Abstract
Aims
Obesity is associated with increased risk for heart failure. We analysed data from the Swedish Obese Subjects (SOS) study, a prospective matched cohort study, to investigate whether bariatric surgery reduces this risk.
Methods and results
From the total SOS population (n = 4047), we identified 4033 obese individuals with no history of heart failure at baseline, of whom 2003 underwent bariatric surgery (surgery group) and 2030 received usual care (control group). First-time principal diagnoses of heart failure were identified by crosschecking the SOS database with the Swedish National Patient Register and the Swedish Cause of Death Register using diagnosis codes. During a median follow-up of 22 years, first-time heart failure occurred in 188 of the participants treated with surgery and in 266 of those receiving usual care. The risk of developing heart failure was lower in the surgery group than in the control group [sub-hazard ratio 0.65, 95% confidence interval (CI) 0.54–0.79; P < 0.001]. After pooling data from the two study groups, the quartile of subjects with the largest weight loss after 1 year (mean −41 kg) displayed the greatest risk reduction (sub-hazard ratio 0.51, 95% CI 0.30–0.70; P < 0.001). This association remained statistically significant after adjustment for surgical intervention and potential baseline confounders (sub-hazard ratio 0.60, 95% CI 0.36–0.97; P = 0.038).
Conclusion
Compared with usual care, bariatric surgery was associated with reduced risk of heart failure among persons being treated for obesity. The risk of heart failure appeared to decline in parallel with a greater degree of weight loss.
ClinicalTrials.gov Identifier
Keywords: Obesity, Bariatric surgery, Weight loss, Heart failure
Introduction
Obesity, with a prevalence above 20% in many European countries1 and above 30% in the US,2 has repeatedly been shown to be associated with heart failure.3,4 In the Framingham cohort, people with obesity had a twofold higher risk of developing heart failure than in those of normal weight; for each increment of body mass index (BMI) (the weight in kilograms divided by the square of the height in meters) the risk of heart failure increased by 5–7%.3
Heart failure is characterized by impaired quality of life, frequent hospitalizations, and poor outcome.5 In developed countries, the prevalence among adults is often reported to be around 2% and for people older than 80 years the occurrence is above 10%.6 Consequently, heart failure is responsible for a significant proportion of healthcare expenditures.7 Although obesity intervention can improve cardiovascular risk factors8 and may have beneficial effects in patients with compromised cardiac function,9 there are no large controlled studies investigating the impact of weight loss on the development of heart failure.
A clinically significant weight loss is difficult to achieve with lifestyle interventions and the results are often temporary. In contrast, bariatric surgery is an effective and safe treatment option resulting in large weight losses that can be maintained over time.10 The Swedish Obese Subjects (SOS) study is an ongoing controlled intervention trial that compares the effects of bariatric surgery and conventional obesity care on morbidity and mortality.11 The SOS study has found that bariatric surgery, as a primary preventive strategy, reduces mortality12 and cardiovascular morbidity13 in obesity. The purpose of this report is to describe the effect of bariatric surgery on the incidence of new-onset heart failure.
Methods
Study design
The ongoing prospective controlled SOS intervention study comparing the effects of weight loss by bariatric surgery and conventional obesity care has previously been described in detail.14 In brief, 4047 obese participants were enrolled at 25 surgical departments and at 480 primary health care centres between 1 September 1987 and 31 January 2001. The surgery group included 2010 individuals who expressed a preference for treatment with bariatric surgery. A matched control group of 2037 participants was created using an automatic matching program and 18 matching variables (sex, age, weight, height, waist-hip ratio, blood pressure, serum cholesterol and triglycerides, smoking, diabetes, menopause, four psychosocial variables associated with risk for death, and personality traits related to treatment preferences). The matching was not performed on an individual basis (i.e. subject by subject); instead the matching algorithm selected controls in such a way that the current mean values of the matching variables in the control group moved as much as possible in the direction of the current mean values in the surgery group.
Subjects eligible for the SOS study were aged between 37 and 60 years and had a BMI of 34 kg/m2 or more for men and 38 kg/m2 or more for women. Subjects were excluded if they had a history of earlier surgery for gastric or duodenal ulcer, earlier bariatric surgery, gastric ulcer during the past 6 months, ongoing malignancy, active malignancy during the past 5 years, myocardial infarction during the past 6 months, bulimic eating pattern, drug or alcohol abuse, psychiatric or cooperative problems contraindicating bariatric surgery, or other contraindicating conditions (such as chronic glucocorticoid or anti-inflammatory treatment). The surgical procedures used in the study were vertical banded gastroplasty (68%), gastric banding (19%), and gastric bypass (13%). The study complied with the Declaration of Helsinki and seven regional ethics review boards in Sweden approved the study protocol. All participants gave written or oral consent.
