules, and regulations relating to the conduct of the clinical study. Informed consent was obtained from all patients for being included in the study and ethical approval was obtained from all participating centers. Further details regarding the ethics committees from all the participating centers who approved this study can be found in the Supplementary Materials.
Randomization
Randomization took place at center level; centers were randomly assigned (with the use of an electronic algorithm) in a ratio of 1:1 to provide either standard-of-care treatment (control group) or standard-of-care along with a systematic patient education program (empowerment group) to patients with T2DM. Investigators decided independently on the patients’ treatment regimen and goals.
In the empowerment group, investigators utilized a sponsor-approved educational material on diabetes which was based on the national and international recommendations. The material included information on disease knowledge, diet and exercise, use of medications and adherence to treatment, adverse events related to treatment, and coping with disease- or treatment-related stress. Investigators were trained on its use for education of their patients during study visits. This material was also provided to patients for further reading following enrollment.
In addition, physicians in the empowerment group were communicating via telephone (approximately bi-weekly) with the patients to support them on the attainment of the treatment goals; predetermined discussion topics included diet, physical activity, adherence to prescribed medication etc. A telephone contact log was used for this purpose.
Procedures
The study follow-up period lasted 8 months. Demographic and biomedical data and patient-reported outcome questionnaires were collected by the investigators at study entry (baseline visit) and at 4- and at 8-month (± 1 week) visits; the two aforementioned visits were held in the context of standard-of-care. Demographic data included age, gender, age at time of T2DM diagnosis, disease duration (recorded only at baseline), body mass index, waist and hip size, smoking habits, alcohol consumption, and physical activity. Biomedical data included measurements of HbA1c, fasting plasma glucose (FPG), postprandial glucose (PPG), blood lipids (total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and triglycerides), microalbuminuria, blood pressure, heart rate, comorbidities, and preventative screening (lower extremity and eye examinations, and assessment for atherosclerotic cardiovascular disease).
The patient-reported outcomes included in the analysis were adherence to treatment, health-related status, and treatment satisfaction. Adherence to treatment was assessed with the four-item Morisky Medication Adherence Scale (MMAS-4; score range 0–4; higher scores indicate higher adherence) [18]; on the basis of their MMAS-4 score, patients were classified into high, medium, or low adherence (MMAS-4 scores 4, 2–3, and 0–1, respectively). Health-related quality of life was assessed with the five-level EQ-5D (EQ-5D-5L) questionnaire and the visual analogue scale (EQ-VAS) [19]. Finally, treatment satisfaction was assessed with the Diabetes Treatment Satisfaction Questionnaire status (DTSQs; score range 0–36; higher scores indicate higher satisfaction) and change (DTSQc) versions [20, 21].
Objectives
The primary objective was to compare the self-reported adherence to the treatment between the empowerment and control groups, on the basis of the proportions of patients with high versus medium/low self-reported adherence to the treatment at 4 months.
The key secondary objectives included (1) the comparison of the proportion of patients with high versus medium/low adherence to treatment at 8 months between the empowerment control groups and (2) the within-group change in the proportion of patients with high versus medium/low adherence from baseline to 4 and 8 months. Other secondary objectives were the comparison of treatment satisfaction and health-related quality of life status between the empowerment and control groups, at 4 and 8 months; the within-group change of treatment satisfaction and health-related quality of life status from baseline to 4 and 8 months; the comparison of the proportion of patients achieving predefined targets for diabetes, lipid, and blood pressure variables between the empowerment and control groups, at 4 and 8 months. These targets were HbA1c < 7.0%; blood glucose < 110 mg/dl; LDL-C < 100 mg/dl (< 70 mg/dl for patients with diabetes and cardiovascular disease); and systolic blood pressure (SBP) < 130 mmHg (default target for blood pressure, systolic/diastolic blood pressure < 125/75 mmHg in case of renal dysfunction, proteinuria > 1 g/24 h).
Statistical Analysis
The sample size was estimated on the assumptions that among patients with poorly controlled diabetes the proportion of patients with high/medium adherence (defined with MMAS-4) is approximately 60%, the difference in the between-group ratio of patients with high/medium adherence would be at least 15%, and the rate of data loss would be at maximum approximately 10% of the required sample. When a two-sided chi-square test was used with type I error 0.05 and power 0.8, the sample size needed to identify the specified difference between the two proportions was 152 for each group. To account for clustering effects from the randomization scheme, an intraclass correlation coefficient of 0.04 was applied; this increased the sample size to 207 patients. Therefore, the required sample size per arm was 230 patients and the total for both groups was 460 patients with 46 research sites recruiting 10 patients each. This assumption was based on available bibliography data suggesting that the prevalence of self-reported medication nonadherence with the use of MMAS-4 in older adults ranges from 33% to 57% [22–24].
Descriptive analysis was performed to summarize demographic, clinical variables and patient-reported outcomes of study patients. Continuous variables are presented as mean with standard deviation (SD) and categorical variables as counts and proportions.
The association between categorical variables was assessed using the chi-square (χ2) test. The McNemar test was used for the association of a categorical variable between two time points. Differences of continuous variables at different time points were evaluated with Wilcoxon signed rank test for related samples, while the differences of continuous variables between two groups were evaluated with the U Mann–Whitney test for independent samples. The Breslow-Day test for homogeneity of odds ratios was used to compare the change in the rates of high adherence or intense physical activity from baseline to 4 or 8 months.
