Abstract
Introduction
Once-weekly semaglutide is a novel glucagon-like peptide-1 (GLP-1) analog for the treatment of type 2 diabetes (T2D) that has been associated with greater reductions in glycated hemoglobin (HbA1c) and body weight versus GLP-1 receptor agonists dulaglutide, exenatide extended-release (ER), liraglutide and lixisenatide in the SUSTAIN trial program and a network meta-analysis (NMA). The aim of the present study was to assess the long-term cost-effectiveness of semaglutide versus all available GLP-1 receptor agonists in Denmark, using a clinically orientated treatment approach.
Methods
Outcomes were projected over patient lifetimes using the IQVIA CORE Diabetes Model. Baseline characteristics and treatment effects were sourced from the corresponding SUSTAIN trials and the NMA. Patients were assumed to initiate GLP-1 receptor agonist therapy and subsequently treatment-intensify according to clinical treatment guidelines, with addition of basal insulin and switching to basal-bolus insulin occurring when HbA1c exceeded recommended targets. Patients were assumed to receive a GLP-1 receptor agonist plus basal insulin therapy once HbA1c levels reached 7.5% and a basal-bolus insulin regimen once HbA1c exceeded 8.0%. Costs were captured in 2017 Danish kroner (DKK), with future costs and outcomes discounted at 3% per annum.
Results
Primary analyses indicated that semaglutide 0.5 mg and 1 mg were associated with improvements in quality-adjusted life expectancy of 0.11 and 0.34 quality-adjusted life years, respectively, versus dulaglutide, achieved at cost savings of DKK 289 and DKK 13,416, respectively. Supporting analyses indicated that both doses of semaglutide were either cost-effective or dominant versus exenatide ER, liraglutide 1.2 mg and 1.8 mg and lixisenatide.
Conclusion
Semaglutide represents a cost-effective alternative to other GLP-1 receptor agonist therapies available in Denmark, demonstrating clinical benefits versus dulaglutide, exenatide ER, liraglutide and lixisenatide for the treatment of patients with T2D.
Funding
Novo Nordisk A/S.
Plain Language Summary
Plain language summary available for this article.
Electronic supplementary material
The online version of this article (10.1007/s13300-019-0630-6) contains supplementary material, which is available to authorized users.
Keywords: Cost, Cost-effectiveness, Denmark, Diabetes mellitus, GLP-1 receptor agonist
Plain Language Summary
As the prevalence and associated costs of diabetes increase, choosing cost-effective treatments is becoming ever more important.
Studies have shown that early, multifactorial treatments that improve glycemic control and body weight reduce the risk of long-term diabetes-related complications.
In the SUSTAIN clinical trial program, once-weekly semaglutide was associated with improved efficacy versus dulaglutide and exenatide extended-release (ER), while in a network meta-analysis, semaglutide was associated with improved efficacy versus liraglutide and lixisenatide in terms of greater HbA1c reductions and weight loss in patients with type 2 diabetes.
The present analysis used a clinical treatment approach to assess the long-term cost-effectiveness of semaglutide versus all available GLP-1 receptor agonists for the treatment of patients with type 2 diabetes not achieving glycemic control on oral anti-diabetic medications from a healthcare payer perspective in Denmark.
Semaglutide 0.5 mg and 1 mg were associated with improved life expectancy and quality-adjusted life expectancy versus all comparators over the 50 years of the analysis. Semaglutide 0.5 mg was associated with delayed treatment intensification by between 1 and 4 years, reduced costs versus dulaglutide, liraglutide 1.8 mg and lixisenatide, and increased costs versus exenatide ER and liraglutide 1.2 mg, while semaglutide 1 mg was associated with delayed intensification by between 3 and 6 years and reduced costs versus all comparators over patient lifetimes.
Both semaglutide doses therefore offer highly cost-effective alternatives to dulaglutide, exenatide ER, liraglutide and lixisenatide for the treatment of patients with type 2 diabetes in Denmark.
Introduction
Diabetes accounts for a substantial clinical and economic burden in Denmark, with 6.4% of the adult population living with diabetes in 2017 and diabetes-related direct and societal costs exceeding EUR 7.0 billion [1, 2]. Almost 60% of diabetes-related healthcare expenditure has been attributed to the 25% of patients who experience major diabetes-related complications, demonstrating the need for treatments that target the risk factors of these events [2]. Recent post hoc analysis of the Danish Steno-2 Study has suggested that intensive therapy targeting multiple short-term risk factors greatly increases life expectancy of patients with type 2 diabetes (T2D), without increasing the current level of expenditure seen with conventional therapy [3]. These findings correlate with several landmark studies, which have indicated that improvements in glycated hemoglobin (HbA1c), body weight, systolic blood pressure, plasma lipids and hypoglycemia risk are associated with a substantially reduced risk of long-term diabetes-related complications [4–14]. Early, multifactorial treatment that improves glycemic control while reducing body weight is therefore imperative to directly improve patients’ quality of life while reducing costs for the healthcare payer [3, 10].
The Danish healthcare system provides healthcare for all Danish citizens and is based on the principles of free and equal access to healthcare for all. In Denmark, approximately 74% of patients with T2D are diagnosed, and among those 90% receive care within the last year of their diagnosis [15]. In newly diagnosed patients, only 30% of those receiving therapy in primary care achieve an HbA1c level ≤ 7.0%, while 12% of patients have an HbA1c level ≥ 8.0% [16]. Moreover, results from a large Danish cohort study indicated that metformin monotherapy is often insufficient to maintain glycemic control, with 9678 out of 45,268 patients (21.4%) requiring treatment intensification with a second anti-diabetic therapy after 3 years, following a mean HbA1c level of 8.0% (64 mmol/mol) prior to intensification [17]. Additionally, patients with T2D often struggle to maintain a healthy body mass index (BMI), with approximately 80% of patients classified as either overweight (BMI 25.0–29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2) [18]. Novel, multifactorial treatments could therefore greatly benefit patients with T2D in Denmark.
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of multifactorial T2D medications that have been shown to improve numerous risk factors for diabetes-related complications, including glycemic control and body weight [19–22]. In addition, recent cardiovascular outcomes trials (CVOTs) have shown that long-acting human GLP-1 receptor agonists, including liraglutide, semaglutide, dulaglutide and albiglutide, are associated with improved cardiovascular outcomes in patients with T2D [23–26]. In Denmark, available GLP-1 receptor agonists include the once-weekly variants semaglutide, dulaglutide and exenatide extended-release (ER) and the once-daily variants liraglutide and lixisenatide. These therapies are recommended as a second-line therapy for patients with T2D with inadequate glycemic control (HbA1c > 7.0%) on metformin, particularly those with established cardiovascular disease, in line with the latest American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) guidelines [27, 28]. Additionally, ADA guidelines recommend considering dual therapy in patients with newly diagnosed type 2 diabetes with an HbA1c ≥ 1.5% above their glycemic target [29].
Once-weekly semaglutide is a novel GLP-1 analog that has been associated with improved glycemic control and weight loss versus all comparators throughout the SUSTAIN trial program, including dulaglutide 0.75 mg and 1.5 mg in the 40-week SUSTAIN 7 trial and exenatide ER in the 56-week SUSTAIN 3 trial [30–32]. In addition, a network meta-analysis (NMA) has shown that semaglutide is associated with similar benefits versus liraglutide 1.2 mg, liraglutide 1.8 mg and lixisenatide [33].