The two study groups underwent identical examinations at the participating surgical departments and primary health care centres both at baseline and during follow-up at 0.5, 1, 2, 3, 4, 6, 8, 10, 15, and 20 years. After bariatric surgery, the recommended daily nutritional supplementation included oral doses of multivitamin and mineral supplements, vitamin B12, and a combination of calcium and vitamin D3. If laboratory findings indicated deficiencies of iron or folate, a replacement therapy was introduced. The matched controls received the standard nonsurgical obesity treatment for their primary health care centres. No attempt was made to standardize the conventional treatment, which ranged from sophisticated lifestyle intervention and behaviour modification to, in some practices, no treatment at all.
Body weight was measured with electronic or calibrated scales at baseline and at regular follow-up appointments. Blood samples were analysed by the Central Laboratory at Sahlgrenska University Hospital (accredited according to the international standard ISO 15189:2012). Self-reported information on cardiovascular disease, medication, smoking, and alcohol intake was obtained through a questionnaire administered at baseline and during follow-up visits. Hypertension was defined as systolic pressure >140 mmHg, or diastolic pressure > 90 mmHg, or self-reported use of anti-hypertensive medication. Diabetes was defined as a fasting blood glucose level of at least 6.1 mmol/L (110 mg/dL) or self-reported use of a prescribed antidiabetic medication.
Heart failure
The main outcome of the present analysis was the first-time detection of heart failure as a principal diagnosis in the Swedish National Patient Register or in the Swedish Cause of Death Register. The National Patient Register has information on diagnoses for all inpatients in Sweden since 1987 and from all hospital-based outpatient visits since 2001; it does not include information from primary care. Reporting to the National Patient Register is mandatory for public hospitals, and since almost all of Swedish health care is included in the national public health care system, the coverage is 99%. The register has been shown to be a powerful tool to study health-related outcomes in the Swedish populations15 and heart failure as a principal diagnosis was found to have a validity of 95%.16 The Swedish Cause of Death Register includes all those who died and were registered in Sweden at the time of death with over 99% population coverage.17 We identified a first-time principal diagnosis of heart failure by crosschecking the SOS database with the National Patient Register and the Cause of Death Register for the following diagnosis codes: 428 (International Classification of Diseases-9 until 1996) and I50 (International Classification of Diseases-10 from 1997).
Statistical analysis
Data are presented as mean values with standard deviations or as percentages. Baseline comparisons between treatment groups used t-tests for continuous variables and a logistic-regression model for dichotomous variables. Participants were followed until the first-time principal diagnosis of heart failure, death or 31 December 2016, at which point the National Patient Register and Cause of Death Register were complete and the registers were linked. Calculation of the median follow-up time was based on the reverse Kaplan–Meier method.
Persons who reported a history of heart failure at baseline were excluded from all analyses (n = 14).
The differences in changes in BMI and in self-reported medication use between the surgery and control groups were analysed with multilevel mixed-effects regression models. The observations were considered nested within the individuals, and the statistical tests and confidence intervals were thus calculated controlling for the repeated measurements. Test for treatment by time interactions were conducted to evaluate between-group differences in changes.
Cumulative incidence of heart failure was estimated with competing-risks regression models, in which deaths without heart failure were treated as competing events. Persons without heart failure who emigrated, altered their obesity intervention, withdrew their consent or were alive at the end of follow-up were treated as censored observations.
Univariable and multivariable models were applied to obtain relative risk estimates expressed as sub-hazard ratios. The treatment effect in the surgery group compared with the control group was evaluated in a primary unadjusted analysis with a single covariate for treatment group (surgery or control) and in a secondary analysis that was adjusted for preselected baseline risk factors considered traditional for heart failure.
After pooling data from the two study groups, patients were divided into quartiles of weight change occurring during the first year of follow-up. The quartiles ranged from weight gain (Quartile 1) to the greatest weight loss (Quartile 4). The association of weight change and heart failure risk was studied: in a primary model with Quartile 1 as reference; in a secondary model adjusted for surgical intervention; and in a tertiary model adjusted for surgical intervention and selected baseline characteristics. Persons who had a follow-up time of <1 year (n = 39) or for whom a weight measurement at the 1-year follow-up was not available (n = 371) were excluded from these analyses.