As a result of the study design, a hierarchical, multilevel, mixed effects model was fitted to further investigate the efficacy of the educational intervention after controlling for baseline differences between the experimental and the control group and for variability in clinical practice among centers. Factors that were associated with a high adherence rate at 4 months (p < 0.1) were included in the multivariate model, with random effects fitted for the study centers (further details are included as Supplementary Material).
All statistical tests were two-sided and were performed at a 0.05 significance level. The p values were reported, even for non-significant results, rounded to three decimals unless the p value was less than 0.001 (in such case p < 0.001 was reported). No adjustment for multiple testing was performed. Analysis was performed with SAS® version 9.4.
Results
Patient Demographic and Biomedical Characteristics at Baseline
Forty-five centers were recruited in the study and a total of 457 patients with T2DM were enrolled (Table 1). Female patients accounted for 43.5% of the entire study population. Mean (SD) baseline characteristics were age, 62.7 (11.4) years; duration of diabetes, 8.5 (6.5) years; and HbA1c, 7.8 (0.9) %. Comorbidities and diabetes complications occurred in 75.7% and 7.7% of patients, respectively. The most common comorbidities were arterial hypertension (44.8%), dyslipidemia (39.0%), ischemic heart disease (4.6%), and myocardial infarction (1.7%), while the most common diabetes-related complications were nephropathy (31.4%), retinopathy (25.7%), and neuropathy (17.1%).
Table 1.
Patient characteristics at baseline, overall and per group
| All patients (N = 457) |
Control group (n = 227) |
Empowerment group (n = 230) |
p value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (SD) | 62.7 (11.4) | 63.8 (10.1) | 61.5 (6.7) | 0.047 |
| Female (%) | 43.5 | 42.3 | 44.8 | 0.591 |
| BMI (kg/m2) | 30.6 (5.3) | 30.3 (5.0) | 30.8 (5.6) | 0.203 |
| Diabetes duration (SD) | 8.5 (6.5) | 8.8 (6.3) | 8.3 (6.7) | 0.152 |
| Diabetes history in 1st degree relatives (%) | 53.8 | 54.2 | 53.5 | 0.952 |
| Biomedical characteristics | ||||
| Comorbidities, all (%) | 75.7 | 78.9 | 72.6 | 0.120 |
| Diabetes complications, all (%) | 7.7 | 8.4 | 7.0 | 0.570 |
| Physical training | ||||
| Intense activity for ≥ 20 min, once or more times per week | 109 (24.0) | 52 (23.0) | 57 (24.9) | 0.638 |
| No or limited weekly activity | 346 (76.0) | 174 (77.0) | 172 (75.1) | |
| Patients on specific diabetes diet (%) | 47.5 | 53.7 | 41.3 | 0.028 |
| Smoking | 0.340 | |||
| Current | 14.4 | 11.9 | 17.0 | |
| Former | 21.0 | 22.5 | 19.6 | |
| Never | 61.7 | 63.4 | 60.0 | |
| Alcohol consumption | 0.004 | |||
| Daily | 4.2 | 4.0 | 4.3 | |
| Never | 56.9 | 65.2 | 48.7 | |
| Occasionally | 36.8 | 29.1 | 44.3 | |
| Diabetes treatments (%) | ||||
| Biguanides | 65.9 | 64.8 | 67.0 | |
| DPP-4 | 46.0 | 43.2 | 48.7 | |
| SGLT-2 | 22.8 | 23.8 | 21.7 | |
| Insulin (all types) | 1.8 | 0.9 | 2.6 | |
| HbA1c | 7.8 (0.9) | 7.7 (0.8) | 8.0 (1.0) | 0.001 |
| FPG (mg/dl) | 149.1 (40.5) | 140.9 (31.0) | 157.0 (46.6) | 0.001 |
| PPG (mg/dl) | 175.1 (50.6) | 162.1 (30.9) | 188.3 (62.2) | 0.001 |
| Triglycerides (mg/dl) | 162.9 (81.3) | 156 (68.3) | 169.8 (92.2) | 0.446 |
| HDL-C (mg/dl) | 45.7 (11.3) | 46.4 (10.9) | 45.0 (11.7) | 0.261 |
| LDL-C (mg/dl) | 103.6 (31.6) | 100.5 (29.7) | 106.6 (33.1) | 0.108 |
| TC (mg/dl) | 183.3 (36.2) | 180.8 (33.6) | 185.8 (38.6) | 0.159 |
| DBP (mmHg) | 79.1 (8.2) | 79.5 (8.4) | 78.8 (8.0) | 0.766 |
| SBP (mmHg) | 131.4 (12.9) | 132.0 (13.3) | 130.9 (12.5) | 0.521 |
| Patient-reported outcomes | ||||
| MMAS-4 score | 3.2 (1.1) | 3.2 (1.1) | 3.2 (1.0) | 0.849 |
| EQ-5D-5L score | 0.81 (0.19) | 0.82 (0.18) | 0.80 (0.20) | 0.376 |
| EQ-VAS score | 75.8 (16.7) | 78.8 (16.1) | 72.9 (16.8) | 0.000 |
| DTSQs score | 27.1 (6.4) | 29.1 (6.7) | 25.0 (6.7) | 0.000 |
All data are mean (SD) unless otherwise shown. p values are for control versus empowerment group comparison
DBP diastolic blood pressure, DTSQs Diabetes Treatment Satisfaction Questionnaire status version, EQ-5D-5L EuroQol 5-dimension, 5-level scale, EQ-VAS EuroQol visual analogue scale, FPG fasting plasma glucose, HbA1c glycated hemoglobin, HDL high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, MMAS-4 4-item Morisky Medication Adherence Scale, N total number of patients, n number of patients in specified group, PPG postprandial glucose, TC total cholesterol, TG triglycerides, SD standard deviation, SBP systolic blood pressure
Per study design, 23 and 22 centers were randomly assigned to the control and empowerment groups, respectively; as a result of this randomization, 227 and 230 patients were allocated in the control and empowerment patient groups, respectively.