The primary aim of the present analysis was to assess the long-term cost-effectiveness of semaglutide using a clinically relevant approach, with treatment intensification occurring at defined HbA1c thresholds. This recognized that glycemic control becomes increasingly difficult over the long term as T2D progresses. Previous long-term cost-effectiveness analyses of GLP-1 receptor agonists have focused on a more simplified treatment switching pattern with a fixed time period for each intervention before intensification to rescue therapy. Although this offers pertinent information for health technology assessments, such a treatment approach is less relevant from a clinical perspective. Primary analyses assessed the long-term cost-effectiveness of semaglutide 0.5 mg and 1 mg versus dulaglutide 1.5 mg for the treatment of patients with T2D not achieving glycemic control on metformin from a healthcare payer perspective in Denmark. Supporting analyses were also prepared to compare semaglutide 0.5 mg and 1 mg with the remaining GLP-1 receptor agonists available in Denmark, including exenatide ER, lixisenatide and liraglutide 1.2 mg and 1.8 mg.
Methods
Model Overview
Analyses were performed using the IQVIA CORE Diabetes Model (version 9.0), a non-product specific diabetes policy analysis tool based on a series of inter-dependent sub-models that simulate short- and long-term diabetes-related complications, with projected outcomes validated against real-life data in 2004 and more recently in 2014 [34, 35]. The model projects outcomes for populations based on user inputs, including baseline cohort characteristics and history of complications; current and future diabetes management and concomitant medications; screening strategies; and changes in physiological parameters over time [36]. Each of the 15 sub-models, which simulate angina, congestive heart failure, myocardial infarction, stroke, peripheral vascular disease, macular edema, cataract, retinopathy, nephropathy, neuropathy, foot ulcer and amputation, hypoglycemia, ketoacidosis, lactic acidosis and non-specific mortality, has a semi-Markov structure and uses time, state, time-in-state and diabetes type-dependent probabilities derived from published sources. Model outputs include life expectancy (measured in years), quality-adjusted life expectancy (measured in quality-adjusted life years [QALYs]), cumulative incidence and time to onset of diabetes-related complications, direct medical costs, and cost-effectiveness scatterplots and acceptability curves. Incremental cost-effectiveness ratios (ICERs), calculated as the incremental cost per unit of effectiveness gained by using the new intervention versus the comparator, can be used as a measure of cost-effectiveness where an intervention is associated with improved clinical outcomes at an increased cost.
All base case analyses were projected over patient lifetimes (up to 50 years) and utilized UK Prospective Diabetes Study (UKPDS) 68 risk equations, with background mortality based on Danish-specific life tables from the World Health Organization (WHO) [37]. This approach was chosen to capture all relevant long-term complications and costs and to assess the impact of each intervention on life expectancy and quality-adjusted life expectancy, in line with previous analyses of GLP-1 receptor agonists and the guidance on assessing the cost-effectiveness of interventions for diabetes [38–41]. All base case and sensitivity analyses were performed using a first-order Monte Carlo approach, with probabilistic sensitivity analyses performed separately using a second-order Monte Carlo approach. Clinical and cost outcomes were discounted at 3.0% annually, consistent with guidelines for health economic analyses in the Danish setting [42].
Clinical Data for the Primary Analysis
Clinical data, including baseline cohort characteristics and treatment effects, were sourced from SUSTAIN 7 for primary comparisons of semaglutide versus dulaglutide (Table 1 and Table S1). Data were obtained from pre-specified end points wherever possible, but to fulfill all of the data requirements for an analysis using the IQVIA CORE Diabetes Model, a number of post hoc analyses of the trial data were required. Pre-specified statistical analyses in SUSTAIN 7 assessed the statistical significance of differences in treatment effects between semaglutide 1 mg and dulaglutide 1.5 mg, but post hoc analyses were required to assess the statistical significance of differences in treatment effects between semaglutide 0.5 mg and dulaglutide 1.5 mg. While dulaglutide 0.75 mg was also assessed in SUSTAIN 7, this is indicated only for monotherapy or in patients aged > 75 years by the European Medicines Agency (EMA), so this dose was not included in the present study [43]. Treatment effects (both statistically and non-statistically significant differences, in line with ISPOR guidance) were applied in the first year of the analysis, while hypoglycemic event rates from SUSTAIN 7 were applied until patients treatment-intensified with the addition of basal insulin [44].
Table 1.
Treatment effects and adverse event rates applied in the analyses
| Parameter | SUSTAIN 7 | SUSTAIN 3 | NMA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Semaglutide 0.5 mg | Semaglutide 1 mg | Dulaglutide 1.5 mg | Semaglutide 1 mg | Exenatide ER | Semaglutide 0.5 mg | Semaglutide 1 mg | Liraglutide 1.2 mg | Liraglutide 1.8 mg | Lixisenatide | |
| Physiological parameters, means (standard errors) | ||||||||||
| HbA1c (%) | − 1.51 (0.06) | − 1.78 (0.06)* | − 1.37 (0.06) | − 1.54 (0.06)* | − 0.92 (0.06) | − 1.23 (0.13)¤,‡ | − 1.47 (0.12)¤,†,‡ | − 0.87 (0.12) | − 1.11 (0.10) | − 0.56 (0.20) |
| Systolic blood pressure (mmHg) | − 2.44 (0.76) | − 4.88 (0.77) | − 2.86 (0.75) | − 4.60 (0.68)* | − 2.23 (0.70) | − 5.41 (1.55) | − 6.28 (1.52)†,‡ | − 4.45 (1.39) | − 4.21 (1.36) | − 3.00 (1.93) |
| Diastolic blood pressure (mmHg) | − 0.57 (0.48) | − 2.05 (0.49)* | − 0.03 (0.47) | − 1.00 (0.45) | − 0.10 (0.46) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total cholesterol (mmol/l) | − 0.18 (0.04) | − 0.14 (0.05) | − 0.07 (0.05) | − 0.24 (0.04) | − 0.15 (0.05) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HDL cholesterol (mmol/l) | − 0.01 (0.01)* | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.00 (0.01) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LDL cholesterol (mmol/l) | − 0.08 (0.04) | − 0.01 (0.04) | 0.02 (0.04) | − 0.12 (0.04) | − 0.09 (0.04) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Triglycerides (mmol/l) | − 0.16 (0.03) | − 0.25 (0.03) | − 0.19 (0.03) | − 0.28 (0.04)* | − 0.05 (0.04) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| BMI (kg/m2) | − 1.63 (0.10)* | − 2.33 (0.10)* | − 1.08 (0.10) | − 2.01 (0.10)* | − 0.65 (0.10) | − 0.86 (0.10)¤,‡ | − 1.35 (0.10)¤,†,‡ | − 0.64 (0.10) | − 0.73 (0.09) | − 0.32 (0.18) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | − 2.42 (0.48) | − 2.80 (0.49) | − 3.51 (0.47) | − 5.83 (0.76) | − 5.27 (0.76) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypoglycemia event rates, per 100 patient-years | ||||||||||
| Non-severe hypoglycemia | 1.28 | 2.60 | 1.26 | 9.66 | 11.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Severe hypoglycemia | 0.00 | 0.43 | 0.84 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Proportion of non-severe events that are nocturnal | 0.00 | 0.00 | 0.00 | 0.10 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Proportion of severe events that are nocturnal | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
BMI body mass index, ER extended-release, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
* Statistically significant difference at 95% confidence level versus dulaglutide 1.5 mg
§Statistically significant difference at 95% confidence level versus exenatide ER
¤Statistically significant difference at 95% confidence level versus liraglutide 1.2 mg
†Statistically significant difference at 95% confidence level versus liraglutide 1.8 mg
‡Statistically significant difference at 95% confidence level versus lixisenatide
Clinical Data for the Supporting Analyses
Baseline cohort characteristics and treatment effects for supporting comparison of semaglutide 1 mg with exenatide ER were sourced from SUSTAIN 3 (Table 1 and Table S1). For supporting comparisons of semaglutide with exenatide ER, liraglutide and lixisenatide, treatment effect data were obtained from the NMA, with baseline patient characteristics based on the SUSTAIN 7 cohort (Table 1 and Table S1). Changes from baseline in HbA1c, systolic blood pressure and BMI (the outcomes included in the NMA that were applicable to an analysis using the IQVIA CORE Diabetes Model) versus placebo were applied in all treatment arms, with both statistically significant and non-statistically significant differences included. Where parameters were not included in the NMA, inputs were assumed to be 0 in all arms to ensure that these did not drive cost-effectiveness outcomes.