The consistency of the treatment effect with respect to the main outcome was assessed in several subgroups defined by baseline characteristics. Homogeneity was evaluated with test of interaction between the indicator for treatment and baseline variables using competing-risks regression models. Continuous variables were dichotomized by a median split to illustrate the effects, but the interaction test was based on the original continuous variable. No adjustment for multiple testing was performed.
All statistical tests were two-tailed and P-values of <0.05 were considered statistically significant. A study flow chart is provided in Supplementary material online, Figure SA.
Results
Baseline characteristics and changes in weight
Of the 4047 participants in the SOS study, 14 were excluded due to a history of heart failure at baseline, resulting in 4033 persons eligible for the present analysis. Of these, 2003 underwent bariatric surgery (surgery group) and 2030 were treated conventionally (control group). Although the two study groups were fairly well balanced with respect to baseline characteristics, BMI was higher and several cardiovascular risk factors were less favourable in the surgery group (Table 1). The differences are mainly explained by disparate weight changes occurring in the two groups during a delay between matching and baseline measurements: during the waiting time for bariatric surgery (on average more than 1 year), participants awaiting surgery tended to gain weight and control subjects tended to lose weight.12
Table 1.
Baseline characteristics of study participants
| Surgery group (n = 2003) | Control group (n = 2030) | P-value | |
|---|---|---|---|
| Age (years) | 47.2 ± 5.9 | 48.7 ± 6.3 | <0.001 |
| Female sex (%) | 70.8 | 71.2 | 0.781 |
| Height (cm) | 169 ± 9.1 | 169 ± 9.2 | 0.655 |
| Weight (kg) | 121 ± 17 | 115 ± 17 | <0.001 |
| Body mass index (kg/m2) | 42.4 ± 4.5 | 40.1 ± 4.7 | <0.001 |
| Waist circumference (cm) | 126 ± 11 | 120 ± 11 | <0.001 |
| Waist/hip ratio | 0.99 ± 0.08 | 0.98 ± 0.07 | <0.001 |
| Systolic blood pressure (mmHg) | 145 ± 19 | 138 ± 18 | <0.001 |
| Diastolic blood pressure (mmHg) | 90 ± 11 | 85 ± 11 | <0.001 |
| Total cholesterol (mmol/L) | 5.86 ± 1.12 | 5.61 ± 1.06 | <0.001 |
| Apo B/Apo A1 ratio | 0.94 ± 0.28 | 0.91 ± 0.28 | <0.001 |
| Blood glucose (mmol/L) | 5.18 ± 2.01 | 4.93 ± 1.81 | <0.001 |
| Insulin (mU/L) | 21.6 ± 13.7 | 18.0 ± 11.3 | <0.001 |
| Creatinine (µmol/L) | 69.2 ± 8.8 | 69.5 ± 9.5 | 0.238 |
| Free thyroxine (pmol/L) | 15.6 ± 3.7 | 15.6 ± 3.6 | 0.634 |
| Thyroid-stimulating hormone (mIU/L) | 2.01 ± 2.34 | 2.04 ± 2.66 | 0.671 |
| Hypertension (%) | 78.4 | 63.7 | <0.001 |
| Diabetes (%) | 17.2 | 12.7 | <0.001 |
| Smoking (%) | 25.8 | 20.9 | <0.001 |
| Alcohol intake (g/daily) | 5.2 ± 7.3 | 5.3 ± 8.0 | 0.715 |
Values are means ± SD or %.
Figure 1 shows changes in BMI in patients without baseline heart failure for up to 20 years of follow-up. Bariatric surgery lowered the mean baseline BMI of 42.4 kg/m2 by 25% at Year 1, by 18% at Year 6, by 16% at Year 15, and by 16% at Year 20. The mean baseline BMI of 40.1 kg/m2 in the control group remained largely unchanged during follow-up. The average of all between-group differences in BMI during a 20-year follow-up was –8.0 kg/m2 (95% CI −7.7 to −8.3); P < 0.001).
Figure 1.
