Abstract
This editorial refers to ‘Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram-based registry study’†, by A. Ghimire et al., on page 2110.
That some achieve great success, is proof to all that others can achieve it as well.
Abraham Lincoln
For many years it has been recognized that a proportion of patients with heart failure with reduced ejection fraction (HFrEF) demonstrate remarkable capacity for improvement in myocardial contractile dysfunction. Studies that describe this phenomenon pre-date many of the landmark trials that have informed the current therapeutic management of heart failure.1 In the wake of progressive refinement in treatment comes an appreciation that improvement in cardiac function, or reverse remodelling, is characterized by reduced symptom burden, a low risk of cardiac hospitalizations, and lower mortality.2–4 It is clear that reverse remodelling should be considered a continuous spectrum rather than a binary phenomenon. Some patients will continue to have abnormal plasma biomarkers, sustained neurohumoral activation, subsequent risk of deterioration in cardiac function, and high rates of relapse following therapy withdrawal.4–7 Others will have normalization of biomarkers and resolution of symptoms, indicating remission of heart failure.
Studying left ventricular reverse remodelling offers important scientific potential. Understanding the process on a mechanistic level may offer new horizons in heart failure therapy, enhanced risk stratification, and individualized patient care. The conditions of left ventricular reverse remodelling are highly heterogenous, occurring in the setting of drug therapy, removal of exogenous stimuli, coronary revascularization, cardiac resynchronization therapy, ventricular assist device implantation, or indeed spontaneously. Accordingly, the ability to capture common underlying pathways remains elusive. To ascertain whether studying this process can provide the key to unlock sustained myocardial remission, there is a need to intricately characterize this cohort with long-term follow-up. In addition, it is equally important to understand why some patients relapse by studying the dynamic changes that occur over the long term.
In the meantime, we face a novel subgroup of heart failure patients shrouded in uncertainty. The existing evidence to guide management of patients with improved function is sparse. International guidelines make recommendations based on a single measurement of left ventricular ejection fraction (LVEF), typically taken at the onset of heart failure, and do not account for the dynamic nature of the condition and heterogeneous disease course.8 Further studies are required to inform the optimal long-term management of these patients.
In this issue of the European Heart Journal, Ghimire et al. add to the expanding body of evidence supporting the existence of a heart failure subgroup with improved cardiac function and additionally reinforce previous studies demonstrating a favourable clinical course compared with HFrEF.9 In this retrospective cohort study, the authors evaluated all patients living in the Canadian province Alberta with a physician-assigned diagnosis of heart failure, who had undergone at least two transthoracic echocardiograms separated by a minimum of 6 months. The cohort included a mix of inpatient and outpatient subjects. The median time between echocardiograms was 17 months, although notably there was considerable variation amongst patients (interquartile range 11–29 months). The analysis focused on patients with an initial LVEF ≤40%, who were subdivided into: (i) ‘heart failure with recovered ejection fraction’ (HFrecEF), where LVEF had improved by ≥10% on the interval echocardiogram; or (ii) persistent HFrEF, where LVEF had not improved to this level. The majority of patients were treated in line with available guideline recommendations (90% on an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, 92% on a beta-blocker, and 45% on a mineralocorticoid receptor antagonist); the study pre-dated the local availability of sacubitril/valsartan. Only a small number of patients (10.7%) received an implantable defibrillator or cardiac resynchronization device, which is perhaps lower than would be expected considering the baseline population. Baseline covariates were extracted from administrative databases. Clinical outcomes were evaluated, focusing on both all-cause events (all-cause mortality, all-cause hospitalization, and all-cause emergency room visits) and heart failure-related events [heart failure hospitalizations, left ventricular assist device (LVAD) implantation, or cardiac transplant]. The major findings were: (i) a high proportion (37.6%) of patients initially diagnosed with HFrEF subsequently evolved into HFrecEF; (ii) LVEF improvement was associated with female sex, younger age, lower baseline LVEF, and a history of atrial fibrillation, cancer, hypertension, or hydralazine use; and (iii) HFrecEF patients had consistently favourable clinical outcomes compared with persistent HFrEF across both all-cause and heart failure-related outcome measures, over a mean 2.7-year follow-up period.