Treatment Effects, Biomarker Progression and Treatment Duration
Following application of the treatment effects in the first year of the analysis, HbA1c was modeled to increase by 0.14% per year, based on the metformin arm of the ADOPT study (as no data on long-term HbA1c changes with GLP-1 receptor agonists are available), and patients were assumed to receive semaglutide or comparator treatment until HbA1c reached 7.5% (Fig. 1) [45]. At this stage, treatment was intensified with addition of basal insulin and continuation of GLP-1 receptor agonist therapy (the most common intensification step from GLP-1 receptor agonists in Denmark), with the reduction in HbA1c based on an insulin-naïve population and derived from the “Core” multivariate equations estimated by Willis et al. [46]. HbA1c was subsequently modeled to follow the UKPDS progression equation until reaching 8.0%, after which patients discontinued GLP-1 receptor agonist therapy and intensified to basal-bolus insulin. HbA1c was modeled to drop according to the multivariate “Core” equations for an insulin-experienced population published by Willis et al. and then to progress according to the UKPDS equation, with patients remaining on basal-bolus insulin for the remainder of their lifetimes [46]. Using this approach, patients received semaglutide 0.5 mg, 1 mg and dulaglutide 1.5 mg for 7, 9 and 6 years, respectively, and once-weekly semaglutide 0.5 mg, 1 mg and dulaglutide 1.5 mg plus basal insulin for 3 years, before intensifying to basal-bolus insulin for the remainder of their lifetimes (Fig. 1). This approach was chosen to reflect common clinical practice, where, due to the progressive nature of T2D, glycemic control cannot be maintained indefinitely with one medication [27]. For the majority of patients, and particularly for those with high cardiovascular disease risk, for whom GLP-1 receptor agonists are recommended following metformin failure, treatment intensification with the addition of basal insulin followed by basal-bolus insulin therapy is required to maintain good glycemic control over the long term [18, 27, 29]. Variations in initial treatment duration, including an analysis with GLP-1 receptor agonist therapy maintained for patient lifetimes, were explored in sensitivity analyses.
Fig. 1.
HbA1c progression in the primary analysis based on SUSTAIN 7. HbA1c glycated hemoglobin. Curves for each medication continue to converge following year 30 until the end of the analysis (year 50). Changes in HbA1c for semaglutide 0.5 mg, 1 mg and dulaglutide 1.5 mg are − 0.75%, − 0.76% and − 0.74%, respectively, on first intensification and − 1.46%, − 1.47% and − 1.45%, respectively, on second intensification, based on the equations published by Willis et al. [46]. Intensification steps were delayed by 1 year with semaglutide 0.5 mg and by 3 years with semaglutide 1 mg
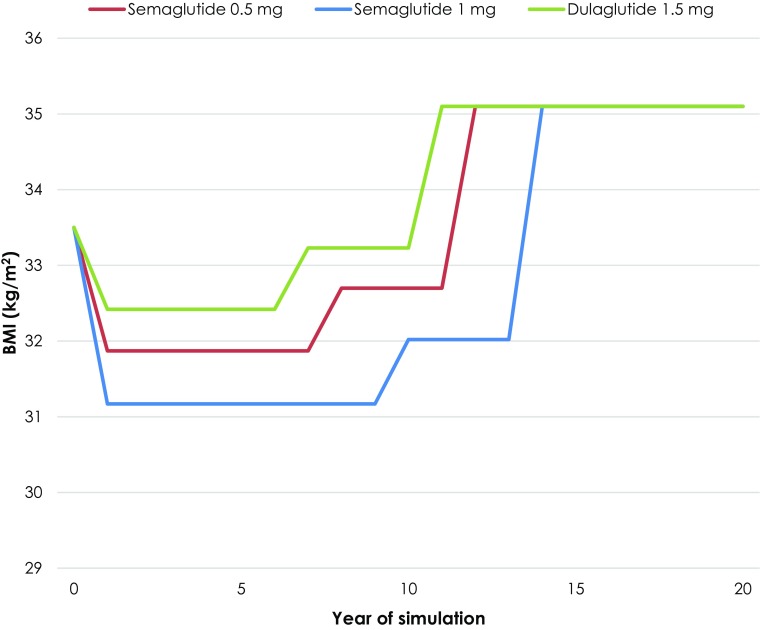
BMI progression was modeled to correlate with treatment switching patterns, taking into account the weight gain often observed in patients receiving basal insulin therapy (Fig. 2). Initial treatment effects with semaglutide or the comparator were maintained until basal insulin was added to the treatment regimen, where BMI was modeled to increase according to the “Core” equations for an insulin-naïve population provided by Willis et al. [46]. BMI was maintained at this level until patients intensified to basal-bolus insulin therapy, after which BMI was further increased according to the equations for an insulin-experienced population, again published by Willis et al., with the differences between the treatment arms abolished [46].
Fig. 2.
BMI progression in the primary analysis based on SUSTAIN 7. BMI body mass index. Curves for each medication remain at the same level following year 20 until the end of the analysis (year 50). Changes in BMI for semaglutide 0.5 mg, 1 mg and dulaglutide 1.5 mg are + 0.83, + 0.85 and + 0.81 kg/m2, respectively, on first intensification and + 2.40, + 3.08 and + 1.87 kg/m2, respectively, on second intensification, based on the equations published by Willis et al. [46]. Intensification steps were delayed by 1 year with semaglutide 0.5 mg and by 3 years with semaglutide 1 mg
Systolic blood pressure and serum lipids were assumed to follow the natural progression algorithms built into the IQVIA CORE Diabetes Model from the start of each analysis, based on the UKPDS or Framingham data (as described by Palmer et al.) [36]. Following the first treatment intensification with the addition of basal insulin, non-severe and severe hypoglycemic events were projected to increase to 10.615 and 0.258 events per patient per year, respectively, based on the Danish Hypoglycemia Assessment Tool study (as the multivariate regression results calculated by Willis et al. had a poor fit) [47]. These were applied in all arms and were maintained for the remainder of the analyses.