At baseline, certain patient characteristics differed between groups (Table 1). Age and occasional alcohol consumption were significantly (p = 0.047 and p = 0.004, respectively) higher in the empowerment group, while the use of specific diabetes diet was more common in the control group (p = 0.028). Regarding other biomedical characteristics, HbA1c, FPG, and PPG levels were significantly higher in the empowerment versus the control group (p = 0.001 for all comparisons; Table 1).
With respect to the patient-reported outcomes at baseline, the MMAS-4 and EQ-5D-5L scores were not statistically different between groups (p = 0.849 and p = 0.376, respectively; Table 1). In contrast, the EQ-VAS and DTSQs scores were significantly higher for the control versus the empowerment group at baseline (p < 0.001 for both comparisons).
Patient Support During Follow-Up Period
Investigators in the empowerment group performed regular (approximately bi-weekly) telephone support sessions with their patients during follow-up. On average, 15.3 (2.8) telephone sessions were performed per patient in this group. The overall frequency of topics discussed during these sessions were diet, 93.0%; physical activity, 88.9%; adherence to prescribed medication, 81.3%; smoking habits were not a frequent subject of discussion (28.8%).
Improvement in Self-Reported Adherence Rates During Follow-Up
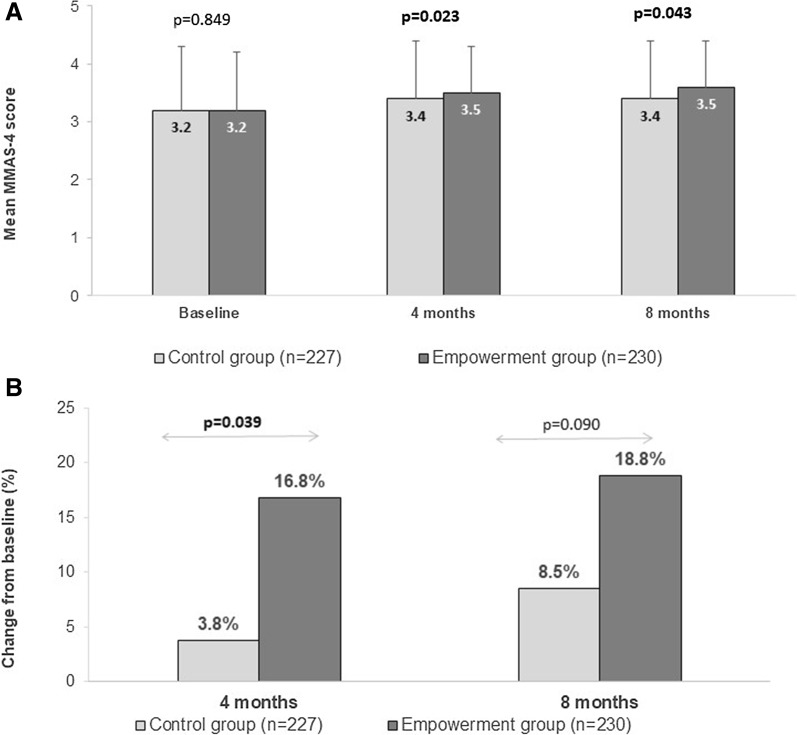
Although the mean baseline MMAS-4 scores were similar between groups, the mean score for the empowerment group was significantly higher compared to control group at 4 months (p = 0.023) and 8 months (p = 0.043) (Fig. 1a).
Fig. 1.
Per group improvements in self-reported adherence from baseline to 4 or 8 months. a Per group improvements in mean MMAS-4 scores from baseline to 4 and 8 months. b Per group change in proportion of patients with high adherence from baseline to 4 or 8 months. High adherence was defined as MMAS-4 score of 4. p values in a are for empowerment versus control group. p values in b are based on Breslow-Day t test for group comparison. p values in bold indicate statistical significance. T bars denote the standard deviation
After controlling for the effect of differences in the clinical practice and baseline covariates, it was observed that the empowerment group was not statistically significantly associated with higher odds of achieving high adherence at 4 months, compared to the control group (OR = 2.1, 95% CI 0.575–7.670; further details are included as Supplementary Material).