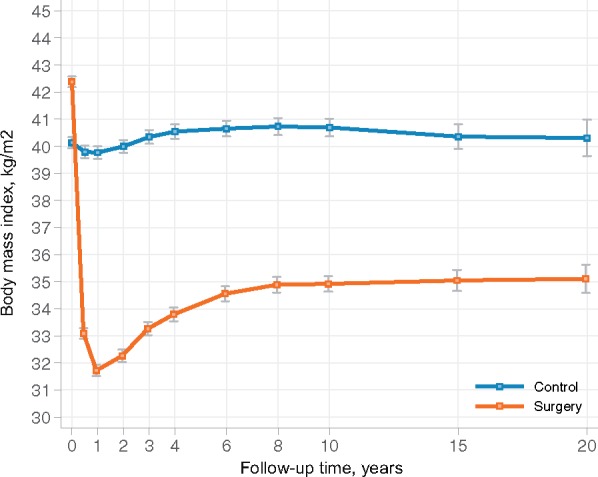
Change in body mass index in patients without baseline heart failure during 20 years of follow-up. Bariatric surgery lowered the mean BMI of 42.4 kg/m2 by 25% at year 1, by 18% at year 6, by 16% at year 15, and by 16% at year 20 whereas the mean BMI of 40.1 in the control group remained largely unchanged during follow-up.
Incidence of heart failure
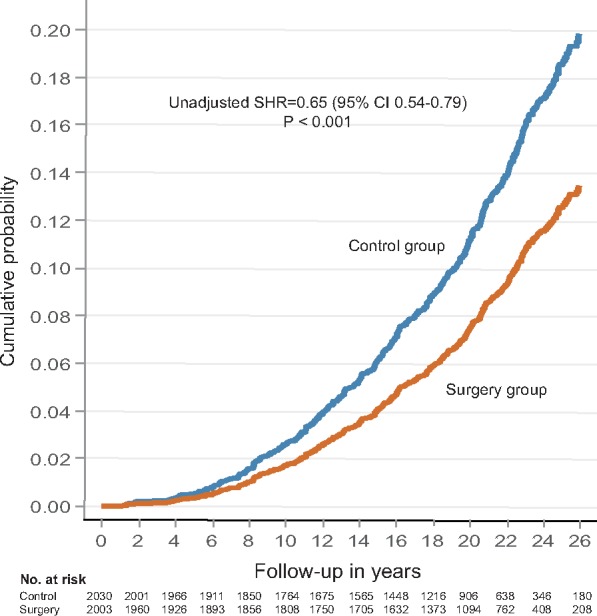
During a median follow-up of 22 (inter-quartile range 18–24) years, a first-time diagnosis of heart failure occurred in 188 patients in the surgery group and in 266 patients in the control group (Take home figure). Thus, patients treated with bariatric surgery had a 35% lower risk for being diagnosed with heart failure than patients in the control group (sub-hazard ratio 0.65, 95% CI 0.54–0.79; P < 0.001). After adjustments for selected baseline conditions, weight loss by bariatric surgery remained associated with reduced incidence of heart failure (adjusted sub-hazard ratio 0.66, 95% CI 0.51–0.81; P < 0.001). The incidence rate for heart failure cases per 1000 patient years was 4.8 (95% CI 4.2–5.6) in the surgery group and 7.2 (95% CI 6.4–8.1) in the control group. Based on the adjusted competing risk regression model, the cumulative incidence rates in the surgery group after 10, 15, and 20 years were 1.3%, 3.1%, and 5.8%, respectively. The corresponding incidence rates for the control group were 2.0%, 4.7%, and 8.7%.
Figure 2.
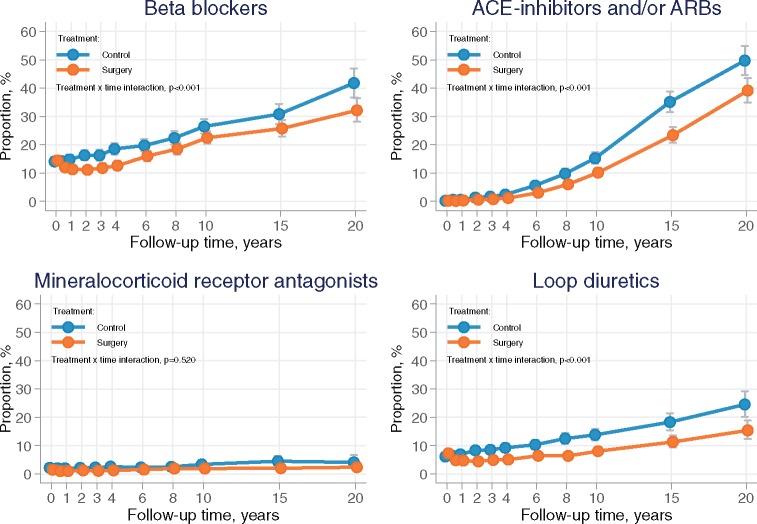
The use of conventional heart failure medication including beta-blockers, renin–angiotensin system inhibitors, and loop-diuretics were lower in the surgery group as compared with control subjects, whereas the usage of mineralocorticoid receptor antagonists did not differ between groups.