Costs and Utilities
Costs were estimated from a Danish healthcare payer perspective and expressed in 2017 Danish kroner (DKK). Pharmacy acquisition costs were sourced from the Danish Medicines Agency in March 2018 [48]. Diabetes medication resource use was based on the relevant clinical trials from which data were taken for each comparison.
Following addition of basal insulin to the treatment regimen, patients were assumed to receive the defined daily dose (DDD) of 40 IU, with the cost weighted to the market share of basal insulin in Denmark (to reflect current practice). Once patients intensified to basal-bolus insulin, patients were assumed to receive the DDDs of both basal insulin and bolus insulin (both 40 IU), with the cost again weighted to the market shares of basal and bolus insulin in Denmark. Costs were the same in all arms following the second intensification step. Resource use was used to calculate annual treatment costs for each arm (Table S2, Table S3 and Table S4). Annual costs also captured concomitant medications, needle use and self-monitoring of blood glucose (SMBG) testing with GLP-1 receptor agonist and intensification treatments.
Costs of treating diabetes-related complications were based on a literature review updated in 2018, with prices inflated to 2017 DKK (Table S5). Utilities relating to quality of life were taken from a 2014 review by Beaudet et al., with the exception of those associated with hypoglycemia [49]. A diminishing approach for disutilities relating to non-severe hypoglycemic events was used, based on a 2014 publication by Lauridsen et al., while a disutility applied following severe events was sourced from a 2013 publication by Evans et al., both of which were published after the literature searches by Beaudet et al. had been completed [50, 51].
Sensitivity Analyses Conducted for the Primary Analysis
Projection of long-term outcomes from short-term data is associated with uncertainty. Therefore, to test the robustness of the primary analysis, several one-way deterministic sensitivity analyses and a probabilistic sensitivity analysis were conducted on key parameters and assumptions of the analyses.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Primary Base Case Analysis
Long-term projections, based on data from SUSTAIN 7, indicated that semaglutide 0.5 mg and 1 mg were associated with improvements in life expectancy of 0.04 and 0.15 years, respectively, and quality-adjusted life expectancy of 0.11 and 0.34 QALYs, respectively, versus dulaglutide (Table 2). Improvements in life expectancy and quality-adjusted life expectancy were a result of the multifactorial risk-reduction effects of semaglutide, delaying the time to treatment intensification and resulting in a delayed time to onset and reduced cumulative incidence of diabetes-relating complications in the long term.
Table 2.
Long-term cost-effectiveness outcomes from the primary analysis based on SUSTAIN 7
| Health outcomes | Semaglutide 0.5 mg | Dulaglutide 1.5 mg | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 14.27 (0.19) | 14.24 (0.20) | + 0.03 |
| Discounted quality-adjusted life expectancy (QALYs) | 9.19 (0.12) | 9.08 (0.12) | + 0.11 |
| Discounted direct costs (DKK) | 415,200 (10,048) | 415,489 (9722) | − 289 |
| ICER for semaglutide 0.5 mg versus dulaglutide 1.5 mg | Semaglutide 0.5 mg dominant | ||
| Health outcomes | Semaglutide 1 mg | Dulaglutide 1.5 mg | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 14.39 (0.20) | 14.24 (0.20) | + 0.15 |
| Discounted quality-adjusted life expectancy (QALYs) | 9.41 (0.12) | 9.08 (0.12) | + 0.34 |
| Discounted direct costs (DKK) | 402,073 (9719) | 415,489 (9722) | − 13,416 |
| ICER for semaglutide 1 mg versus dulaglutide 1.5 mg | Semaglutide 1 mg dominant | ||
Values are means (standard deviations)
DKK 2017 Danish kroner, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life years
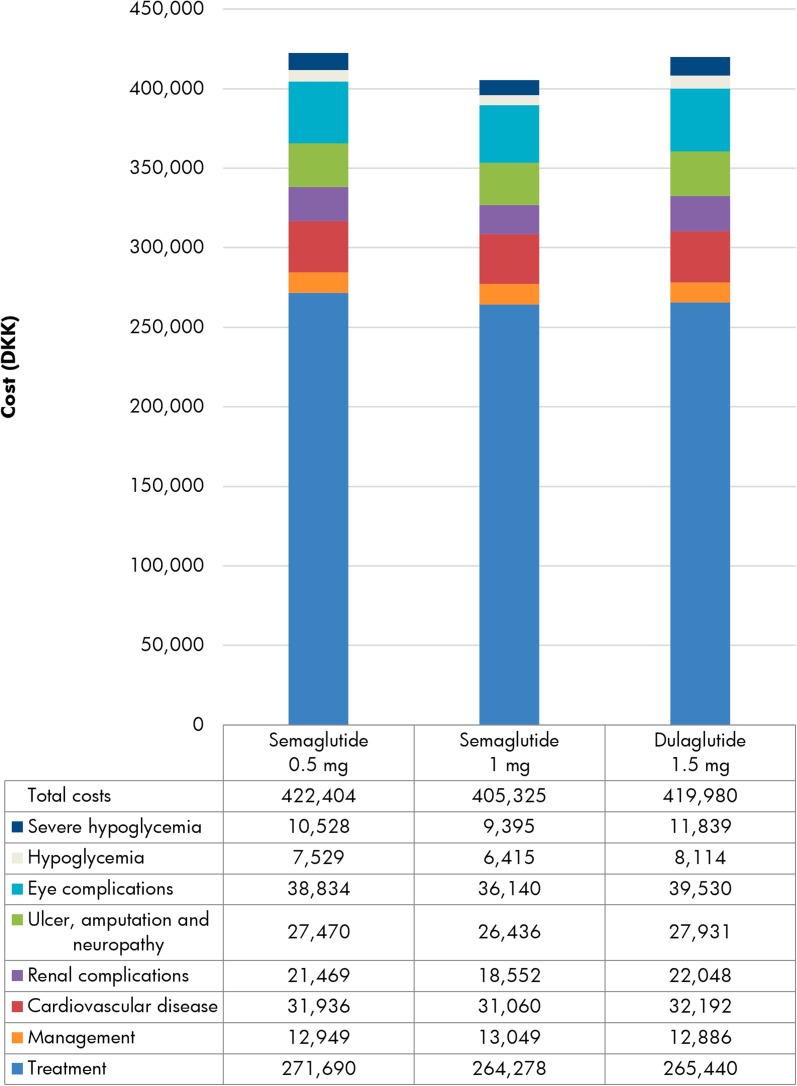
Semaglutide 0.5 mg and 1 mg were associated with cost savings of DKK 289 and DKK 13,416 per patient, respectively, versus dulaglutide over the lifetime course of the analysis (Fig. 3). This was primarily due to improved glycemic control with semaglutide, delaying the first intensification step to the costlier treatment combination of a GLP-1 receptor agonist plus basal insulin (Table S2). Further cost savings were achieved through avoidance of diabetes-related complications, most notably severe hypoglycemia (mean cost savings of DKK 2719 per patient with semaglutide 0.5 mg and DKK 6158 per patient with semaglutide 1 mg). With clinical benefits achieved at cost savings, both semaglutide 0.5 mg and 1 mg were considered dominant versus dulaglutide.