For the assessment of the self-reported adherence levels, patients were classified into high (MMAS-4 score 4) or medium/low (MMAS-4 score 0–3) adherence subgroups (Table 2). At baseline, the proportions of patients with high adherence were similar between the control and empowerment groups (57.3% and 53.9%, respectively; p = 0.470). At 4 months (primary objective), the proportion of patients with high adherence was significantly greater in the empowerment than the control group (70.7% versus 61.1%, respectively; p = 0.032; Table 2); compared to baseline, the increase in the proportion of patients with high adherence was significantly greater for the empowerment than the control group (16.8% and 3.8%, respectively; Breslow-Day test p = 0.04; Fig. 1b).
Table 2.
Classification of patients into high or medium/low adherence per group, at baseline, 4 and 8 months
| Control group (n = 227) |
Empowerment group (n = 230) |
p value | |
|---|---|---|---|
| Baseline | |||
| High adherence | 130 (57.3) | 124 (53.9) | 0.470 |
| Medium/low adherence | 97 (42.7) | 106 (46.1) | |
| 4 months | |||
| High adherence | 135 (61.1) | 157 (70.7) | 0.032 |
| Medium/low adherence | 86 (38.9) | 65 (29.3) | |
| 8 months | |||
| High adherence | 144 (65.8) | 157 (72.7) | 0.117 |
| Medium/low adherence | 75 (34.2) | 59 (27.3) | |
All data are n (%). Patients were classified into high or medium/low adherence subgroups by means of their baseline MMAS-4 score (high adherence, MMAS-4 score 4; medium/low adherence, MMAS-4 score 0 to < 3). p values are for control versus empowerment group comparison
MMAS-4 four-item Morisky Medication Adherence Scale, n number of patients in specified group
At 8 months, the proportions of patients with high adherence were not significantly different between groups (p = 0.117; Table 2); similarly, the increases in the proportions of patients with high adherence from baseline were non-significant between the empowerment and control groups (18.8% versus 8.5%, respectively; Breslow-Day test p = 0.09; Fig. 1b).
Improvement in Biomedical Characteristics During Follow-Up
Assessment of other biomedical variables is shown in Table 3. Although the mean HbA1c levels were significantly higher for the empowerment versus the control group at baseline, the between-group differences at 4 and 8 months were non-significant (p = 0.724 and p = 0.114, respectively). For the empowerment group, the mean HbA1c was reduced to 7.1% at 4 months (− 0.9% from baseline) and to 7.0% at 8 months (− 1.0% from baseline); for the control group, the respective mean HbA1c levels and reductions from baseline were 7.0% (− 0.7%) and 6.9 (− 0.8%). Similarly, the between-group differences in the levels of PPG and FPG at 4 and 8 months were non-significant, albeit that the respective baseline levels were significantly higher for the empowerment versus the control group. No significant between-group differences were observed in other clinical characteristics such as blood pressure, heart rate, and lipidemic profile at 4 and 8 months.
Table 3.
Development of key biomedical characteristics from baseline through to 4 and 8 months per group
| Patient group | p value | ||
|---|---|---|---|
| Control group (n = 227) |
Empowerment group (n = 230) |
||
| HbA1c (%) | |||
| Baseline | 7.7 (0.8) | 8.0 (1.0) | 0.001 |
| 4 months | 7.0 (0.6) | 7.1 (0.6) | 0.724 |
| 8 months | 6.9 (0.6) | 7.0 (0.6) | 0.114 |
| FPG (mg/dl) | |||
| Baseline | 140.9 (31.0) | 157.0 (46.6) | 0.001 |
| 4 months | 125.7 (21.6) | 128.1 (23.5) | 0.318 |
| 8 months | 120.6 (21.6) | 123.1 (21.9) | 0.294 |
| PPG (mg/dl) | |||
| Baseline | 162.1 (30.9) | 188.3 (62.2) | 0.001 |
| 4 months | 141.1 (20.4) | 154.8 (28.8) | 0.001 |
| 8 months | 141.4 (24.5) | 149.2 (24.5) | 0.016 |
| TC (mg/dl) | |||
| Baseline | 180.8 (33.6) | 185.8 (38.6) | 0.159 |
| 4 months | 177.3 (26.3) | 178.3 (32.7) | 0.769 |
| 8 months | 172.8 (25.7) | 179.7 (32.1) | 0.088 |
| TG (mg/dl) | |||
| Baseline | 156.0 (68.3) | 169.8 (92.2) | 0.446 |
| 4 months | 140.2 (45.8) | 148.4 (61.4) | 0.362 |
| 8 months | 137.1 (47.6) | 144.4 (60.6) | 0.271 |
| HDL-C (mg/dl) | |||
| Baseline | 46.4 (10.9) | 45.0 (11.7) | 0.261 |
| 4 months | 47.7 (10.2) | 47.2 (11.7) | 0.643 |
| 8 months | 47.6 (9.8) | 47.2 (10.8) | 0.726 |
| LDL-C (mg/dl) | |||
| Baseline | 100.5 (29.7) | 106.6 (33.1) | 0.108 |
| 4 months | 100.0 (25.1) | 97.9 (28.5) | 0.365 |
| 8 months | 94.8 (26.3) | 101.1 (30.1) | 0.183 |