Apart from analysing the total incidence of new onset heart failure, we also studied the detection rate of heart failure in different clinical settings. Hospitalization for new onset heart failure occurred in 115 surgery patients and 171 control subjects (sub-hazard ratio 0.63, 95% CI 0.49–0.79; P < 0.001) (Supplementary material online, Figure SB), whereas fatal heart failure was diagnosed in 22 surgery patients and 33 controls (sub-hazard ratio 0.62, 95% CI 0.36–1.07; P = 0.087) (Supplementary material online, Figure SC).
During follow-up, self-reported use of conventional heart failure medication including beta-blockers, agents acting on the renin–angiotensin system (ACE-inhibitors and/or ARBs) and loop-diuretics was significantly lower in the surgery group, whereas the usage of mineralocorticoid receptor antagonists did not differ between the two groups (Figure 2). There was no difference in the use of thyroid preparations between study groups during follow-up (Supplementary material online, Figure SD).
Multivariate and interaction analysis
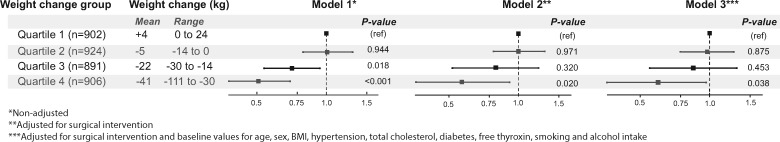
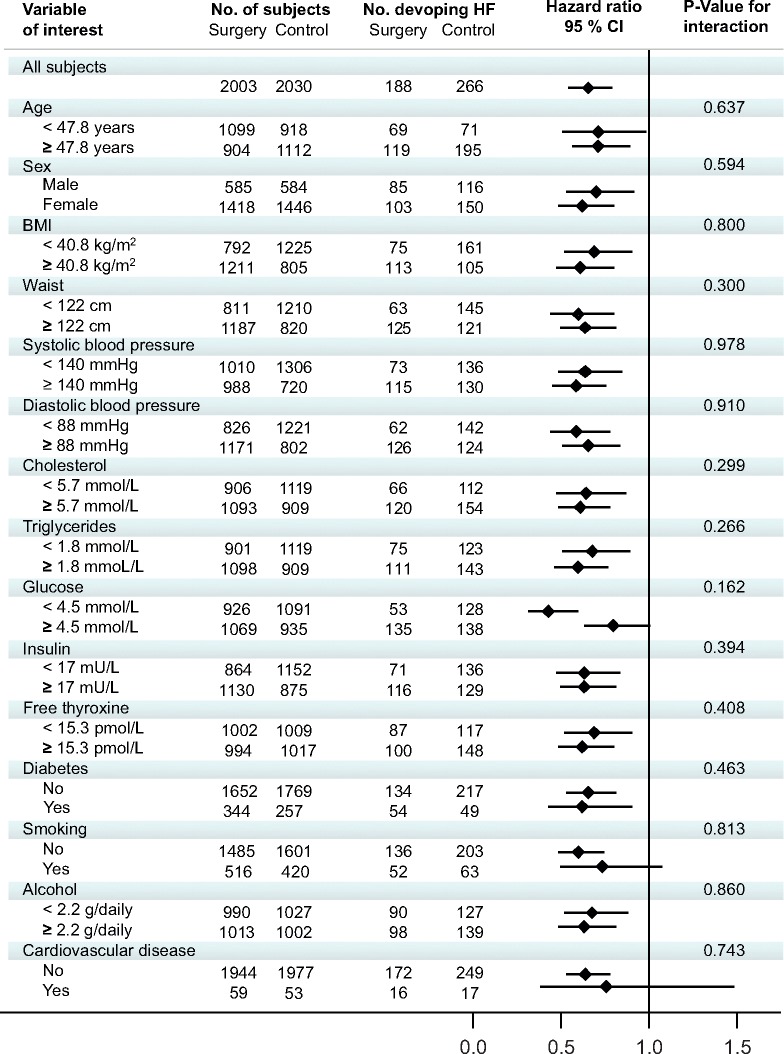
In the pooled population, baseline conditions that were independently associated with increased risk of heart failure included advancing age, male sex, hypertension, diabetes, elevated cholesterol levels, smoking, and increasing thyroxine levels (Table 2). The risk of heart failure declined across quartiles with a greater degree of weight loss (Figure 3). The quartile of subjects with the largest weight loss after 1 year (mean −41 kg) displayed the greatest risk reduction (sub-hazard ratio 0.51, 95% CI 0.30–0.70; P < 0.001). This association remained statistically significant after adjustment for surgical intervention (sub-hazard ratio 0.57, 95% CI 0.36–0.92; P = 0.020) and after further adjustment for potential baseline confounders (sub-hazard ratio 0.60, 95% CI 0.36–0.97; P = 0.038). In an interaction analysis, the effect of bariatric surgery on incident heart failure was similar in subgroups defined by a median split of a wide range of baseline characteristics (Figure 4).