Fig. 3.
Discounted direct costs over patient lifetimes in the primary analysis based on SUSTAIN 7. DKK 2017 Danish kroner
Sensitivity Analyses
Sensitivity analyses, conducted for the primary comparisons of semaglutide 0.5 mg and 1 mg versus dulaglutide, showed that the results of the base case analysis were robust to changes in the input parameters and assumptions used (Table 3 and Table S6). Shortening the time horizon to 10 and 20 years resulted in reduced incremental clinical benefits for semaglutide, but the 1 mg dose remained cost saving and dominant versus dulaglutide, while the 0.5 mg dose was associated with increased costs and ICERs of DKK 12,793 and DKK 24,490 per QALY gained, respectively.
Table 3.
Sensitivity analyses results for semaglutide 1 mg versus dulaglutide 1.5 mg based on SUSTAIN 7
| Analysis | Discounted quality-adjusted life expectancy (QALYs) | Discounted direct costs (DKK) | ICER (DKK per QALY gained) | ||||
|---|---|---|---|---|---|---|---|
| Semaglutide 1 mg | Dulaglutide 1.5 mg | Difference | Semaglutide 1 mg | Dulaglutide 1.5 mg | Difference | ||
| Base case | 9.41 | 9.08 | + 0.34 | 402,073 | 415,489 | − 13,416 | Semaglutide dominant |
| 20-year time horizon | 8.17 | 7.88 | + 0.28 | 295,697 | 306,919 | − 11,222 | Semaglutide dominant |
| 10-year time horizon | 5.48 | 5.28 | + 0.20 | 152,959 | 167,743 | − 14,785 | Semaglutide dominant |
| Statistically significant differences only | 9.40 | 9.08 | + 0.32 | 403,334 | 415,489 | − 12,156 | Semaglutide dominant |
| First treatment intensification at HbA1c ≥ 7.0% and second intensification at HbA1c ≥ 7.5% | 9.16 | 8.88 | + 0.28 | 427,307 | 438,257 | − 10,950 | Semaglutide dominant |
| First treatment intensification at HbA1c ≥ 8.0% and second intensification at HbA1c ≥ 8.5% | 9.59 | 9.24 | + 0.34 | 383,083 | 390,248 | − 7165 | Semaglutide dominant |
| Treatment intensification at 3 years | 9.65 | 9.54 | + 0.11 | 386,173 | 382,932 | + 3242 | 30,408 |
| Treatment intensification at 5 years | 9.72 | 9.60 | + 0.12 | 375,320 | 371,568 | + 3753 | 31,721 |
| GLP-1 receptor agonist treatment continued for patient lifetimes | 9.45 | 9.08 | + 0.37 | 476,223 | 502,717 | − 26,494 | Semaglutide dominant |
| Cost of complications + 25% | 9.41 | 9.08 | + 0.34 | 436,659 | 454,138 | − 17,479 | Semaglutide dominant |
| Cost of complications − 25% | 9.41 | 9.08 | + 0.34 | 367,496 | 376,849 | − 9353 | Semaglutide dominant |
| All costs + 25% | 9.41 | 9.08 | + 0.34 | 499,312 | 516,115 | − 16,803 | Semaglutide dominant |
| All costs − 25% | 9.41 | 9.08 | + 0.34 | 304,842 | 314,872 | − 10,030 | Semaglutide dominant |
| Lee et al. BMI disutility applied | 8.96 | 8.57 | + 0.40 | 402,073 | 415,489 | − 13,416 | Semaglutide dominant |
| Evans et al. hypoglycemia disutilities applied | 9.30 | 8.94 | + 0.37 | 402,073 | 415,489 | − 13,416 | Semaglutide dominant |
| Currie et al. hypoglycemia disutilities applied | 9.49 | 9.17 | + 0.32 | 402,073 | 415,489 | − 13,416 | Semaglutide dominant |
BMI body mass index, DKK 2017 Danish kroner, HbA1c glycated hemoglobin, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year
Maintaining GLP-1 therapy for patient lifetimes led to increased clinical benefits for both doses of semaglutide, with the 1 mg dose remaining cost saving and dominant but the 0.5 mg dose associated with slight cost increases and an ICER of DKK 6264 per QALY gained.
Including only statistically significant treatment effects between the treatment arms led to only slightly reduced clinical benefits and cost savings for semaglutide 1 mg, and it remained dominant versus dulaglutide, while semaglutide 0.5 mg was associated with reduced clinical benefits and increased incremental costs, yielding an ICER of DKK 683,944 per QALY gained. This was driven by treatment switching occurring simultaneously, as HbA1c progression was equal in both treatment arms.
Exploration of alternative treatment switching approaches, with a set period of 3 or 5 years for GLP-1 receptor agonist treatment followed by intensification to basal insulin (aligned with a recent cost-effectiveness analysis of semaglutide published in the UK setting), resulted in reduced clinical benefits and increased incremental costs for semaglutide 0.5 mg and 1 mg, yielding ICERs of DKK 68,414 and DKK 30,408 per QALY gained, respectively, when treatment switching occurred after 3 years, and DKK 96,326 and DKK 31,721 per QALY gained, respectively, when treatment switching occurred after 5 years [52]. There were only minor changes to the overall results when altering HbA1c thresholds for treatment switching, changing costs by ± 25% or using alternative BMI and hypoglycemia disutilities versus dulaglutide [51, 53, 54].
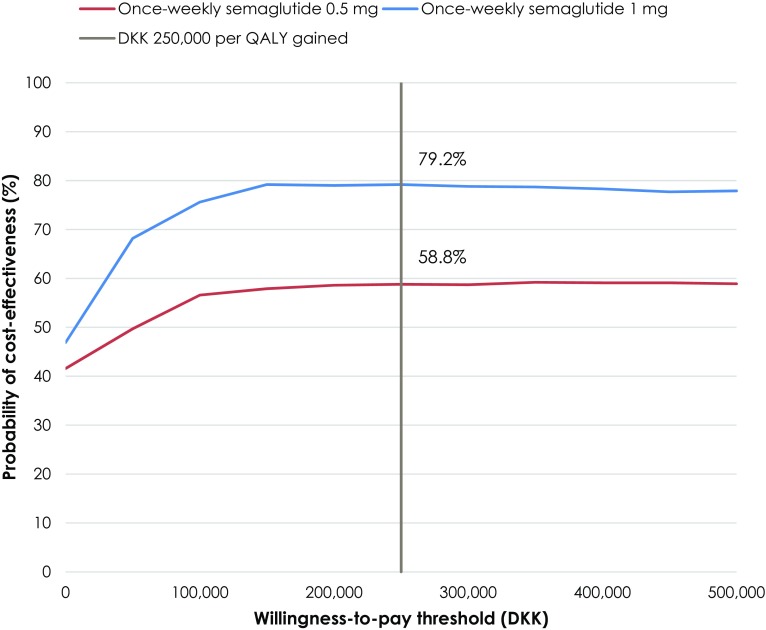
Clinical outcomes from the PSA were similar to those from the base case analysis, but semaglutide 0.5 mg and 1 mg were associated with increased incremental costs and reduced cost savings, respectively, with greater variance shown around the mean outcomes (Fig. 4). Semaglutide 0.5 mg and 1 mg were associated with increased quality-adjusted life expectancy of 0.10 and 0.30 QALYs, respectively, versus dulaglutide. Mean costs were DKK 3668 higher with semaglutide 0.5 mg but DKK 6718 lower with semaglutide 1 mg versus dulaglutide over patient lifetimes. Semaglutide 0.5 mg was therefore associated with an ICER of DKK 37,197 per QALY gained, while semaglutide 1 mg was dominant versus dulaglutide in the PSA. At a willingness-to-pay threshold of DKK 250,000 per QALY gained (a representative value based on GBP 20,000 in the UK), the probabilities of once-weekly semaglutide 0.5 mg and 1 mg being considered cost-effective were 58.8% and 79.2%, respectively, versus dulaglutide 1.5 mg (Fig. 5).