| DBP (mmHg) | |||
| Baseline | 79.5 (8.4) | 78.8 (8.0) | 0.766 |
| 4 months | 78.9 (7.7) | 78.2 (7.2) | 0.301 |
| 8 months | 78.3 (7.2) | 78.5 (7.9) | 0.740 |
| SBP (mmHg) | |||
| Baseline | 132.0 (13.3) | 130.9 (12.5) | 0.521 |
| 4 months | 129.6 (12.1) | 128.3 (10.7) | 0.708 |
| 8 months | 129.7 (12.4) | 129.0 (10.7) | 0.778 |
| Intense physical activity, n (%) | |||
| Baseline | 52 (23.0) | 57 (24.9) | 0.638 |
| 4 months | 56 (25.2) | 86 (38.7) | 0.002 |
| 8 months | 59 (26.9) | 103 (47.2) | < 0.001 |
All data are mean (SD) unless otherwise shown. p values are for the control versus the empowerment group comparison
DBP diastolic blood pressure, FPG fasting plasma glucose, HbA1c glycated hemoglobin, HDL high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, n number of patients in specified group, PPG postprandial glucose, TC total cholesterol, TG triglycerides, SD standard deviation, SBP systolic blood pressure
The rates of intense physical activity (≥ 20 min once or more times per week) were significantly higher in the empowerment group versus the control group at both 4 months (p = 0.002) and 8 (p < 0.001) months (Table 3); compared to baseline, the increases in the proportions of patients with intense physical activity were significantly higher for the empowerment group compared with the control group at 4 months (13.9% and 2.3%, respectively; Breslow-Day test p = 0.082) and 8 months (22.5% and 4%, respectively; Breslow-Day test p < 0.001) (Fig. 2).
Fig. 2.
Per group change in the proportion of patients with intense physical activity (defined as ≥ 20 min once or more times per week) from baseline to 4 or 8 months. p values are based on Breslow-Day t test for group comparison
Improvements in Treatment Satisfaction and Other Patient-Reported Outcomes During Follow-Up
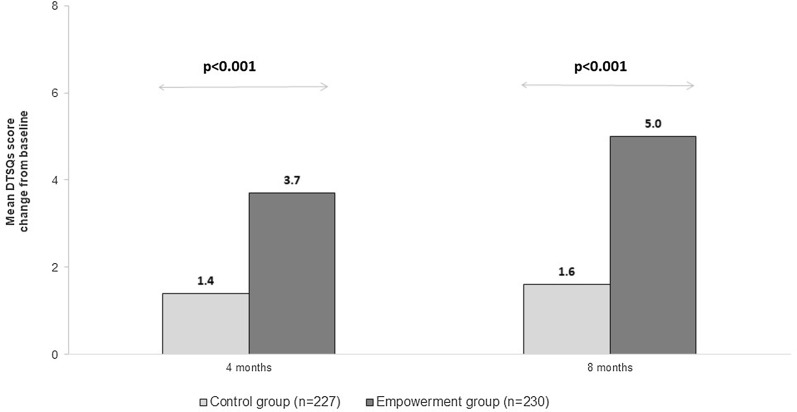
The significantly higher treatment satisfaction levels observed at baseline for the control versus the empowerment group were maintained at 4 months (p = 0.001) and 8 months (p = 0.011). The mean (SD) change from baseline to 4 and 8 months assessed with the DTSQs questionnaire was significantly (p < 0.001) higher for the empowerment versus the control group (Fig. 3).
Fig. 3.
Per group change in treatment satisfaction from baseline to 4 or 8 months. DTSQs Diabetes Treatment Satisfaction Questionnaire status. p values are for empowerment versus control group
Compared to baseline, the EQ-5D-5L mean score remained stable for both groups, with no significant between-group differences at 4 months (p = 0.38) or 8 months (p = 0.66). The significantly lower EQ-VAS mean score for the empowerment versus the control group, observed at baseline, was maintained at 4 months (p < 0.001) and 8 months (p = 0.001).
Discussion
The ADVICE study assessed the impact of a structured educational program and telephone support on the self-reported adherence to oral glucose-lowering treatment in patients with T2DM. The main finding was that the increase in the proportion of patients with high adherence from baseline to 4 months was significantly (p = 0.04) greater for patients who participated in the education program compared with patients who received only standard-of-care. This between-group difference was maintained at 8 months, albeit at not statistically significant levels.
To our knowledge, only a small number of randomized clinical studies have addressed the impact of structured patient education on adherence to treatment. Tan et al. [25] assessed the impact of an education program on adherence to treatment in a population of patients with T1DM and T2DM over 3 months versus standard care. Patients in the intervention group received two individual education sessions followed by a telephone follow-up. The educational intervention focused on self-care practices (including medication adherence) and problem-solving skills. Consistent with the main findings of this study, it was reported that patients in the intervention group presented significantly higher adherence rates than patients in the control group, with a greater improvement in adherence rates from baseline. Other randomized clinical studies have also reported that diabetes education improves adherence to treatment in the short term (≤ 6 months) in patients with T2DM [26–29]. It is unclear whether this favorable impact of education on adherence to treatment is sustained in the long term.