Table 2.
Association of bariatric surgery and selected baseline characteristics with the risk of heart failure based on 188 events in the surgery group and 266 events in the control group
| SHR | 95% CI | P-value | |
|---|---|---|---|
| Surgery (vs. control) | 0.67 | 0.55–0.81 | <0.001 |
| Age per 5 years | 1.51 | 1.3–1.6 | <0.001 |
| Male (vs. female) | 2.03 | 1.65–2.51 | <0.001 |
| Body mass index per 5 kg/m2 | 1.08 | 0.91–1.19 | 0.156 |
| Hypertension | 1.44 | 1.12–1.86 | 0.005 |
| Total cholesterol per mmol/L | 1.10 | 1.01–1.19 | 0.020 |
| Diabetes | 1.37 | 1.09–1.73 | 0.006 |
| Free thyroxine per 5 pmol/L | 1.26 | 1.14–1.39 | <0.001 |
| Smoking | 1.26 | 1.01–1.57 | 0.037 |
| Alcohol intake per 10 g/daily | 1.02 | 0.91–1.14 | 0.737 |
Data from the surgery and control groups are pooled in this analysis.
CI, confidence interval; SHR, sub-hazard ratio.
Figure 3.
Forest plot of hazard ratios and 95% confidence intervals for the risk of heart failure by 1-year weight change quartiles. The study groups were pooled in these analyses. The risk of heart failure declined across quartiles with a greater degree of weight loss. This association remained statistically significant after adjustment for surgical intervention (Model 2) and, in addition, potential baseline confounders (Model 3).
Figure 4.
Hazard ratios for the risk of heart failure in subgroups. There were no significant interactions between treatment and subgroups.
Take home figure.

Surgical obesity treatment and the risk of heart failure during long-term follow-up.
Adverse events
Adverse events in the SOS study have been described previously.11 Bariatric procedures were performed with open surgery in 89% of cases. There were 5 individuals (0.2%) in the surgery group and 2 (0.1%) in the control group who died within 90 days of surgery/inclusion. In the surgery group, 151 (13.0%) of the participants had 193 postoperative complications. Of these, 46 persons (2.8%) needed additional surgery.
Discussion
Among participants in the SOS study, persons treated with bariatric surgery had a lower risk of developing heart failure during long-term follow-up than did those receiving usual care. The risk of heart failure was reduced by 35% in the surgery group, despite a less favourable cardiovascular risk factor profile at baseline and a lower use of beta-blockers, renin–angiotensin system inhibitors and loop-diuretics during follow-up. The risk of heart failure appeared to decline in parallel with greater degrees of weight loss.
The results from our prospective controlled intervention study are in agreement with findings from a previous study that retrospectively compared two large registries, the Scandinavian Obesity Surgery Registry and a Swedish registry of obese people treated with a lifestyle program.18 In this earlier study, gastric bypass resulted in a 1-year weight loss that was 18.8 kg larger than that attained by lifestyle intervention; during a median follow-up of 4.1 years, surgically treated patients had a significantly lower risk of heart failure, but the incidence rates were low, 0.41% and 0.76%, respectively.18 In the SOS study, separation of the cumulative incidence curves, reflecting a beneficial effect of bariatric surgery, did not occur until 5 years after the intervention, but increased thereafter. Thus, the primary preventive design of the SOS study required a long-term follow-up to enable the favourable effects of weight loss on heart failure risk to become apparent.