Fig. 4.
Probabilistic sensitivity analysis scatterplot for the primary analysis versus dulaglutide, based on SUSTAIN 7. DKK 2017 Danish kroner, QALYs quality-adjusted life years
Fig. 5.
Cost-effectiveness acceptability curve for the primary analysis versus dulaglutide, based on SUSTAIN 7. DKK 2017 Danish kroner, QALY quality-adjusted life year
Supporting Analyses
In the supporting analysis based on SUSTAIN 3, semaglutide 1 mg was projected to improve life expectancy by 0.24 years, and quality-adjusted life expectancy by 0.47 QALYs, versus exenatide ER (Table 4). Clinical benefits were achieved at a cost saving from a healthcare payer perspective, with semaglutide 1 mg costing DKK 18,088 less over a patient’s lifetime than exenatide ER. Clinical benefits and cost savings were a result of a reduced incidence and delayed time to onset of diabetes-related complications.
Table 4.
Long-term cost-effectiveness outcomes from the supporting analyses based on SUSTAIN 3
| Health outcomes | Semaglutide 1 mg | Exenatide ER | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 14.79 (0.18) | 14.55 (0.20) | + 0.24 |
| Discounted quality-adjusted life expectancy (QALYs) | 9.46 (0.12) | 8.98 (0.12) | + 0.47 |
| Discounted direct costs (DKK) | 461,487 (10,962) | 479,575 (11,829) | − 18,088 |
| ICER for semaglutide 1 mg versus exenatide ER | Semaglutide 1 mg dominant | ||
Values are means (standard deviations)
DKK 2017 Danish kroner, ER extended-release, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life years
In the supporting analyses based on the NMA, semaglutide 0.5 mg and 1 mg were associated with clinical benefits versus all comparators (Table 5). Over patient lifetimes, semaglutide 0.5 mg was associated with higher costs of DKK 3218 versus exenatide ER and DKK 1518 versus liraglutide 1.2 mg, but cost savings of DKK 30,970 versus liraglutide 1.8 mg and DKK 16,961 versus lixisenatide. This yielded ICERs of DKK 17,024 and DKK 7390 per QALY gained for semaglutide 0.5 mg versus exenatide ER and liraglutide 1.2 mg, respectively, while it was considered dominant versus liraglutide 1.8 mg and lixisenatide. Semaglutide 1 mg was associated with cost savings of DKK 11,884, DKK 13,583, DKK 46,071 and DKK 32,063 versus exenatide ER, liraglutide 1.2 mg, liraglutide 1.8 mg and lixisenatide, respectively, and was therefore considered dominant versus all comparators.
Table 5.
Long-term cost-effectiveness outcomes from the supporting analyses based on the NMA
| Health outcomes | Semaglutide 0.5 mg | Semaglutide 1 mg | Exenatide ER | Liraglutide 1.2 mg | Liraglutide 1.8 mg | Lixisenatide |
|---|---|---|---|---|---|---|
| Discounted life expectancy (years) | 14.16 (0.21) | 14.26 (0.19) | 14.07 (0.21) | 14.04 (0.20) | 14.12 (0.20) | 13.98 (0.19) |
| Discounted quality-adjusted life expectancy (QALYs) | 8.94 (0.13) | 9.14 (0.12) | 8.75 (0.13) | 8.73 (0.12) | 8.80 (0.12) | 8.55 (0.12) |
| Discounted direct costs (DKK) | 431,248 (10,024) | 416,147 (9771) | 428,031 (10,687) | 429,730 (9883) | 462,218 (10,721) | 448,210 (11,094) |
| ICER for semaglutide 0.5 mg (DKK per QALY gained) | – | – | 17,024 | 7390 | Semaglutide 0.5 mg dominant | Semaglutide 0.5 mg dominant |
| ICER for semaglutide 1 mg (DKK per QALY gained) | – | – | Semaglutide 1 mg dominant | Semaglutide 1 mg dominant | Semaglutide 1 mg dominant | Semaglutide 1 mg dominant |
Values are means (standard deviations)
DKK 2017 Danish kroner, ER extended-release, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life years
Discussion
Recent studies have emphasized the importance of early, intensive glycemic control to reduce the risk of long-term complications, thereby drastically increasing patients’ quality of life and life expectancy while minimizing healthcare payer expenditure [3, 9, 10]. Moreover, multifactorial treatments that target improvements in blood pressure, cholesterol level and body weight, as well as HbA1c, have been shown to reduce the risk of death, myocardial infarction and stroke in patients with T2D [55]. Semaglutide represents a novel treatment option in the class of GLP-1 receptor agonists and has been associated with greater reductions in HbA1c and body weight throughout the SUSTAIN clinical trial program and several NMAs versus medications used throughout the T2D treatment algorithm [30, 32, 33]. The present analysis exemplifies the legacy effect of these short-term improvements, translating to a substantial benefit over patient lifetimes in terms of life expectancy and quality-adjusted life expectancy. Moreover, the greater clinical efficacy of semaglutide 1 mg led to delayed intensification with basal and bolus insulins by 3 years compared with dulaglutide. While there is no officially recognized willingness-to-pay threshold in Denmark, an estimate can be based on the threshold of between GBP 20,000 and 30,000 per QALY gained set by the National Institute for Health and Care Excellence (NICE) in the UK. Based on a representative threshold of DKK 250,000 per QALY gained, semaglutide 0.5 mg and 1 mg were found to be dominant versus dulaglutide 1.5 mg for the treatment of patients with T2D with inadequate glycemic control on metformin. Supporting analyses showed that semaglutide 0.5 mg was cost-effective, and semaglutide 1 mg was dominant, versus exenatide ER and liraglutide 1.2 mg, while both doses of semaglutide were dominant versus liraglutide 1.8 mg and lixisenatide. The consistently higher effectiveness associated with the 1 mg dose of semaglutide highlights the need for patients to receive early and aggressive treatment to stem the risk of long-term complications, and patients should ideally be up-titrated as soon as possible provided they do not experience any serious treatment-related adverse events.