With respect to glycemic control, the present study shows that both groups attained similar mean HbA1c levels at 4 and 8 months. As already stated, the HbA1c levels at baseline, along with other variables such as age and alcohol consumption, were significantly lower versus the empowerment group; this difference may have masked any comparative effectiveness of the educational program on glycemic control. Previous studies have shown that face-to-face education (the delivery mode used in the present study) was significantly more effective in the reduction of HbA1c levels and other diabetes-related biomedical characteristics compared with standard-of-care [30–32]. On the contrary, telephone contacts as a means of reinforcement of a previous educational program do not appear to have an additional effect on the HbA1c levels [33]. With respect to the long-term maintenance of glycemic control, Khunti et al. [34] reported that most of the improvements seen at 12 months in HbA1c levels were not sustained after 3 years. Clearly, the evaluation of the long-term effect of education on adherence to treatment would be an interesting research topic for future trials.
Another finding of the present study was the significant increase in the proportion of patients in the empowerment group performing intense physical activity at 8 months versus those in the control group. Physical activity is a key constituent of diabetes management as it contributes to improved glycemic control [35]. A recent study assessing the impact of education on perceived benefits, barriers, and self-efficacy reported significant improvements in the mean metabolic equivalent of task in the intervention versus the control group [36]. In the present study, this increase in physical activity did not appear to translate into biochemical improvements; however, it is considered that there may be a lag effect and therefore potential improvements would have been apparent beyond 8 months.
Finally, regarding treatment satisfaction, patients in the empowerment group had a significantly lower DTSQs score at baseline versus patients in the control group. As treatment satisfaction is inversely related to HbA1c levels [37], it is possible that the observed difference in satisfaction levels at baseline may be related to the significantly higher HbA1c levels of the empowerment group. As already stated, the significant difference of the control versus the empowerment group persisted throughout the observation period. Despite this, the change from baseline to 4 or 8 months in terms of treatment satisfaction was significantly higher for the empowerment versus the control group. This finding concurs with the findings of the BENCH-D study [38], which showed that higher empowerment levels in patients with T2DM were associated with improved treatment satisfaction. The results regarding the quality of life are presented with caution, since the statistically significant difference of EQ-5D VAS might depict the baseline differences between the two groups.
The present study has several limitations. Physicians in the control group may have inadvertently treated their patients in a more than standard-of-care manner with regards to providing relevant information. Despite study protocol clarity, variability in participating physicians’ practices, measurements, and delivery of education cannot be ruled out. Selection bias cannot be ruled out with respect to participating centers.
It is possible that the differences in key diabetes variables (HbA1c, FPG, and PPG) at baseline between patients allocated in the empowerment and control groups may have masked the impact of education on these variables. However this study design implies that the empowerment and the control groups may differ in terms of baseline characteristics, and thus this was an expected study feature that was further evaluated using a multilevel mixed model analytical approach. After taking into consideration the variation of clinical practices between the participating sites and baseline covariates, the multivariate analysis showed that the empowerment group was not statistically significantly associated with higher odds of achieving high adherence at 4 months, compared to the control group, even though the estimate of the OR remained favorable for the empowerment group (OR 2.1, 95% CI 0.575–7.670).
Conclusions
This cluster randomized real-world study provides evidence that a structured educational program may lead to improved short-term adherence to treatment in patients with T2DM. Patients who participated in the educational program experienced greater treatment satisfaction and increased their physical activity levels compared with patients receiving standard care.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and article processing charges were funded by MSD, Greece. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
List of Investigators
The authors wish to thank the investigators, staff, and participants in the ADVICE trial: Spyridon Karamagkiolis, Vasilios Christakis, Stathoula Theiakogiorgou, Foteini Karachaliou, Evangelos Voliotis, Panagiota Paraskevopoulou, Theodoros Feloukas, Athanasios Mitromaras, Georgios Kapetanos, Christos Giannoutsos, Michail Papas, Panagiota-Nektaria Fardi, Vasilios Sideris, Elisavet Iliadou, Ilias Pelekanos, Ioannis Douloumpakas, Dimitrios Skoutas, Konstantinos Dolianitis, Savvas Papadopoulos, Stavros Tsibos, Athanasios Zervas, Areti Korakovouni, Kyros Siomos, Athanasios Gianasmidis, Spyridon Fotiadis, Konstantinos Kairis, Ioannis Katsakoulas, Eleni Batzili, Georgios Kaslamas, Konstantinos Ziambaras, Nikolaos Lathourakis, Theocharoula Milonaki, Georgios Chaliotis, Athanasios Tsekouras, Vasilios Lekakis, Konstantinos Anyfantakis, Theodoros Angeolopoulos, Vasilios Andreadis, Thekla Chatziadamidou.