American College of Cardiology–American Heart Association and European Society of Cardiology guidelines19,20 advocate that obesity should be treated to prevent or delay the onset of heart failure (Class of recommendation I and IIa, respectively). In the absence of controlled clinical trials, these recommendations have been based on expert opinion and consensus (level of evidence C). In a similar manner, 2016 European Guidelines on cardiovascular disease prevention in clinical practice21 advocate BMI 20–25 kg/m2 as an optimal target level to reduce cardiovascular risk, but at the same time acknowledge gaps in evidence on how to achieve a long-term healthy weight and whether this will improve outcome. By demonstrating that sustained weight loss in obesity reduces the risk of incident heart failure, the present study provides robust verification for the use of viable strategies to prevent or treat obesity in this respect. However, it is important to note that our results relate to surgical obesity treatment in men with BMI ≥34 kg/m2 and in women with BMI ≥38 kg/m2.
The mechanism by which surgically induced weight loss may reduce the risk of heart failure is probably multifactorial. Bariatric surgery improves heart failure risk factors including hypertension, diabetes, and dyslipidaemia22 and lowers the incidence of cardiovascular events.13 The reversal of haemodynamic overload leads to regress of left ventricular hypertrophy23 and improves diastolic dysfunction.24 Previously, we have shown that obesity surgery lowers the incidence of atrial fibrillation,25 alleviates sleep apnoea,26 and promotes leisure time physical activity.27 In addition, weight loss lessens several adverse metabolic, hormonal, and inflammatory stimuli that may contribute to the development of heart failure.28
The associations of baseline characteristics with increased heart failure risk were similar to those previously reported and included: advancing age, male sex, hypertension, hypercholesterolaemia, diabetes, and smoking. We also observed that higher free thyroxine was independently associated with risk for heart failure in both univariate and multivariate analyses. Obesity has been shown to be associated with disturbances in thyroid function,29 including higher free thyroxine levels within the normal range, which in turn might predispose to atrial fibrillation30 and subsequent heart failure. The use of thyroid preparations was similar in both surgery and control patients and, therefore, unlikely to explain to the difference in heart failure risk between groups. Weight loss can affect thyroid hormone levels31,32 and whether this may have beneficial effects on cardiac energy homeostasis and impede heart failure onset deserves further research. The effect of surgical obesity treatment was consistent across all subgroups defined by a median split of baseline characteristics. Hence, bariatric surgery did not show a greater benefit among patients with a higher BMI at baseline as compared to those with a lesser degree of obesity, which has been a constant finding for other clinical endpoints in the SOS study.11
The risk of heart failure decreased across quartiles of increasing weight loss, even after adjusting for surgical intervention and potential baseline confounders. However, these findings should be interpreted with caution due to the strong relationship between surgical treatment and weight loss. One-year weight change in control subjects is very different from that in surgical cases; and due to the collinearity between bariatric surgery and weight loss it is difficult disentangle their effects on outcome in statistical models.
Heart failure is a major and growing public health problem worldwide resulting in poor quality of life, frequent hospitalizations and high mortality rates.6 The American College of Cardiology–American Heart Association heart failure model emphasizes the progressive pathophysiology of the syndrome through four stages (A, B, C, and D),33 which motivates early detection and prevention efforts. Obesity has been identified as an important risk factor for heart failure and is frequently associated with asymptomatic disturbances in cardiac structure and function. As a consequence, weight reduction has been proposed as a potential preventive measure.34 In this context, the findings of the present study are of significant value. The successful prevention of heart failure through obesity surgery suggests that this treatment may have a positive impact on disease-related morbidity and mortality and may also reduce health care utilization.
The strengths of the SOS trial include the large study cohort, the prospective controlled design, and the long period of follow-up. The major limitation is the non-random treatment assignment, in which the two study groups expressed a different preference towards obesity surgery. However, the Swedish ethical review boards did not approve a randomized trial design when the study was introduced in the 1980s because of the high post-operative mortality at that time. In addition, it is unlikely that a randomized trial of this kind would be considered feasible today. Heart failure was not a pre-specified endpoint of the in the SOS study; instead the diagnosis was collected by crosslinking the SOS database with the National Patient Register on inpatient and outpatient diagnosis codes and the Swedish Cause of Death Register. Ascertainment of heart failure as a principal diagnosis using the National Patient Register has a validity of 95% and the Cause of Death Register covers 99% of deaths in the Swedish population.
In conclusion, surgically induced weight loss in subjects with severe obesity is associated with a reduced risk for heart failure during long-term follow-up. A greater weight loss appeared to result in a larger risk reduction. Primary prevention of heart failure by bariatric surgery is likely to reduce cardiovascular morbidity and mortality.