Previous long-term cost-effectiveness analyses of semaglutide have reported similar outcomes to this study, but these used distinct periods of GLP-1 receptor agonist treatment before simultaneous treatment intensification to basal insulin therapy in all arms [52, 56, 57]. While this approach allows a more direct comparison of any two interventions, this treatment progression is less representative of real-world practice, where patients with T2D have tailored treatment regimens and would continue to receive a given treatment as long as they remain within glycemic target. Additionally, analyses performed with fixed 3- or 5-year periods of initial treatment, such as the sensitivity analyses conducted for this study, yielded lower health outcomes and increased incremental costs for semaglutide compared with the base case analysis, highlighting the importance of a clinically oriented modeling approach in capturing all relevant clinical and economic benefits. The present study is among the first to use such an approach for GLP-1 receptor agonists, which recognizes the progressive nature of T2D and the difficulty of maintaining glycemic control in the long term. Moreover, the use of the multivariate prediction equations published by Willis et al., which provide estimates of changes in HbA1c and BMI when basal and basal-bolus insulin therapies are initiated, represents a key strength of the present analysis [46]. As the equations are based on a review of the published literature, the subsequent calculated treatment effects are informed by data from a variety of sources, allowing the present study to avoid the use of specific treatment effects designed to artificially improve modeling outcomes.
Alternative treatment options for patients with T2D with inadequate glycemic control on metformin in Denmark not assessed in the present study include dipeptidyl peptidase-4 (DPP-4) inhibitors, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones and basal insulin therapy [58]. Semaglutide has been shown to improve short-term clinical outcomes of HbA1c and body weight versus DPP-4 inhibitor sitagliptin in SUSTAIN 2, basal insulin glargine U100 in SUSTAIN 4 and SGLT-2 inhibitor empagliflozin in an NMA [59–61]. Moreover, treatment with sulfonylureas or thiazolidinediones is often associated with weight gain, contrasting with the weight loss associated with semaglutide therapy [58]. Considering that greater reductions in HbA1c are associated with a delayed time to treatment intensification in the present analysis, treatment with semaglutide is likely to result in a delayed time to intensification versus all currently available second-line therapies, yielding both clinical benefits for patients and cost savings for the healthcare payer. However, further analyses are required before the long-term benefits versus all second-line comparators are fully elucidated. Nonetheless, among current GLP-1 receptor agonists, a once-weekly injection of semaglutide provides clear benefits over the once-daily injectables liraglutide and lixisenatide, while improving patient outcomes versus existing once-weekly variants dulaglutide and exenatide ER.
One aspect of care the present analyses were unable to incorporate was the additional short-term cardiovascular benefits GLP-1 receptor agonists offer. Recent evidence has suggested that GLP-1 receptor agonists as a class are potentially beneficial for patients at risk of cardiovascular disease, with semaglutide, liraglutide and albiglutide demonstrating significant benefits versus placebo in SUSTAIN 6, LEADER and HARMONY Outcomes, respectively, while lixisenatide and exenatide ER have displayed cardiovascular safety versus placebo in ELIXA and EXSCEL, respectively [23, 24, 26, 62, 63]. Current guidelines for the treatment of T2D in Denmark recommend treatment with SGLT-2 inhibitors or GLP-1 receptor agonists for patients with established cardiovascular disease. However, considering that most patients with T2D eventually develop micro- and macrovascular disease, and that 22% of newly diagnosed patients in Denmark have existing macrovascular disease, a sensitivity analysis was included to explore patients remaining on GLP-1 receptor agonist therapy for their lifetimes, which confirmed that semaglutide improved health outcomes for patients while minimizing costs for the healthcare payer [16, 58]. Given the results of SUSTAIN 6 and the clinical benefits displayed in this analysis, semaglutide is considered a valuable second-line treatment option for patients with T2D [58, 64].
The use of data from an NMA for supporting analyses could be seen as a potential limitation of the study. However, these analyses aimed to compare both doses of semaglutide with the most relevant comparators in Denmark, namely all available GLP-1 receptor agonists. Additionally, the NMA data were shown to be robust through the two comparisons of semaglutide 1 mg with exenatide ER, one of which used data from the head-to-head SUSTAIN 3 trial and one that used data from the NMA, which both yielded similar outcomes. Moreover, the use of methodologically guided evidence synthesis such as this is becoming increasingly central to decision making and accepted by health technology assessment agencies worldwide [65, 66].
Modeling HbA1c increases during the first phase of the analyses based on data from the metformin arm of ADOPT could also be seen as a drawback [45]. However, patients were at a similar stage in the T2D treatment algorithm to patients receiving GLP-1 receptor agonists in Denmark. The data from ADOPT could therefore be argued to be applicable to patients receiving GLP-1 receptor agonists. Indeed, the progression of HbA1c observed in SUSTAIN 6, where patients received semaglutide, correlates with the increases seen in ADOPT, adding credence to this assumption [24, 47].
A further limitation is the use of short-term data to project long-term outcomes. However, this remains one of the essential tenets of health economic modeling and arguably one of the best available options to inform decision making in the absence of long-term clinical trial data [38]. Additionally, projecting outcomes over patient lifetimes is recommended in guidelines for economic evaluation of interventions for patients with diabetes mellitus [38]. While there is always an element of clinical doubt around the accuracy of such an approach, every effort was made in the present analysis to minimize this, primarily by using a model of diabetes that has been extensively published and validated against real-life data both on first publication and recently following a series of model updates, in addition to extensive sensitivity analyses that test the robustness of the base case findings [34, 35].
Conclusions
Semaglutide represents a highly cost-effective treatment option within the class of GLP-1 receptor agonists available in Denmark, with the present analyses demonstrating clinical benefits versus dulaglutide, exenatide ER, liraglutide 1.2 mg and 1.8 mg and lixisenatide for the treatment of patients with T2D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The present cost-effectiveness analysis and article processing charges were supported by funding from Novo Nordisk A/S. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
The authors thank Johannes Pöhlmann at Ossian Health Economics and Communications GmbH for medical writing assistance. Ossian received funding from Novo Nordisk A/S to support this analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Peter Gæde has nothing to disclose. Pierre Johansen is an employee of Novo Nordisk A/S. Christian Tikkanen is an employee of Novo Nordisk Scandinavia AB. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. Richard Pollock was an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis, during preparation of the manuscript, and is now an employee of Covalence Research Limited. Samuel Malkin is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8040746.
Change history
5/31/2019
In the original publication, Figs. 3 and 5 and the final sentence in the final paragraph of Results/Sensitivity Analyses were incorrectly published. The corrected statement and the figures are given below.
References
- 1.International Diabetes Federation (IDF). IDF Diabetes Atlas, 8th edn. http://www.diabetesatlas.org/across-the-globe.html. Accessed May 8, 2018.