Disclosures
Eugenia Karamousouli is an MSD employee. Bernd Voss is an MSD employee. John Doupis has participated in advisory panels for MSD, AstraZeneca, Novo Nordisk, Eli Lilly, Abbott, Johnson & Johnson, and Boehringer Ingelheim and he has received research support from MSD, Eli Lilly, Novo Nordisk, Sanofi, Boehringer Ingelheim, AstraZeneca, and Angelini. Theodore Alexandrides has participated in advisory panels for Merck Sharp Dohme (MSD), AstraZeneca, Sanofi, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and Novartis and has received research support and honoraria as a speaker from MSD, Eli Lilly, Elpen, Novo Nordisk, Sanofi, Pfizer, AstraZeneca, Ricordati, Vianex, WinMedica, Angelini, and Novartis. Moses Elisaf reports honoraria from MSD, Novartis, Chiesi, Bayer, Astra Zeneca, Pfizer, Abbott, Mylan, Sanofi, Amgen, Boehringer Ingelheim, Eli Lilly, GSK, Angelini, WinMedica, and grants and personal fees from MSD and Astra Zeneca. Professor MS Elisaf have given talks and attended conferences sponsored by various pharmaceutical companies, including Bristol-Myers Squibb, Novartis, Chiesi, Bayer, Astra Zeneca, Pfizer, Abbott, Mylan, Sanofi, Amgen, Boehringer Ingelheim, Eli Lilly, GSK, Angelini, WinMedica, and MSD. Andreas Melidonis has received research support and honoraria as a speaker from MSD, Eli Lilly, Servier, Novo Nordisk, Galenica, Vianex, Sanofi, AstraZeneca, Libytec, Novartis, ELPEN, and Boehringer Ingelheim. Stavros Bousboulas has participated in advisory panels for Merck Sharp Dohme (MSD), AstraZeneca, Sanofi, Novo Nordisk, and Boehringer Ingelheim. Except that he has received research support and speaker’s fees from MSD, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, AstraZeneca, Janssen, and Novartis. Anastasia Thanopoulou has received Honoraria and Travel Grants from Eli-Lilly, Sanofi, Novo, and AstraZeneca. Emmanouil Pagkalos reports contribution in lectures, clinical trials, and advisory boards for the following companies MSD, Eli Lilly, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim, Bayer, GSK, Winmedica, Novartis, Vianex, Sanofi, Galenica, Jansen, Abbott, Medtronic, and Roche. Iakovos Avramidis has received speaker honoraria, consulting fees, clinical trials with Sanofi-Aventis, Eli Lilly, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim, Novartis, MSD/Vianex, Elpen, and GSK. Angelos Pappas has participated in advisory boards for MSD, Sanofi Aventis, Novo-Nordisk and received honoraria from Eli Lilly, Novo-NordisK, Boehringer Ingelheim, Elpen, MSD, and Sanofi Aventis. Eleni Arvaniti has participated in educational and research activities sponsored by MSD, Novo Nordisk, and SMB SA. Nikolaos Tentolouris has participated in advisory panels for Merck Sharp Dohme (MSD), AstraZeneca, Sanofi, Novo Nordisk, ELPEN, Eli Lilly, Servier, Boehringer Ingelheim, and Novartis, and has received research support from MSD, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, AstraZeneca, Janssen, Cilag, GlaxoSmithKline, and Novartis.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013, and with the standards of Good Pharmacoepidemiology Practice; all applicable local laws, rules, and regulations relating to the conduct of the clinical study. Informed consent was obtained from all patients for being included in the study and ethical approval was obtained from all participating centers. Further details regarding the ethics committees from all the participating centers who approved this study can be found in the Supplementary Materials.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8204378.
References
- 1.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604–609. doi: 10.2337/dc14-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes 2017, glycemic targets. Diabetes Care. 2017;40(Suppl. 1):S6–S10. doi: 10.2337/dc17-S004. [DOI] [PubMed] [Google Scholar]
- 3.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi: 10.1016/S0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 4.Moser A, van der Bruggen H, Widdershoven G, Spreeuwenberg C. Self-management of type 2 diabetes mellitus: a qualitative investigation from the perspective of participants in a nurse-led, shared-care programme in the Netherlands. BMC Public Health. 2008;18(8):91. doi: 10.1186/1471-2458-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellenic Diabetes Association. 2017. https://www.ede.gr/wp-content/uploads/2017/odigies.pdf. Accessed Sept 05, 2018.