The main strength of this study lies in the broad spectrum and large number of patients sampled (1174 with HFrecEF and 1950 with persistent HFrEF). Pre-existing studies have largely comprised retrospective analyses of randomized controlled trials or registry data from specialized tertiary centre cohorts, both of which lead to selection bias.4,10 The population-based strategy adopted in this study undoubtedly improves patient coverage and may reduce selection bias, although by no means eliminate it. The other strength of this study is the post-hoc analysis demonstrating that females with HFrEF have a significantly lower risk of death than males, irrespective of subsequent remodelling status. This supports previous findings of a sex disparity amongst clinical outcomes across a spectrum of patients with heart failure.11,12
As acknowledged by the authors, the major limitation of this study relates to the lack of clinical data. The study design utilized administrative data sets lacking NYHA class, blood pressure, biomarker data, revascularization status, QRS duration, and symptom duration, all of which impact remodelling and clinical outcomes and would have strengthened the analysis. The lack of an inception cohort may have resulted in patient misclassification had remodelling occurred either before or following the two echocardiograms. The inclusion criteria of two echocardiograms separated by at least 6 months represents a separate source of both selection and survival bias. The latter is a point that requires further clarification to ensure that this is not simply a study of survivors living beyond 6 months. Further analysis of the subset of patients who had undergone ≥3 echocardiograms for evaluation of the longer term remodelling trajectory would have been a valuable addition, given the non-linear clinical course associated with this cohort.5,6
The major commentary from this study relates to the high proportion of HFrEF patients that transitioned to HFrecEF (37.6%). This is higher than the rate observed by Wilcox et al., who evaluated patients from the IMPROVE HF registry, in which 28.6% of patients demonstrated an improvement in LVEF of >10%.13 Other studies have observed lower rates.3,4,10 Several potential explanations may underlie this finding. First, the retrospective data collection and selection criteria used by Ghimire et al. may have enriched this population. Secondly, the use of evidence-based drug therapy was very high, which may have been contributory. Thirdly, significantly more patients with HFrecEF had atrial fibrillation than the sustained HFrEF subgroup (35.1% vs. 22.9%, P < 0.0001). This raises the possibility of there existing a higher proportion of tachycardia-related cardiomyopathy in this cohort, which is associated with higher rates of reverse remodelling following treatment of the underlying arrhythmia.14
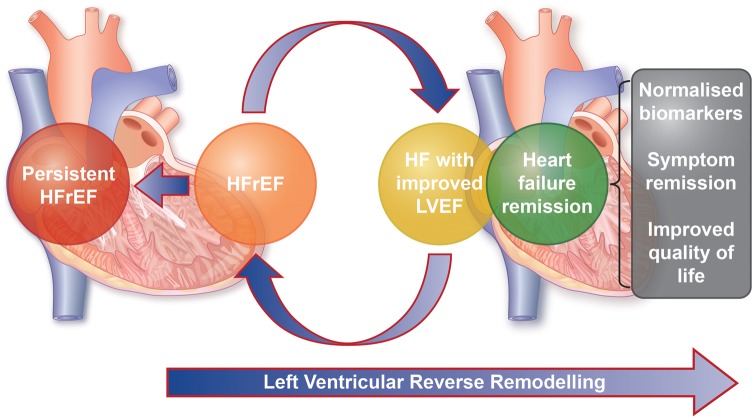
The authors illustrate that HFrecEF is far from a status that is free from morbidity, observing two patients in this subgroup who ultimately underwent cardiac transplantation or LVAD implantation. This emphasizes the previous recognition that HFrecEF is a misnomer. A move towards the use of the term ‘remission’ rather than ‘recovery’ appears appropriate until the latter can be defined more precisely. Defining remission as a binary phenomenon based on LVEF is also inadequate. Reverse remodelling and heart failure remission should be recognized as a spectrum and incorporate patient-centred variables, such as symptom status and quality of life, and natriuretic peptide levels to define the different stages of this process. In patients with improved LVEF without normalization of these variables, heart failure with improved ejection fraction may be a more appropriate term than HFrecEF (see Take home figure).