Supplementary Material
Acknowledgements
We thank Professor Lars Sjöström for initiating the SOS study, the patients who participated, and Dr Rosie Perkins Institute of Medicine, University of Gothenburg for editing the manuscript.
Funding
Research reported in this publication was supported by and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Award Number R01DK105948); and the Swedish Heart-Lung Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The SOS study is supported by the Swedish Research Council (K2013-99X-22279-01, K2013-54X-11285-19, and K2015-55X-22082-04); the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-596701 and ALFGBG-775351); and the Swedish Diabetes Foundation.
Conflict of interest: L.C. has received lecture fees from AstraZeneca and Johnson & Johnson. K.K. has received lecture fees from AstraZeneca and Orion Pharma. There are no financial conflicts of interests among the other authors.
See page 2139 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz406)
References
- 1.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL.. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS.. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 4. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ.. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 5. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ.. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315–322. [DOI] [PubMed] [Google Scholar]
- 6. Savarese G, Lund LH.. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook C, Cole G, Asaria P, Jabbour R, Francis DP.. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368–376. [DOI] [PubMed] [Google Scholar]
- 8. Beamish AJ, Olbers T, Kelly AS, Inge TH.. Cardiovascular effects of bariatric surgery. Nat Rev Cardiol 2016;13:730–743. [DOI] [PubMed] [Google Scholar]
- 9. McDowell K, Petrie MC, Raihan NA, Logue J.. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obes Rev 2018;19:1189–1204. [DOI] [PubMed] [Google Scholar]
- 10. DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 2007;356:2176–2183. [DOI] [PubMed] [Google Scholar]
- 11. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219–234. [DOI] [PubMed] [Google Scholar]
- 12. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 13. Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM.. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 14. Sjostrom L, Larsson B, Backman L, Bengtsson C, Bouchard C, Dahlgren S, Hallgren P, Jonsson E, Karlsson J, Lapidus L.. Swedish Obese Subjects (SOS). Recruitment for an interventional study and a selected description of the obese state. Int J Obes 1992;465–479. [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ingelsson E, Arnlov J, Sundstrom J, Lind L.. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005;7:787–791. [DOI] [PubMed] [Google Scholar]
- 17.The Cause of Death Register. http://www.socialstyrelsen.se/statistics/statisticaldatabase/help/causeofdeath (3 May 2019).
- 18. Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M.. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL.. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 21. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 23. Karason K, Wallentin I, Larsson B, Sjostrom L.. Effects of obesity and weight loss on left ventricular mass and relative wall thickness: survey and intervention study. BMJ 1997;315:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karason K, Wallentin I, Larsson B, Sjostrom L.. Effects of obesity and weight loss on cardiac function and valvular performance. Obes Res 1998;6:422–429. [DOI] [PubMed] [Google Scholar]
- 25. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjostrom L, Karason K.. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol 2016;68:2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L.. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep 2007;30:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karason K, Lindroos AK, Stenlof K, Sjostrom L.. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med 2000;160:1797–1802. [DOI] [PubMed] [Google Scholar]
- 28. Purkayastha S, Cai D.. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2013;2:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol 2010;316:165–171. [DOI] [PubMed] [Google Scholar]
- 30. Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FD, Wilson S, Sheppard MC, Franklyn JA.. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 2007;167:928–934. [DOI] [PubMed] [Google Scholar]
- 31. Dall'Asta C, Paganelli M, Morabito A, Vedani P, Barbieri M, Paolisso G, Folli F, Pontiroli AE.. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring) 2010;18:854–857. [DOI] [PubMed] [Google Scholar]
- 32. Neves JS, Castro Oliveira S, Souteiro P, Pedro J, Magalhaes D, Guerreiro V, Bettencourt-Silva R, Costa MM, Cristina Santos A, Queiros J, Varela A, Freitas P, Carvalho D; AMTCO Group. Effect of weight loss after bariatric surgery on thyroid-stimulating hormone levels in patients with morbid obesity and normal thyroid function. Obes Surg 2018;28:97–103. [DOI] [PubMed] [Google Scholar]
- 33. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ.. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–1570. [DOI] [PubMed] [Google Scholar]
- 34. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y; American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on High Blood Pressure Research, Quality of Care and Outcomes Research Interdisciplinary Working Group, Functional Genomics and Translational Biology Interdisciplinary Working Group. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008;117:2544–2565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.