- 6.Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes: a Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. J Acad Nutr Diet. 2015;115(8):1323–1334. doi: 10.1016/j.jand.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis J, Skinner TC, Carey ME, Davies MJ. How can structured self-management patient education improve outcomes in people with type 2 diabetes? Diabetes Obes Metab. 2010;12(1):12–19. doi: 10.1111/j.1463-1326.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 8.Coppola A, Sasso L, Bagnasco A, Giustina A, Gazzaruso C. The role of patient education in the prevention and management of type 2 diabetes: an overview. Endocrine. 2016;53(1):18–27. doi: 10.1007/s12020-015-0775-7. [DOI] [PubMed] [Google Scholar]
- 9.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926–943. doi: 10.1016/j.pec.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn DF, Swidrovich J, Lemstra M. Non-adherence in type 2 diabetes: practical considerations for interpreting the literature. Patient Prefer Adherence. 2013;7:183–189. doi: 10.2147/PPA.S30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(Suppl 5A):27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Liatis S, Dafoulas GE, Kani C, et al. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res Clin Pract. 2016;118:162–167. doi: 10.1016/j.diabres.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Tsirona S, Katsaros F, Bargiota A, Polyzos SA, Arapoglou G, Koukoulis GN. Prevalence and determinants of type 2 diabetes mellitus in a Greek adult population. Hormones (Athens) 2016;15(1):88–98. doi: 10.14310/horm.2002.1662. [DOI] [PubMed] [Google Scholar]
- 15.Poulimeneas D, Grammatikopoulou MG, Bougioukli V, et al. Diabetes knowledge among Greek type 2 diabetes mellitus patients. Endocrinol Nutr. 2016;63(7):320–326. doi: 10.1016/j.endonu.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Merakou K, Knithaki A, Karageorgos G, Theodoridis D, Barbouni A. Group patient education: effectiveness of a brief intervention in people with type 2 diabetes mellitus in primary health care in Greece: a clinically controlled trial. Health Educ Res. 2015;30(2):223–232. doi: 10.1093/her/cyv001. [DOI] [PubMed] [Google Scholar]
- 17.Zioga E, Kazakos K, Dimopoulos E, et al. Adherence and quality of life in patients with type II diabetes mellitus in Northern Greece. Mater Sociomed. 2016;28(4):258–262. doi: 10.5455/msm.2016.28.258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7(5):445–451. doi: 10.1111/j.1464-5491.1990.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 21.Bradley C, Plowright R, Stewart J, Valentine J, Witthaus E. The Diabetes Treatment Satisfaction Questionnaire change version (DTSQc) evaluated in insulin glargine trials shows greater responsiveness to improvements than the original DTSQ. Health Qual Life Outcomes. 2007;5:57. doi: 10.1186/1477-7525-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MR, Reid CM, Ryan P, Wilson K, Yelland L. Self-reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2) Med J Aust. 2006;185:487–489. doi: 10.5694/j.1326-5377.2006.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 23.Unni EJ, Farris KB. Unintentional nonadherence and belief in medicines in older adults. Patient Educ Couns. 2011;83:265–268. doi: 10.1016/j.pec.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Marcum ZA, Zheng Y, Perera S, et al. Prevalence and correlates of self-reported medication non-adherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Res Soc Adm Pharm. 2013;9(6):817–827. doi: 10.1016/j.sapharm.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan MY, Magarey JM, Chee SS, Lee LF, Tan MH. A brief structured education programme enhances self-care practices and improves glycemic control in Malaysians with poorly controlled diabetes. Health Educ Res. 2011;26(5):896–907. doi: 10.1093/her/cyr047. [DOI] [PubMed] [Google Scholar]
- 26.Negarandeh R, Mahmoodi H, Noktehdan H, Heshmat R, Shakibazadeh E. Teach back and pictorial image educational strategies on knowledge about diabetes and medication/dietary adherence among low health literate patients with type 2 diabetes. Prim Care Diabetes. 2013;7(2):111–118. doi: 10.1016/j.pcd.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Aliha JM, Asgari M, Khayeri F, Ramazani M, Farajzadegan Z, Javaheri J. Group education and nurse-telephone follow-up effects on blood glucose control and adherence to treatment in type 2 diabetes patients. Int J Prev Med. 2013;4(7):797–802. [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Haj Mohd MM, Phung H, Sun J, Morisky DE. Improving adherence to medication in adults with diabetes in the United Arab Emirates. BMC Public Health. 2016;16(1):857. doi: 10.1186/s12889-016-3492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caetano IRCES, Santiago LM, Marques M. Impact of written information on control and adherence in type 2 diabetes. Rev Assoc Med Bras (1992) 2018;64(2):140–147. doi: 10.1590/1806-9282.64.02.140. [DOI] [PubMed] [Google Scholar]
- 30.Sturt JA, Whitlock S, Fox C, et al. Effects of the diabetes manual 1:1 structured education in primary care. Diabet Med. 2008;25(6):722–731. doi: 10.1111/j.1464-5491.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 31.Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;21(1):CD005268. doi: 10.1002/14651858.cd005268.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011;171(22):2001–2010. doi: 10.1001/archinternmed.2011.507. [DOI] [PubMed] [Google Scholar]
- 33.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care. 2008;31(3):408–414. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]
- 34.Khunti K, Gray LJ, Skinner T, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ. 2012;344:e2333. doi: 10.1136/bmj.e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Pablos-Velasco P, Ricart W, Monereo S, Moreno B, Marrugat J, KAREN Investigators The relation between physical activity and metabolic control in type 2 diabetes with < 20 years of evolution. Diabetes Care. 2003;26(5):1648–1650. doi: 10.2337/diacare.26.5.1648. [DOI] [PubMed] [Google Scholar]
- 36.Lari H, Tahmasebi R, Noroozi A. Effect of electronic education based on health promotion model on physical activity in diabetic patients. Diabetes Metab Syndr. 2018;12(1):45–50. doi: 10.1016/j.dsx.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25(3):458–463. doi: 10.2337/diacare.25.3.458. [DOI] [PubMed] [Google Scholar]
- 38.Rossi MC, Lucisano G, Funnell M, et al. Interplay among patient empowerment and clinical and person-centered outcomes in type 2 diabetes. The BENCH-D study. Patient Educ Couns. 2015;98(9):1142–1149. doi: 10.1016/j.pec.2015.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.