- 2.Sortsø C, Green A, Jensen PB, Emneus M. Societal costs of diabetes mellitus in Denmark. Diabet Med. 2016;33(7):877–885. doi: 10.1111/dme.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gæde J, Oellgaard J, Ibsen R, et al. A cost analysis of intensified vs conventional multifactorial therapy in individuals with type 2 diabetes: a post hoc analysis of the Steno-2 study. Diabetologia. 2019;62(1):147–55. doi: 10.1007/s00125-018-4739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismail-Beigi F, Craven T, Banerji MA, ACCORD trial group et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, ADVANCE Collaborative Group et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMicm066227. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 8.Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 11.Kearney PM, Blackwell L, Collins R, Cholesterol Treatment Trialists’ Collaborators et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care. 2008;31(8):1510–1515. doi: 10.2337/dc07-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman WH, Ye W, Griffin SJ, et al. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe) Diabetes Care. 2015;38(8):1449–1455. doi: 10.2337/dc14-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm AL, Andersen GS, Jørgensen ME, et al. Is the rule of halves framework relevant for diabetes care in Copenhagen today? A register-based cross-sectional study. BMJ Open. 2018;8:e023211. doi: 10.1136/bmjopen-2018-023211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open. 2018;8:e017273. doi: 10.1136/bmjopen-2017-017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen RW, Baggesen LM, Søgaard M, et al. Early glycaemic control in metformin users receiving their first add-on therapy: a population-based study of 4,734 people with type 2 diabetes. Diabetologia. 2015;58(10):2247–2253. doi: 10.1007/s00125-015-3698-1. [DOI] [PubMed] [Google Scholar]
- 18.Inzucchi SE, Bergenstal RM, Buse JB, American Diabetes Association [ADA] and European Association for the Study of Diabetes [EASD] et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a Randomized Controlled Trial (AWARD-5) Diabetes Care. 2014;37(8):2149–58. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 21.Russell-Jones D, Vaag A, Schmitz O, Liraglutide Effect and Action in Diabetes 5 [LEAD-5] met + SU Study Group et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152–160. doi: 10.2337/diaspect.29.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 25.Eli Lilly. Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. 2018. https://investor.lilly.com/node/39796/pdf. Accessed Nov 22, 2018.
- 26.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 27.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;2018(61):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 28.Dansk Sesklab for Almen Medicin (DSAM) [Danish Company for General Medicine]. Farmakologisk behandling af type 2-diabetes [Pharmacological treatment of type 2 diabetes]. 2018. https://vejledninger.dsam.dk/media/files/4/guidelines-2018-final.pdf. Accessed Jan 23, 2019.
- 29.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S90–S102. [DOI] [PubMed]
- 30.European Medicines Agency. EPAR summary for the public: Ozempic/semaglutide. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004174/WC500244166.pdf. Accessed June 1, 2018.
- 31.Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 32.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 33.Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving 1–2 oral anti-diabetic drugs. Diabetes Ther. 2018;9(3):1149–1167. doi: 10.1007/s13300-018-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–S40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 35.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Global Health Observatory data repository: Life tables by country—Denmark. 2015. http://apps.who.int/gho/data/view.main.60450?lang=en. Accessed June 1, 2018.
- 38.Hunt B, Ye Q, Valentine WJ, Ashley D. Evaluating the long-term cost-effectiveness of daily administered GLP-1 receptor agonists for the treatment of type 2 diabetes in the United Kingdom. Diabetes Ther. 2017;8(1):129–147. doi: 10.1007/s13300-016-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt B, Mocarski M, Valentine WJ, Langer J. Evaluation of the long-term cost-effectiveness of IDegLira versus liraglutide added to basal insulin for patients with type 2 diabetes failing to achieve glycemic control on basal insulin in the USA. J Med Econ. 2017;20(7):663–670. doi: 10.1080/13696998.2017.1301943. [DOI] [PubMed] [Google Scholar]
- 40.Hunt B, Vega-Hernandez G, Valentine WJ, Kragh N. Evaluation of the long-term cost-effectiveness of liraglutide versus lixisenatide for treatment of type 2 diabetes mellitus in the UK setting. Diabetes Obes Metab. 2017;19(6):842–849. doi: 10.1111/dom.12890. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 42.Lægemiddelstyrelsen [Danish Medicines Agency]. Erfaringer med sundhedsøkonomiske analyser i ansøgninger om generelt tilskud til lægemidler [Guidelines for health-economic analyses for novel pharmaceuticals]. 2004. https://laegemiddelstyrelsen.dk/da/tilskud/generelle-tilskud/ansoegning/sundhedsoekonomiske-analyser/erfaringer-med-sundhedsoekonomiske-analyser-i-ansoegninger-om-generelt-tilskud-til-laegemidler#47. Accessed Nov 1, 2018.
- 43.European Medicines Agency. Trulicity. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002825/human_med_001821.jsp&mid=WC0b01ac058001d124. Accessed June 1, 2018.
- 44.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Viberti G, Kahn SE, Greene DA, et al. A Diabetes Outcome Progression Trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care. 2002;25(10):1737–1743. doi: 10.2337/diacare.25.10.1737. [DOI] [PubMed] [Google Scholar]
- 46.Willis M, Asseburg C, Nilsson A, Johnsson K, Kartman B. Multivariate prediction equations for HbA1c lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20:357–371. doi: 10.1016/j.jval.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Khunti K, Alsifri S, Aronson R, Berković MC, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907–915. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lægemiddelstyrelsen [Danish Medicines Agency]. Medicinpriser. 2018. https://www.medicinpriser.dk/?lng=2. Accessed June 13, 2018.
- 49.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–470. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 51.Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for the treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–21. doi: 10.1111/dom.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 54.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 55.Rawshani AI, Rawshani AR, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 56.Johansen P, Håkan-Bloch J, Liu AR, Bech PG, Persson S, Leiter LA. Cost Effectiveness of Once-Weekly Semaglutide Versus Once-Weekly Dulaglutide in the Treatment of Type 2 Diabetes in Canada. Pharmacoecon Open. 2019. 10.1007/s41669-019-0131-6 (epub ahead of print). [DOI] [PMC free article] [PubMed]
- 57.Tikkanen CK, Johansen P, Hunt B, Malkin S, Pollock RF. Once-weekly semaglutide provides better health outcomes compared to dulaglutide as dual therapy in the treatment of type 2 diabetes: a cost-effectiveness analysis. Diabetologia. 2018;61(Supplement 1):S426–S427. [Google Scholar]
- 58.Snorgaard O, Kristensen JK, Balasubramaniam K et al. Farmakologisk behandling af type 2-diabetes [Pharmacological treatment of type 2 diabetes]. 2018. https://vejledninger.dsam.dk/media/files/4/guidelines-2018-final.pdf. Accessed Nov 27, 2018.
- 59.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 60.Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 61.Sharma R, Wilkinson L, Vrazic H, et al. Comparative efficacy of once-weekly semaglutide and SGLT-2 inhibitors in type 2 diabetic patients inadequately controlled with metformin monotherapy: a systematic literature review and network meta-analysis. Curr Med Res Opin. 2018;34(9):1595–1603. doi: 10.1080/03007995.2018.1476332. [DOI] [PubMed] [Google Scholar]
- 62.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 63.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aagaard DT, Nielsen Christiansen M, Mogensen M, et al. Cardiovascular risk according to add-on therapy in patients with type 2 diabetes. Eur Heart J. 2018;39:492. doi: 10.1093/eurheartj/ehy565.P2523. [DOI] [Google Scholar]
- 65.EUnetHTA. European Network for Health Technology. Guideline—comparators & comparisons: direct and indirect comparisons. February 2013. http://www.eunethta.eu/sites/5026.fedimbo.belgium.be/files/Direct%20and%20indirect%20comparisons.pdf. Accessed Dec 13, 2017.
- 66.Dias SW, N J, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, 2011; last updated April 2012. http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/TSD1-Introduction.final_.08.05.12.pdf. Accessed Dec 13, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.