Take home figure.
Patients with heart failure undergo dynamic transition between disease subgroups due to left ventricular remodelling. Heart failure remission requires normalization of cardiac function, cardiac biomarkers, and patient-centred factors. HFrEF, heart failure with reduced ejection fraction; HF, heart failure; LVEF, left ventricular ejection fraction.
We take forward two key points from this article: first a high proportion of HFrEF patients have the potential for left ventricular reverse remodelling; and, secondly, this is associated with favourable clinical outcomes. The unmet needs are first to unpick the molecular pathways that accompany this process; secondly to study the long-term natural history of heart failure remission and the optimal management needed to sustain it; and thirdly to define the stages of reverse remodelling, incorporating symptom status, quality of life, measures of cardiac function, and natriuretic peptide concentrations. Future research should focus on prospective longitudinal characterization of recent-onset HFrEF patients during the process of left ventricular reverse remodelling. The integration of advanced imaging techniques with state-of-the-art ‘omics’ technologies offers ever-expanding mechanisms to explore the molecular landscape of the remodelling heart, unveiling novel markers of remission, theranostic targets, and insights into underlying pathways. The ultimate goal is to transition from ‘heart failure’ to ‘heart success’. Currently we remain some way from achieving this, but the present study and future research directions provide us with hope that this is not beyond the realms of possibility in the future.
Acknowledgements
The authors acknowledge support from the British Heart Foundation, Cardiomyopathy UK, and the Alexander Jansons Fund.
Conflict of interest: none declared.
Footnotes
doi:10.1093/eurheartj/ehz233.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD.. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol 1994;23:553–559. [DOI] [PubMed] [Google Scholar]
- 2. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS.. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail 2011;17:527–532. [DOI] [PubMed] [Google Scholar]
- 3. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J.. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
- 4. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC.. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 2014;129:2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groote P De, Fertin M, Pentiah AD, Goémine C, Lamblin N, Bauters C.. Long-term functional and clinical follow-up of patients with heart failure with recovered left ventricular ejection fraction after β-blocker therapy. Circ Heart Fail 2014;7:434–439. [DOI] [PubMed] [Google Scholar]
- 6. Merlo M, Stolfo D, Anzini M, Negri F, Pinamonti B, Barbati G, Ramani F, Lenarda AD, Sinagra G.. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long‐term follow‐up: does real healing exist? J Am Heart Assoc 2015;4:e001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK.. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 9. Ghimire A, Fine N, Ezekowitz JA, Howlett J, Youngson E, McAlister FA.. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram-based registry study. Eur Heart J 2019;40:2110--2117. [DOI] [PubMed] [Google Scholar]
- 10. Florea VG, Rector TS, Anand IS, Cohn JN.. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival. Circ Heart Fail 2016;9:e003123. [DOI] [PubMed] [Google Scholar]
- 11. Gustafsson F, Torp-Pedersen C, Burchardt H, Buch P, Seibaek M, Kjøller E, Gustafsson I, Køber L.. Female sex is associated with a better long-term survival in patients hospitalized with congestive heart failure. Eur Heart J 2004;25:129–135. [DOI] [PubMed] [Google Scholar]
- 12. Halliday BP, Gulati A, Ali A, Newsome S, Lota A, Tayal U, Vassiliou VS, Arzanauskaite M, Izgi C, Krishnathasan K, Singhal A, Chiew K, Gregson J, Frenneaux MP, Cook SA, Pennell DJ, Collins P, Cleland JGF, Prasad SK.. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur J Heart Fail 2018;20:1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilcox JE, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Heywood JT, Inge PJ, McBride ML, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Gheorghiade M.. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J 2012;163:49–56. [DOI] [PubMed] [Google Scholar]
- 14. Calvo N, Bisbal F, Guiu E, Ramos P, Nadal M, Tolosana JM, Arbelo E, Berruezo A, Sitges M, Brugada J, Mont L.. Impact of atrial fibrillation-induced tachycardiomyopathy in patients undergoing pulmonary vein isolation. Int J Cardiol 2013;168:4093–4097. [DOI] [PubMed] [Google Scholar]